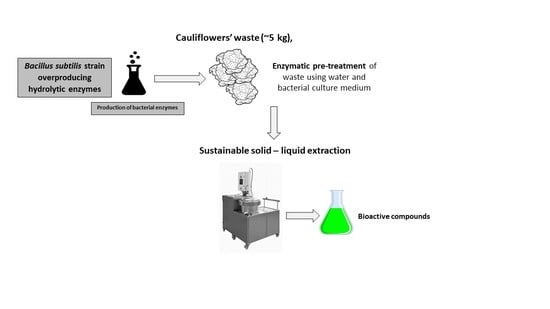
Bacterial-Assisted Extraction of Bioactive Compounds from Cauliflower
Abstract
:1. Introduction
2. Results
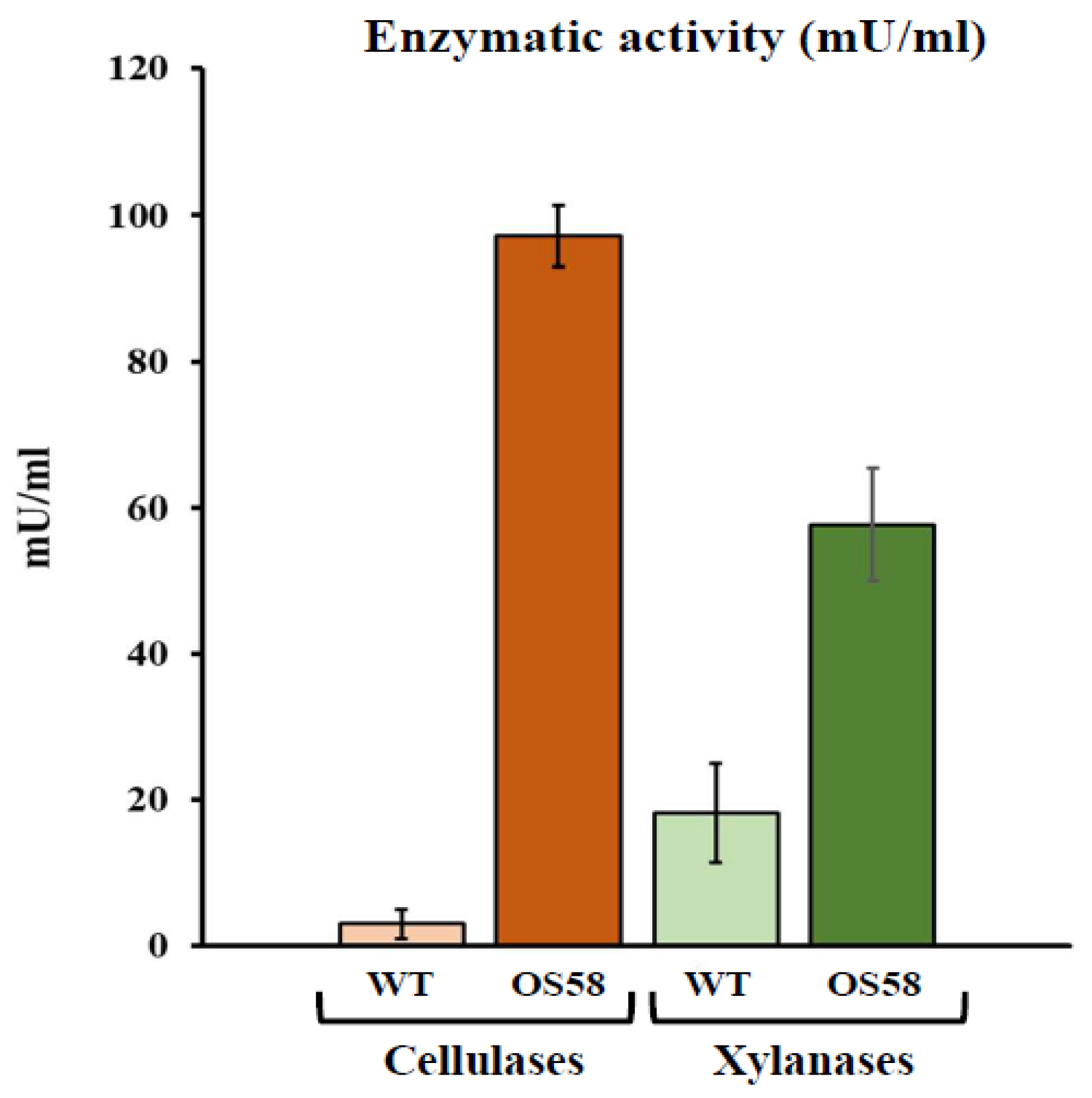
2.1. Bacillus subtilis Enzymes and Pretreatment
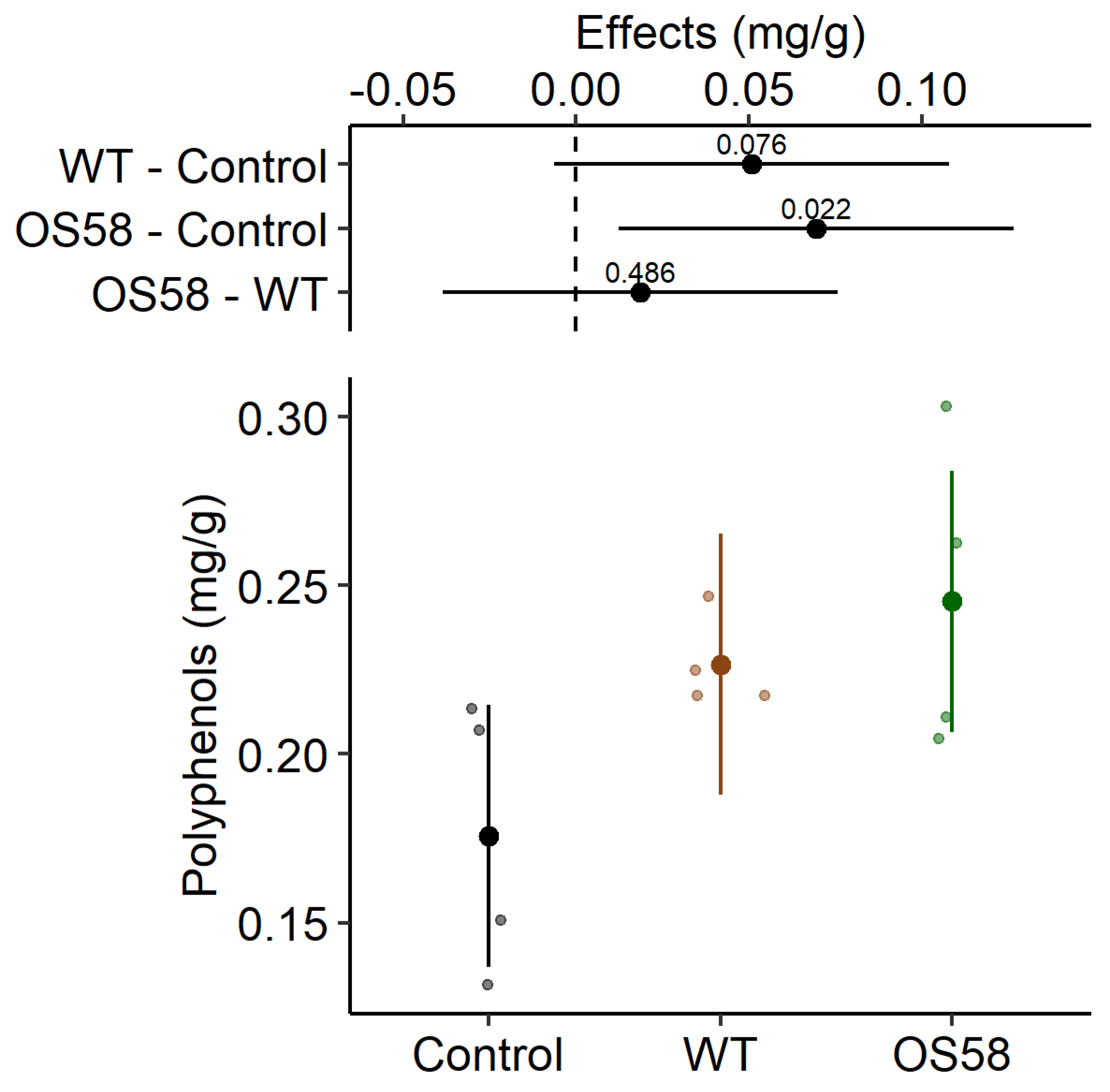
2.2. Recovery of Phenolic Compounds
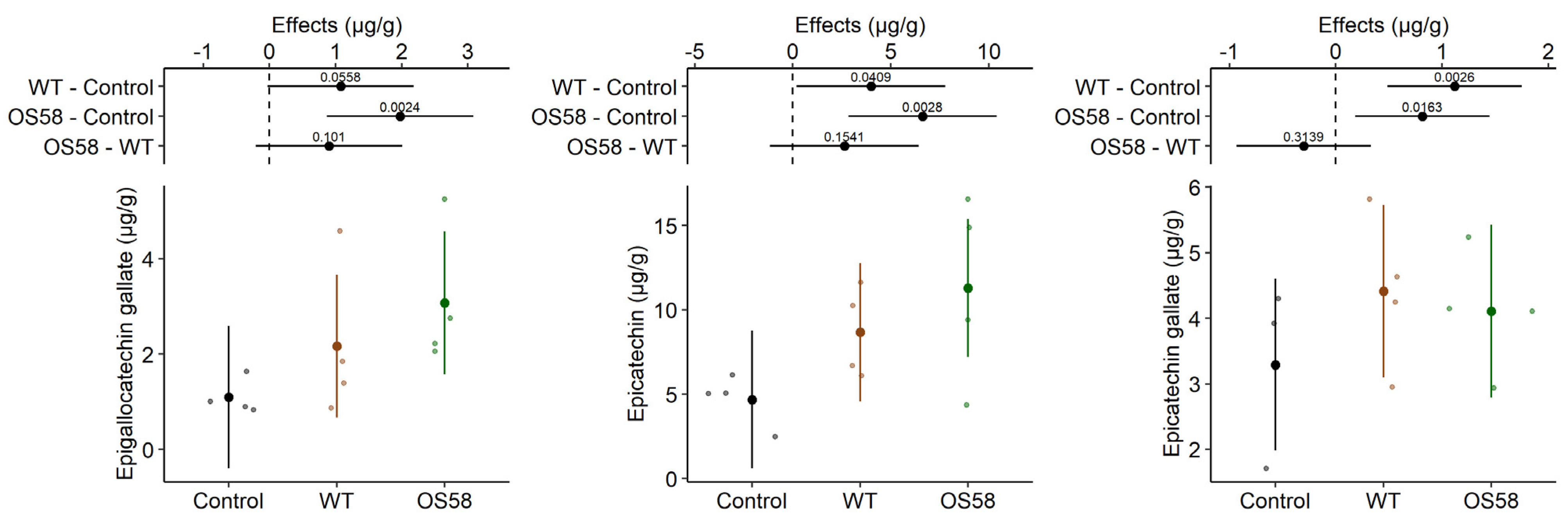
2.2.1. Catechins
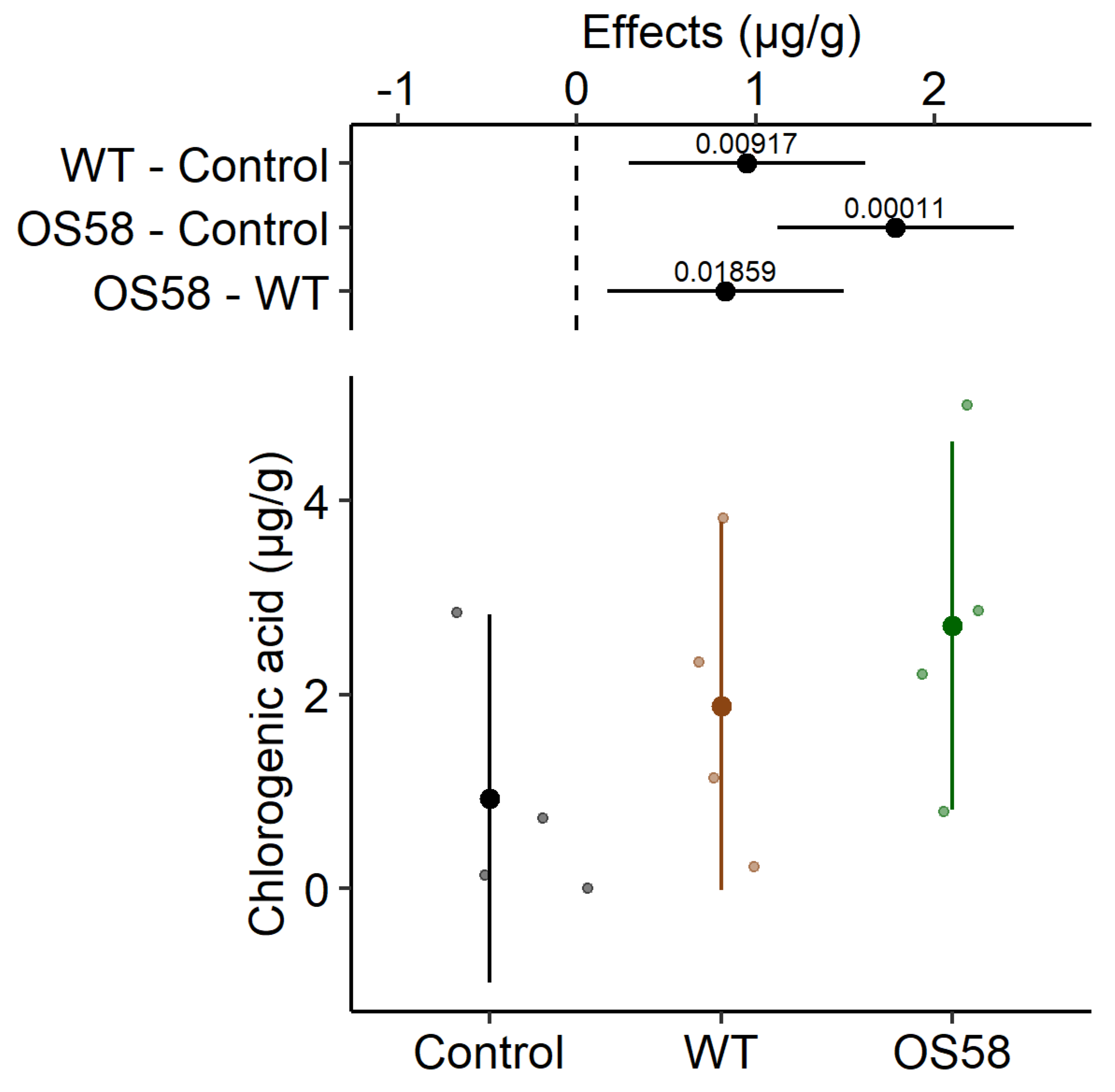
2.2.2. Chlorogenic Acid
2.3. Recovery of Sulphur-Containing Plant Secondary Metabolites
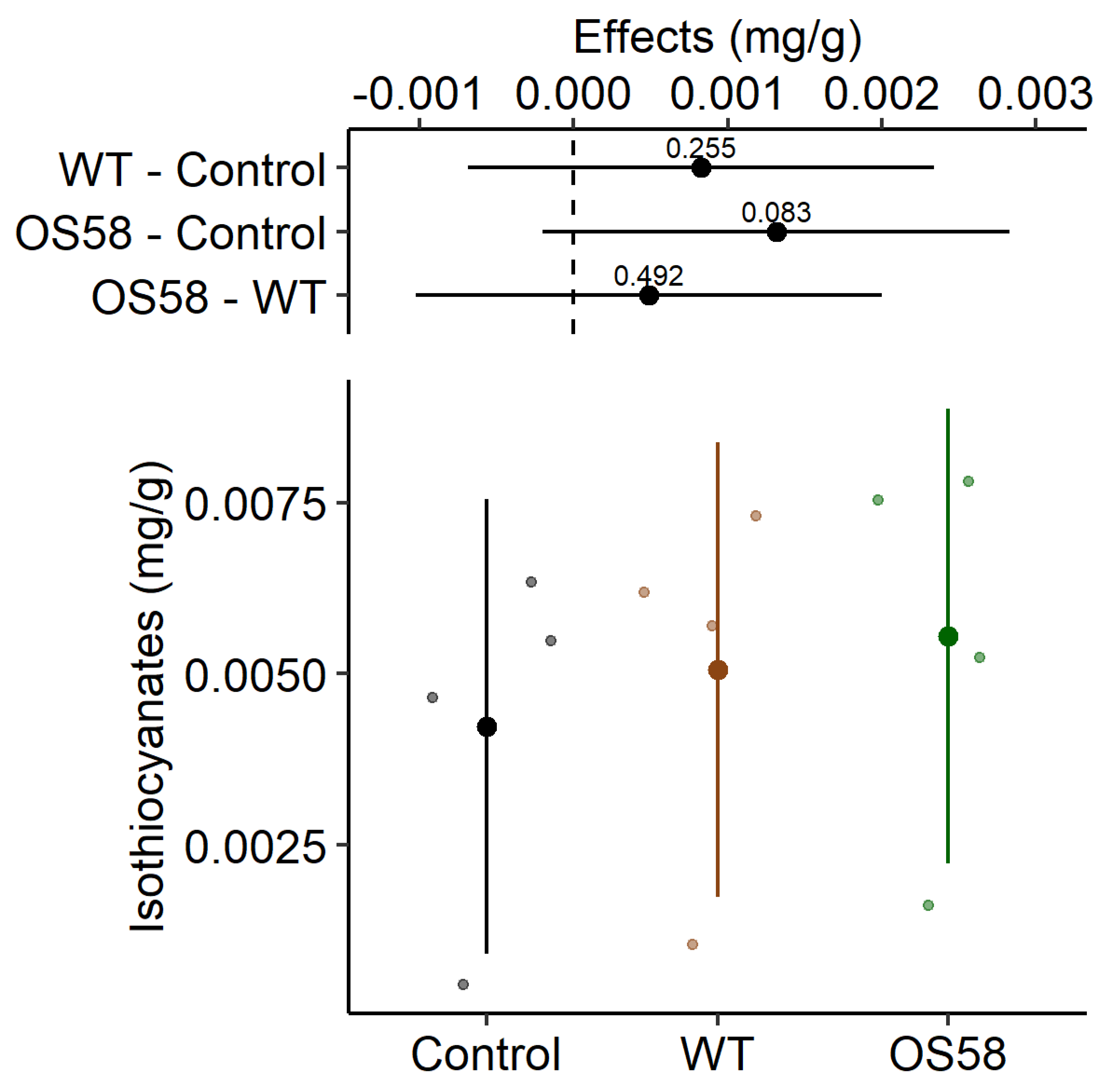
Isothiocyanates
3. Discussion and Conclusions
4. Materials and Methods
4.1. Bacterial Strains
4.2. Bacterial Growth
4.3. Cellulase and Xylanase Activity Assays
4.4. Plant Material
4.5. EAE Pretreatment Procedure of the Cauliflower Waste Material
4.6. Extraction Process
4.7. Total Polyphenol Content
4.8. Catechins and Chlorogenic Acid HPLC Analyses
4.9. Isothiocyanates HPLC Analyses
4.10. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Springmann, M.; Clark, M.; Mason-D’Croz, D.; Wiebe, K.; Bodirsky, B.L.; Lassaletta, L.; De Vries, W.; Vermeulen, S.J.; Herrero, M.; Carlson, K.M.; et al. Options for keeping the food system within environmental limits. Nature 2018, 562, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Vilariño, M.V.; Franco, C.; Quarrington, C. Food loss and Waste Reduction as an Integral Part of a Circular Economy. Front. Environ. Sci. 2017, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Wadhwa, M.; Bakshi, M.P.S. Utilization of Fruit and Vegetable Wastes as Livestock Feed and as Substrates for Generation of Other Value-Added Products; RAP Publication, FAO: Rome, Italy, 2013; pp. 1–59. Available online: https://www.fao.org/3/i3273e/i3273e.pdf (accessed on 7 February 2022).
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Galanakis, C.M. Food Waste Recovery: Processing Technologies and Industrial Techniques; Elsevier: New York, NY, USA, 2015; pp. 381–392. [Google Scholar]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Marathe, S.J.; Jadhav, S.B.; Bankar, S.B.; Singhal, R.S. Enzyme-Assisted Extraction of Bioactives. In Food Bioactives; Puri, M., Ed.; Springer: Cham, Switzerland, 2017; pp. 171–201. [Google Scholar] [CrossRef]
- Nadar, S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef]
- Cui, W.; Han, L.; Suo, F.; Liu, Z.; Zhou, L.; Zhou, Z. Exploitation of Bacillus subtilis as a robust workhorse for production of heterologous proteins and beyond. World J. Microbiol. Biotechnol. 2018, 34, 145. [Google Scholar] [CrossRef]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D. Bacillus subtilis: A universal cell factory for industry, agriculture, biomaterials and medicine. Microb. Cell Factories 2020, 19, 173. [Google Scholar] [CrossRef]
- CAZy Carbohydrate Active enZYmes. Available online: http://www.cazy.org/b67.html (accessed on 7 February 2022).
- Van Dijl, J.M.; Hecker, M. Bacillus subtilis: From soil bacterium to super-secreting cell factory. Microb. Cell Factories 2013, 12, 3. [Google Scholar] [CrossRef] [Green Version]
- Danilova, I.; Sharipova, M. The Practical Potential of Bacilli and Their Enzymes for Industrial Production. Front. Microbiol. 2020, 11, 1782. [Google Scholar] [CrossRef]
- Srivatsan, A.; Han, Y.; Peng, J.; Tehranchi, A.K.; Gibbs, R.; Wang, J.D.; Chen, R. High-Precision, Whole-Genome Sequencing of Laboratory Strains Facilitates Genetic Studies. PLoS Genet. 2008, 4, e1000139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tjalsma, H.; Antelmann, H.; Jongbloed, J.D.; Braun, P.G.; Darmon, E.; Dorenbos, R.; Dubois, J.Y.F.; Westers, H.; Zanen, G.; Quax, W.J.; et al. Proteomics of protein secretion by Bacillus subtilis: Separating the “secrets” of the secretome. Microbiol. Mol. Biol. Rev. 2004, 68, 207–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Comprehensive R Archive Network-Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.7.2. Available online: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (accessed on 7 February 2022).
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C. Enzyme-assisted extractions of polyphenols—A comprehensive review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Lenucci, M.S.; De Caroli, M.; Marrese, P.P.; Iurlaro, A.; Rescio, L.; Böhm, V.; Dalessandro, G.; Piro, G. Enzyme-aided extraction of lycopene from high-pigment tomato cultivars by supercritical carbon dioxide. Food Chem. 2015, 170, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.G.; Martínez-Ávila, G.C.G.; Aguilar, C.N. Enzyme-assisted extraction of antioxidative phenolics from grape (Vitis vinifera L.) residues. 3 Biotech 2012, 2, 297–300. [Google Scholar] [CrossRef] [Green Version]
- Rane, A.N.; Baikar, V.V.; Ravi Kumar, V.; Deopurkar, R.L. Agro-Industrial Wastes for Production of Biosurfactant by Bacillus subtilis ANR 88 and Its Application in Synthesis of Silver and Gold Nanoparticles. Front. Microbiol. 2017, 8, 492. [Google Scholar] [CrossRef] [Green Version]
- Radhakrishnan, R.; Abeer, H.; Abd Allah, E.F. Bacillus: A Biological Tool for Crop Improvement through Bio-Molecular Changes in Adverse Environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef]
- Heimler, D.; Isolani, L.; Vignolini, P.; Tombelli, S.; Romani, A. Polyphenol Content and Antioxidative Activity in Some Species of Freshly Consumed Salads. J. Agric. Food Chem. 2007, 55, 1724–1729. [Google Scholar] [CrossRef]
- Koh, E.; Wimalasiri, K.; Chassy, A.; Mitchell, A. Content of ascorbic acid, quercetin, kaempferol and total phenolics in commercial broccoli. J. Food Compos. Anal. 2009, 22, 637–643. [Google Scholar] [CrossRef]
- Valverde, J.; Reilly, K.; Villacreces, S.; Gaffney, M.; Grant, J.; Brunton, N. Variation in bioactive content in broccoli (Brassica oleracea var. italica) grown under conventional and organic production systems. J. Sci. Food Agric. 2015, 95, 1163–1171. [Google Scholar] [CrossRef]
- Soengas, P.; Cartea, M.E.; Velasco, P.; Francisco, M. Endogenous Circadian Rhythms in Polyphenolic Composition Induce Changes in Antioxidant Properties in Brassica Cultivars. J. Agric. Food Chem. 2018, 66, 5984–5991. [Google Scholar] [CrossRef] [PubMed]
- Lawson, A.P.; Long, M.; Coffey, R.T.; Qian, Y.; Weerapana, E.; El Oualid, F.; Hedstrom, L. Naturally Occurring Isothiocyanates Exert Anticancer Effects by Inhibiting Deubiquitinating Enzymes. Cancer Res. 2015, 75, 5130–5142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.; Paonessa, J.D.; Zhang, Y.; Ambrosone, C.B.; McCann, S.E. Total isothiocyanate yield from raw cruciferous vegetables commonly consumed in the United States. J. Funct. Foods 2013, 5, 1996–2001. [Google Scholar] [CrossRef] [Green Version]
- ToTušek, J.; TříSka, J.; LeFNeroVá, D.; STrohaLm, J.; Vrchotová, N.; Zendulka, O.; Průchová, J.; Chaloupková, J.; Novotná, P.; Houška, M. Contents of sulforaphane and total isothiocyanates, antimutagenic activity, and inhibition of clastogenicity in pulp. Czech J. Food Sci. 2011, 29, 548–556. [Google Scholar] [CrossRef] [Green Version]
- Karanikolopoulou, S.; Revelou, P.-K.; Xagoraris, M.; Kokotou, M.G.; Constantinou-Kokotou, V. Current Methods for the Extraction and Analysis of Isothiocyanates and Indoles in Cruciferous Vegetables. Analytica 2021, 2, 93–120. [Google Scholar] [CrossRef]
- Wang, Z.; Kwan, M.L.; Pratt, R.; Roh, J.M.; Kushi, L.H.; Danforth, K.N.; Zhang, Y.; Ambrosone, C.B.; Tang, L. Effects of cooking methods on total isothiocyanate yield from cruciferous vegetables. Food Sci. Nutr. 2020, 8, 5673–5682. [Google Scholar] [CrossRef]
- Mordini, S.; Osera, C.; Marini, S.; Scavone, F.; Bellazzi, R.; Galizzi, A.; Calvio, C. The role of SwrA, DegU and PD3 in fla/che expression in B. subtilis. PLoS ONE 2013, 8, e85065. [Google Scholar] [CrossRef] [Green Version]
- Ermoli, F.; Bontà, V.; Vitali, G.; Calvio, C. SwrA as global modulator of the two-component system DegSU in Bacillus subtilis. Res. Microbiol. 2021, 172, 103877. [Google Scholar] [CrossRef]
- Naviglio, D.; Formato, A.; Gallo, M. Comparison between 2 Methods of Solid-Liquid Extraction for the Production ofCinchona calisayaElixir: An Experimental Kinetics and Numerical Modeling Approach. J. Food Sci. 2014, 79, E1704–E1712. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y. The 1,2-Benzenedithiole-Based Cyclocondensation Assay: A Valuable Tool for the Measurement of Chemopreventive Isothiocyanates. Crit. Rev. Food Sci. Nutr. 2012, 52, 525–532. [Google Scholar] [CrossRef] [Green Version]
- The R Project for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 7 February 2022).
| Control | WT | OS58 | |
|---|---|---|---|
| Polyphenols (mg/g) | 0.176 ± 0.020 | 0.227 ± 0.007 | 0.245 ± 0.023 * |
| Epigallocatechin gallate (g/g) | 1.094 ± 0.185 | 2.170 ± 0.829 | 3.071 ± 0.740 ** |
| Epicatechin (g/g) | 4.685 ± 0.778 | 8.670 ± 1.346 * | 11.30 ± 2.769 ** |
| Epicatechin gallate (g/g) | 3.293 ± 0.573 | 4.411 ± 0.590 ** | 4.108 ± 0.469 * |
| Chlorogenic acid (g/g) | 0.928 ± 0.658 | 1.880 ± 0.776 ** | 2.711 ± 0.871 *** |
| Isothiocyanates (g/g) | 0.004 ± 0.001 | 0.005 ± 0.001 | 0.006 ± 0.001 |
| Time (Min) | A (%) | B (%) |
|---|---|---|
| 1 | 90 | 10 |
| 5 | 90 | 10 |
| 7 | 80 | 20 |
| 8 | 80 | 20 |
| 10 | 75 | 25 |
| 15 | 70 | 30 |
| 20 | 20 | 80 |
| 25 | 50 | 50 |
| 28 | 70 | 30 |
| 30 | 90 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doria, E.; Buonocore, D.; Marra, A.; Bontà, V.; Gazzola, A.; Dossena, M.; Verri, M.; Calvio, C. Bacterial-Assisted Extraction of Bioactive Compounds from Cauliflower. Plants 2022, 11, 816. https://doi.org/10.3390/plants11060816
Doria E, Buonocore D, Marra A, Bontà V, Gazzola A, Dossena M, Verri M, Calvio C. Bacterial-Assisted Extraction of Bioactive Compounds from Cauliflower. Plants. 2022; 11(6):816. https://doi.org/10.3390/plants11060816
Chicago/Turabian StyleDoria, Enrico, Daniela Buonocore, Antonio Marra, Valeria Bontà, Andrea Gazzola, Maurizia Dossena, Manuela Verri, and Cinzia Calvio. 2022. "Bacterial-Assisted Extraction of Bioactive Compounds from Cauliflower" Plants 11, no. 6: 816. https://doi.org/10.3390/plants11060816
APA StyleDoria, E., Buonocore, D., Marra, A., Bontà, V., Gazzola, A., Dossena, M., Verri, M., & Calvio, C. (2022). Bacterial-Assisted Extraction of Bioactive Compounds from Cauliflower. Plants, 11(6), 816. https://doi.org/10.3390/plants11060816