Effect of Jasmonic Acid Foliar Spray on the Morpho-Physiological Mechanism of Salt Stress Tolerance in Two Soybean Varieties (Glycine max L.)
Abstract
1. Introduction
2. Results
2.1. Changes in Seedlings Growth
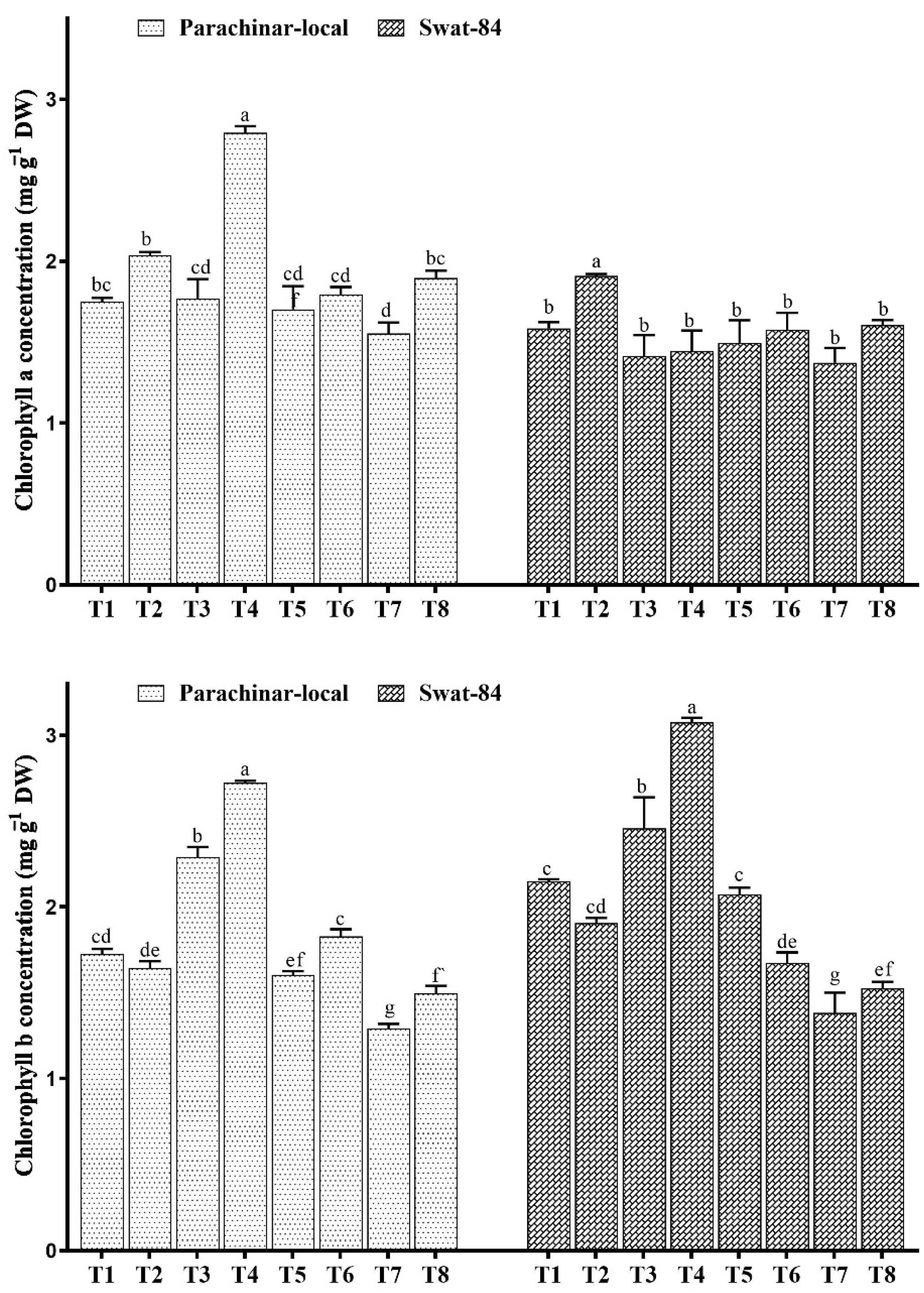
2.2. Changes in Photosynthetic Pigments
2.3. Changes in Foliar Ions Accumulation
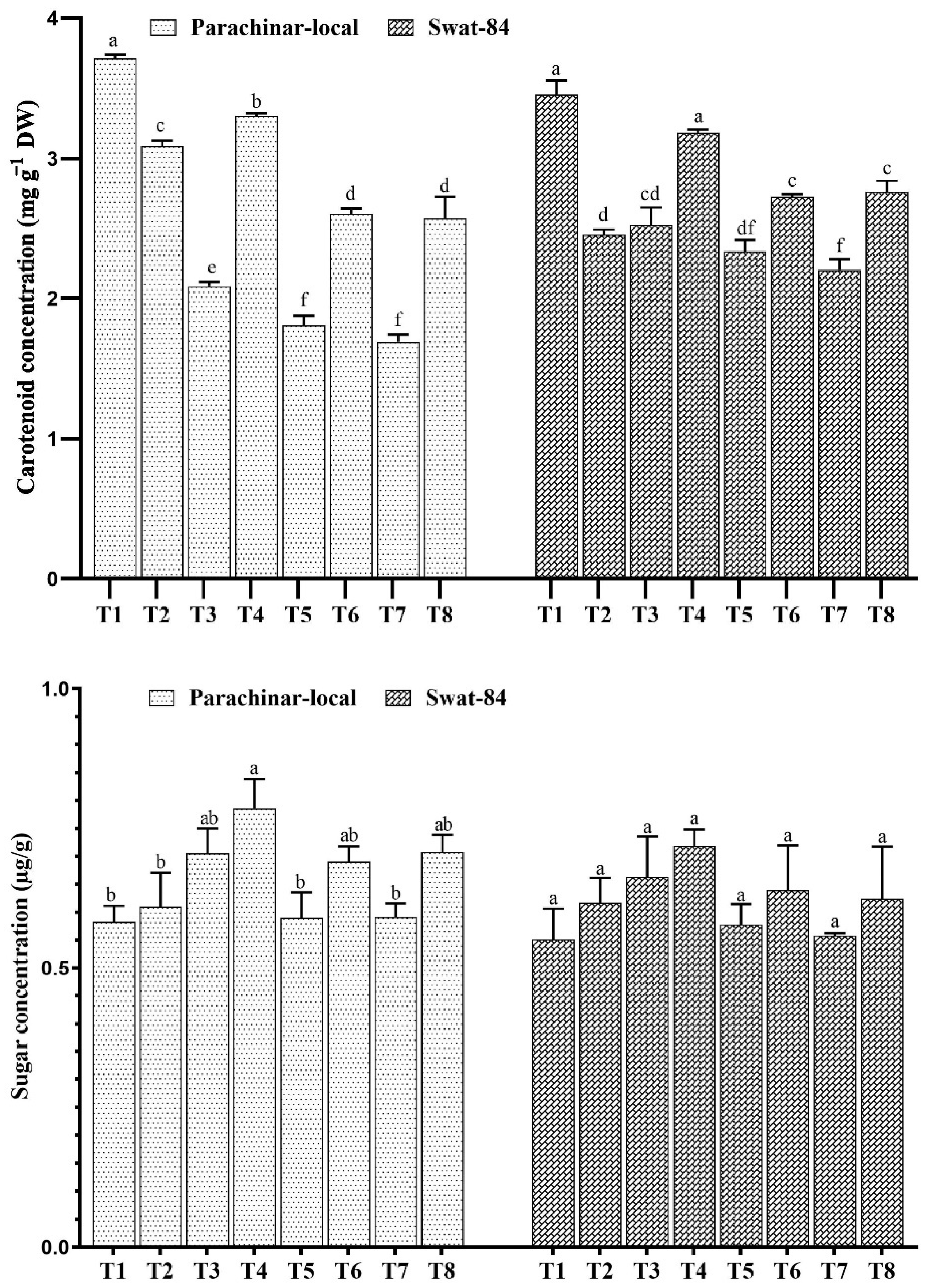
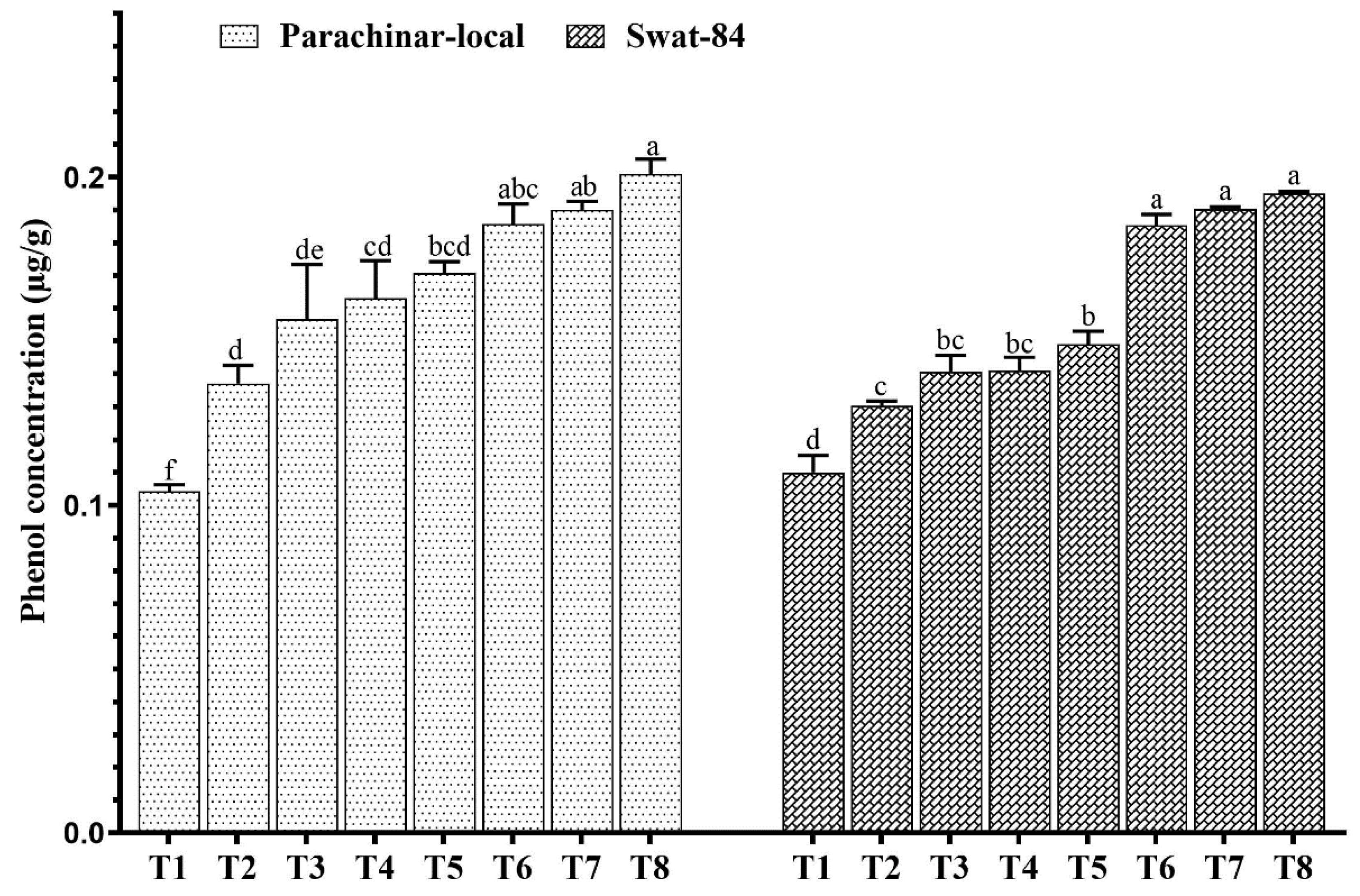
2.4. Changes in Foliar Sugar, Protein, Phenol, and Vitamin A Concentration
3. Discussion
4. Materials and Methods
4.1. Experimental Setup
4.2. Growth Parameters
4.3. Determination of Photosynthetic Pigments
4.4. Determination of Foliar Mineral Nutrients Concentration
4.5. Determination of Foliar Sugar, Protein, Total Phenol, and Vitamin A
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, M.; Kamran, M.; Abbasi, G.H.; Saleem, M.H.; Ahmad, S.; Parveen, A.; Malik, Z.; Afzal, S.; Ahmar, S.; Dawar, K.M.; et al. Melatonin-Induced Salinity Tolerance by Ameliorating Osmotic and Oxidative Stress in the Seedlings of Two Tomato (Solanum lycopersicum L.) Cultivars. J. Plant Growth Regul. 2020, 40, 2236–2248. [Google Scholar] [CrossRef]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.-T. An Overview of Hazardous Impacts of Soil Salinity in Crops, Tolerance Mechanisms, and Amelioration through Selenium Supplementation. Int. J. Mol. Sci. 2019, 21, 148. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.; Serralheiro, R. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Chen, P.; Yan, K.; Shao, H.; Zhao, S. Physiological mechanisms for high salt tolerance in wild soybean (Glycine soja) from Yellow River Delta, China: Photosynthesis, osmotic regulation, ion flux and antioxidant capacity. PLoS ONE 2013, 8, e83227. [Google Scholar] [CrossRef]
- Alam, H.; Khattak, J.Z.; Ksiksi, T.S.; Saleem, M.H.; Fahad, S.; Sohail, H.; Ali, Q.; Zamin, M.; El-Esawi, M.A.; Saud, S. Negative impact of long-term exposure of salinity and drought stress on native Tetraena mandavillei L. Physiol. Plant. 2021, 172, 1336–1351. [Google Scholar] [CrossRef]
- Teakle, N.L.; Tyerman, S.D. Mechanisms of Cl-transport contributing to salt tolerance. Plant Cell Environ. 2010, 33, 566–589. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Zhang, Z.; Chai, X.; Tariq, A.; Zeng, F.; Graciano, C.; Li, X.; Gao, Y.; Ullah, A. Coordinated Patterns in the Allocation, Composition, and Variability of Multiple Elements Among Organs of Two Desert Shrubs Under Nitrogen Addition and Drought. J. Soil Sci. Plant Nutr. 2021, 21, 1–12. [Google Scholar] [CrossRef]
- Ullah, A.; Li, M.; Noor, J.; Tariq, A.; Liu, Y.; Shi, L. Effects of salinity on photosynthetic traits, ion homeostasis and nitrogen metabolism in wild and cultivated soybean. PeerJ 2019, 7, e8191. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Amjad, S.F.; Saleem, M.H.; Yasmin, H.; Imran, M.; Riaz, M.; Ali, Q.; Joyia, F.A.; Ahmed, S.; Ali, S. Foliar application of ascorbic acid enhances salinity stress tolerance in barley (Hordeum vulgare L.) through modulation of morpho-physio-biochemical attributes, ions uptake, osmo-protectants and stress response genes expression. Saudi J. Biol. Sci. 2021, 28, 4276–4290. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, R.; Aziz, O.; Saleem, M.H.; Riaz, M.; Zafar-ul-Hye, M.; Rehman, M.; Ali, S.; Rizwan, M.; Nasser Alyemeni, M.; El-Serehy, H.A. Ameliorating the Drought Stress for Wheat Growth through Application of ACC-Deaminase Containing Rhizobacteria along with Biogas Slurry. Sustainability 2020, 12, 6022. [Google Scholar] [CrossRef]
- Guo, Q.; Major, I.T.; Howe, G.A. Resolution of growth–defense conflict: Mechanistic insights from jasmonate signaling. Curr. Opin. Plant Biol. 2018, 44, 72–81. [Google Scholar] [CrossRef]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth–defense tradeoffs in plants: A balancing act to optimize fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef]
- Jin, S.; Chen, C.; Plant, A. Regulation by ABA of osmotic-stress-induced changes in protein synthesis in tomato roots. Plant Cell Environ. 2000, 23, 51–60. [Google Scholar] [CrossRef]
- Hoyos, M.E.; Zhang, S. Calcium-independent activation of salicylic acid-induced protein kinase and a 40-kilodalton protein kinase by hyperosmotic stress. Plant Physiol. 2000, 122, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.S.; Gu, Y.-Q.; Pautot, V.; Bray, E.A.; Walling, L.L. Leucine aminopeptidase RNAs, proteins, and activities increase in response to water deficit, salinity, and the wound signals systemin, methyl jasmonate, and abscisic acid. Plant Physiol. 1999, 120, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.S. Jasmonate-inducible plant defences cause increased parasitism of herbivores. Nature 1999, 399, 686–688. [Google Scholar] [CrossRef]
- Yan, Z.; Li, X.; Chen, J.; Tam, N.F.-Y. Combined toxicity of cadmium and copper in Avicennia marina seedlings and the regulation of exogenous jasmonic acid. Ecotoxicol. Environ. Saf. 2015, 113, 124–132. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Hamayun, M.; Lee, S.-K.; Lee, I.-J. Methyl jasmonate alleviated salinity stress in soybean. J. Crop Sci. Biotechnol. 2009, 12, 63–68. [Google Scholar] [CrossRef]
- Avalbaev, A.; Yuldashev, R.; Fedorova, K.; Somov, K.; Vysotskaya, L.; Allagulova, C.; Shakirova, F. Exogenous methyl jasmonate regulates cytokinin content by modulating cytokinin oxidase activity in wheat seedlings under salinity. J. Plant Physiol. 2016, 191, 101–110. [Google Scholar] [CrossRef]
- Qiu, Z.; Guo, J.; Zhu, A.; Zhang, L.; Zhang, M. Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotoxicol. Environ. Saf. 2014, 104, 202–208. [Google Scholar] [CrossRef]
- de Ollas, C.; Hernando, B.; Arbona, V.; Gómez-Cadenas, A. Jasmonic acid transient accumulation is needed for abscisic acid increase in citrus roots under drought stress conditions. Physiol. Plant. 2013, 147, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chi, H.; Yue, M.; Zhang, X.; Li, W.; Jia, E. The regulation of exogenous jasmonic acid on UV-B stress tolerance in wheat. J. Plant Growth Regul. 2012, 31, 436–447. [Google Scholar] [CrossRef]
- Ouli-Jun, Z.; Zhou-Bin, L.; Ge, W.; Bo-Zhi, Y.; Xue-Xiao, Z. Mitigation of waterlogging-induced damages to pepper by exogenous MeJA. Pak. J. Bot. 2017, 49, 1127–1135. [Google Scholar]
- Sheteiwy, M.S.; Gong, D.; Gao, Y.; Pan, R.; Hu, J.; Guan, Y.J.E.; Botany, E. Priming with methyl jasmonate alleviates polyethylene glycol-induced osmotic stress in rice seeds by regulating the seed metabolic profile. Environ. Exp. Bot. 2018, 153, 236–248. [Google Scholar] [CrossRef]
- Nawaz, A.; Sheteiwy, M.S.; Khan, S.M.; Hu, Q.; Guan, Y.; Bukhari, S.A.H.; Luo, Y.; Hu, J. Optimization of germination inhibitors for controlling pre-harvest sprouting in hybrid rice. Pak. J. Agric. Sci. 2017, 54, 261–270. [Google Scholar]
- Sheteiwy, M.S.; Shao, H.; Qi, W.; Daly, P.; Sharma, A.; Shaghaleh, H.; Hamoud, Y.A.; El-Esawi, M.A.; Pan, R.; Wan, Q. Seed priming and foliar application with jasmonic acid enhance salinity stress tolerance of soybean (Glycine max L.) seedlings. J. Sci. Food Agric. 2021, 101, 2027–2041. [Google Scholar] [CrossRef]
- Mir, M.A.; John, R.; Alyemeni, M.N.; Alam, P.; Ahmad, P. Jasmonic acid ameliorates alkaline stress by improving growth performance, ascorbate glutathione cycle and glyoxylase system in maize seedlings. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Kang, D.J.; Seo, Y.J.; Lee, J.D.; Ishii, R.; Kim, K.; Shin, D.; Park, S.; Jang, S.; Lee, I.J. Jasmonic acid differentially affects growth, ion uptake and abscisic acid concentration in salt-tolerant and salt-sensitive rice cultivars. J. Agron. Crop Sci. 2005, 191, 273–282. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Q.; Cao, J.; Yu, B. Differential cl−/salt tolerance and NaCl-induced alternations of tissue and cellular ion fluxes in Glycine max, Glycine soja and their hybrid seedlings. J. Agron. Crop Sci. 2011, 197, 329–339. [Google Scholar] [CrossRef]
- Wang, K.J.; Li, X.H. Interspecific gene flow and the origin of semi-wild soybean revealed by capturing the natural occurrence of introgression between wild and cultivated soybean populations. Plant Breed. 2011, 130, 117–127. [Google Scholar] [CrossRef]
- Ashrafi, E.; Razmjoo, J.; Zahedi, M.; Pessarakli, M. Selecting alfalfa cultivars for salt tolerance based on some physiochemical traits. Agron. J. 2014, 106, 1758–1764. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Ullah, A.; Sadaf, S.; Ullah, S.; Alshaya, H.; Okla, M.K.; Alwasel, Y.A.; Tariq, A.J.L. Using Halothermal Time Model to Describe Barley (Hordeum vulgare L.) Seed Germination Response to Water Potential and Temperature. Life 2022, 12, 209. [Google Scholar] [CrossRef]
- Ali, B.; Wang, X.; Saleem, M.H.; Azeem, M.A.; Afridi, M.S.; Nadeem, M.; Ghazal, M.; Batool, T.; Qayyum, A.; Alatawi, A.; et al. Bacillus mycoides PM35 Reinforces Photosynthetic Efficiency, Antioxidant Defense, Expression of Stress-Responsive Genes, and Ameliorates the Effects of Salinity Stress in Maize. Life 2022, 12, 219. [Google Scholar] [CrossRef]
- Tsonev, T.; Lazova, G.; Stoinova, Z.G.; Popova, L. A possible role for jasmonic acid in adaptation of barley seedlings to salinity stress. J. Plant Growth Regul. 1998, 17, 153–159. [Google Scholar] [CrossRef]
- Javid, M.G.; Sorooshzadeh, A.; Moradi, F.; Modarres Sanavy, S.A.M.; Allahdadi, I. The role of phytohormones in alleviating salt stress in crop plants. Aust. J. Crop Sci. 2011, 5, 726–734. [Google Scholar]
- Raeder, J.; Larson, D.; Li, W.; Kepko, E.L.; Fuller-Rowell, T. OpenGGCM simulations for the THEMIS mission. Space Sci. Rev. 2008, 141, 535–555. [Google Scholar] [CrossRef]
- Walia, H.; Wilson, C.; Condamine, P.; Liu, X.; Ismail, A.M.; Close, T.J. Large-scale expression profiling and physiological characterization of jasmonic acid-mediated adaptation of barley to salinity stress. Plant Cell Environ. 2007, 30, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Nazar, R.; Iqbal, N.; Anjum, N.A. Phytohormones and Abiotic Stress Tolerance in Plants; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Farhangi-Abriz, S.; Ghassemi-Golezani, K. How can salicylic acid and jasmonic acid mitigate salt toxicity in soybean plants? Ecotoxicol. Environ. Saf. 2018, 147, 1010–1016. [Google Scholar] [CrossRef]
- Sirhindi, G.; Mushtaq, R.; Gill, S.S.; Sharma, P.; Abd_Allah, E.F.; Ahmad, P. Jasmonic acid and methyl jasmonate modulate growth, photosynthetic activity and expression of photosystem II subunit genes in Brassica oleracea L. Sci. Rep. 2020, 10, 9322. [Google Scholar] [CrossRef]
- Cenzano, A.; Vigliocco, A.; Kraus, T.; Abdala, G. Exogenously applied jasmonic acid induces changes in apical meristem morphology of potato stolons. Ann. Bot. 2003, 91, 915–919. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, H.; Liu, B.; Liu, L.; Song, S. Jasmonate action in plant growth and development. J. Exp. Bot. 2017, 68, 1349–1359. [Google Scholar] [CrossRef]
- Gao, Z.; Gao, S.; Li, P.; Zhang, Y.; Ma, B.; Wang, Y. Exogenous methyl jasmonate promotes salt stress-induced growth inhibition and prioritizes defense response of Nitraria tangutorum Bobr. Physiol. Plant. 2021, 172, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; An, J.; Yin, M.; Jia, X.; Guan, Y.; He, F.; Hu, J. Cold plasma treatment and exogenous salicylic acid priming enhances salinity tolerance of Oryza sativa seedlings. Protoplasma 2019, 256, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Sajjad, F. The Promotive Effects of Nephthyl Acetic Acid on Maize Cultivars Grown Under Saline Conditions. Commun. Soil Sci. Plant Anal. 2017, 48, 2155–2169. [Google Scholar] [CrossRef]
- Poonam, S.; Kaur, H.; Geetika, S. Effect of jasmonic acid on photosynthetic pigments and stress markers in Cajanus cajan (L.) Millsp. seedlings under copper stress. Am. J. Plant Sci. 2013, 4, 29827. [Google Scholar] [CrossRef]
- Li, M.; Xu, J.; Guo, R.; Liu, Y.; Wang, S.; Wang, H.; Ullah, A.; Shi, L. Identifying the metabolomics and physiological differences among Soja in the early flowering stage. Plant Physiol. Biochem. 2019, 139, 82–91. [Google Scholar] [CrossRef]
- Kaur, H.; Sirhindi, G.; Sharma, P. Effect of jasmonic acid on some biochemical and physiological parameters in salt-stressed Brassica napus seedlings. Int. J. Plant Physiol. Biochem. 2017, 9, 36–42. [Google Scholar]
- Rezai, S.; Orojloo, M.; Bidabadi, S.S.; Soleimanzadeh, M. Possible Role of Methyl Jasmonate in Protection to NaCl-Induced Salt Stress in Pepper cv. Green Hashemi. Int. J. Agric. Crop Sci. 2013, 6, 1235. [Google Scholar]
- Wu, Y.; Jin, X.; Liao, W.; Hu, L.; Dawuda, M.M.; Zhao, X.; Tang, Z.; Gong, T.; Yu, J. 5-Aminolevulinic acid (ALA) alleviated salinity stress in cucumber seedlings by enhancing chlorophyll synthesis pathway. Front. Plant Sci. 2018, 9, 635. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, L.; Li, M.; Su, L.; Lian, S.; Zhang, B.; Li, X.; Ge, K.; Li, L. AhGLK1 affects chlorophyll biosynthesis and photosynthesis in peanut leaves during recovery from drought. Sci. Rep. 2018, 8, 2250. [Google Scholar] [CrossRef] [PubMed]
- Reinbothe, S.; Reinbothe, C. The regulation of enzymes involved in chlorophyll biosynthesis. Eur. J. Biochem. 1996, 237, 323–343. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Wang, X.; Saleem, M.H.; Sumaira Hafeez, A.; Afridi, M.S.; Khan, S.; Zaib-Un-Nisa; Ullah, I.; Amaral Júnior, A.T.d.; Alatawi, A.; et al. PGPR-Mediated Salt Tolerance in Maize by Modulating Plant Physiology, Antioxidant Defense, Compatible Solutes Accumulation and Bio-Surfactant Producing Genes. Plants 2022, 11, 345. [Google Scholar] [CrossRef]
- Sirhindi, G.; Mir, M.A.; Abd-Allah, E.F.; Ahmad, P.; Gucel, S. Jasmonic acid modulates the physio-biochemical attributes, antioxidant enzyme activity, and gene expression in Glycine max under nickel toxicity. Front. Plant Sci. 2016, 7, 591. [Google Scholar] [CrossRef]
- Sheteawi, S.A. Improving growth and yield of salt-stressed soybean by exogenous application of jasmonic acid and ascobin. Int. J. Agric. Biol. 2007, 9, 473–478. [Google Scholar]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Bio Technol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Nemati, I.; Moradi, F.; Gholizadeh, S.; Esmaeili, M.; Bihamta, M. The effect of salinity stress on ions and soluble sugars distribution in leaves, leaf sheaths and roots of rice (Oryza sativa L.) seedlings. Plant Soil Environ. 2011, 57, 26–33. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Peshev, D.; Zinta, G.; Van den Ende, W.; Janssens, I.A.; Asard, H. Climate extreme effects on the chemical composition of temperate grassland species under ambient and elevated CO2: A comparison of fructan and non-fructan accumulators. PLoS ONE 2014, 9, e92044. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.Y.; Li, Y.; Yan, S.G. Effects of salinity on germination of six salt-tolerant forage species and their recovery from saline conditions. N. Z. J. Agric. Res. 2003, 46, 263–269. [Google Scholar] [CrossRef][Green Version]
- Amor, N.B.; Hamed, K.B.; Debez, A.; Grignon, C.; Abdelly, C. Physiological and antioxidant responses of the perennial halophyte Crithmum maritimum to salinity. Plant Sci. 2005, 168, 889–899. [Google Scholar] [CrossRef]
- Netondo, G.W.; Onyango, J.C.; Beck, E. Sorghum and salinity: II. Gas exchange and chlorophyll fluorescence of sorghum under salt stress. Crop Sci. 2004, 44, 806–811. [Google Scholar] [CrossRef]
- Li, M.X.; Xu, M.; Xu, J.S.; Ullah, A.; Shi, L.X. Gas Exchange and Ionomic Changes in Wild and Cultivated Soybean Seedlings under Salt Stress. Int. J. Agric. Biol. 2019, 22, 1627–1635. [Google Scholar]
- Gao, S.; Liu, K.T.; Chung, T.W.; Chen, F. The effects of NaCl stress on Jatropha cotyledon growth and nitrogen metabolism. J. Soil Sci. Plant Nutr. 2013, 13, 99–113. [Google Scholar] [CrossRef]
- Chunwu, Y.; Changyou, L.; Hongjuan, Y.; Miao, J.; Decheng, S. Physiological response of xiaobingmai (Triticum aestivum-Agropyron intermedium) to salt-stress and alkali-stress. Acta Agron Sin 2010, 33, 1255–1261. [Google Scholar]
- Queiroz, H.M.; Sodek, L.; Haddad, C.R.B. Effect of salt on the growth and metabolism of Glycine max. Braz. Arch. Biol. Technol. 2012, 55, 809–817. [Google Scholar] [CrossRef][Green Version]
- Anderson, J.M. Jasmonic acid-dependent increase in vegetative storage protein in soybean tissue cultures. J. Plant Growth Regul. 1991, 10, 5–10. [Google Scholar] [CrossRef]
- Kumari, G.J.; Reddy, A.M.; Naik, S.T.; Kumar, S.G.; Prasanthi, J.; Sriranganayakulu, G.; Reddy, P.C.; Sudhakar, C. Jasmonic acid-induced changes in protein pattern, antioxidative enzyme activities and peroxidase isozymes in peanut seedlings. Biol. Plant. 2006, 50, 219–226. [Google Scholar] [CrossRef]
- Malekpoor, F.; Salimi, A.; Pirbalouti, A.G. Effect of jasmonic acid on total phenolic content and antioxidant activity of extract from the green and purple landraces of sweet basil. Acta Pol. Pharm 2016, 73, 1229–1234. [Google Scholar] [PubMed]
- Govindarajan, R.; Singh, D.; Rawat, A. High-performance liquid chromatographic method for the quantification of phenolics in ‘Chyavanprash’a potent Ayurvedic drug. J. Pharm. Biomed. Anal. 2007, 43, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Memelink, J. Regulation of gene expression by jasmonate hormones. Phytochemistry 2009, 70, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in plant regulation and response to abiotic stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Li, M.; Yang, D.; Zhang, J.; Shi, L. The physiological variations of adaptation mechanism in Glycine soja seedlings under saline and alkaline stresses. Pak. J. Bot. 2016, 48, 2183–2193. [Google Scholar]
- Holm, G. Chlorophyll mutations in barley. Acta Agric. Scand. 1954, 4, 457–471. [Google Scholar] [CrossRef]
- Jiao, Y.; Bai, Z.; Xu, J.; Zhao, M.; Khan, Y.; Hu, Y.; Shi, L. Metabolomics and its physiological regulation process reveal the salt-tolerant mechanism in Glycine soja seedling roots. Plant Physiol. Biochem. 2018, 126, 187–196. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.t.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Malik, C.P.S.M.B. Plant Enzymology and Histo-Enzymology: A Text Manual; Kalyani Publishers: New Delhi, India, 1980. [Google Scholar]
- Bayfield, R.; Cole, E. [24] Colorimetric estimation of vitamin A with trichloroacetic acid. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1980; Volume 67, pp. 189–195. [Google Scholar]

| Soybean Variety | Treatments | Shoot Height (cm) | Shoot Fresh Weight (g) | Shoot Dry Weight (g) | Shoot Moisture Content |
|---|---|---|---|---|---|
| Parachinar-local | T1 | 29.00 ± 5.27 ab | 7.79 ± 0.27 abc | 2.93 ± 1.25 ab | 4.86 ± 1.14 a |
| T2 | 32.67 ± 2.95 a | 13.88 ± 4.50 a | 3.59 ± 0.89 a | 10.29 ± 3.62 a | |
| T3 | 31.43 ± 4.46 ab | 14.00 ± 2.58 a | 2.83 ± 0.54 ab | 11.17 ± 3.09 a | |
| T4 | 30.67 ± 2.49 ab | 14.17 ± 1.83 a | 3.64 ± 0.28 a | 10.53 ± 1.55 a | |
| T5 | 22.00 ± 1.15 ab | 6.03 ± 1.90 bc | 1.37 ± 0.46 b | 4.66 ± 1.46 a | |
| T6 | 27.90 ± 0.49 ab | 12.97 ± 2.52 ab | 2.34 ± 0.29 ab | 10.63 ± 2.81 a | |
| T7 | 21.40 ± 1.70 b | 5.35 ± 0.62 c | 1.39 ± 0.51 b | 3.96 ± 1.08 a | |
| T8 | 27.03 ± 4.23 ab | 11.46 ± 1.42 abc | 2.57 ± 0.13 ab | 8.89 ± 1.37 a | |
| Swat-84 | T1 | 35.10 ± 0.95 a | 17.60 ± 2.92 a | 4.82 ± 0.55 a | 12.77 ± 2.37 a |
| T2 | 31.07 ± 2.10 ab | 13.90 ± 3.18 ab | 3.56 ± 0.76 ab | 10.33 ± 2.42 ab | |
| T3 | 27.70 ± 0.42 bc | 9.56 ± 2.75 ab | 2.35 ± 0.73 ab | 7.21 ± 2.03 ab | |
| T4 | 30.33 ± 2.42 bc | 11.33 ± 2.32 ab | 3.04 ± 0.86 ab | 8.29 ± 2.75 ab | |
| T5 | 29.13 ± 0.52 bc | 11.08 ± 3.55 ab | 2.50 ± 0.76 ab | 8.58 ± 2.79 ab | |
| T6 | 26.33 ± 0.88 cd | 11.44 ± 0.86 ab | 2.98 ± 0.52 ab | 8.46 ± 0.34 ab | |
| T7 | 23.47 ± 0.69 d | 6.55 ± 0.70 b | 2.09 ± 0.21 b | 4.46 ± 0.63 a | |
| T8 | 31.50 ± 0.75 ab | 16.43 ± 4.27 a | 4.50 ± 1.14 ab | 11.93 ± 3.22 ab |
| Soybean Variety | Treatments | Root Length (cm) | Root Fresh Weight(g) | Root Dry Weight (g) | Root Moisture (g) |
|---|---|---|---|---|---|
| Parachinar-local | T1 | 20.67 ± 0.67 ab | 1.19 ± 0.18 abc | 0.52 ± 0.10 a | 0.67 ± 0.08 abc |
| T2 | 16.60 ± 3.15 b | 1.31 ± 0.31 abc | 0.65 ± 0.14 a | 0.66 ± 0.18 abc | |
| T3 | 17.43 ± 1.34 ab | 2.11 ± 0.44 a | 0.71 ± 0.26 a | 1.40 ± 0.18 ab | |
| T4 | 19.33 ± 3.38 ab | 1.81 ± 0.64 ab | 0.67 ± 0.05 a | 1.13 ± 0.59 ab | |
| T5 | 17.03 ± 3.07 b | 0.79 ± 0.21 bc | 0.35 ± 0.11 a | 0.45 ± 0.10 bc | |
| T6 | 24.43 ± 1.84 a | 1.84 ± 0.46 ab | 0.36 ± 0.08 a | 1.48 ± 0.38 a | |
| T7 | 16.33 ± 1.20 b | 0.61 ± 0.06 c | 0.48 ± 0.01 a | 0.12 ± 0.06 c | |
| T8 | 22.67 ± 1.01 ab | 1.30 ± 0.14 abc | 0.49 ± 0.12 a | 0.81 ± 0.26 abc | |
| Swat-84 | T1 | 26.23 ± 0.77 a | 1.80 ± 0.30 a | 0.78 ± 0.10 a | 1.03 ± 0.21 a |
| T2 | 14.33 ± 1.20 cd | 1.13 ± 0.29 a | 0.51 ± 0.11 a | 0.62 ± 0.19 a | |
| T3 | 17.00 ± 1.53 bcd | 0.63 ± 0.17 a | 0.38 ± 0.08 a | 0.24 ± 0.10 a | |
| T4 | 22.67 ± 5.21 ab | 1.14 ± 0.26 a | 0.44 ± 0.13 a | 0.70 ± 0.23 a | |
| T5 | 19.77 ± 0.83 abc | 1.52 ± 0.61 a | 0.43 ± 0.15 a | 1.09 ± 0.49 a | |
| T6 | 19.30 ± 0.69 abc | 1.47 ± 0.31 a | 0.67 ± 0.17 a | 0.80 ± 0.15a | |
| T7 | 12.03 ± 1.74 d | 1.01 ± 0.27 a | 0.45 ± 0.08 a | 0.56 ± 0.20 a | |
| T8 | 24.00 ± 1.15 ab | 1.66 ± 0.63 a | 0.80 ± 0.29 a | 0.86 ± 0.34 a |
| Soybean Variety | Treatments | NO3− | Cl− | SO42− | H2PO4− |
|---|---|---|---|---|---|
| Parachinar-local | T1 | 10.26 ± 0.84 a | 8.61 ± 0.54 d | 17.46 ± 0.51 f | 10.87 ± 0.47 a |
| T2 | 9.06 ± 0.70 ab | 6.06 ± 0.30 c | 23.69 ± 2.91d e | 10.45 ± 0.55 a | |
| T3 | 6.92 ± 0.23 c | 12.85 ± 0.42 bc | 22.58 ± 2.74 ef | 9.82 ± 0.51 ab | |
| T4 | 7.98 ± 0.37 bc | 12.21 ± 0.55 b | 21.56 ± 2.42 ef | 10.16 ± 0.58 ab | |
| T5 | 6.58 ± 0.23 cd | 14.29 ± 0.15 a | 28.93 ± 0.98 cd | 8.84 ± 0.34 bc | |
| T6 | 7.41 ± 0.41 c | 12.15 ± 1.07 b | 31.85 ± 0.69 bc | 9.81 ± 0.39 ab | |
| T7 | 5.15 ± 0.31 d | 14.11 ± 0.15 a | 36.08 ± 0.58 ab | 7.44 ± 0.30 d | |
| T8 | 5.37 ± 0.29 d | 12.27 ± 0.27 b | 37.46 ± 1.12 a | 7.83 ± 0.19 cd | |
| Swat-84 | T1 | 7.27 ± 0.17 a | 8.88 ± 0.88 e | 17.32 ± 1.04 g | 10.60 ± 0.53 a |
| T2 | 6.62 ± 0.31 b | 6.47 ± 0.36 f | 24.39 ± 0.12 e | 9.70 ± 0.48 ab | |
| T3 | 5.37 ± 0.31 c | 14.69 ± 0.70 d | 20.69 ± 2.00 f | 9.13 ± 0.49 abc | |
| T4 | 5.99 ± 0.23 c | 15.35 ± 0.58 cd | 24.72 ± 0.75 de | 9.46 ± 0.50 abc | |
| T5 | 4.29 ± 0.15 d | 17.29 ± 0.46 b | 29.32 ± 1.77 bc | 8.77 ± 0.42 bcd | |
| T6 | 4.73 ± 0.18 d | 16.74 ± 0.51 bc | 27.94 ± 0.40 cd | 8.97 ± 0.48 bcd | |
| T7 | 3.03 ± 0.04 e | 19.33 ± 0.28 a | 31.72 ± 0.48 b | 7.48 ± 0.56 d | |
| T8 | 3.36 ± 0.14 e | 18.03 ± 0.47 ab | 36.97 ± 0.97 a | 7.70 ± 0.48 cd |
| Soybean Variety | Treatments | Na+ | K+ | Mg2+ | P3+ |
|---|---|---|---|---|---|
| Parachinar-local | T1 | 20.36 ± 0.79 d | 177.98 ± 3.85 a | 42.14 ± 2.50 a | 21.19 ± 1.45 b |
| T2 | 18.21 ± 0.67 cd | 167.31 ± 5.84 ab | 34.85 ± 1.29 b | 24.61 ± 1.37 a | |
| T3 | 22.71 ± 0.54 cd | 161.48 ± 5.01 bc | 29.38 ± 0.77 cd | 20.82 ± 1.02 b | |
| T4 | 20.93 ± 1.00 cd | 152.94 ± 3.69 cd | 31.42 ± 0.97 bcd | 22.48 ± 1.22 ab | |
| T5 | 29.93 ± 1.10 b | 140.76 ± 6.59 de | 33.59 ± 1.73 bc | 16.15 ± 1.13 c | |
| T6 | 22.90 ± 1.13 c | 128.66 ± 3.07 ef | 34.04 ± 1.04 b | 14.35 ± 0.39 cd | |
| T7 | 33.04 ± 0.97 a | 118.92 ± 3.88 f | 28.04 ± 0.66 d | 12.48 ± 1.18 d | |
| T8 | 28.66 ± 0.68 b | 128.53 ± 2.92 ef | 30.68 ± 0.85b cd | 11.78 ± 0.26 d | |
| Swat-84 | T1 | 22.56 ± 1.23 d | 163.02 ± 6.47 a | 41.64 ± 2.55 a | 14.06 ± 0.40 a |
| T2 | 19.51 ± 0.38 d | 139.57 ± 8.57 b | 37.57 ± 0.92 ab | 15.52 ± 0.87 a | |
| T3 | 36.08 ± 1.33 c | 141.49 ± 5.45 b | 33.84 ± 1.04 bc | 13.91 ± 0.61 a | |
| T4 | 34.59 ± 1.09 c | 91.63 ± 5.91 d | 35.69 ± 0.34 b | 13.13 ± 0.61 a | |
| T5 | 40.02 ± 1.09 b | 120.26 ± 3.12 c | 29.11 ± 1.49 de | 9.64 ± 0.66 a | |
| T6 | 32.58 ± 1.13 c | 85.60 ± 7.18d e | 30.97 ± 2.07 cd | 10.52 ± 1.05 a | |
| T7 | 48.59 ± 2.02 a | 65.60 ± 4.20 f | 24.25 ± 0.42 f | 10.12 ± 0.59 a | |
| T8 | 42.64 ± 1.63 b | 69.20 ± 2.12 ef | 25.26 ± 1.03 ef | 10.19 ± 1.27 a |
| Soybean Variety | Treatments | Mn2+ | B3+ | Zn3+ | Fe3+ |
|---|---|---|---|---|---|
| Parachinar-local | T1 | 0.08 ± 0.00 a | 0.34 ± 0.03 b | 0.08 ± 0.00 a | 1.44 ± 0.10 a |
| T2 | 0.10 ± 0.01 a | 0.43 ± 0.02 a | 0.08 ± 0.00 ab | 1.60 ± 0.16 a | |
| T3 | 0.07 ± 0.00 a | 0.31 ± 0.02 bc | 0.07 ± 0.00 cd | 1.47 ± 0.09 a | |
| T4 | 0.10 ± 0.01 a | 0.32 ± 0.01 bc | 0.07 ± 0.00 bc | 1.70 ± 0.14 a | |
| T5 | 0.06 ± 0.01 a | 0.27 ± 0.03 cde | 0.05 ± 0.00 ef | 1.72 ± 0.32 a | |
| T6 | 0.10 ± 0.03 a | 0.28 ± 0.01b cd | 0.06 ± 0.00 de | 2.06 ± 0.43 a | |
| T7 | 0.06 ± 0.01 a | 0.22 ± 0.02 d | 0.04 ± 0.00 f | 1.96 ± 0.36 a | |
| T8 | 0.07 ± 0.00 a | 0.24 ± 0.01 de | 0.05 ± 0.00 ef | 2.26 ± 0.43 a | |
| Swat-84 | T1 | 0.14 ± 0.01 a | 0.48 ± 0.01 ab | 0.09 ± 0.01 a | 1.48 ± 0.18 a |
| T2 | 0.14 ± 0.00 a | 0.52 ± 0.08 a | 0.08 ± 0.00 ab | 1.70 ± 0.43 a | |
| T3 | 0.09 ± 0.00 cd | 0.37 ± 0.01 c | 0.06 ± 0.00 bcd | 1.80 ± 0.39 a | |
| T4 | 0.10 ± 0.01 bc | 0.39 ± 0.01 bc | 0.07 ± 0.01 bc | 1.64 ± 0.27 a | |
| T5 | 0.11 ± 0.00 b | 0.32 ± 0.01 c | 0.05 ± 0.00 cd | 1.50 ± 0.19 a | |
| T6 | 0.11 ± 0.00 b | 0.35 ± 0.02 c | 0.06 ± 0.00 bcd | 1.65 ± 0.26 a | |
| T7 | 0.08 ± 0.00 d | 0.29 ± 0.01 c | 0.05 ± 0.01 d | 1.59 ± 0.41 a | |
| T8 | 0.09 ± 0.00 cd | 0.30 ± 0.01 c | 0.05 ± 0.01 cd | 1.80 ± 0.43 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noor, J.; Ullah, A.; Saleem, M.H.; Tariq, A.; Ullah, S.; Waheed, A.; Okla, M.K.; Al-Hashimi, A.; Chen, Y.; Ahmed, Z.; et al. Effect of Jasmonic Acid Foliar Spray on the Morpho-Physiological Mechanism of Salt Stress Tolerance in Two Soybean Varieties (Glycine max L.). Plants 2022, 11, 651. https://doi.org/10.3390/plants11050651
Noor J, Ullah A, Saleem MH, Tariq A, Ullah S, Waheed A, Okla MK, Al-Hashimi A, Chen Y, Ahmed Z, et al. Effect of Jasmonic Acid Foliar Spray on the Morpho-Physiological Mechanism of Salt Stress Tolerance in Two Soybean Varieties (Glycine max L.). Plants. 2022; 11(5):651. https://doi.org/10.3390/plants11050651
Chicago/Turabian StyleNoor, Javaria, Abd Ullah, Muhammad Hamzah Saleem, Akash Tariq, Sami Ullah, Abdul Waheed, Mohammad K. Okla, Abdulrahman Al-Hashimi, Yinglong Chen, Zeeshan Ahmed, and et al. 2022. "Effect of Jasmonic Acid Foliar Spray on the Morpho-Physiological Mechanism of Salt Stress Tolerance in Two Soybean Varieties (Glycine max L.)" Plants 11, no. 5: 651. https://doi.org/10.3390/plants11050651
APA StyleNoor, J., Ullah, A., Saleem, M. H., Tariq, A., Ullah, S., Waheed, A., Okla, M. K., Al-Hashimi, A., Chen, Y., Ahmed, Z., & Ahmad, I. (2022). Effect of Jasmonic Acid Foliar Spray on the Morpho-Physiological Mechanism of Salt Stress Tolerance in Two Soybean Varieties (Glycine max L.). Plants, 11(5), 651. https://doi.org/10.3390/plants11050651








