Thermotherapy Followed by Shoot Tip Cryotherapy Eradicates Latent Viruses and Apple Hammerhead Viroid from In Vitro Apple Rootstocks
Abstract
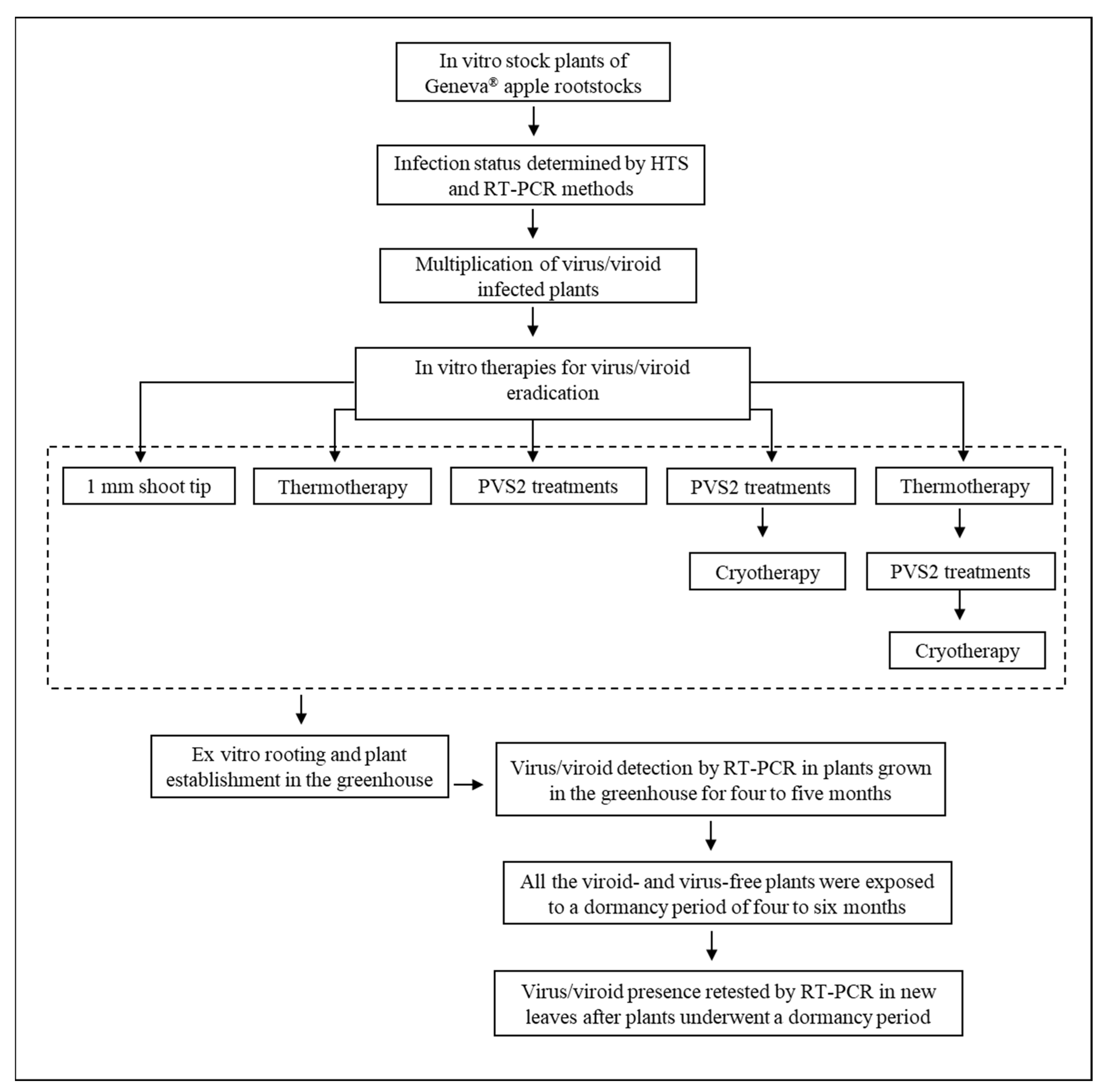
1. Introduction
2. Results
2.1. Survival and Regrowth of Shoot Tips
2.2. Effect of Treatments on Virus and Viroid Eradication
| A. Shoot tip survival after treatment (% ± SE) | ||||||||||
| Rootstock | No Liquid Nitrogen | Liquid Nitrogen | ||||||||
| Identity | Shoot Tips | Thermotherapy | PVS2 60 Min | PVS2 75 min | TT + PVS2 60 min | TT + PVS2 75 min | PVS2 60 min | PVS2 75 min | TT + PVS2 60 min | TT + PVS2 75 min |
| CG 2034 (ACLSV) | 100 ± 0 a | 78 ± 3 abcdef | 88 ± 3 abc | 88 ± 3 abcd | 68 ± 3 bcdefgh | 50 ± 5 ghijkl | 80 ± 5 abcde | 73 ± 3 bcdefg | 53 ± 3 fghijkl | 30 ± 5 kl |
| CG 4213 (AHVd) | 100 ± 0 a | 88 ± 3 abc | 88 ± 3 abc | 78 ± 8 abcdef | 55 ± 5 efghijk | 38 ± 3 ijkl | 80 ± 0 abcde | 68 ± 3 bcdefgh | 48 ± 3 ghijkl | 30 ± 10 kl |
| CG 5257 (AHVd) | 100 ± 0 a | 90 ± 0 ab | 93 ± 3 ab | 80 ± 5 abcde | 63 ± 3 cdefghi | 53 ± 8 fghijkl | 88 ± 3 abc | 73 ± 3 bcdefg | 55 ± 5 efghijk | 28 ± 3 l |
| CG 6006 (ASGV) | 100 ± 0 a | 88 ± 3 abc | 63 ± 8 cdefghi | 60 ± 10 defghij | 70 ± 5 bcdefg | 42 ± 2 hijkl | 55 ± 5 efghijk | 35 ± 6 jkl | 50 ± 7 ghijkl | 34 ± 6 jkl |
| Mean | 100 ± 0 | 86 ± 3 | 83 ± 7 | 75 ± 5 | 64 ± 3 | 46 ± 3 | 76 ± 7 | 62 ± 9 | 52 ± 2 | 31 ± 1 |
| B. Shoot tip regrowth after treatment (% ± SE) | ||||||||||
| Rootstock | No Liquid Nitrogen | Liquid Nitrogen | ||||||||
| Identity | Shoot Tips | Thermotherapy | PVS2 60 min | PVS2 75 min | TT + PVS2 60 min | TT + PVS2 75 min | PVS2 60 min | PVS2 75 min | TT + PVS2 60 min | TT + PVS2 75 min |
| CG 2034 (ACLSV) | 100 ± 0 a | 60 ± 10 cd | 58 ± 8 cd | 48 ± 3 cdefg | 28 ± 3 efghijk | 15 ± 5 hijk | 43 ± 8 cdefgh | 40 ± 5 cdefgh | 15 ± 5 hijk | 5 ± 5 jk |
| CG 4213 (AHVd) | 95 ± 5 ab | 55 ± 5 cde | 43 ± 3 cdefgh | 35 ± 10 defghi | 25 ± 5 fghijk | 15 ± 5 hijk | 43 ± 3 cdefgh | 28 ± 3 efghijk | 10 ± 5 ijk | 0 ± 0 k |
| CG 5257 (AHVd) | 98 ± 3 a | 58 ± 3 cd | 48 ± 3 cdefg | 43 ± 8 cdefgh | 28 ± 3 efghijk | 15 ± 0 hijk | 50 ± 5 cdef | 25 ± 5 fghijk | 20 ± 5 ghijk | 3 ± 3 k |
| CG 6006 (ASGV) | 98 ± 3 a | 68 ± 8 bc | 43 ± 8 cdefgh | 33 ± 3 defghij | 55 ± 5 cde | 24 ± 4 fghijk | 40 ± 0 cdefgh | 25 ± 5 fghijk | 15 ± 3 hijk | 6 ± 2 jk |
| Mean | 98 ± 1 | 60 ± 3 | 48 ± 4 | 39 ± 3 | 34 ± 7 | 17 ± 2 | 44 ± 2 | 30 ± 4 | 15 ± 2 | 4 ± 1 |
| C. Percent of non-infected plants obtained (number uninfected/total number assessed in parentheses) after treatment | ||||||||||
| Rootstock | No Liquid Nitrogen | Liquid Nitrogen | ||||||||
| Identity | Shoot Tips | Thermotherapy | PVS2 60 min | PVS2 75 min | TT + PVS2 60 min | TT + PVS2 75 min | PVS2 60 min | PVS2 75 min | TT + PVS2 60 min | TT + PVS2 75 min |
| CG 2034 (ACLSV) | 31 (4/13) | 5 (1/19) | 67 (8/12) | 23 (3/13) | 9 (1/11) | 0 (0/6) | 79 (11/14) | 69 (11/19) | 100 (6/6) | 100 (2/2 |
| CG 4213 (AHVd) | 0 (0/6) | 0 (0/20) | 0 (0/2) | 0 (0/4) | 0 (0/6) | 0 (0/6) | 0 (0/7) | 0 (0/9) | 25 (1/4) | 0 (0/0) |
| CG 5257 (AHVd) | 0 (0/4) | 0 (0/19) | 0 (0/6) | 0 (0/4) | 0 (0/10) | 0 (0/6) | 0 (0/20) | 0 (0/7) | 75 (6/8) | 0 (0/1) |
| CG 6006 (ASGV) | 0 (0/5) | 8 (1/12) | 0 (0/5) | 0 (0/7) | 11 (1/9) | 14 (1/7) | 0 (0/10) | 0 (0/3) | 100 (6/6) | 50 (1/2) |
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. In Vitro Therapies for Virus/Viroid Eradication
4.2.1. Thermotherapy Treatments
4.2.2. PVS2 and Droplet-Vitrification Cryotherapy Treatments
4.2.3. Thermotherapy and Droplet-Vitrification Cryotherapy
4.3. Shoot Tip Recovery and Data Analyses
4.4. Ex Vitro Rooting, Acclimatization and Plant Maintenance
4.5. Virus/Viroid Detection
4.5.1. HTS-Based Diagnostic in Stock Plants
4.5.2. Virus/Viroid Diagnostics Using RT-PCR
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACLSV | Apple chlorotic leaf spot virus |
| AGCaV | Apple green crinkle associated virus |
| AHVd | Apple hammerhead viroid |
| ApMV | Apple mosaic virus |
| ARWaV-1 | Apple rubbery wood-associated viruses 1 |
| ARWaV-2 | Apple rubbery wood-associated viruses 2 |
| ASGV | Apple stem grooving virus |
| ASPV | Apple stem pitting virus |
| BA | 6-benzyl aminopurine |
| BM | Basal medium |
| CCGaV | Citrus concave gum-associated virus |
| DMSO | Dimethyl sulfoxide |
| GA3 | Gibberellic acid |
| HTS | High-throughput sequencing |
| IBA | Indole-3-butyric acid |
| LN | Liquid nitrogen |
| MS | Murashige and Skoog medium |
| NAD5 | NADH-ubiquinone oxidoreductase chain 5 |
| PVS2 | Plant vitrification solution 2 |
| RBDV | Raspberry bushy dwarf virus |
| RT-PCR | Reverse transcription polymerase chain reaction |
| RVDB | Reference viral database |
| SMM | Shoot multiplication medium |
References
- Bramel, P.J.; Volk, G. A Global Strategy for the Conservation and Use of Apple Genetic Resources; Global Crop Diversity Trust: Bonn, Germany, 2019; 52p. [Google Scholar]
- FAOSTAT (Food and Agriculture Organization of the United Nations). Crops and Livestock Products. Available online: www.fao.org/faostat/en/#data (accessed on 2 November 2021).
- Fazio, G.; Robinson, T.L.; Aldwinckle, H.S. The Geneva apple rootstock breeding program. In Plant Breeding Reviews; Janick, J., Ed.; Wiley-Blackwell: New York, NY, USA, 2015; pp. 379–424. [Google Scholar] [CrossRef]
- Robinson, T. Advances in apple culture worldwide. Rev. Bras. Frutic. 2011, 33, 37–47. [Google Scholar] [CrossRef]
- Fazio, G. Genetics, breeding, and genomics of apple rootstocks. In The Apple Genome, Compendium of Plant Genomes; Korban, S.S., Ed.; Springer: Cham, Switzerland, 2021; pp. 105–130. [Google Scholar] [CrossRef]
- Rufato, L.; da Silva, P.S.; Kretzschmar, A.A.; Bogo, A.; de Macedo, T.A.; Welter, J.F.; Fazio, G.; Petry, D. Geneva® series rootstocks for apple trees under extreme replanting conditions in Southern Brazil. Front. Plant. Sci 2021, 12, 712162. [Google Scholar] [CrossRef]
- Marini, R.P.; Fazio, G. Apple rootstocks: History, physiology, management, and breeding. In Horticultural Reviews; Warrington, I., Ed.; Wiley-Blackwell: New York, NY, USA, 2018; pp. 197–312. [Google Scholar] [CrossRef]
- Tworkoski, T.; Fazio, G. Hormone and growth interactions of scions and size-controlling rootstocks of young apple trees. Plant. Growth Regul. 2016, 78, 105–119. [Google Scholar] [CrossRef]
- Fazio, G.; Robinson, T. Modification of nursery tree architecture with apple rootstocks: A breeding perspective. In New York Fruit Quarterly 16; Robinson, T., Hoying, S., Eds.; New York State Horticultural Society: New York, NY, USA, 2008; pp. 13–16. [Google Scholar]
- Cummins, J.N.; Aldwinckle, H.S. Breeding apple rootstocks. In Plant Breeding Reviews; Janick, J., Ed.; Springer: Boston, MA, USA, 1983; pp. 294–394. [Google Scholar] [CrossRef]
- Aldwinckle, H.S.; Borejsza-Wysocka, E.E.; Malnoy, M.; Brown, S.K.; Beer, S.V.; Meng, X.; Norelli, J.L.; He, S.Y.; Jin, Q.-L. Development of fire blight resistant apple cultivars by genetic engineering. Acta Hortic. 2003, 622, 105–111. [Google Scholar] [CrossRef]
- Fazio, G.; Kviklys, D.; Grusak, M.A.; Robinson, T. Soil pH, soil type and replant disease affect growth and nutrient absorption in apple rootstocks. In New York Fruit Quarterly; Robinson, T., Hoying, S., Eds.; New York State Horticultural Society: New York, NY, USA, 2012; pp. 22–28. [Google Scholar]
- Gardiner, S.E.; Norelli, J.L.; de Silva, N.; Fazio, G.; Peil, A.; Malnoy, M.; Horner, M.; Bowatte, D.; Carlisle, C.; Wiedow, C.; et al. Putative resistance gene markers associated with quantitative trait loci for fire blight resistance in Malus ‘Robusta 5’ accessions. BMC Genet. 2012, 13, 25. [Google Scholar] [CrossRef]
- Robinson, T.; Aldwinckle, H.S.; Fazio, G.; Holleran, T. The Geneva series of apple rootstocks from Cornell: Performance, disease resistance, and commercialization. Acta Hortic. 2003, 622, 513–520. [Google Scholar] [CrossRef]
- Fazio, G.; Robinson, T.; Aldwinckle, H. Geneva® Apple Rootstocks Comparison Chart v.2. 2016. Available online: https://www.canr.msu.edu/uploads/236/100348/GENEVA-Apple-Rootstocks-Comparison-Chart.pdf (accessed on 2 November 2021).
- Fazio, G.; Aldwinckle, H.; Robinson, T. Unique characteristics of Geneva® apple rootstocks. In New York Fruit Quarterly; Robinson, T., Hoying, S., Eds.; New York State Horticultural Society: New York, NY, USA, 2013; pp. 25–28. [Google Scholar]
- Robinson, T. The evolution towards more competitive apple orchard systems in the USA. Acta Hortic. 2008, 772, 491–500. [Google Scholar] [CrossRef]
- Rubio, L.; Galipienso, L.; Ferriol, I. Detection of plant viruses and disease management: Relevance of genetic diversity and evolution. Front. Plant. Sci. 2020, 11, 1092. [Google Scholar] [CrossRef]
- Barba, M.; Ilardi, V.; Pasquini, G. Control of pome and stone fruit virus diseases. In Advances in Virus Research; Loebenstein, G., Katis, N.I., Eds.; Academic Press/Elsevier: Cambridge, UK, 2015; pp. 47–83. [Google Scholar] [CrossRef]
- Umer, M.; Liu, J.; You, H.; Xu, C.; Dong, K.; Luo, N.; Kong, L.; Li, X.; Hong, N.; Wang, G.; et al. Genomic, morphological and biological traits of the viruses infecting major fruit trees. Viruses 2019, 11, 515. [Google Scholar] [CrossRef]
- Nickel, O.; Fajardo, T.V.M. Novas Viroses Diagnosticadas em Macieiras no Brasil Por Sequenciamento de Alto Desempenho (HTS), 1st ed.; Circular técnica 161; Embrapa Uva e Vinho: Bento Gonçalves, Brazil, 2021; p. 18. [Google Scholar]
- Kumar, S.; Singh, L.; Ram, R.; Zaidi, A.A.; Hallan, V. Simultaneous detection of major pome fruit viruses and a viroid. Indian J. Microbiol. 2014, 54, 203–210. [Google Scholar] [CrossRef]
- Guerra, D.S.; Nickel, O.; Del Ponte, E.M.; Valdebenito-Sanhueza, R.M.; Fajardo, T.V.M.; Marodin, G. Development of glomerella leaf spot is enhanced in virus-infected maxi gala apples. Plant. Pathol. J. 2012, 94, 237–241. [Google Scholar] [CrossRef]
- Cieślińska, M.; Rutkowski, K.P. Effect of Apple chlorotic leaf spot virus on yield and quality of fruits from ‘Golden Delicious’ and ‘Sampion’ apple trees. Acta Hortic. 2008, 781, 119–124. [Google Scholar] [CrossRef]
- Hadidi, A.; Barba, M. Economic impact of pome and stone fruit viruses and viroids. In Virus and Virus-Like Diseases of Pome and Stone Fruits; Hadidi, A., Barba, M., Candresse, T., Jelkmann, W., Eds.; APS Press: Saint Paul, MN, USA, 2011; pp. 1–7. [Google Scholar] [CrossRef]
- Maliogka, V.I.; Minafra, A.; Saldarelli, P.; Ruiz-García, A.B.; Glasa, M.; Katis, N.; Olmos, A. Recent advances on detection and characterization of fruit tree viruses using high-throughput sequencing technologies. Viruses 2018, 10, 436. [Google Scholar] [CrossRef]
- Mushtaq, M.; Dar, A.A.; Basu, U.; Bhat, B.A.; Mir, R.A.; Vats, S.; Dar, M.S.; Tyagi, A.; Ali, S.; Bansal, M.; et al. Integrating CRISPR-Cas and Next Generation Sequencing in Plant Virology. Front. Genet. 2021, 12, 735489. [Google Scholar] [CrossRef]
- Hou, W.; Li, S.; Massart, S. Is There a “Biological Desert” With the Discovery of New Plant Viruses? A Retrospective Analysis for New Fruit Tree Viruses. Front. Microbiol. 2020, 11, 592816. [Google Scholar] [CrossRef]
- Al Rwahnih, M.; Turturo, C.; Minafra, A.; Salsarelli, P.; Myrta, A.; Pallás, V.; Savino, V. Molecular variability of Apple chlorotic leaf spot virus in different hosts and geographical regions. J. Plant. Pathol. 2004, 86, 117–122. [Google Scholar]
- Massart, S.; Jijakli, M.H.; Kummert, J. Apple stem grooving virus. In Virus and Virus-Like Diseases of Pome and Stone Fruits; Hadidi, A., Barba, M., Candresse, T., Jelkmann, W., Eds.; APS Press: Saint Paul, MN, USA, 2011; pp. 85–90. [Google Scholar] [CrossRef]
- Serra, P.; Messmer, A.; Sanderson, D.; James, D.; Flores, R. Apple hammerhead viroid-like RNA is a bona fide viroid: Autonomous replication and structural features support its inclusion as a new member in the genus Pelamoviroid. Virus Res. 2018, 2, 8–15. [Google Scholar] [CrossRef]
- Zhang, Z.; Qi, S.; Tang, N.; Zhang, X.; Chen, S.; Zhu, P.; Ma, L.; Cheng, J.; Xu, Y.; Lu, M.; et al. Discovery of replicating circular RNAs by RNA-seq and computational algorithms. PLoS Pathog. 2004, 10, e1004553. [Google Scholar] [CrossRef]
- Nabi, S.U.; Baranwal, V.K. First Report of Apple Hammerhead Viroid infecting apple cultivars in India. Plant. Dis 2020, 104, 3086. [Google Scholar] [CrossRef]
- Wright, A.A.; Cross, A.R.; Harper, S.J. A bushel of viruses: Identification of seventeen novel putative viruses by RNA-seq in six apple trees. PLoS ONE 2020, 15, e0227669. [Google Scholar] [CrossRef]
- Szostek, S.A.; Wright, A.A.; Harper, S.J. First report of Apple Hammerhead Viroid in the United States, Japan, Italy, Spain, and New Zealand. Plant. Dis. 2018, 102, 2670. [Google Scholar] [CrossRef]
- Messmer, A.; Sanderson, D.; Braun, G.; Serra, P.; Flores, R.; James, D. Molecular and phylogenetic identification of unique isolates of hammerhead viroid-like RNA from ‘Pacific Gala’ apple (Malus domestica) in Canada. Can. J. Plant. Pathol. 2017, 39, 342–353. [Google Scholar] [CrossRef]
- Nickel, O.; Fajardo, T.V.M.; Candresse, T. First report on occurrence of apple hammerhead viroid in apples in Brazil. In Proceedings of the XXXII Congresso Brasileiro de Virologia: Virologia em Casa, 32, Belo Horizonte, Brazil, 19–23 October 2021; Even3 CBV: Belo Horizonte, Brazil, 2021. [Google Scholar]
- Lim, S.; Moon, J.S.; Cho, I.S.; Kim, H.R.; Lee, S.H. First Report of Apple Hammerhead Viroid infecting apple trees in South Korea. Plant. Dis. 2019, 103, 2700. [Google Scholar] [CrossRef]
- Sanderson, D.; James, D. Analysis of the genetic diversity of genome sequences of variants of apple hammerhead viroid. Can. J. Plant. Pathol. 2019, 41, 551–559. [Google Scholar] [CrossRef]
- Di Serio, F.; Ambrós, S.; Sano, T.; Flores, R.; Navarro, B. Viroid diseases in pome and stone fruit trees and Koch’s postulates: A critical assessment. Viruses 2018, 10, 612. [Google Scholar] [CrossRef]
- Nazarov, P.A.; Baleev, D.N.; Ivanova, M.I.; Sokolova, L.M.; Karakozova, M.V. Infectious plant diseases: Etiology, current status, problems and prospects in plant protection. Acta Nat. 2020, 12, 46–59. [Google Scholar] [CrossRef]
- Romadanova, N.V.; Mishustina, S.A.; Gritsenko, D.A.; Omasheva, M.Y.; Galiakparov, N.N.; Reed, B.M.; Kushnarenko, S.V. Cryotherapy as a method for reducing the virus infection of apples (Malus sp.). Cryo Lett. 2016, 37, 1–9. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Dalla Costa, M.; Souza, J.A.; Volk, G.; Nickel, O.; da Silva, F.N.; Kretzschmar, A.A. Cryotherapy by encapsulation-dehydration is effective for in vitro eradication of latent viruses from Marubakaido apple rootstock. J. Biotechnol. 2018, 269, 1–7. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Souza, J.A.; Volk, G.M.; Dalla Costa, M.; da Silva, F.N.; Kretzschmar, A.A. Eradication of latent viruses from apple cultivar ’Monalisa’ shoot tips using droplet-vitrification cryotherapy. Sci. Hortic. 2019, 250, 12–18. [Google Scholar] [CrossRef]
- Souza, J.A.; Bogo, A.; Bettoni, J.C.; Dalla Costa, M.; da Silva, F.N.; Casa, R.T.; Rufato, L. Droplet-vitrification cryotherapy for eradication of Apple stem grooving virus and apple stem pitting virus from “Marubakaido” apple rootstock. Trop. Plant. Pathol. 2020, 45, 148–152. [Google Scholar] [CrossRef]
- Li, B.Q.; Feng, C.H.; Hu, L.Y.; Wang, M.R.; Wang, Q.C. Shoot tip culture and cryopreservation for eradication of Apple stem pitting virus (ASPV) and Apple stem grooving virus (ASGV) from apple rootstocks ‘M9’ and ‘M26’. Ann. Appl. Biol. 2016, 168, 1–9. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Yan, L.; Jin, Y.; Sun, L.; Yang, Y.; Wang, Y.; Zhao, Z. Different eradication effects of latent viruses by combining thermotherapy with shoot tip culture or cryotherapy in four apple cultivars. Sci. Hortic. 2021, 288, 110356. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, W.R.; Cui, Z.H.; Chen, L.; Volk, G.M.; Wang, Q.C. Combining thermotherapy with cryotherapy for efficient eradication of Apple stem grooving virus from infected in-vitro-cultured apple shoots. Plant. Dis. 2018, 102, 1574–1580. [Google Scholar] [CrossRef]
- Kumar, P.L.; Cuervo, M.; Kreuze, J.F.; Muller, G.; Kulkarni, G.; Kumari, S.G.; Massart, S.; Mezzalama, M.; Alakonya, A.; Muchugi, A.; et al. Phytosanitary interventions for safe global germplasm exchange and the prevention of transboundary pest spread: The role of CGIAR germplasm health units. Plants 2021, 10, 328. [Google Scholar] [CrossRef]
- Wang, M.-R.; Li, B.Q.; Feng, C.-H.; Wang, Q.C. Culture of shoot tips from adventitious shoots can eradicate Apple stem pitting virus but fails in Apple stem grooving virus. Plant. Cell Tiss. Organ. Cult. 2016, 125, 283–291. [Google Scholar] [CrossRef]
- Wang, M.R.; Cui, Z.H.; Li, J.W.; Hao, X.Y.; Zhao, L.; Wang, Q.C. In vitro thermotherapy-based methods for plant virus eradication. Plant Methods 2018, 14, 87. [Google Scholar] [CrossRef]
- Faccioli, G.; Marani, F. Virus elimination by meristem tip culture and tip micrografting. In Plant Virus Diseases Control; Hadid, A., Dhetarpal, A.R.K., Koganezawa, H., Eds.; APS Press: Saint Paul, MN, USA, 1998; pp. 346–380. [Google Scholar]
- Wang, Q.; Cuellar, W.J.; Rajamaki, M.-L.; Hirata, Y.; Valkonen, J.P.T. Combined thermotherapy and cryotherapy for efficient virus eradication: Relation of virus distribution, subcellular changes, cell survival and viral RNA degradation in shoot tips. Mol. Plant. Pathol. 2008, 9, 237–250. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Zhang, F.; Hong, N.; Wang, G.; Wang, A.; Wang, L. Identification and characterization of microRNAs from in vitro-grown pear shoots infected with Apple stem grooving virus in response to high temperature using small RNA sequencing. BMC Genom. 2015, 16, 945. [Google Scholar] [CrossRef]
- Panattoni, A.; Luvisi, A.; Triolo, E. Elimination of viruses in plants: Twenty years of progress. Span. J. Agric. Res. 2013, 11, 173–188. [Google Scholar] [CrossRef]
- Hu, G.-J.; Dong, Y.-F.; Zhang, Z.-P.; Fan, X.-D.; Ren, F.; Li, Z.-N. Efficacy of virus elimination from apple by thermotherapy coupled with in vivo shoot-tip grafting and in vitro meristem culture. J. Phytopathol. 2017, 165, 701–706. [Google Scholar] [CrossRef]
- Paprštein, F.; Sedlák, J.; Polak, J.; Svobodova, L.; Hassan, M.; Bryxiova, M. Results of in vitro thermotherapy of apple cultivars. Plant. Cell Tissue Organ. Cult. 2008, 94, 347–352. [Google Scholar] [CrossRef]
- Magyar-Tábori, K.; Mendler-Drienyovszki, N.; Hanász, A.; Zsombik, L.; Dobránszki, J. Phytotoxicity and other adverse effects on the in vitro shoot cultures caused by virus elimination treatments: Reasons and solutions. Plants 2021, 10, 670. [Google Scholar] [CrossRef] [PubMed]
- Farhadi-Tooli, S.; Ghanbari, A.; Kermani, M.J.; Zeinalabedini, M.; Bettoni, J.C.; Naji, A.M.; Kazemi, N. Droplet-vitrification cryotherapy and thermotherapy as efficient tools for the eradication of apple chlorotic leaf spot virus and Apple stem grooving virus from virus-infected quince in vitro cultures. Eur. J. Plant. Pathol. 2021, 162, 31–43. [Google Scholar] [CrossRef]
- Hu, G.; Dong, Y.; Zhang, Z.; Fan, X.; Ren, F.; Zou, J. Virus elimination from in vitro apple by thermotherapy combined with chemotherapy. Plant. Cell Tissue Organ. Cult. 2015, 121, 435–443. [Google Scholar] [CrossRef]
- Griffiths, H.M.; Slack, S.A.; Dodds, J.H. Effect of chemical and heat therapy on virus concentration in in vitro plantlets. Can. J. Bot. 1990, 68, 1515–1521. [Google Scholar] [CrossRef]
- Faccioli, G. Control of potato viruses using meristem and stem-cuttings cultures, thermotherapy and chemotherapy. In Virus and Virus-Like Diseases of Potato and Production of Seed-Potatoes; Loebenstein, G., Berger, P.H., Brunt, A.A., Lawson, R.H., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 365–390. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Brison, M.; Boucaud, M.T.; Pierronnet, A.; Dosba, F. Effect of cryopreservation on the sanitary state of a cv. Prunus rootstock experimentally contaminated with Plum. Pox Potyvirus. Plant. Sci. 1997, 123, 189–196. [Google Scholar] [CrossRef]
- Wang, Q.C.; Panis, B.; Engelmann, F.; Lambardi, M.; Valkonen, J.P.T. Cryotherapy of shoot tips: A technique for pathogen eradication to produce healthy planting materials and prepare healthy plant genetic resources for cryopreservation. Ann. Appl. Biol. 2009, 154, 351–363. [Google Scholar] [CrossRef]
- Wang, B.; Wang, R.-R.; Cui, Z.-H.; Bi, W.-L.; Li, J.-W.; Li, B.-Q.; Ozudogru, E.A.; Volk, G.M.; Wang, Q.-C. Potential applications of cryogenic technologies to plant genetic improvement and pathogen eradication. Biotech. Adv. 2014, 32, 583–595. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Dalla Costa, M.; Gardin, J.P.P.; Kretzschmar, A.A.; Pathirana, R. Cryotherapy: A new technique to obtain grapevine plants free of viruses. Rev. Bras. Frutic. 2016, 38, e-833. [Google Scholar] [CrossRef]
- Bi, W.L.; Hao, X.Y.; Cui, Z.H.; Pathirana, R.; Volk, G.M.; Wang, Q.C. Shoot tip cryotherapy for efficient eradication of grapevine leafroll-associated virus-3 from diseased grapevine in vitro plants. Ann. Appl. Biol. 2018, 173, 261–270. [Google Scholar] [CrossRef]
- Pathirana, R.; McLachlan, A.; Hedderley, D.; Carra, A.; Carimi, F.; Panis, B. Removal of leafroll viruses from infected grapevine plants by droplet vitrification. Acta Hortic. 2015, 1083, 491–498. [Google Scholar] [CrossRef]
- Vieira, R.L.; da Silva, A.L.; Zaffari, G.R.; Steinmacher, A.; Fraga, H.P.F.; Guerra, M.P. Efficient elimination of virus complex from garlic (Allium sativum L.) by cryotherapy of shoot tips. Acta Physiol. Plant. 2015, 37, 1–11. [Google Scholar] [CrossRef]
- Kaya, E. Comparison of three different techniques for eradication of Apple mosaic virus (ApMV) from hazelnut (Corylus avellana L.). J. Plant. Prot. Res. 2021, 61, 11–19. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Marković, Z.; Bi, W.; Volk, G.M.; Matsumoto, T.; Wang, Q.-C. Grapevine shoot tip cryopreservation and cryotherapy: Secure storage of disease-free plants. Plants 2021, 10, 2190. [Google Scholar] [CrossRef]
- Wang, Q.C.; Valkonen, J.P.T. Cryotherapy of shoot tips: Novel pathogen eradication method. Trend Plant. Sci. 2009, 14, 119–122. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Volk, G.M. Challenges in implementing plant shoot tip cryopreservation technologies. Plant. Cell Tiss Organ. Cult. 2021, 144, 21–34. [Google Scholar] [CrossRef]
- Di Serio, F.; Martínez de Alba, A.E.; Navarro, B.; Gisel, A.; Flores, R. RNA-dependent RNA polymerase 6 delays accumulation and precludes meristem invasion of a nuclear-replicating viroid. J. Virol. 2010, 84, 2477–2489. [Google Scholar] [CrossRef]
- Rodio, M.E.; Delgado, S.; De Stradis, A.; Gomez, M.D.; Flores, R.; Di Serio, F. A viroid RNA with a specific structural motif inhibits chloroplast development. Plant. Cell 2007, 19, 3610–3626. [Google Scholar] [CrossRef]
- Zhang, Z.; Lee, Y.; Spetz, C.; Clarke, J.L.; Wang, Q.; Blystad, D.-R. Invasion of shoot apical meristems by Chrysanthemum stunt viroid differs among Argyranthemum cultivars. Front. Plant. Sci. 2015, 6, 53. [Google Scholar] [CrossRef][Green Version]
- Zhao, L.; Wang, M.; Li, J.; Cui, Z.; Volk, G.M.; Wang, Q. Cryobiothechnology: A double-edged sword for obligate plant pathogens. Plant. Dis. 2019, 103, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Mathew, L.; Tiffin, H.; Erridge, Z.; McLachlan, A.; Hunter, D.; Pathirana, R. Efficiency of eradication of Raspberry bushy dwarf virus from infected raspberry (Rubus idaeus) by in vitro chemotherapy, thermotherapy and cryotherapy and their combinations. Plant. Cell Tissue Organ. Cult. 2021, 144, 133–141. [Google Scholar] [CrossRef]
- Wang, M.-R.; Hamborg, Z.; Ma, X.-Y.; Blystad, D.-R.; Wang, Q.-C. Double-edged effects of the cryogenic technique for virus eradication and preservation in shallot shoot tips. Plant. Pathol. 2021, 71, 494–504. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Planta 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Li, B.Q.; Feng, C.H.; Wang, M.R.; Hu, L.Y.; Volk, G.; Wang, Q.C. Recovery patters, histological observations and genetic integrity in Malus shoot tips cryopreserved using droplet-vitrification and encapsulation-dehydration procedures. J. Biotechnol. 2015, 214, 182–191. [Google Scholar] [CrossRef]
- Sakai, A.; Kobayashi, S.; Oiyama, I. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant. Cell Rep. 1990, 9, 30–33. [Google Scholar] [CrossRef]
- Malapi-Wight, M.; Adhikari, B.; Zhou, J.; Hendrickson, L.; Maroon-Lango, C.J.; McFarland, C.; Foster, J.; Hurtado-Gonzales, O.P. HTS-based diagnostics of sugarcane viruses: Seasonal variation and its implications for accurate detection. Viruses 2021, 13, 1627. [Google Scholar] [CrossRef]
- Andrews, S. FASTQC: A Quality Control Tool for High throughput Sequence Data. Babraham Bioinforma, 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 2 October 2021).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinform 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Dmitry, A.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Goodacre, N.; Aljanahi, A.; Nandakumar, S.; Mikailov, M.; Khan, A.S. A Reference Viral Database (RVDB) To Enhance Bioinformatics Analysis of High-Throughput Sequencing for Novel Virus Detection. mSphere 2018, 3, 1–18. [Google Scholar] [CrossRef]
- Menzel, W.; Jelkmann, W.; Maiss, E. Detection of four apple viruses by multiplex RT-PCR assays with coamplification of plant mRNA as internal control. J. Virol. Methods 2002, 99, 81–92. [Google Scholar] [CrossRef]

| Primer | Sequence | Product Size | Source |
|---|---|---|---|
| ACLSV-F | 5′-TTCATGGAAAGACAGGGGCAA-3′ | 677 bp | [89] |
| ACLSV-R | 5′-AAGTCTACAGGCTATTTATTATAAGTCTAA-3′ | ||
| ASGV-F ASGV-R | 5′-GCCACTTCTAGGCAGAACTCTTTGAA-3′ | 273 bp | [89] |
| 5′-AACCCCTTTTTGTCCTTCAGTACGAA-3′ | |||
| AHVd-F AHVd-R | 5′-CCCTCCGGTCKTRTCCAACC-3′ | 92 bp | Costa et al., unpublished |
| 5′-GCGAGAGAGAGCGACTTCTC-3′ | |||
| NAD5-F NAD5-R | 5′-GATGCTTCTTGGGGCTTCTTGTT-3′ | 181 bp | [89] |
| 5′-CTCCAGTCACCAACATTGGCATAA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bettoni, J.C.; Fazio, G.; Carvalho Costa, L.; Hurtado-Gonzales, O.P.; Rwahnih, M.A.; Nedrow, A.; Volk, G.M. Thermotherapy Followed by Shoot Tip Cryotherapy Eradicates Latent Viruses and Apple Hammerhead Viroid from In Vitro Apple Rootstocks. Plants 2022, 11, 582. https://doi.org/10.3390/plants11050582
Bettoni JC, Fazio G, Carvalho Costa L, Hurtado-Gonzales OP, Rwahnih MA, Nedrow A, Volk GM. Thermotherapy Followed by Shoot Tip Cryotherapy Eradicates Latent Viruses and Apple Hammerhead Viroid from In Vitro Apple Rootstocks. Plants. 2022; 11(5):582. https://doi.org/10.3390/plants11050582
Chicago/Turabian StyleBettoni, Jean Carlos, Gennaro Fazio, Larissa Carvalho Costa, Oscar P. Hurtado-Gonzales, Maher Al Rwahnih, Abby Nedrow, and Gayle M. Volk. 2022. "Thermotherapy Followed by Shoot Tip Cryotherapy Eradicates Latent Viruses and Apple Hammerhead Viroid from In Vitro Apple Rootstocks" Plants 11, no. 5: 582. https://doi.org/10.3390/plants11050582
APA StyleBettoni, J. C., Fazio, G., Carvalho Costa, L., Hurtado-Gonzales, O. P., Rwahnih, M. A., Nedrow, A., & Volk, G. M. (2022). Thermotherapy Followed by Shoot Tip Cryotherapy Eradicates Latent Viruses and Apple Hammerhead Viroid from In Vitro Apple Rootstocks. Plants, 11(5), 582. https://doi.org/10.3390/plants11050582







