5-Aminolevulinic Acid and 24-Epibrassinolide Improve the Drought Stress Resilience and Productivity of Banana Plants
Abstract
1. Introduction
2. Results
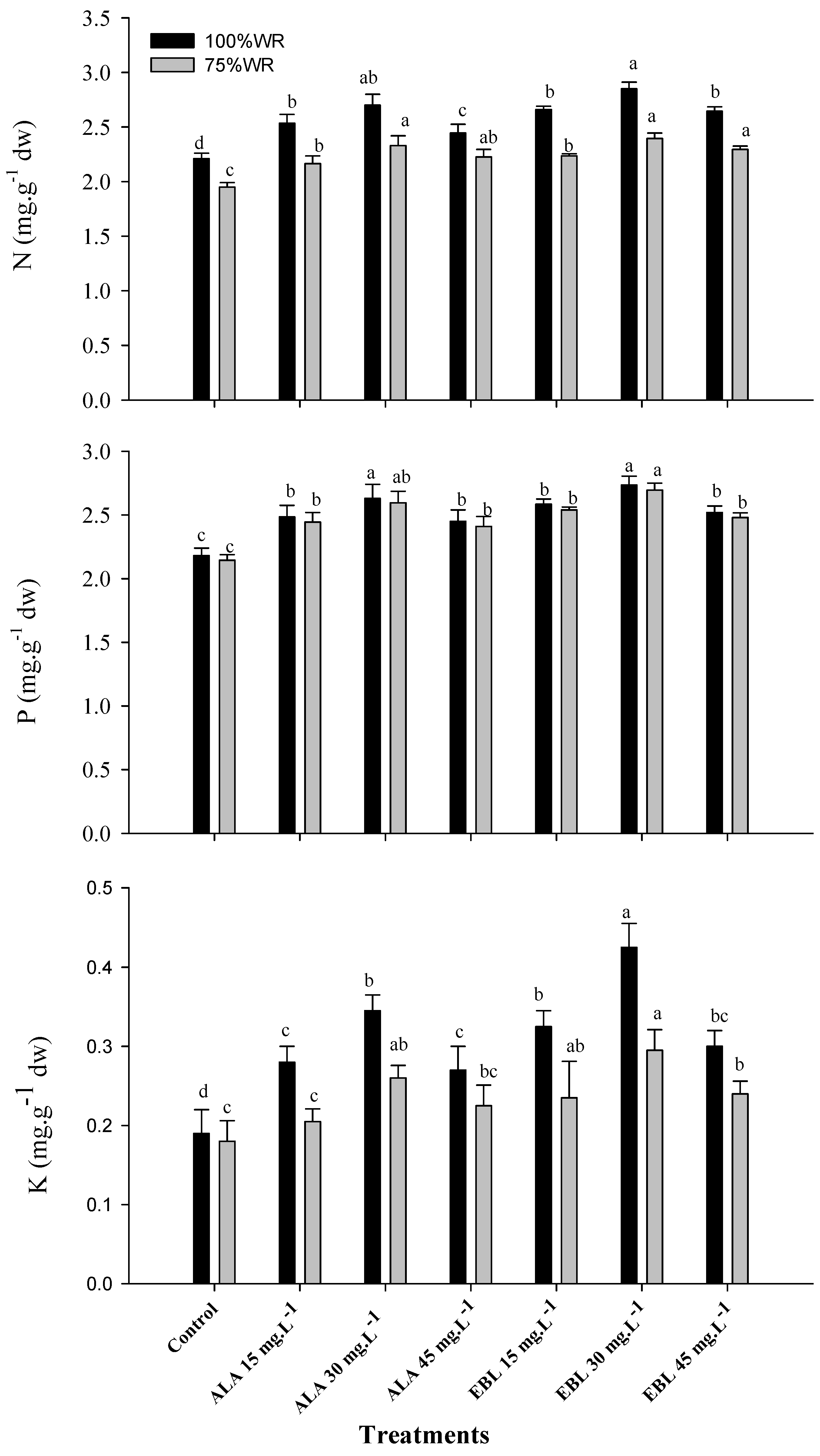
2.1. The Application of ALA or EBL Increased the NPK Content under Drought
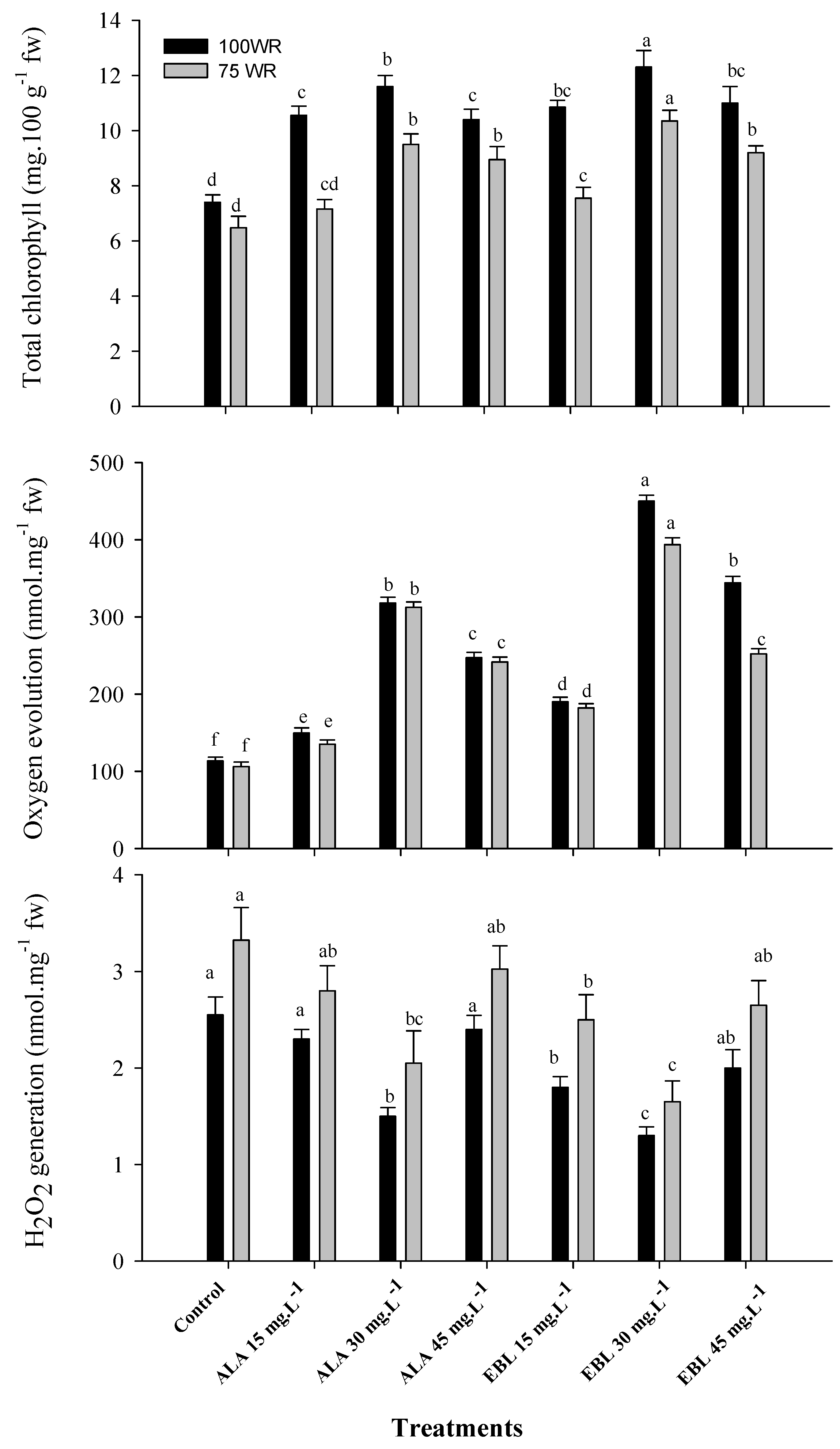
2.2. The Application of ALA or EBL Suppressed Chloroplast Degeneration under Drought
2.3. The Application of ALA or EBL Maintained Membrane Integrity under Drought
2.4. The Application of ALA or EBL Activated the Antioxidant Enzyme System under Drought
2.5. The Application of ALA or EBL Altered Phytohormone Levels under Drought
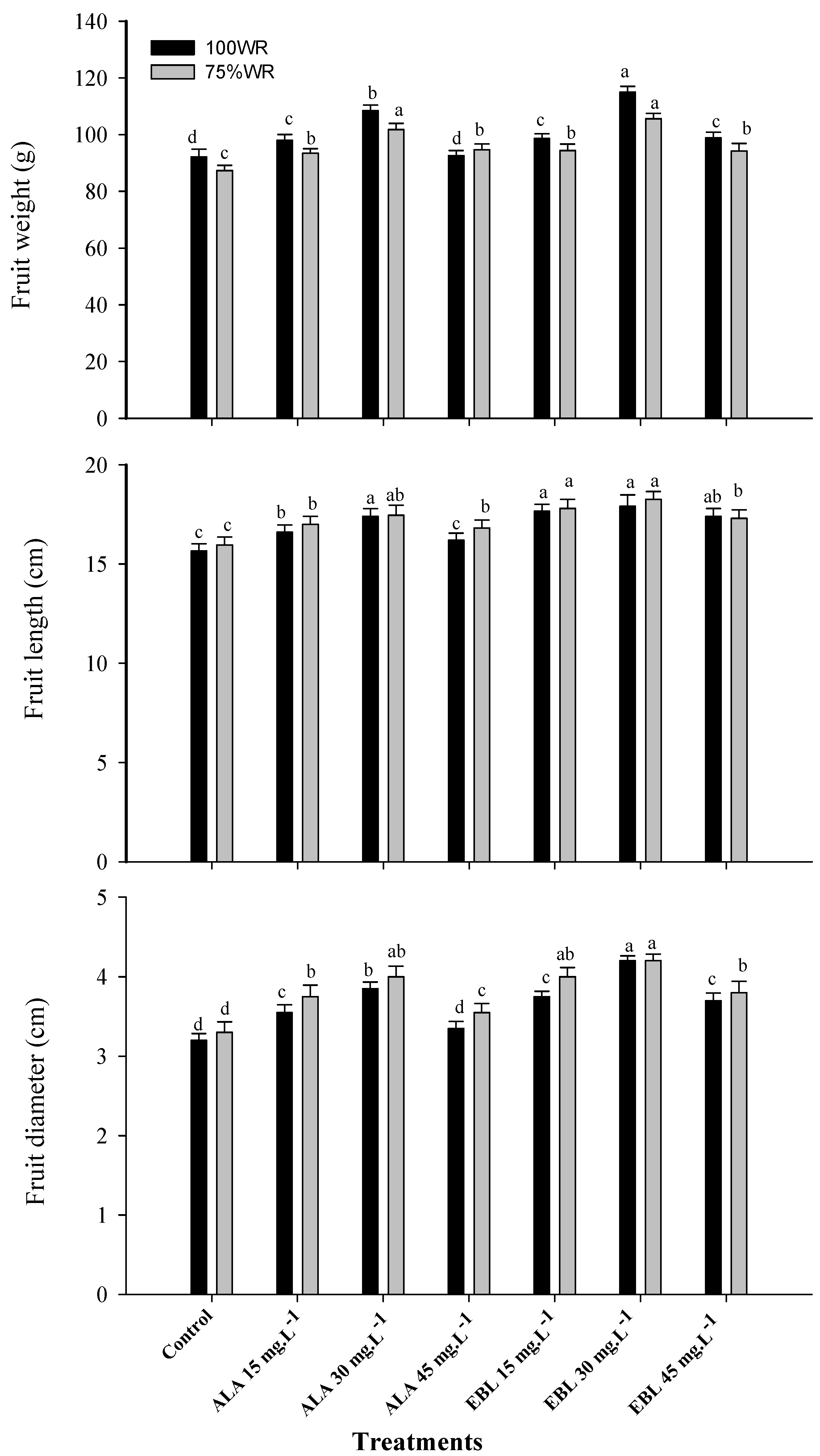
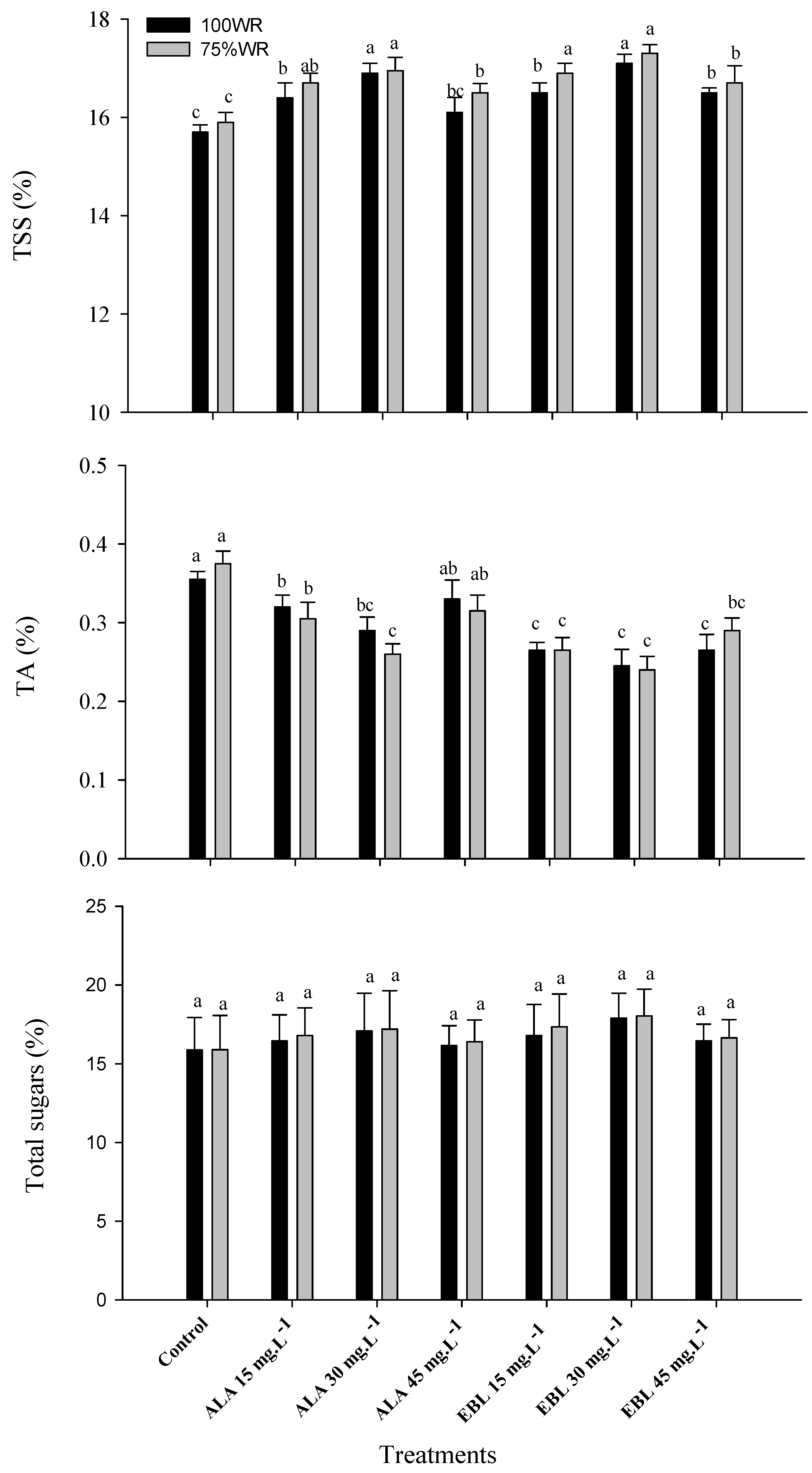
2.6. The Application of ALA or EBL Improved Yield and Fruit Quality under Drought
2.7. The Application of ALA or EBL Enhanced Fruit Physical Characteristics under Drought
2.8. The Application of ALA or EBL Enhanced Fruit Chemical Characteristics under Drought
3. Discussion
4. Materials and Methods
4.1. Experiment
4.2. Leaf Analysis
4.3. Yield and Fruit Quality
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinson, J.C.; Sauco, V.G. Banana and Plantains, 2nd ed.; CAB International: Wallingford, UK, 2010; p. 320. [Google Scholar]
- International Network for the Improvement of Banana and Plantain (INIBAP). Banana and Plantain—Food for Thought; Annual Report; International Network for the Improvement of Banana and Plantain: Montpellier, France, 1992; pp. 7–11. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Banana Facts and Figures; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2021; Available online: http://http://www.fao.org/economic/est/est-commodities/bananas/bananafacts/en/#.YDJBwC1h0U0 (accessed on 21 February 2021).
- Food and Agriculture Organization of the United Nations (FAO). FAO Statistics; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2019; Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 21 December 2021).
- Helgi Analytics. Banana Production in Egypt; Helgi Library: Prague, Czech Republic, 2020; Available online: https://www.helgilibrary.com/indicators/banana-production/egypt/ (accessed on 23 November 2021).
- El-Shereif, A.R.; Abou Elyazid, D.M. Tropical and subtropical fruits in Egypt. Chron. Hortic. 2016, 56, 28–31. [Google Scholar]
- Selina Wamucii. Egypt Bananas Prices; Selina Wamucii for Food and Agricultureal Produce in Africa: Nairobi, Kenya, 2021; Available online: https://www.selinawamucii.com/insights/prices/egypt/bananas/ (accessed on 26 October 2021).
- Stover, R.H.; Simmonds, N.W. Classification of banana cultivars. In Bananas, 3rd ed.; Stover, R.H., Simmonds, N.W., Eds.; Longman Scientific and Technical: London, UK, 1987; pp. 97–103. [Google Scholar]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Ravi, I.; Uma, S.; Vaganan, M.M.; Mustaffa, M.M. Phenotyping bananas for drought resistance. Front. Physiol. 2013, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jin, Z.Q.; Zhao, J.; Zhang, G.P.; Wu, F.B. Physiological and biochemical responses to drought stress in cultivated and Tibetan wild barley. Plant Growth Regul. 2015, 75, 567–574. [Google Scholar] [CrossRef]
- Dudal, R. Inventory of the major soild of the world with special reference to mineral stress hazards. In Plant Adaptation to Mineral Stress in Problem Soils, 1st ed.; Wright, M.J., Ed.; Cornell University Press: Beltsville, MD, USA, 1977; pp. 3–13. [Google Scholar]
- Salekdeh, G.H.; Reynolds, M.; Bennett, J.; Boyer, J. Conceptual framework for drought phenotyping during molecular breeding. Trends Plant Sci. 2009, 14, 488–496. [Google Scholar] [CrossRef]
- Abobatta, W.F. Drought mechanisms of plants—A review. Adv. Agric. Environ. Sci. 2019, 2, 42–45. [Google Scholar]
- Kumawat, K.R.; Sharma, N.K. Effect of drought stress on plants growth. Pop. Kheti 2018, 6, 239–241. [Google Scholar]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Surendar, K.K.; Devi, D.D.; Ravi, I.; Jeyakumar, P.; Velayudham, K. Studies on the impact of water deficit on morphological, physiological and yield of banana (Musa spp.) cultivars and hybrids. Int. J. Agric. Sci. 2013, 3, 473–482. [Google Scholar]
- Hazzati, S.; Tahmasebi-Sarvestani, Z.; Mokhtassi-Bidgoli, A.; Modarres-Sanavy, S.A.M.; Mohammadi, H.; Nicola, S. Effects of zeolite and water stress on growth, yield and chemical compositions of Aloe vera L. Agric. Water Manag. 2017, 181, 66–72. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.F.; Paton, N.W.; Lilley, K.S.; Binz, P.A.; Julian, R.K.; Jones, A.R.; Zhu, W.; Apweiler, R.; Aebersold, R.; Deutsch, E.W.; et al. The minimum information about a proteomic experiment (MIAPE). Nat. Biotechnol. 2007, 25, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Kavar, T.; Maras, M.; Kidric, M.; Sustar Vozlic, V.; Meglic, V. Identification of genes involved in the response of leaves of Phaseolus vulgris to drought stress. Mol. Breed. 2007, 21, 159–172. [Google Scholar] [CrossRef]
- Tasiu, I.T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar]
- Helaly, M.N.; El-Hoseiny, H.; El-Sheery, N.I.; Anshu, R.; Hazem, M.; Kalaji, H.M. Regulation and physiological role of silicon in alleviating drought stress of mango. Plant Physiol. Biochem. 2017, 118, 31–44. [Google Scholar] [CrossRef]
- Agricultural Statistics of Egypt. Water Scarcity in Egypt: The Urgent Need for Regional Cooperation among the Nile Basin Countries; Report of the Ministry of Water Resources and Irrigation; Government of Egypt: Cairo, Egypt, 2014; p. 5. [Google Scholar]
- Cosgrove, W.J.; Loucks, D.P. Water management: Current and future challenges and research directions. Water Resour. Res. 2015, 51, 4823–4839. [Google Scholar] [CrossRef]
- Young, S.C.H.; Sammis, T.W.; Wu, I.-P. Banana yield as affected by deficit irrigation and pattern of lateral layouts. Trans. Am. Soc. Agric. Biol. Eng. 1985, 28, 507-0510. [Google Scholar] [CrossRef]
- Santana-Ojeda, J.; Suárez-Sánchez, C.L. Response of the banana plant to deficit irrigation in the Canary Islands. Acta Hort. 1998, 490, 167–174. [Google Scholar] [CrossRef]
- Surendar, K.K.; Rajendran, V.; Devi, D.D.; Jeyakumar, P.; Ravi, I.; Velayudham, K. Impact of water deficit on growth attributes and yields of banana cultivars and hybrids. Afric. J. Agric. Res. 2013, 8, 6116–6125. [Google Scholar]
- Nadyitegeye, O.; Onyando, J.O.; Okwany, R.O.; Kwach, J.K. Vegetative growth of banana as influenced by deficit irrigation and irrigation interval. Fund. Appl. Agric. 2019, 4, 1047–1053. [Google Scholar] [CrossRef]
- El Namas, A.E. Effect of Deficit irrigation and biochar application on growth, yield components, water use efficiency and water productivity of banana (Musa sapientum) grown in sandy soil under drip irrigation. J. Soil Sci. Agric. Eng. 2020, 11, 163–175. [Google Scholar]
- Ravi, I.; Vaganan, M. Abiotic stress tolerance in banana. In Abiotic Stress Physiology of Horticultural Crops, 1st ed.; Rao, N.K.S., Shivashankara, K.S., Laxman, R.H., Eds.; Springer India: New Delhi, India, 2016; Chapter 12; pp. 207–222. [Google Scholar]
- English, M. Deficit irrigation. I: Analytical framework. J. Irrig. Drain. Eng. 1990, 116, 399–412. [Google Scholar] [CrossRef]
- Akram, N.A.; Ashraf, M. Regulation in plant stress tolerance by a potential plant growth regulator, 5-aminolevulinic acid. J. Plant Growth Regul. 2013, 32, 663–679. [Google Scholar] [CrossRef]
- Naeem, M.S.; Jin, Z.L.; Wan, G.L.; Liu, D.; Liu, H.B.; Yoneyama, K.; Zhou, W.J. 5-Aminolevulinic acid improves photosynthetic gas exchange capacity and ion uptake under salinity stress in oilseed rape (Brassica napus L.). Plant Soil 2010, 332, 405–415. [Google Scholar] [CrossRef]
- Hotta, Y.; Tanaka, T.; Takaoka, H.; Takeuchi, Y.; Konnai, M. New physiological effects of 5-aminolevulinic acid in plants: The increase of photosynthesis, chlorophyll content, and plant growth. Biosci. Biotechnol. Biochem. 1997, 61, 2025–2028. [Google Scholar] [CrossRef]
- Uehlinger, P.; Zellweger, M.; Wagnieres, G.; Juillerat-Jeanneret, L.; van den Bergh, H.; Lange, N. 5-Aminolevulinic acid and its derivatives: Physical chemical properties and protoporphyrin IX formation in cultured cells. J. Photochem. Photobiol. B: Biol. 2000, 54, 72–80. [Google Scholar] [CrossRef]
- Balestrasse, K.B.; Tomaro, M.L.; Batlle, A.; Noriega, G.O. The role of 5-aminolevulinic acid in the response to cold stress in soybean plants. Phytochemistry 2010, 71, 2038–2045. [Google Scholar] [CrossRef]
- Xia, X.J.; Wang, Y.J.; Zhou, Y.H.; Yuan, T.; Mao, W.H.; Kai, S.; Asami, T.; Chen, Z.X.; Yu, J.Q. Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol. 2009, 150, 801–814. [Google Scholar] [CrossRef]
- Kuiju, N.; Xiang, M.; Guoling, L.; Huiling, M.; Zhifeng, J.; Wenhui, L.; Qianqian, Y. 5-Aminolevulinic acid modulates antioxidant defense systems and mitigates drought-induced damage in Kentucky bluegrass seedlings. Protoplasma 2017, 254, 2083–2094. [Google Scholar]
- Wang, L.; Jiang, W.; Huang, B. Promotion of photosynthesis by 5-aminolevulinic acid during and after chilling stress in melon seedlings grown under low light condition. Acta Hortic. Sin. 2004, 31, 321–326. [Google Scholar]
- Wu, Y.; Jin, X.; Liao, W.; Hu, L.; Dawuda, M.M.; Zhao, X.; Tang, Z.; Gong, T.; Yu, J. 5-Aminolevulinic Acid (ALA) alleviated salinity stress in cucumber seedlings by enhancing chlorophyll synthesis pathway. Front. Plant Sci. 2018, 9, 635. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wu, L.; Naeem, M.S.; Liu, H.; Deng, X.; Xu, L.; Zhang, F.; Zhou, W. 5-Aminolevulinic acid enhances photosynthetic gas exchange, chlorophyll fluorescence and antioxidant system in oilseed rape under drought stress. Acta Physiol. Plant 2013, 35, 2747–2759. [Google Scholar] [CrossRef]
- He, J.; Li, M.-F.; Wu, F.; Li, S.P.; Zhou, S.Y. Physiological response of banana seedling to exogenous ALA under drought stress. J. South. Agric. 2013, 44, 745–750. [Google Scholar]
- Zhang, M.Z.; Zhai, X.; Tian, X.; Duan, L.; Li, Z. Brassinolide alleviated the adverse effect of water deficits on photosynthesis and the antioxidant of soybean (Glycine max L.). Plant Growth Regul. 2008, 56, 257–264. [Google Scholar] [CrossRef]
- Yang, Z.; Chang, Z.; Sun, L.; Yu, J.; Huang, B. Physiological and metabolic effects of 5-aminolevulinic acid for mitigating salinity stress in creeping bentgrass. PLoS ONE 2014, 9, 116283. [Google Scholar] [CrossRef]
- Divi, U.K.; Rahman, T.; Krishna, P. Gene expression and functional analyses in brassinosteroid-mediated stress tolerance. Plant Biotechnol. 2016, 14, 419–432. [Google Scholar] [CrossRef]
- Peres, A.L.G.L.; Soares, J.S.; Tavares, R.G.; Righetto, G.; Zullo, M.A.; Mandava, N.B.; Menossi, M. Brassinosteroids, the sixth class of phytohormones: A molecular view from the discovery to hormonal interactions in plant development and stress adaptation. Int. J. Mol. Sci. 2019, 20, 331. [Google Scholar] [CrossRef]
- Choudhary, S.P.; Yu, J.Q.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S. Benefits of brassinosteroids crosstalk. Trends Plant Sci. 2012, 17, 594–605. [Google Scholar] [CrossRef]
- Bhandari, S.; Nailwal, T.K. Role of brassinosteroids in mitigating abiotic stresses in plants. Biologia 2020, 75, 2203–2230. [Google Scholar] [CrossRef]
- Coban, O.; Baydar, N.G. Brassinosteroid effects on some physical and biochemical properties and secondary metabolite accumulation in peppermint (Mentha piperita L.) under salt stress. Ind. Crop. Prod. 2016, 86, 251–258. [Google Scholar] [CrossRef]
- Lima, J.V.; Lobato, A.K.S. Brassinosteroids improve photosystem II efficiency, gas exchange, antioxidant enzymes and growth of cowpea plants exposed to water deficit. Physiol. Mol. Biol. Plants 2017, 23, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Antonio, M.; Zullo, T.; Adam, G. Brassinosteroids phytohormones—Structure, bioactivity and applications. Braz. J. Plant Physiol. 2002, 14, 143–181. [Google Scholar]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech 2015, 4, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, Z.; Qin, G.; Tian, S. Effects of brassinosteroids on postharvest disease and senescence of jujube fruit in storage. Postharvest Biol. Technol. 2010, 56, 50–55. [Google Scholar] [CrossRef]
- Shamon, M.S.; El-Awadi, M.E.; Gergis, M.D.; El-Rorkiek, G.A. Physiological Role of Brassinosteroids and Cauliflower Extract on Quinoa Plant Grown under Sandy Soil. Asian J. Appl. Sci. 2020, 13, 68–76. [Google Scholar] [CrossRef]
- Sudarshna, K.; Anju, T. The Effects of Water Stress and Brassinosteroid on Apple Varietes. Int. J. Econ. Plants 2019, 6, 1–6. [Google Scholar]
- Anwar, A.; Wang, J.; Yu, X.; He, C.; Li, Y. Substrate application of 5-aminolevulinic acid enhanced low-temperature and weak-light stress tolerance in cucumber (Cucumis sativus L.). Agronomy 2020, 10, 472. [Google Scholar] [CrossRef]
- Aksakal, O.; Algur, O.; Aksakal, F.; Aysin, F. Exogenous 5-aminolevulinic acid alleviates the detrimental effects of UV-B stress on lettuce (Lactuca sativa L.) seedlings. Acta Physiol. Plant. 2017, 39, 55. [Google Scholar] [CrossRef]
- Xi, Z.; Wang, Z.; Fang, Y.; Hu, Z.; Hu, Y.; Deng, M.; Zhang, Z. Effects of 24-epibrassinolide on autoxidation defense and osmoregulation systems of young grapevines (V. vinifera L.) under chilling stress. Plant Growth Regul. 2013, 71, 57–65. [Google Scholar] [CrossRef]
- Nunkaew, T.; Kantachote, D.; Kanzaki, H.; Nitoda, T.; Ritchie, R. Effects of 5-aminolevulinic acid containing supernatants from selected Rhodopseudomonas palustris strains on rice growth under NaCl stress, with mediating effects on chlorophyll, photosynthetic electron transport and antioxidative enzymes. Electron. J. Biotechnol. 2014, 17, 1. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Yan, F.; Hu, L.P.; Zhou, X.T.; Zou, Z.R.; Cui, L.R. Effects of exogenous 5-aminolevulinic acid on photosynthesis, stomatal conductance, transpiration rate, and PIP gene expression of tomato seedlings subject to salinity stress. Genet. Mol. Res. 2015, 14, 6401–6412. [Google Scholar] [CrossRef] [PubMed]
- Ogweno, J.O.; Song, X.S.; Shi, K.; Hu, W.H.; Mao, W.H.; Zhou, Y.H.; Yu, J.Q.; Nogués, S. Brassinosteroids Alleviate Heat-Induced Inhibition of Photosynthesis by Increasing Carboxylation Efficiency and Enhancing Antioxidant Systems in Lycopersicon esculentum. J. Plant Growth Regul. 2008, 27, 49–57. [Google Scholar] [CrossRef]
- Korkmaz, A.; Korkmaz, Y.; Demirkıran, A.R. Enhancing chilling stress tolerance of pepper seedlings by exogenous application of 5-aminolevulinic acid. Environ. Exp. Bot. 2010, 67, 495–501. [Google Scholar] [CrossRef]
- Liu, D.; Hu, L.Y.; Ali, B.; Yang, A.G.; Wan, G.L.; Xu, L.; Zhou, W.J. Influence of 5-aminolevulinic acid on photosynthetically related parameters and gene expression in Brassica napus L. under drought stress. Soil Sci. Plant Nutr. 2016, 62, 254–262. [Google Scholar] [CrossRef]
- Nassar, A.H. Effect of homobrassinolide on in vitro growth of apical meristems and heat tolerance of banana shoots. Int. J. Agric. Biol. 2004, 6, 771–776. [Google Scholar]
- Jury, W.A.; Vaux, J.H. The role of science in solving the world’s emerging water problems. Proc. Natl. Acad. Sci. USA 2005, 102, 15715–15720. [Google Scholar] [CrossRef]
- Ashraf, M.; Akram, N.A.; Al-Qurainy, F.; Foolad, M.R. Drought tolerance: Roles of organic osmolytes, growth regulators, and mineral nutrients. Adv. Agron. 2011, 111, 249–296. [Google Scholar]
- Kallarackal, J.; Milburn, J.A.; Baker, D.A. Water relations of the banana. III effects of controlled water stress on water potential, transpiration, photosynthesis and leaf growth. Aust. J. Plant Physiol. 1990, 17, 79–90. [Google Scholar] [CrossRef]
- Turner, D.W. The Impact of Environmental Factors on the Development and Productivity of Bananas and Plantains. In Proceedings of the 13th ACORBAT Meeting, Guayaquil, Ecuador, 13–16 July 1998; pp. 635–663. [Google Scholar]
- Turner, D.W.; Thomas, D.S. Measurements of plant and soil water status and their association with leaf gas exchange in banana (Musa spp.): A laticiferous plant. Sci. Hort. 1998, 77, 177–193. [Google Scholar] [CrossRef]
- Ghavami, M. Determining water needs of the banana plant. Trans. Am. Soc. Agric. Eng. 1973, 16, 598–600. [Google Scholar] [CrossRef]
- Shu, S.; Tang, Y.; Yuan, Y.; Sun, J.; Zhong, M.; Guo, S. The role of 24-epibrassinolide in the regulation of photosynthetic characteristics and nitrogen metabolism of tomato seedlings under a combined low temperature and weak light stress. Plant Physiol. Biochem. 2016, 107, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Grotjohann, I.; Jolley, C.; Fromme, P. Evolution of photosynthesis and oxygen evolution: Implications from the structural comparison of photosystem I and II. Phys. Chem. Chem. Phys. 2004, 6, 4743–4753. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species, metabolism, oxidative stress and signal transduction. Ann. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Sen, A. Oxidative stress studies in plant tissue culture. In Antioxidant Enzyme; World’s Largest Science, Technology and Medicine Open Access Book; El-Missiry, M.A., Ed.; INTECH: Rijeka, Croatia, 2012; Chapter 3; pp. 59–88. [Google Scholar] [CrossRef]
- Gadallah, M.A.A. Effect of proline and glycinebetaine on Vicia faba responses to salt stress. Biol. Plant. 1999, 42, 249–257. [Google Scholar] [CrossRef]
- Al-Wasfy, M.M. Response of Sakkoti date palms to foliar application of royal jelly, silicon and vitamins B. J. Am. Sci. 2013, 9, 315–321. [Google Scholar]
- Ma, C.; Liu, H.; Guo, H.; Musante, C.; Coskun, S.H.; Nelson, B.C.; White, J.C.; Xing, B.; Dhankher, O.P. Defense mechanisms and nutrient displacement in Arabidopsis thaliana upon exposure to CeO2 and In2O3 nanoparticles. Environ. Sci. Nano 2016, 3, 1369–1379. [Google Scholar] [CrossRef]
- Garg, N.; Manchanda, G. ROS generation in plants: Boon or bane? Plant Biosyst. 2009, 143, 81–96. [Google Scholar] [CrossRef]
- Nxele, X.A.; Klein, A.B.; Ndimba, B.K. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S. Afr. J. Bot. 2017, 108, 261–266. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutase in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Sajedi, N.A.; Ferasat, M.; Mirzakhani, M.; Mashhadi, M.; Boojar, A. Impact of water deficit stress on biochemical characteristics of sunflower cultivars. Physiol. Mol. Biol. Plants 2012, 18, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, F.; Ahmadi, A.; Poustini, K.; Alhzadeh, H. Survey of relation activity of antioxidant enzyme with cell membrane stability and chlorophyll of bread wheat cultivars of resistance and sensitive to drought stress. J. Agric. Sci. 2006, 2, 50–56. [Google Scholar]
- Omar, S.A.; Elsheery, N.I.; Kalaji, H.M.; Xu, Z.F.; Song-Quan, S.; Carpentier, R.; Lee, C.H.; Allakhverdiev, S.I. Dehydroascorbate reductase and glutathione reductase play an important role in scavenging hydrogen peroxide during natural and artificial dehydration of Jatropha curcas seeds. J. Plant Biol. 2013, 55, 469–480. [Google Scholar] [CrossRef][Green Version]
- Babar, S.; Siddiqui, E.H.; Hussain, I.; Bhati, K.H.; Rasheed, R. Mitigating the effects of salinity by foliar application of Salicylic acid in fenugreek. Physiol. J. 2014, 14, 869058. [Google Scholar] [CrossRef]
- Sharma, P.; Bhushan, A.J.; Shanker, R.D.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Varjovi, M.B.; Valizadeh, M.; Bandehagh, A. Primary Antioxidant Enzymes and their Important Role in Oxidative Stress in Plants and Mammalian. Biol. Forum Int. J. 2015, 7, 148–154. [Google Scholar]
- Akram, N.A.; Ashraf, M.; Al-Qurainy, F. Aminolevulinic acid-induced regulation in some key physiological attributes and activities of antioxidant enzymes in sunflower (Helianthus annus L.) under saline regimes. Sci. Hort. 2012, 142, 143–148. [Google Scholar] [CrossRef]
- Xu, F.; Zhu, J.; Cheng, S.; Zhang, W.; Wang, Y. Effect of 5-aminolevulinic acid on photosynthesis, yield, nutrition and medicinal values of kudzu (Pueraria phaseoloides). Trop. Grassl. 2010, 44, 260–265. [Google Scholar]
- Maruyama-Nakashita, A.; Hira, M.Y.; Funada, S.; Fuek, S. Exogenous application of 5-aminolevulinic acid increases the transcript levels of sulfur transport and assimilatory genes, sulfate uptake, and cysteine and glutathione contents in Arabidopsis thaliana. Soil Sci. Plant Nutr. 2010, 56, 281–288. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tanaka, A.; Tsuji, H. Stabilization of apoproteins of light-harvesting chlorophyll-a/b protein complex by feeding 5-aminolevulinic acid under intermittent illumination. Plant Physiol. Biochem. 1992, 30, 365–370. [Google Scholar]
- Chakraborty, N.; Tripathy, B.C. Involvement of singlet oxygen in 5-aminolevulinic acid-induced photodynamic damage of cucumber (Cucumis sativus L.) chloroplasts. Plant Physiol. 1992, 98, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Hudson, D.; Guevara, D.; Yaish, M.W.; Hannam, C.; Long, N. GNC and CGA1 modulate chlorophyll biosynthesis and glutamate synthase (GLU1/Fd-GOGAT) expression in Arabidopsis. PLoS ONE 2011, 6, 26765. [Google Scholar] [CrossRef] [PubMed]
- Akram, N.A.; Ashraf, M.; Al-Qurainy, F. Aminolevulinic acid-induced changes in yield and seed-oil characteristics of sunflower (Helianthus annus L.) plants under salt stress. Pak. J. Bot. 2011, 43, 2845–2852. [Google Scholar]
- Youssef, T.; Awad, M.A. Mechanisms of enhancing photosynthetic gas exchange in date palm seedlings (Phoenix dactylifera L.) under salinity stress by a 5-aminolevulinic acid-based fertilizer. J. Plant Growth Regul. 2008, 27, 1–9. [Google Scholar] [CrossRef]
- Rasheed, R.; Yasmeen, H.; Hussain, I.; Iqbal, M.; Ashraf, M.A.; Parveen, A. Exogenously applied 5-aminolevulinic acid modulates growth, secondary metabolism and oxidative defense in sunflower under water deficit stress. Physiol. Mol. Biol. Plants. 2020, 26, 489–499. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Sun, W.; Li, Q.; Dai, A.; Bai, J. 5-Aminolevulinic acid pretreatment mitigates drought stress of cucumber leaves through altering antioxidant enzyme activity. Sci. Hort. 2011, 130, 820–828. [Google Scholar] [CrossRef]
- Memon, S.A.; Hou, X.; Wang, L.; Li, Y. Promotive effect of 5-aminolevulinic acid on chlorophyll, antioxidative enzymes and photosynthesis of Pakchoi (Brassica campestris ssp. Chinensis var. communis Tsen et Lee). Acta Physiol. Plant 2009, 31, 51–57. [Google Scholar] [CrossRef]
- Phung, T.; Jung, H.I.; Park, J.; Kim, J.; Back, K.; Jung, S. Porphyrin biosynthesis control under water stress: Sustained porphyrin status correlates with drought tolerance in transgenic rice. Plant Physiol. 2011, 157, 1746–1764. [Google Scholar] [CrossRef]
- Al-Khateeb, S.A. Promotive effect of 5-aminolevulinic acid on growth, yield and gas exchange capacity of barley (Hordeum Vulgare L.) grown under different irrigation regimes. J. King Saud Univ. Agric. Sci. 2006, 18, 103–111. [Google Scholar]
- D’Auzac, J.; Cretin, H.; Marin, B.; Lioret, C. A plant vacuolar system: The lutoids from Hevea brasiliensis latex. Physiol. Veg. 1982, 20, 311–331. [Google Scholar]
- Sunil, B.; Rajsheel, P.; Aswani, V.; Bapatla, R.B.; Talla, S.K.; Raghavendra, S.A. Photosynthesis is sensitive to nitric oxide and respiration sensitive to hydrogen peroxide: Studies with pea mesophyll protoplasts. J. Plant Physiol. 2020, 246–247, 153133. [Google Scholar] [CrossRef] [PubMed]
- Maodzeka, A.; Wang, Q.; Chen, X.; Hussain, N.; Wu, D.; Jiang, L. Effects of 5-aminolevulinic acid on the bioactive compounds and seedling growth of oilseed rape (Brassica napus L.). J. Plant Biol. 2019, 62, 181–194. [Google Scholar] [CrossRef]
- Kallarackal, J.; Milburn, J.A.; Baker, D.A. Transpiration characteristics of banana leaves in response to progressive depletion of available soil moisture. Sci. Hortic. 1986, 30, 289–300. [Google Scholar]
- An, Y.; Cheng, D.; Rao, Z.; Sun, Y.; Tang, Q.; Wang, L. 5-Aminolevulinic acid (ALA) promotes primary root elongation through modulation of auxin transport in Arabidopsis. Acta Physiol. Plant. 2019, 41, 85. [Google Scholar] [CrossRef]
- An, Y.; Liu, L.; Chen, L.; Wang, L. ALA inhibits ABA-induced stomatal closure via reducing H2O2 and Ca2+ levels in guard cells. Front. Plant Sci. 2016, 7, 482. [Google Scholar] [CrossRef]
- Song, X.G.; She, X.P.; He, J.M.; Huang, C.; Song, T.S. Cytokinin- and auxin-induced stomatal opening involves a decrease levels of hydrogen peroxide in guard cells of Vicia faba. Funct. Plant Biol. 2006, 33, 573–583. [Google Scholar] [CrossRef]
- Wang, Q.N.; An, B.; Wei, Y.X.; Reiter, E.J.; Shi, H.T.; Luo, H.L.; He, C.Z. Melatonin regulates root meristem by repressing auxin synthesis and polar auxin transport in Arabidopsis. Front. Plant Sci. 2016, 7, 1882. [Google Scholar] [CrossRef]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005, 433, 39–44. [Google Scholar] [CrossRef]
- Ruzicka, K.; Ljung, K.; Vanneste, S.; Podhorska, R.; Beeckman, T.; Friml, J.; Benkova, E. Ethylene tegulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 2007, 19, 2197–2212. [Google Scholar] [CrossRef]
- Al-Thabet, S.S. Promotive effect of 5-aminolevulinic acid on growth and yield of wheat grown under dry conditions. J. Agron. 2006, 5, 45–49. [Google Scholar] [CrossRef]
- Al-Khateeb, A.A.; Al-Khateeb, S.A.; Okawara, R.; Al-Abdoulhady, I.A. Promotive effects of 5-aminolevulinic acid (5-ALA) on fruit yield and quality of date palm cv Khalas. J. Biol. Sci. 2006, 6, 1118–1121. [Google Scholar]
- Cai, C.; He, S.; An, Y.; Wang, L. Exogenous 5-aminolevulinic acid improves strawberry tolerance to osmotic stress and its possible mechanisms. Physiol. Plant. 2020, 168, 948–962. [Google Scholar] [CrossRef]
- Johnson, R.S.; Handley, D.F.; Day, K.R. Postharvest water stress of an early maturing plum. J. Hortic. Sci. 1994, 69, 1035–1041. [Google Scholar] [CrossRef]
- Jiménez, S.; Fattahi, M.; Bedis, K.; Nasrolahpour-moghadam, S.; Irigoyen, J.J.; Gogorcena, Y. Interactional Effects of Climate Change Factors on the Water Status, Photosynthetic Rate, and Metabolic Regulation in Peach. Front. Plant Sci. 2020, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Vu, J.C.V.; Yelenosky, G. Non-structural carbohydrate concentrations in leaves ‘Valencia’ orange subjected to water deficits. Environ. Expt. Bot. 1989, 29, 149–154. [Google Scholar] [CrossRef]
- Yuan, X.K.; Yang, Z.Q.; Li, Y.X.; Liu, Q.; Han, W. Effects of different levels of water stress on leaf photosynthetic characteristics and antioxidant enzyme activities of greenhouse tomato. Photosynthetica 2016, 54, 28–39. [Google Scholar] [CrossRef]
- Anwar, A.; Liu, Y.; Dong, R.; Bai, L.; Yu, X.; Li, Y. The physiological and molecular mechanism of brassinosteroids in response to stress: A review. Biol. Res. 2018, 51, 46. [Google Scholar] [CrossRef]
- Catterou, M.; Dubois, F.; Schaller, H.; Aubanelle, L.; Vilcot, B.; Sangwan-Norreel, B.S.; Sangwan, R.S. Brassinosteroids, microtubules and cell elongation in Arabidopsis thaliana. I. Molecular, cellular and physiological characterization of the Arabidopsis bull mutant, defective in the Δ7-sterol- C5-desaturation step leading to brassinosteroid biosynthesis. Planta 2001, 212, 659–972. [Google Scholar] [CrossRef]
- Vilarrasa-Blasi, J.; Gonzalez-Garcia, M.P.; Frigola, D.; Fabregas, N.; Alexiou, K.G.; Lopez-Bigas, N.; Rivas, S.; Jauneau, A.; Lojmann, J.U.; Benfey, P.N. Regulation f plant stem cell quiescence by a brassinosteroid signaling module. Dev. Cell 2014, 30, 36–47. [Google Scholar] [CrossRef]
- Grzesiak, S.; Hordynska, N.; Szczyrek, P.; Grzesiak, M.T.; Noga, A.; Szechynska-Hebda, M. Variation among wheat (Triticum easativum L.) genotypes in response to the drought stress: I–selection approaches. J. Plant. Interact. 2019, 14, 30–44. [Google Scholar] [CrossRef]
- Caño-Delgado, A.; Yin, Y.; Yu, C.; Vafeados, D.; Mora-García, S.; Cheng, J.C.; Nam, K.H.; Li, J.; Chory, J. BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis. Development 2004, 131, 5341–5351. [Google Scholar] [CrossRef] [PubMed]
- Zaharah, S.S.; Singh, Z.; Symons, G.M.; Reid, J.B. Role of brassinosteroids, ethylene, abscisic acid, and indole-3-acetic acid in mango fruit ripening. J. Plant Growth Regul. 2012, 31, 363–372. [Google Scholar] [CrossRef]
- Yuldashev, R.; Avalbaev, A.; Bezrukova, M.; Vysotskaya, L.; Khripach, V.; Shakirova, F. Cytokinin oxidase is involved in the regulation of cytokinin content by 24-epibrassinolide in wheat seedlings. Plant Physiol. Biochem. 2012, 55, 1–6. [Google Scholar] [CrossRef]
- Bajguz, A.; Hayat, S. Efects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009, 47, 1–8. [Google Scholar] [CrossRef]
- Zhao, G.; Xu, H.; Zhang, P.; Su, X.; Zhao, H. Effects of 24-epibrassinolide on photosynthesis and Rubisco activase gene expression in Triticum aestivum L. seedlings under a combination of drought and heat stress. Plant Growth Regul. 2017, 81, 377–384. [Google Scholar] [CrossRef]
- Xia, X.-J.; Huang, L.-F.; Zhou, Y.-H.; Mao, W.-H.; Shi, K.; Wu, J.-X.; Asami, T.; Chen, Z.; Yu, J.-Q. Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus L. Planta 2009, 230, 1185. [Google Scholar] [CrossRef]
- Li, J.; Yang, P.; Kang, J.; Gan, Y.; Yu, J.; Calderón-Urrea, A.; Jian, L.; Zhang, G.; Feng, Z.; Xie, J. Transcriptome analysis of pepper revealed a role of 24-epibrassinolide in response to chilling. Front. Plant Sci. 2016, 7, 01281. [Google Scholar] [CrossRef]
- Liu, J.; Gao, H.; Wang, X.; Zheng, Q.; Wang, C.; Wang, X.; Wang, Q. Effects of 24-epibrassinolide on plant growth, osmotic regulation and ion homeostasis of salt-stressed canola. Plant Biol. 2014, 16, 440–450. [Google Scholar] [CrossRef]
- Steber, C.M.; Mccourt, P. A role for brassinosteroids in germination in Arabidopsis. Plant Physiol. 2001, 125, 763–769. [Google Scholar] [CrossRef]
- Talaat, N.B. 24-Epibrassinolide alleviates salt-induced inhibition of productivity by increasing nutrients and compatible solutes accumulation and enhancing antioxidant system in wheat (Triticum aestivum L.). Acta Physiol. Plant. 2013, 35, 729–740. [Google Scholar] [CrossRef]
- Br, A.; Sm, A. Hormone interactions in stomatal function. Plant Mol. Biol. 2009, 69, 451–462. [Google Scholar]
- Zhang, S.; Cai, Z.; Wang, X. The primary signaling outputs of brassinosteroids are regulated by abscisic acid signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 4543–4548. [Google Scholar] [CrossRef] [PubMed]
- Aman, V. Effect of Brassinosteroid, Sodium Nitroprusside and Cadmium on Antioxidative Metabolism in Chickpea Seedlings. Int. J. Plant Res. 2018, 31, 27–33. [Google Scholar]
- Liu, Z.; Li, L.; Luo, Z.; Zeng, F.; Jiang, L.; Tang, K. Effect of brassinolide on energy status and proline metabolism in postharvest bamboo shoot during chilling stress. Postharvest Biol. Technol. 2016, 111, 240–246. [Google Scholar] [CrossRef]
- Xi, Z.M.; Zhang, Z.W.; Huo, S.S.; Luan, L.Y.; Gao, X.; Ma, L.N.; Fang, Y.L. Regulating the secondary metabolism in grape berry using exogenous 24-epibrassinolide for enhanced phenolics content and antioxidant capacity. Food Chem. 2013, 141, 3056–3065. [Google Scholar] [CrossRef]
- Sidhu, J.S.; Zafar, T.A. Bioactive compounds in banana fruits and their health benefits. Food Qual. Saf. 2018, 2, 183–188. [Google Scholar] [CrossRef]
- Boud, A. Evolution and current status of research in phenolic compounds. Phytochemistry 2007, 68, 2722–2735. [Google Scholar]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Alesiani, D.; Canini, A.; D’Abroca, B.; DellaGreca, M.; Fiorentino, A.; Mastellone, C.; Monaco, P.; Pacifico, S. Antioxidant and antiproliferative activities of phytochemicals from Quince (Cydonia vulgaris) peels. Food Chem. 2010, 118, 199–207. [Google Scholar] [CrossRef]
- Sharp, R.E.; LeNoble, M.E.; Else, M.A.; Thome, E.T.; Gherardi, F. Endogenous ABA maintains shoot growth in tomato independently of effects on plant water balance: Evidence for an interaction with ethylene. J. Exp. Bot. 2000, 51, 1575–1584. [Google Scholar] [CrossRef]
- Hegazi, E.S.; El-Motaium, R.A.; Yehia, T.A.; Hashem, M.E. Effect of foliar boron application on boron, chlorophyll, phenol, sugars and hormones concentration of olive (Olea europea L.) buds, leaves, and fruits. J. Plant Nutr. 2018, 41, 749–765. [Google Scholar] [CrossRef]
- Conesa, M.R.; Conejero, W.; Vera, J.; Ruiz-Sánchez, M.C. Effects of postharvest water deficits on the physiological behavior of early-maturing nectarine trees. Plants 2020, 9, 1104. [Google Scholar] [CrossRef] [PubMed]
- Aung, L.H.; Houch, L.G.; Norman, S.M. The abscisic acid content of citrus with special references to lemon. J. Exp. Bot. 1991, 42, 1083–1088. [Google Scholar] [CrossRef]
- Valero, D.; Romero, D.M.; Serrano, M.; Riquelme, F. Postharvest gibberellin and heat treatment effects on polyamines, abscisic acid and firmness in lemon. J. Food Sci. 1998, 63, 611–615. [Google Scholar] [CrossRef]
- Okuda, H.; Noda, K.; Hirabayashi, T. Free ABA concentration in fruit from water-stressed Satsuma mandarin trees did not increase at night. J. Hortic. Sci. Biotechnol. 2002, 77, 674–676. [Google Scholar] [CrossRef]
- García-Mariño, N.; De la Torre, F.; Matilla, A.J. Organic acids and soluble sugars in edible and nonedible parts of damson plum (Prunus domestica L. subsp. insititia cv. Syriaca) fruits during development and ripening. Food Sci. Technol. Int. 2008, 14, 187–193. [Google Scholar] [CrossRef]
- Zhu, T.; Deng, X.; Zhou, X.; Zhu, L.; Zou, L.; Li, P.; Zhang, D.; Lin, H. Ethylene and hydrogen peroxide are involved in brassinosteroid-induced salt tolerance in tomato. Sci. Rep. 2016, 6, 35392. [Google Scholar] [CrossRef]
- Grierson, W. Maturity and grade standards. In Fresh Citrus Fruits; Wardowski, W.F., Miller, W.M., Hall, D.J., Grierson, W., Eds.; Florida Science Source, Inc.: Longboat Key, FL, USA, 2006; pp. 23–48. [Google Scholar]
- Sun, Y.P.; Zhang, Z.P.; Wang, L.S. Promotion of 5-aminolevulinic acid treatment on leaf photosynthesis is related with increase of antioxidant enzyme activity in watermelon seedlings grown under shade condition. Potosynthetica 2009, 47, 347–354. [Google Scholar] [CrossRef]
- Huang, F.; Li, M.; Wang, L.; Li, S.; He, J. Mitigative effects of foliage spraying and root irrigation of ALA plantlets exposed to cold stress. Agric. Sci. Technol. 2013, 14, 858–862. [Google Scholar]
- Yin, X.; Sun, Z.; Struik, P.C.; Gu, J. Evaluating a new method to estimate the rate of leaf respiration in the light by analysis of combined gas exchange and chlorophyll fluorescence measurements. J. Exp. Bot. 2011, 62, 3489–3499. [Google Scholar] [CrossRef] [PubMed]
- Crisosto, C.H.; Johnson, R.S.; Luza, J.G.; Crisosto, G. Irrigation regimes affect fruit soluble solid concentration and rate of water loss of ‘O’Henry’ peaches. Hortic. Sci. 1994, 29, 1169–1171. [Google Scholar] [CrossRef]
- Xu, F.; Xi, Z.-M.; Zhang, H.; Zhang, C.-I.; Zhang, Z.-W. Brassinosteroids are involved in controlling sugar uploading in Vitis vinifera ‘Cabernet Sauvignon’ berries during veraison. Plant Physiol. Biochem. 2015, 94, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Yakushiji, H.; Nonami, H.; Fukuyama, T.; Ono, S.; Takagi, N.; Hashimoto, Y. Sugar accumulation enhanced by osmoregulation in Satsuma Mandarin fruit. J. Am. Soc. Hortic. Sci. 1996, 121, 466–472. [Google Scholar] [CrossRef]
- Thakur, A.; Zora, S. Responses of ‘Spring Bright’ and ‘Summer Bright’ nectarines to deficit irrigation: Fruit growth and concentration of sugars and organic acids. Sci. Hort. 2012, 135, 112–119. [Google Scholar] [CrossRef]
- Cohen, A.; Goell, A. Fruit growth and dry matter accumulation in grapefruit during periods of water withholding after irrigation. Aust. J. Plant Physiol. 1988, 15, 633–639. [Google Scholar]
- World Weather Online. El-khatatba Historical Weather; Egypt Historical Weather Almanac: Manchester, UK, 2020; Available online: https://www.worldweatheronline.com/ezbet-mahattet-el-khatatba-weather/al-minufiyah/eg.aspx (accessed on 23 February 2021).
- Wilde, S.A.; Corey, R.B.; Layer, J.G.; Voigt, G.K. Soils and Plant Analysis for Tree Culture, 3rd ed.; Oxford and IBH Publishing Co.: New Delhi, India, 1985; pp. 529–546. [Google Scholar]
- Wettstein, D. Chlorophyll—Letale und der submikroskopische Formwechsel der Plastiden. Exp. Cell Res. 1957, 12, 427–487. [Google Scholar] [CrossRef]
- Radmer, R. Studies of O2 evolution using H2O analogs and mass spectrometry. In The Oxygen Evolving System of Photosynthesis, Proceeding of the International Symposium on Photosynthetic Water Oxidation and Photosystem II Photochemistry, Wako, Japan, 15–17 March 1983; Inoue, Y., Crofts, R.A., Murata, N., Renger, G., Satoh, K., Eds.; Elsevier Inc.: Philadelphia, PA, USA, 1983; Volume 3, pp. 135–144. [Google Scholar]
- Madhava, R.K.V.; Sresty, T.V. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan L. Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Ilik, P.; Spundova, M.; Sicner, M.; Melkovicova, H.; Kucerova, Z.; Krchnak, P.; Furst, T.; Vecerova, K.; Panzarova, K.; Benediktyova, Z.; et al. Estimating heat tolerance of plants by ion leakage: A new method based on gradual heating. New Phytol. 2018, 218, 1278–1287. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Herzog, V.; Fahimi, H.D. Intracellular distinction between peroxidase andcatalase in exocrine cells of rat lacrimal gland: A biochemical and cytochemical study. Histochemistry 1976, 46, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Bergmeyer, H.-U. Methods of Enzymatic Analysis, 2nd ed.; Academic Press: Berlin, Germany, 1974; Volume 4, p. 800. [Google Scholar]
- Koshioka, M.; Harada, J.M.; Noma, T.; Sassa, T.; Ogiama, K.; Taylor, S.; Rood, S.B.; Legge, R.L.; Pharis, R.P.K. Reversed-phase C18 high performance liquid chromatography of acidic and conjugated gibberellins. J. Chromatogr. 1983, 256, 101–115. [Google Scholar] [CrossRef]
- Nicander, B.; Stahl, U.; Bjorkman, P.O.; Tillberg, E. Immunoaffinity co-purification of cytokinies and analysis by high-performance liquid chromatography with ultra violet spectrum detection. Plant J. 1993, 189, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.F. Banana. In Fruits of Warm Climates, 1st ed.; Morton, J.F., Ed.; Florida Flair Books: Miami, FL, USA, 1987; Chapter 4; pp. 29–46. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000; pp. 490–510. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1980; p. 507. [Google Scholar]

| Foliar Spray | Water Requirements (WR) | |||
|---|---|---|---|---|
| 2018/2019 | 2019/2020 | |||
| 100% WR | 75% WR | 100% WR | 75% WR | |
| MDA (nmol·mg−1 fw) | ||||
| Control | 0.11 ± 0.040 a | 0.17 ± 0.033 a | 0.11 ± 0.013 a | 0.19 ± 0.015 a |
| ALA 15 mg·L−1 | 0.09 ± 0.018 b | 0.13 ± 0.024 b | 0.11 ± 0.018 a | 0.17 ± 0.023 ab |
| ALA 30 mg·L−1 | 0.05 ± 0.006 d | 0.11 ± 0.005 c | 0.05 ± 0.005 c | 0.14 ± 0.080 c |
| ALA 45 mg·L−1 | 0.11 ± 0.028 a | 0.17 ± 0.060 a | 0.12 ± 0.014 a | 0.19 ± 0.061 a |
| EBL 15 mg·L−1 | 0.05 ± 0.008 d | 0.12 ± 0.004 b | 0.07 ± 0.006 b | 0.13 ± 0.029 c |
| EBL 30 mg·L−1 | 0.03 ± 0.004 e | 0.08 ± 0.009 d | 0.04 ± 0.004 d | 0.09 ± 0.009 d |
| EBL 45 mg·L−1 | 0.07 ± 0.003 c | 0.12 ± 0.017 b | 0.07 ± 0.008 b | 0.16 ± 0.018 b |
| EL (%) | ||||
| Control | 54 ± 4.35 b | 60.5 ± 4.71 a | 57.5 ± 2.03 b | 64 ± 2.13 b |
| ALA 15 mg·L−1 | 50 ± 5.11 b | 62 ± 6.28 a | 56 ± 1.90 b | 61 ± 4.21 b |
| ALA 30 mg·L−1 | 42 ± 3.82 c | 45 ± 5.04 bc | 41 ± 2.41 c | 46 ± 1.80 c |
| ALA 45 mg·L−1 | 62 ± 3.49 a | 64 ± 6.00 a | 67 ± 3.11 a | 71 ± 3.65 a |
| EBL 15 mg·L−1 | 34 ± 4.11 d | 43 ± 3.78 c | 37 ± 2.08 d | 42 ± 3.00 d |
| EBL 30 mg·L−1 | 26 ± 4.00 e | 32 ± 5.26 d | 27 ± 2.63 e | 32 ± 2.18 e |
| EBL 45 mg·L−1 | 38 ± 3.99 d | 48 ± 3.88 b | 42 ± 1.98 c | 47 ± 2.07 c |
| Foliar Spray | Water Requirements (WR) | |||
|---|---|---|---|---|
| 2018/2019 | 2019/2020 | |||
| 100% WR | 75% WR | 100% WR | 75% WR | |
| IAA (μg·g−1 fw) | ||||
| Control | 22.9 ± 0.78 e | 20 ± 0.77 e | 22.8 ± 0.8 e | 19.7 ± 0.95 e |
| ALA 15 mg·L−1 | 26.1 ± 0.61 d | 21.6 ± 1 d | 25.9 ± 0.74 d | 21.4 ± 1.11 d |
| ALA 30 mg·L−1 | 29.7 ± 89 b | 27.8 ± 1.08 b | 29.5 ± 1.4 b | 27.6 ± 0.91 b |
| ALA 45 mg·L−1 | 26.8 ± 9 d | 25.8 ± 0.92 c | 26.5 ± 0.5 d | 25.5 ± 0.88 c |
| EBL 15 mg·L−1 | 27.7 ± 55 c | 24.5 ± 0.85 c | 27.5 ± 0.67 c | 24.3 ± 1.27 c |
| EBL 30 mg·L−1 | 32.2 ± 1.02 a | 30.1 ± 1.2 a | 32.1 ± 1.44 a | 29.9 ± 1.25 a |
| EBL 45 mg·L−1 | 27.9 ± 0.87 c | 27.3 ± 0.67 b | 27.6 ± 0.6 c | 27.0 ± 0.89 b |
| CKs (μg·g−1 fw) | ||||
| Control | 7.0 ± 0.24 e | 5.9 ± 0.33 e | 6.9 ± 0.12 d | 5.8 ± 0.37 e |
| ALA 15 mg·L−1 | 8.3 ± 0.35 d | 7.4 ± 0.19 d | 8.1 ± 0.17 c | 7.3 ± 0.15 d |
| ALA 30 mg·L−1 | 9.4 ± 0.29 b | 8.5 ± 0.31 b | 9.2 ± 0.28 b | 8.5 ± 0.19 b |
| ALA 45 mg·L−1 | 8.6 ± 0.33 d | 7.4 ± 0.24 d | 8.3 ± 0.35 c | 7.5 ± 0.34 c |
| EBL 15 mg·L−1 | 8.9 ± 0.4 d | 8.0 ± 0.36 c | 8.6 ± 0.41 c | 7.7 ± 0.49 c |
| EBL 30 mg·L−1 | 10.3 ± 0.42 a | 9.0 ± 0.51 a | 9.9 ± 0.48 a | 8.9 ± 0.17 a |
| EBL 45 mg·L−1 | 9.0 ± 0.56 c | 8.3 ± 0.39 b | 8.9 ± 0.4 b | 7.9 ± 0.23 c |
| ABA (μg·g−1 fw) | ||||
| Control | 0.61 ± 0.09 a | 0.75 ± 0.07 a | 0.64 ± 0.02 a | 0.77 ± 0.03 a |
| ALA 15 mg·L−1 | 0.57 ± 0.06 a | 0.66 ± 0.04 b | 0.59 ± 0.02 b | 0.69 ± 0.06 b |
| ALA 30 mg·L−1 | 0.52 ± 0.04 b | 0.55 ± 0.08 c | 0.55 ± 0.04 b | 0.59 ± 0.03 c |
| ALA 45 mg·L−1 | 0.54 ± 0.09 b | 0.63 ± 0.05 b | 0.58 ± 0.05 b | 0.67 ± 0.04 b |
| EBL 15 mg·L−1 | 0.53 ± 0.03 b | 0.61 ± 0.04 b | 0.56 ± 0.04 b | 0.63 ± 0.03 b |
| EBL 30 mg·L−1 | 0.48 ± 0.04 c | 0.51 ± 0.03 d | 0.50 ± 0.03 c | 0.53 ± 0.02 d |
| EBL 45 mg·L−1 | 0.51 ± 0.04 b | 0.59 ± 0.03 c | 0.54 ± 0.03 b | 0.61 ± 0.05 c |
| Foliar Spray | Water Requirements (WR) | |||
|---|---|---|---|---|
| 2018/2019 | 2019/2020 | |||
| 100% WR | 75% WR | 100% WR | 75% WR | |
| Bunch weight (kg) | ||||
| Control | 28.8 ± 0.7 f | 25.2 ± 0.88 c | 28.0 ± 0.4 e | 24.6 ± 0.67 d |
| ALA 15 mg·L−1 | 33.6 ± 0.47 c | 31.6 ± 0.3 b | 29.8 ± 0.7 e | 32.4 ± 0.51 c |
| ALA 30 mg·L−1 | 34.4 ± 1 b | 32.0 ± 0.41 b | 34.5 ± 1.2 a | 34.8 ± 0.4 ab |
| ALA 45 mg·L−1 | 31.2 ± 0.5 e | 32.5 ± 0.5 b | 31.4 ± 0.3 c | 32.5 ± 0.71 c |
| EBL 15 mg·L−1 | 31.6 ± 0.25 e | 34.5 ± 0.32 a | 30.2 ± 0.65 d | 34.0 ± 0.39 b |
| EBL 30 mg·L−1 | 36.0 ± 0.7 a | 35.2 ± 0.4 a | 34.4 ± 0.81 a | 35.5 ± 0.53 a |
| EBL 45 mg·L−1 | 32.2 ± 0.39 d | 32.8 ± 1.03 b | 32.5 ± 0.36 b | 32.5 ± 0.37 c |
| Number of hands·bunch−1 | ||||
| Control | 10.6 ± 0.33 d | 10.0 ± 0.29 d | 10.3 ± 0.19 d | 9.5 ± 0.34 d |
| ALA 15 mg·L−1 | 11.5 ± 0.28 c | 11.4 ± 0.36 c | 11.0 ± 1.0 c | 11.0 ± 0.7 c |
| ALA 30 mg·L−1 | 12.5 ± 0.31 b | 12.0 ± 0.22 b | 12.2 ± 0.78 b | 11.8 ± 0.42 b |
| ALA 45 mg·L−1 | 11.8 ± 0.4 c | 11.2 ± 0.3 c | 11.6 ± 0.27 c | 11.0 ± 0.35 c |
| EBL 15 mg·L−1 | 12.5 ± 0.72 b | 12.0 ± 0.48 b | 12.2 ± 1.03 b | 11.8 ± 0.47 b |
| EBL 30 mg·L−1 | 14.0 ± 0.85 a | 13.5 ± 0.27 a | 13.8 ± 0.061 a | 13.2 ± 0.401 a |
| EBL 45 mg·L−1 | 12.2 ± 0.78 b | 12.0 ± 0.38 b | 12.5 ± 0.23 b | 11.6 ± 0.17 b |
| Number of fruits·hand−1 | ||||
| Control | 15.5 ± 0.25 e | 14.1 ± 0.12 d | 15.2 ± 0.21 e | 13.7 ± 0.27 d |
| ALA 15 mg·L−1 | 16.0 ± 0.21 d | 15.3 ± 0.09 c | 15.8 ± 0.32 d | 15.0 ± 0.28 c |
| ALA 30 mg·L−1 | 17.2 ± 0.2 b | 16.5 ± 0.3 b | 16.8 ± 0.19 b | 16.5 ± 0.31 a |
| ALA 45 mg·L−1 | 16.5 ± 0.42 c | 16.2 ± 0.19 b | 15.2 ± 0.24 e | 16.0 ± 0.19 b |
| EBL 15 mg·L−1 | 16.8 ± 0.31 c | 16.1 ± 0.22 b | 16.2 ± 0.19 c | 16.0 ± 0.8 b |
| EBL 30 mg·L−1 | 17.9 ± 0.27 a | 17.4 ± 0.1 a | 17.8 ± 0.5 a | 16.8 ± 0.24 a |
| EBL 45 mg·L−1 | 16.6 ± 0.15 c | 16.2 ± 0.09 b | 16.0 ± 0.23 c | 15.8 ± 0.41 b |
| Temperature | Humidity | Rainfall | Wind Speed | Cloud | Sun | UV Index | |
|---|---|---|---|---|---|---|---|
| (°C) | (%) | (mm·monh−1) | (km·h−1) | (%) | (days·month−1) | ||
| Winter | 17.0 | 56.0 | 4.7 | 14.0 | 22.0 | 24.1 | 4.9 |
| Spring | 25.6 | 44.9 | 3.3 | 15.6 | 13.8 | 27.7 | 6.8 |
| Summer | 32.9 | 50.7 | 0.0 | 14.5 | 5.1 | 30.7 | 8.0 |
| Fall | 27.2 | 56.8 | 2.3 | 13.0 | 11.4 | 28.8 | 6.7 |
| Characteristics | Soil Depth (0–30 cm) |
|---|---|
| Texture | Sandy |
| Coarse + fine sand (%) | 89.7 |
| Silt (%) | 6.0 |
| Clay (%) | 4.3 |
| Organic matter (%) | 0.65 |
| N (%) | 0.88 |
| P (%) | 0.25 |
| K (%) | 0.41 |
| CaCO3 (%) | 1.65 |
| HCO3− (meq·L−1) (1:20 extract) | 2.1 |
| CO32− (meq·L−1) (1:20 extract) | 0.0 |
| Cl− (meq·L−1) (1:20 extract) | 2.2 |
| SO42− (meq·L−1) (1:20 extract) | 2.8 |
| Ca2+ (meq·L−1) (1:20 extract) | 2.8 |
| Mg2+ (meq·L−1) (1:20 extract) | 1.1 |
| Na+ (meq·L−1) (1:20 extract) | 0.5 |
| K+ (meq·L−1) (1:20 extract) | 1.6 |
| Fe (mg·L−1) | 2.5 |
| Mn (mg·L−1) | 1.58 |
| Zn (mg·L−1) | 0.23 |
| EC (ds·m−1) (1:5 extract) | 4.5 |
| pH (1:5 extract) | 8.1 |
| Bulk density (g·cm−3) | 1.55 |
| Field capacity | 13.7 |
| Wilting point | 4.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helaly, M.N.; El-Hoseiny, H.M.; Elsheery, N.I.; Kalaji, H.M.; de los Santos-Villalobos, S.; Wróbel, J.; Hassan, I.F.; Gaballah, M.S.; Abdelrhman, L.A.; Mira, A.M.; et al. 5-Aminolevulinic Acid and 24-Epibrassinolide Improve the Drought Stress Resilience and Productivity of Banana Plants. Plants 2022, 11, 743. https://doi.org/10.3390/plants11060743
Helaly MN, El-Hoseiny HM, Elsheery NI, Kalaji HM, de los Santos-Villalobos S, Wróbel J, Hassan IF, Gaballah MS, Abdelrhman LA, Mira AM, et al. 5-Aminolevulinic Acid and 24-Epibrassinolide Improve the Drought Stress Resilience and Productivity of Banana Plants. Plants. 2022; 11(6):743. https://doi.org/10.3390/plants11060743
Chicago/Turabian StyleHelaly, Mohamed N., Hanan M. El-Hoseiny, Nabil I. Elsheery, Hazem M. Kalaji, Sergio de los Santos-Villalobos, Jacek Wróbel, Islam F. Hassan, Maybelle S. Gaballah, Lamyaa A. Abdelrhman, Amany M. Mira, and et al. 2022. "5-Aminolevulinic Acid and 24-Epibrassinolide Improve the Drought Stress Resilience and Productivity of Banana Plants" Plants 11, no. 6: 743. https://doi.org/10.3390/plants11060743
APA StyleHelaly, M. N., El-Hoseiny, H. M., Elsheery, N. I., Kalaji, H. M., de los Santos-Villalobos, S., Wróbel, J., Hassan, I. F., Gaballah, M. S., Abdelrhman, L. A., Mira, A. M., & Alam-Eldein, S. M. (2022). 5-Aminolevulinic Acid and 24-Epibrassinolide Improve the Drought Stress Resilience and Productivity of Banana Plants. Plants, 11(6), 743. https://doi.org/10.3390/plants11060743








