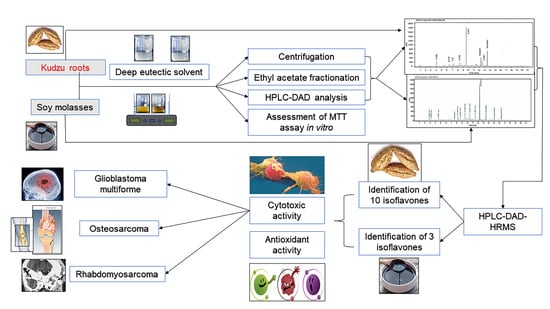
Antioxidant and Cytotoxic Activities of Kudzu Roots and Soy Molasses against Pediatric Tumors and Phytochemical Analysis of Isoflavones Using HPLC-DAD-ESI-HRMS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Equipment
2.2. Plant Materials
2.3. Preparation of Natural Deep Eutectic Solvents (NADESs)
2.4. NADES-Based Ultrasound-Assisted Extraction of KR and SM
2.5. Quantitative Determination of Isoflavones Using HPLC-DAD Method
2.6. Analysis of Isoflavones Using HPLC-ESI-HRMS Method
2.7. DPPH Radical Scavenging Activity (Spectrophotometry and Electron Spin Resonance Spectroscopy)
2.8. Total Polyphenol Content
2.9. Total Flavonoid Content (NaNO2–Al (NO3)3–NaOH Colorimetric Method)
2.10. Assessment of Biological Activity
2.10.1. Cell Lines and Culturing
2.10.2. Cell Viability Assessment
2.11. Statistical Analysis
3. Results and Discussion
3.1. Extraction, Recovery, and Quantification of Isoflavones
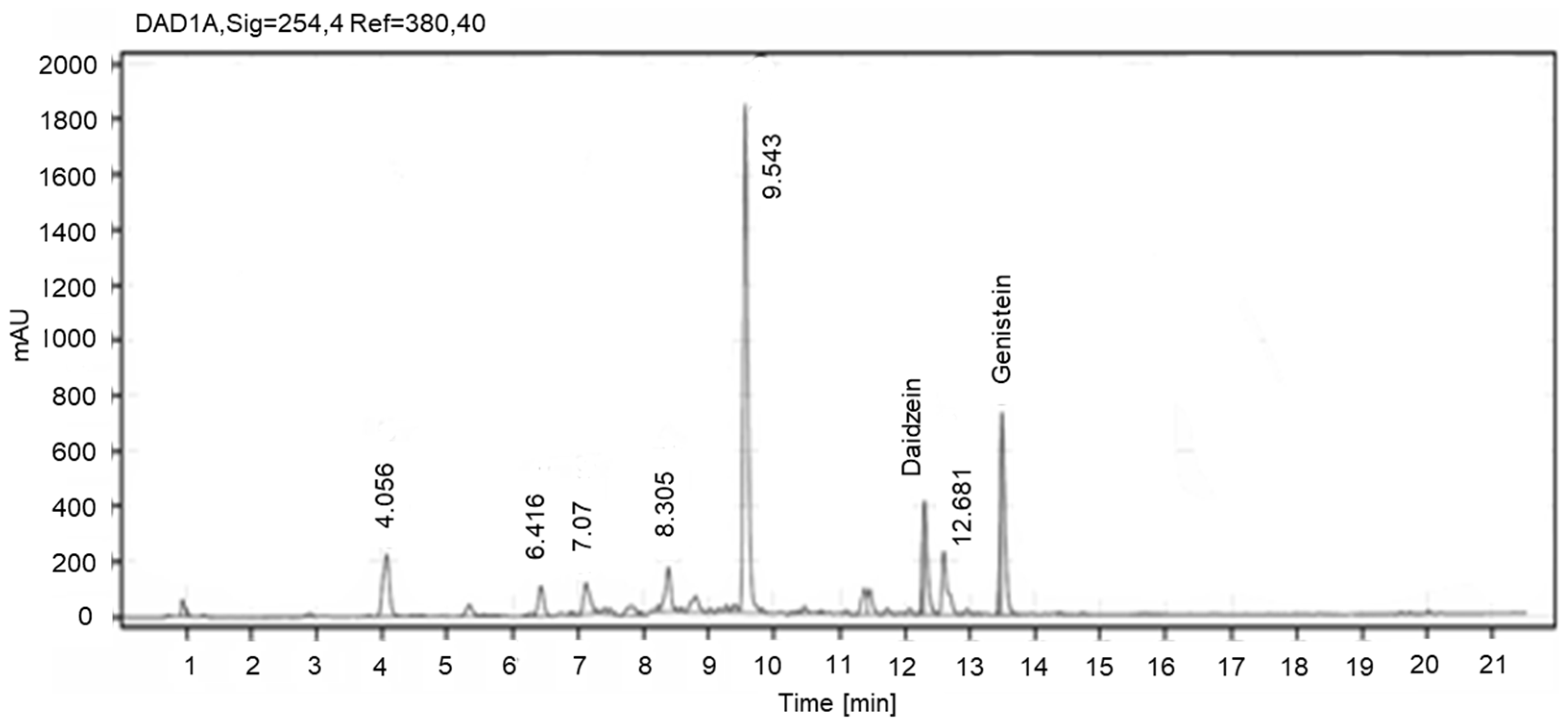
3.2. Quantification of Isoflavones in KR and SM Extracts Using HPLC-DAD
3.3. HPLC-ESI-HRMS Analysis
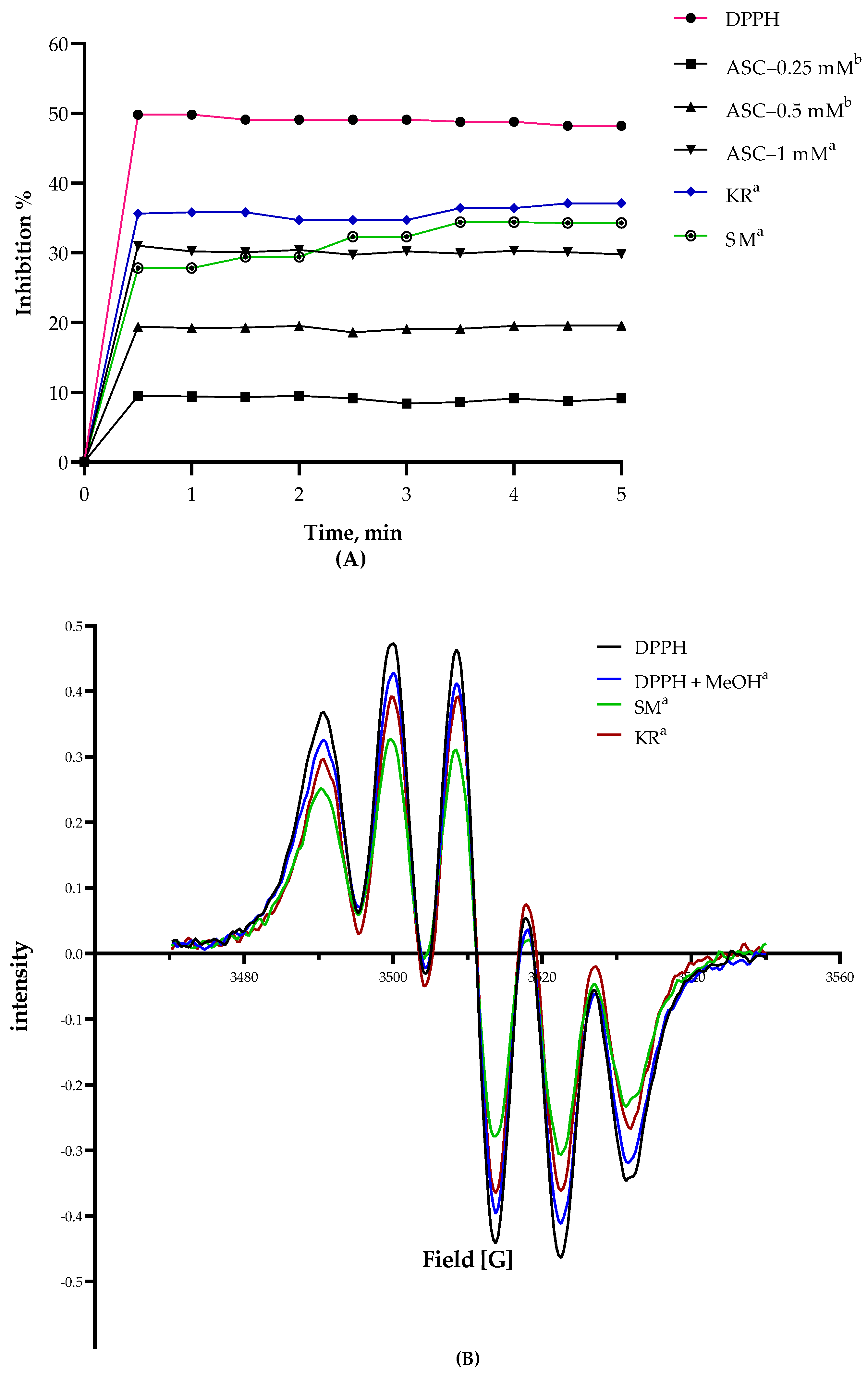
3.4. DPPH Radical Scavenging Activity
3.5. Total Polyphenol and Total Flavonoid Contents
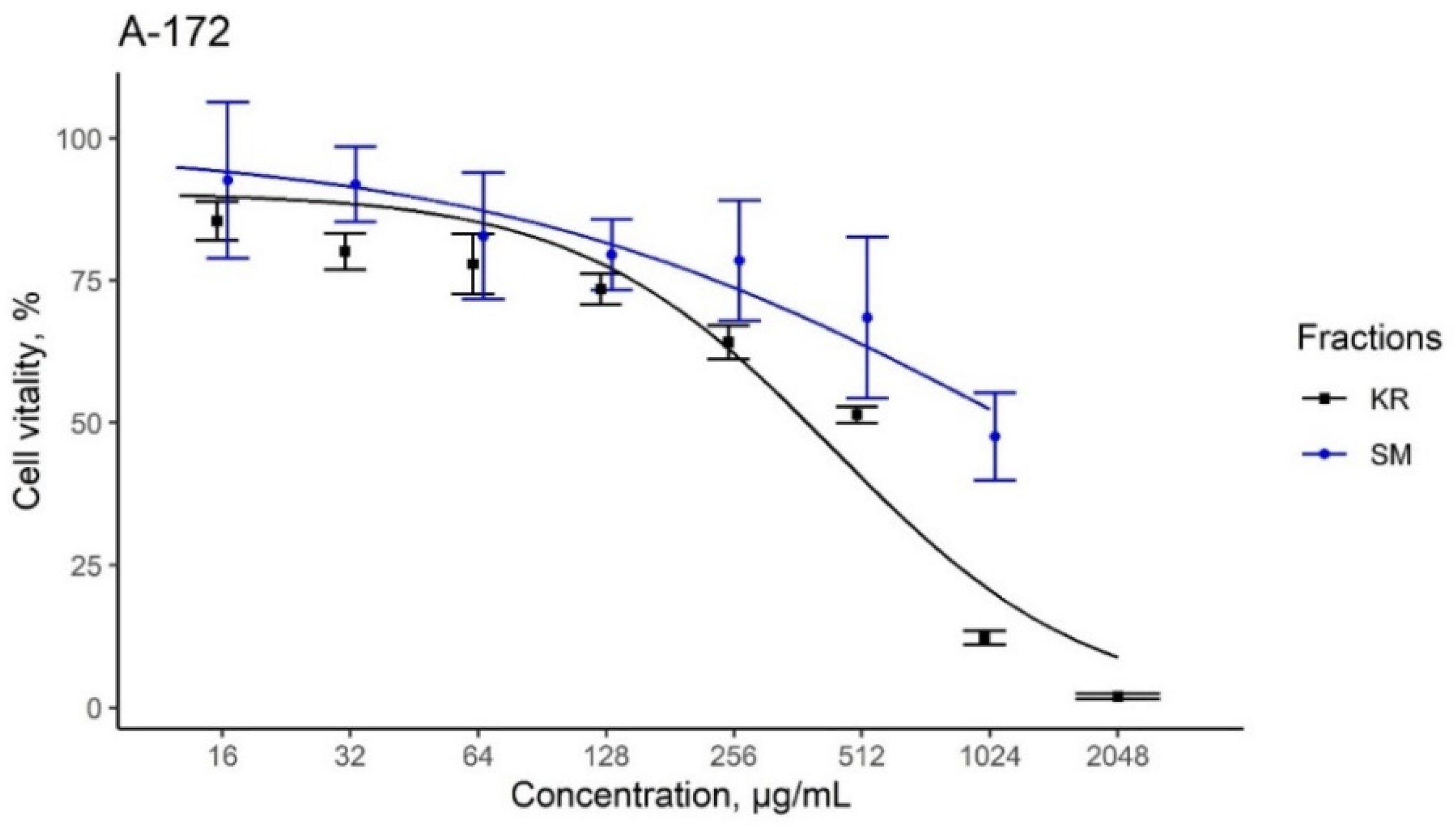
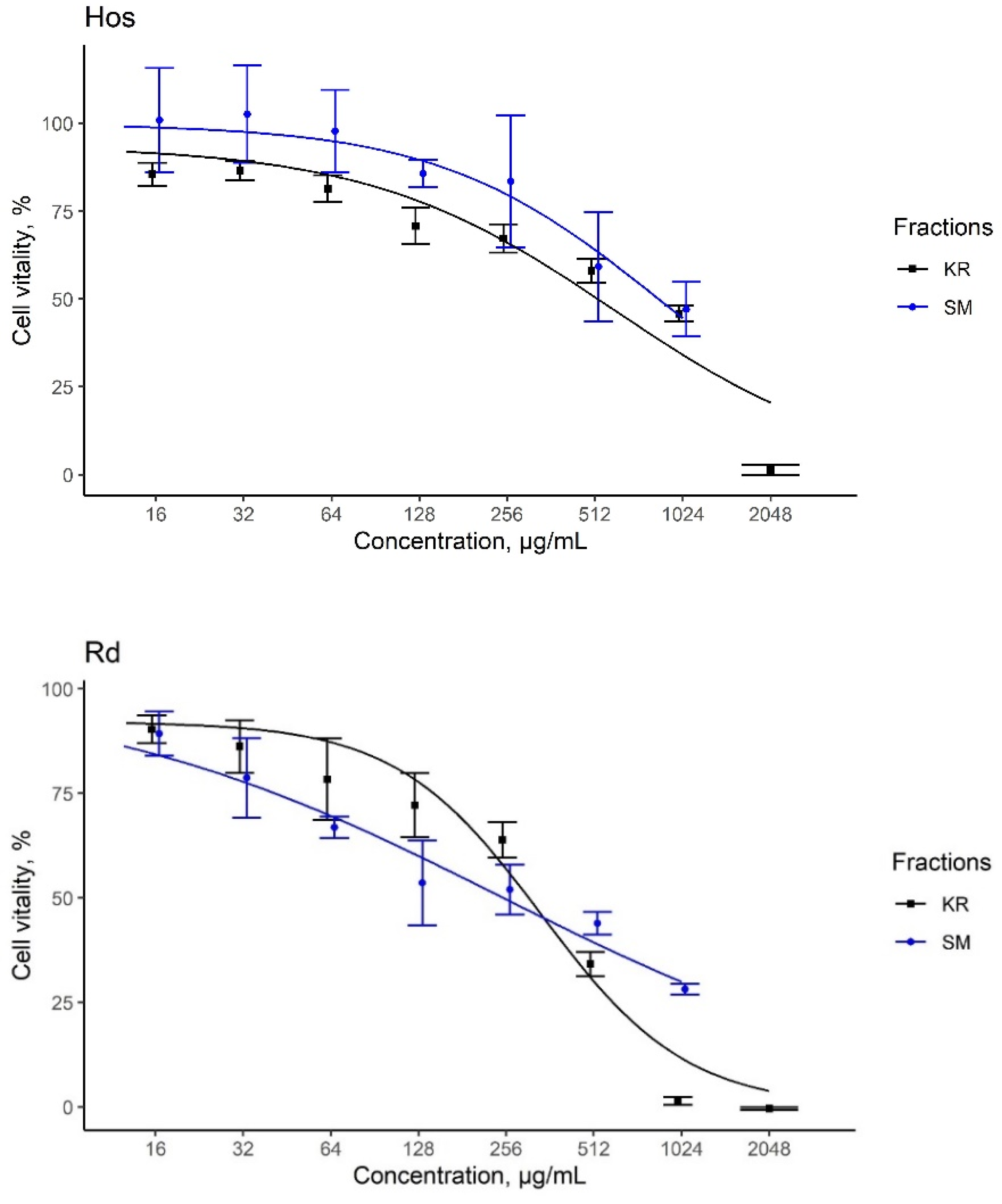
3.6. Assessment of Cytotoxic Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jones, D.T.W.; Banito, A.; Grünewald, T.G.P.; Haber, M.; Jäger, N.; Kool, M.; Milde, T.; Molenaar, J.J.; Nabbi, A.; Pugh, T.J.; et al. Molecular characteristics and therapeutic vulnerabilities across paediatric solid tumours. Nat. Rev. Cancer 2019, 19, 420–438. [Google Scholar] [CrossRef]
- Meng, J.; Bai, Z.; Huang, W.; Liu, Y.; Wang, P.; Nie, S.; Huang, X. Polysaccharide from white kidney bean can improve hyperglycemia and hyperlipidemia in diabetic rats. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100222. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Serre, D.; Reichardt, P.; Martín-Broto, J.; Bauer, S. Options for treating different soft tissue sarcoma subtypes. Future Oncol. 2018, 14, 25–49. [Google Scholar] [CrossRef]
- Patrikidou, A.; Domont, J.; Cioffi, A.; Le Cesne, A. Treating soft tissue sarcomas with adjuvant chemotherapy. Curr. Treat. Options Oncol. 2011, 12, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, A.; Citrin, D. The Role of Radiation Therapy in the Management of Sarcomas. Surg. Clin. N. Am. 2008, 88, 629–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karavasilis, V.; Seddon, B.M.; Ashley, S.; Al-Muderis, O.; Fisher, C.; Judson, I. Significant clinical benefit of first-line palliative chemotherapy in advanced soft-tissue sarcoma: Retrospective analysis and identification of prognostic factors in 488 patients. Cancer 2008, 112, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Italiano, A.; Toulmonde, M.; Cioffi, A.; Penel, N.; Isambert, N.; Bompas, E.; Duffaud, F.; Patrikidou, A.; Lortal, B.; Le cesne, A.; et al. Advanced well-differentiated/dedifferentiated liposarcomas: Role of chemotherapy and survival. Ann. Oncol. 2012, 23, 1601–1607. [Google Scholar] [CrossRef]
- Jones, R.L.; Fisher, C.; Al-Muderis, O.; Judson, I.R. Differential sensitivity of liposarcoma subtypes to chemotherapy. Eur. J. Cancer 2005, 41, 2853–2860. [Google Scholar] [CrossRef]
- Torrens-Mas, M.; Roca, P. Phytoestrogens for cancer prevention and treatment. Biology 2020, 9, 427. [Google Scholar] [CrossRef]
- Mojica, L.; de Mejía, E.G. Characterization and Comparison of Protein and Peptide Profiles and their Biological Activities of Improved Common Bean Cultivars (Phaseolus vulgaris L.) from Mexico and Brazil. Plant Foods Hum. Nutr. 2015, 70, 105–112. [Google Scholar] [CrossRef]
- Rau, K.M.; Kang, H.Y.; Cha, T.L.; Miller, S.A.; Hung, M.C. The mechanisms and managements of hormone-therapy resistance in breast and prostate cancers. Endocr. Relat. Cancer 2005, 12, 511–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthuramalingan, S.R.; Patel, K.; Protheroe, A. Management of patients with hormone refractory prostate cancer. Clin. Oncol. 2004, 16, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Son, E.; Yoon, J.M.; An, B.J.; Lee, Y.M.; Cha, J.; Chi, G.Y.; Kim, D.S. Comparison among Activities and Isoflavonoids from Pueraria thunbergiana Aerial Parts and Root. Molecules 2019, 24, 912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okekunle, A.P.; Gao, J.; Wu, X.; Feng, R.; Sun, C. Higher dietary soy intake appears inversely related to breast cancer risk independent of estrogen receptor breast cancer phenotypes. Heliyon 2020, 6, e04228. [Google Scholar] [CrossRef] [PubMed]
- Batista, V.S.F.; Nunes, G.L.; Viegas, G.I.; Lucas, B.N.; Bochi, V.C.; Emanuelli, T.; Barin, J.S.; de Menezes, C.R.; da Rosa, C.S. Extraction, characterization and microencapsulation of isoflavones from soybean molasses. Ciencia Rural 2020, 50. [Google Scholar] [CrossRef]
- Tanaka, T.; Yokota, Y.; Tang, H.; Zaima, N.; Moriyama, T.; Kawamura, Y. Anti-hyperglycemic effect of a kudzu (Pueraria lobata) vine extract in ovariectomized mice. J. Nutr. Sci. Vitaminol. 2016, 62, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Chen, J.; Lu, H.; Lai, J.; He, Y.; Liu, S.; Guo, X. Pueraria lobata for Diabetes Mellitus: Past, Present and Future. Am. J. Chin. Med. 2019, 47, 1419–1444. [Google Scholar] [CrossRef] [PubMed]
- Vacek, J.; Klejdus, B.; Lojková, L.; Kubán, V. Current trends in isolation, separation, determination and identification of isoflavones: A review. J. Sep. Sci. 2008, 31, 2054–2067. [Google Scholar] [CrossRef] [PubMed]
- Aboushanab, S.A.; Khedr, S.M.; Gette, I.F.; Danilova, I.G.; Kolberg, N.A.; Ravishankar, G.A.; Ambati, R.R.; Kovaleva, E.G. Isoflavones derived from plant raw materials: Bioavailability, anti-cancer, anti-aging potentials, and microbiome modulation. Crit. Rev. Food Sci. Nutr. 2021, 1–27. [Google Scholar] [CrossRef]
- Benlhabib, E.; Baker, J.I.; Keyler, D.E.; Singh, A.K. Quantitative analysis of phytoestrogens in kudzu-root, soy and spiked serum samples by high-pressure liquid chromatography. Biomed. Chromatogr. 2004, 18, 367–380. [Google Scholar] [CrossRef]
- Drašar, P.; Moravcova, J. Recent advances in analysis of Chinese medical plants and traditional medicines. J. Chromatogr. B 2004, 812, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S.; Prasain, J.; D’Alessandro, T.; Arabshahi, A.; Botting, N.; Lila, M.A.; Jackson, G.; Janle, E.M.; Weaver, C.M. The metabolism and analysis of isoflavones and other dietary polyphenols in foods and biological systems. Food Funct. 2011, 2, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Palma-Duran, S.A.; Caire-Juvera, G.; Campa-Siqueiros, M.M.; Chávez-Suárez, K.M.; Robles-Burgueño, M.R.; Gutiérrez-Coronado, M.L.; Bermúdez-Almada, M.C.; Saucedo-Tamayo, M.S.; Grajeda-Cota, P.; Valenzuela-Quintanar, A.I. A comprehensive HPLC-DAD-ESI-MS validated method for the quantification of 16 phytoestrogens in food, serum and urine. Appl. Sci. 2020, 10, 8147. [Google Scholar] [CrossRef]
- Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Biocompatible Deep Eutectic Solvents Based on Choline Chloride: Characterization and Application to the Extraction of Rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Sulaiman, C.T.; Arun, A.; Anandan, E.M.; Sandhya, C.R.; Balachandran, I. Isolation and identification of phytoestrogens and flavonoids in an Ayurvedic proprietary medicine using chromatographic and Mass Spectroscopic analysis. Asian Pac. J. Reprod. 2015, 4, 153–156. [Google Scholar] [CrossRef]
- Achaintre, D.; Buleté, A.; Cren-Olivé, C.; Li, L.; Rinaldi, S.; Scalbert, A. Differential Isotope Labeling of 38 Dietary Polyphenols and Their Quantification in Urine by Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry. Anal. Chem. 2016, 88, 2637–2644. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, A.; Butcher, P.; Maden, K.; Walker, S.; Widmer, M. Comprehensive comparison of liquid chromatography selectivity as provided by two types of liquid chromatography detectors (high resolution mass spectrometry and tandem mass spectrometry): “Where is the crossover point?”. Anal. Chim. Acta 2010, 673, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, S.; Patra, A.; Hussain, M.D.; Kazi, M.; Aldughaim, M.S.; Ahirwar, B. Antioxidant enriched fraction from Pueraria tuberosa alleviates ovariectomized-induced osteoporosis in rats, and inhibits growth of breast and ovarian cancer cell lines in vitro. BioRxiv 2020. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.; Dong, M. Flavonoids of kudzu root fermented by Eurtotium cristatum protected rat pheochromocytoma line 12 (PC12) cells against H2O2-induced apoptosis. Int. J. Mol. Sci. 2017, 18, 2754. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Luo, Q.; Nie, R.; Yang, X.; Tang, Z.; Chen, H. Potential implications of polyphenols on aging considering oxidative stress, inflammation, autophagy, and gut microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 2175–2193. [Google Scholar] [CrossRef]
- Bharti, R.; Chopra, B.S.; Raut, S.; Khatri, N. Pueraria tuberosa: A Review on Traditional Uses, Pharmacology, and Phytochemistry. Front. Pharmacol. 2021, 11, 2014. [Google Scholar] [CrossRef] [PubMed]
- Maji, A.K.; Pandit, S.; Banerji, P.; Banerjee, D. Pueraria tuberosa: A review on its phytochemical and therapeutic potential. Nat. Prod. Res. 2014, 28, 2111–2127. [Google Scholar] [CrossRef]
- Duru, K.C.; Mukhlynina, E.A.; Moroz, G.A.; Gette, I.F.; Danilova, I.G.; Kovaleva, E.G. Anti-diabetic effect of isoflavone rich kudzu root extract in experimentally induced diabetic rats. J. Funct. Foods 2020, 68, 103922. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Slesarev, G.P.; Glukhareva, T.V.; Duru, K.C.; Shevyrin, V.A.; Lyubyakina, P.N.; Kovaleva, E.G. Comparative study of extraction of soy molasses isoflavones and in vivo bioconversion of daidzein into s-equol in rats models. Agron. Res. 2021, 19, 1167–1178. [Google Scholar] [CrossRef]
- Shang, X.; Dou, Y.; Zhang, Y.; Tan, J.N.; Liu, X.; Zhang, Z. Tailor-made natural deep eutectic solvents for green extraction of isoflavones from chickpea (Cicer arietinum L.) sprouts. Ind. Crops Prod. 2019, 140, 111724. [Google Scholar] [CrossRef]
- Chen, T.R.; Shih, S.C.; Ping, H.P.; Wei, Q.K. Antioxidant activity and isoflavonoid components in different sections of Pueraria lobata root. J. Food Drug Anal. 2012, 20, 681–685. [Google Scholar] [CrossRef]
- Tian, F.; Li, B.; Ji, B.; Yang, J.; Zhang, G.; Chen, Y.; Luo, Y. Antioxidant and antimicrobial activities of consecutive extracts from Galla chinensis: The polarity affects the bioactivities. Food Chem. 2009, 113, 173–179. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin-Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Huang, R.; Wu, W.; Shen, S.; Fan, J.; Chang, Y.; Chen, S.; Ye, X. Evaluation of colorimetric methods for quantification of citrus flavonoids to avoid misuse. Anal. Methods 2018, 10, 2575–2587. [Google Scholar] [CrossRef]
- Manzella, G.; Schreck, L.D.; Breunis, W.B.; Molenaar, J.; Merks, H.; Barr, F.G.; Sun, W.; Römmele, M.; Zhang, L.; Tchinda, J.; et al. Phenotypic profiling with a living biobank of primary rhabdomyosarcoma unravels disease heterogeneity and AKT sensitivity. Nat. Commun. 2020, 11, 4629. [Google Scholar] [CrossRef] [PubMed]
- Grube, S.; Freitag, D.; Kalff, R.; Ewald, C.; Walter, J. Characterization of adherent primary cell lines from fresh human glioblastoma tissue, defining glial fibrillary acidic protein as a reliable marker in establishment of glioblastoma cell culture. Cancer Rep. 2021, 4, e1324. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yan, Z.; Liu, Y.; Choy, E.; Hornicek, F.J.; Mankin, H.; Duan, Z. CRISPR-Cas9-Mediated Silencing of CD44 in Human Highly Metastatic Osteosarcoma Cells. Cell. Physiol. Biochem. 2018, 46, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, M.J.; Cruz, A.; Faber, M.; Oldham, R.A.A.; Wang, D.; Medin, J.A.; Schloemer, N.J. Sarcoma IL-12 overexpression facilitates NK cell immunomodulation. Sci. Rep. 2021, 11, 8321. [Google Scholar] [CrossRef] [PubMed]
- Somaida, A.; Tariq, I.; Ambreen, G.; Abdelsalam, A.M.; Ayoub, A.M.; Wojcik, M.; Dzoyem, J.P.; Bakowsky, U. Potent cytotoxicity of four cameroonian plant extracts on different cancer cell lines. Pharmaceuticals 2020, 13, 357. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, J.; Zhang, C.; Wu, W.; Liang, X. Analysis of the estrogenic components in kudzu root by bioassay and high performance liquid chromatography. J. Steroid Biochem. Mol. Biol 2005, 94, 375–381. [Google Scholar] [CrossRef]
- Gu, L.; Gu, W. Characterisation od soy isoflavones and screening for novel malonyl glycosides using high-performance liquid chromatography-electrospray ionisation-mass spectrometry. Phytochem. Anal. 2001, 12, 377–382. [Google Scholar] [CrossRef]
- Zorzi, M.; Gai, F.; Medana, C.; Aigotti, R.; Peiretti, P.G. Identification of Polyphenolic Compounds in Edible Wild Fruits Grown in the North-West of Italy by Means of HPLC-DAD-ESI HRMS. Plant Foods Hum. Nutr. 2020, 75, 420–426. [Google Scholar] [CrossRef]
- Cuyckens, F.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef]
- Montenegro-Burke, J.R.; Guijas, C.; Siuzdak, G. METLIN: A Tandem Mass Spectral Library of Standards. Methods Mol. Biol. 2020, 2104, 149–163. [Google Scholar] [CrossRef]
- Ganai, A.A.; Khan, A.A.; Malik, Z.A.; Farooqi, H. Genistein modulates the expression of NF-κB and MAPK (p-38 and ERK1/2), thereby attenuating d-Galactosamine induced fulminant hepatic failure in Wistar rats. Toxicol. Appl. Pharmacol. 2015, 283, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franski, R.; Gierczyk, B.; Kozik, T.; Popenda, L.; Beszterda, M. Signals of diagnostic ions in the product ion spectra of [M − H](-) ions of methoxylated flavonoids. Rapid Commun. Mass Spectrom. 2019, 33, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Vessecchi, R.; Zocolo, G.J.; Gouvea, D.R.; Hubner, F.; Cramer, B.; de Marchi, M.R.; Humpf, H.U.; Lopes, N.P. Re-examination of the anion derivatives of isoflavones by radical fragmentation in negative electrospray ionization tandem mass spectrometry: Experimental and computational studies. Rapid Commun. Mass Spectrom. 2011, 25, 2020–2026. [Google Scholar] [CrossRef]
- Ablajan, K. A study of characteristic fragmentation of isoflavonoids by using negative ion ESI-MSn. J. Mass Spectrom. 2011, 46, 77–84. [Google Scholar] [CrossRef] [PubMed]
- March, R.E.; Miao, X.-S.; Metcalfe, C.D.; Stobiecki, M.; Marczak, L. A fragmentation study of an isoflavone glycoside, genistein-7-O-glucoside, using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. Int. J. Mass Spectrom. 2004, 232, 171–183. [Google Scholar] [CrossRef]
- Waridel, P.; Wolfender, J.-L.; Ndjoko, K.; Hobby, K.R.; Major, H.J.; Hostettmann, K. Evaluation of quadrupole time-of-flight tandem mass spectrometry and ion-trap multiple-stage mass spectrometry for the differentiation of C-glycosidic flavonoid isomers. J. Chromatogr. A 2001, 926, 29–41. [Google Scholar] [CrossRef]
- McCullagh, M.; Goshawk, J.; Eatough, D.; Mortishire-Smith, R.J.; Pereira, C.A.M.; Yariwake, J.H.; Vissers, J.P.C. Profiling of the known-unknown Passiflora variant complement by liquid chromatography—Ion mobility—Mass spectrometry. Talanta 2021, 221, 121311. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yan, M.; Jiang, H.; Wang, Q.; Guan, X.; Chen, J.; Wang, C. Protective effects of puerarin on acute lung and cerebrum injury induced by hypobaric hypoxia via the regulation of aquaporin (AQP) via NF-κB signaling pathway. Int. Immunopharmacol. 2016, 40, 300–309. [Google Scholar] [CrossRef]
- Liu, B.; Kongstad, K.T.; Qinglei, S.; Nyberg, N.T.; Jäger, A.K.; Staerk, D. Dual High-Resolution α-Glucosidase and Radical Scavenging Profiling Combined with HPLC-HRMS-SPE-NMR for Identification of Minor and Major Constituents Directly from the Crude Extract of Pueraria lobata. J. Nat. Prod. 2015, 78, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Yu-Yang, L.; Lin, S.; Xiu-Li, X.; Xiao-Gang, C. Non-directive Screening for Functional Components in Food Supplements of Pueraria Based on Characteristic Fragmentation. J. Ins. Anal. 2019, 38, 635–642. [Google Scholar]
- Bourgou, S.; Ksouri, R.; Bellila, A.; Skandrani, I.; Falleh, H.; Marzouk, B. Phenolic composition and biological activities of Tunisian Nigella sativa L. shoots and roots. C. R. Biol. 2008, 331, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Jun, M.; Fu, H.Y.; Hong, J.; Wan, X.; Yang, C.S.; Ho, C.T. Comparison of antioxidant activities of isoflavones from kudzu root (Pueraria lobata Ohwi). J. Food Sci. 2003, 68, 2117–2122. [Google Scholar] [CrossRef]
- Kaurinovic, B.; Popovic, M.; Vlaisavljevic, S.; Schwartsova, H.; Vojinovic-Miloradov, M. Antioxidant profile of Trifolium pratense L. Molecules 2012, 17, 11156–11172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harati, K.; Behr, B.; Daigeler, A.; Hirsch, T.; Jacobsen, F.; Renner, M.; Harati, A.; Wallner, C.; Lehnhardt, M.; Becerikli, M. Curcumin and Viscum album Extract Decrease Proliferation and Cell Viability of Soft-Tissue Sarcoma Cells: An In Vitro Analysis of Eight Cell Lines Using Real-Time Monitoring and Colorimetric Assays. Nutr Cancer. 2017, 69, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xiao, A.; Mei, S.; Tang, P.; Ren, L.; Liu, L. Pueraria lobata root constituents as xanthine oxidase inhibitors and protective agents against oxidative stress induced in ges-1 cells. J. Braz. Chem. Soc. 2020, 31, 2071–2081. [Google Scholar] [CrossRef]
- Jeon, G.C.; Park, M.S.; Yoon, D.Y.; Shin, C.H.; Sin, H.S.; Um, S.J. Antitumor activity of spinasterol isolated from Pueraria roots. Exp. Mol. Med. 2005, 37, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Xiao, Z. Formononetin induces apoptosis of human osteosarcoma cell line U2OS by regulating the expression of Bcl-2, Bax and MiR-375 in vitro and in vivo. Cell. Physiol. Biochem. 2015, 37, 933–939. [Google Scholar] [CrossRef]
- Basu, P.; Maier, C. Phytoestrogens and breast cancer: In vitro anticancer activities of isoflavones, lignans, coumestans, stilbenes and their analogs and derivatives. Biomed. Pharmacother. 2018, 107, 1648–1666. [Google Scholar] [CrossRef] [PubMed]
- Mace, T.A.; Ware, M.B.; King, S.A.; Loftus, S.; Farren, M.R.; McMichael, E.; Scoville, S.; Geraghty, C.; Young, G.; Carson, W.E.; et al. Soy isoflavones and their metabolites modulate cytokine-induced natural killer cell function. Sci. Rep. 2019, 9, 5068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Liu, X.; Zhang, J.; Zhou, W.; Lu, J.; Wang, Q.; Hu, R. Daidzein derivative daid002 inhibits glioblastoma growth via disrupting the interaction between moesin and CD44. Oncotarget 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Satpathy, S.; Patra, A.; Hussain, M.D.; Kazi, M.; Aldughaim, M.S.; Ahirwar, B. A fraction of Pueraria tuberosa extract, rich in antioxidant compounds, alleviates ovariectomized-induced osteoporosis in rats and inhibits growth of breast and ovarian cancer cells. PLoS ONE 2021, 16, e0240068. [Google Scholar] [CrossRef]


| Isoflavones | Regression Equation | R2 |
|---|---|---|
| Puerarin | Y = 156.3464X+ 287792 | 0.99966 |
| Daidzein | Y = 236.9975X + 9.2923 | 0.99995 |
| Genistein | Y = 276.2041X + 19.7115 | 0.99979 |
| Formonentin | Y = 202.7583X + 21.7709 | 0.99980 |
| Biochanin A | Y = 256.2404X + 13.2775 | 0.99983 |
| Parameters | KR (%) | SM (%) |
|---|---|---|
| Puerarin | 0.5 ± 0.002 | NI |
| Daidzein | 12 ± 0.004 a | 5.2 ± 0.005 b |
| Genistein | 2 ± 0.021 b | 5.7 ± 0.007 a |
| Formonentin | 0.2 ± 0.008 | NI |
| Biochanin A | 0.2 ± 0.001 | NI |
| Sum | 14.8 ± 0.078 a | 10.9 ± 0.006 b |
| No. | Retention Time (tR, min) | Molecular Formula | Peak Area (EIC) % of Daidzein | Compound Name |
|---|---|---|---|---|
| 1 | 6.1 | C21H20O9 | 1.9 | Puerarin * |
| 2 | 7.1 | C21H20O10 | 4.2 | genistein-8-C-glucoside ** |
| 3 | 7.9 | C21H20O10 | 1.7 | genistein-7-O-glucoside ** |
| 4 | 9.6 | C15H10O5 | 2.2 | isomer of genistein ** |
| 5 | 10.4 | C15H10O4 | 100 | Daidzein * |
| 6 | 10.6 | C16H12O5 | 1.9 | dihydroxy-methoxyisoflavone ** |
| 7 | 11.5 | C15H10O5 | 15.8 | Genistein * |
| 8 | 11.6 | C16H12O6 | 2.6 | Tectorigenin ** |
| 9 | 12.5 | C17H14O5 | 1.6 | hydroxy-dimethoxyisoflavone ** |
| 10 | 12.9 | C16H12O4 | 2.0 | Formononetin * |
| No. | Retention Time (tR, min) | Molecular Formula | Peak Area (EIC) % of Daidzein | Compounds Name |
|---|---|---|---|---|
| 1 | 7.9 | C21H20O10 | 33.7 | genistein-7-O-glucoside ** |
| 2 | 10.4 | C15H10O4 | 100 | Daidzein * |
| 3 | 11.5 | C15H10O5 | 114 | Genistein * |
| Parameters | KR | SM |
|---|---|---|
| DPPH Inhibition (%) | 94.14 ± 0.85 a | 91.37 ± 0.27 b |
| Ascorbic acid (mM equ.) | 2.20 ± 0.05 a | 2.03 ± 0.02 b |
| TPC GA equ. (mmol/L) | 223.1 ± 19.07 b | 330.5 ± 81.45 a |
| TFC Quercetin equ. (mmol/L) | 201.2 ± 10.35 a | 133.1 ± 11.3 b |
| A-172-IC50 (μg/mL) | Hos-IC50 (μg/mL) | Rd-IC50 (μg/mL) | |
|---|---|---|---|
| KR extracts | 440.4 | 597.2 | 337.4 |
| SM extracts | 1212.9 | 847.6 | 244.4 |
| Phytoestrogens/Bioactive Compounds | Concentrations/Cell Lines | Cytotoxic Effect | References |
|---|---|---|---|
| Curcuma longa/curcumin | Fibrosarcoma, liposarcoma, synovial sarcoma, and malignant fibrous histiocytoma/pleomorphic sarcoma/20 μM | ↓ proliferation and viability of soft tissue sarcoma cells | [67] |
| Pueraria lobata/puerarin, daidzin, daidzein, and genistein | Gastric epithelial cell lines (GES-1)/10–100 μmol/L | ↑ cell viability Protect GES-1 cells from injury induced by oxidative stress | [68] |
| Pueraria mirifica and Pueraria lobata/Eight isolated sub-fractions | breast, cervical, ovarian, colon, and liver cancer cell lines/0–5 μg/mL | Potential anti-proliferative effect on different cell lines No effect on normal human fibroblasts or Chang liver cells | [69] |
| Pueraria lobata (fermented vs. non-fermented)/7 different isoflavones | rat pheochromocytoma line 12/0–10 mg/mL | Protect against injury mediated by H2O2-induced oxidative stress | [29] |
| Pueraria tuberosa/genistein and daidzein | breast and ovarian cancer cell lines/31.5 to 500 μg/mL | In vitro cytotoxicity and anticancer activities | [28] |
| Formononetin | human osteosarcoma cell lines (U2OS)/0–80 μM | ↓ proliferation of cancer cells activates apoptotic mechanisms against U2OS | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboushanab, S.A.; Shevyrin, V.A.; Slesarev, G.P.; Melekhin, V.V.; Shcheglova, A.V.; Makeev, O.G.; Kovaleva, E.G.; Kim, K.H. Antioxidant and Cytotoxic Activities of Kudzu Roots and Soy Molasses against Pediatric Tumors and Phytochemical Analysis of Isoflavones Using HPLC-DAD-ESI-HRMS. Plants 2022, 11, 741. https://doi.org/10.3390/plants11060741
Aboushanab SA, Shevyrin VA, Slesarev GP, Melekhin VV, Shcheglova AV, Makeev OG, Kovaleva EG, Kim KH. Antioxidant and Cytotoxic Activities of Kudzu Roots and Soy Molasses against Pediatric Tumors and Phytochemical Analysis of Isoflavones Using HPLC-DAD-ESI-HRMS. Plants. 2022; 11(6):741. https://doi.org/10.3390/plants11060741
Chicago/Turabian StyleAboushanab, Saied A., Vadim A. Shevyrin, Grigory P. Slesarev, Vsevolod V. Melekhin, Anna V. Shcheglova, Oleg G. Makeev, Elena G. Kovaleva, and Ki Hyun Kim. 2022. "Antioxidant and Cytotoxic Activities of Kudzu Roots and Soy Molasses against Pediatric Tumors and Phytochemical Analysis of Isoflavones Using HPLC-DAD-ESI-HRMS" Plants 11, no. 6: 741. https://doi.org/10.3390/plants11060741
APA StyleAboushanab, S. A., Shevyrin, V. A., Slesarev, G. P., Melekhin, V. V., Shcheglova, A. V., Makeev, O. G., Kovaleva, E. G., & Kim, K. H. (2022). Antioxidant and Cytotoxic Activities of Kudzu Roots and Soy Molasses against Pediatric Tumors and Phytochemical Analysis of Isoflavones Using HPLC-DAD-ESI-HRMS. Plants, 11(6), 741. https://doi.org/10.3390/plants11060741