Effects of Different Inter-Row Soil Management and Intra-Row Living Mulch on Spontaneous Flora, Beneficial Insects, and Growth of Young Olive Trees in Southern Italy
Abstract
1. Introduction
2. Results
2.1. Entomological Report
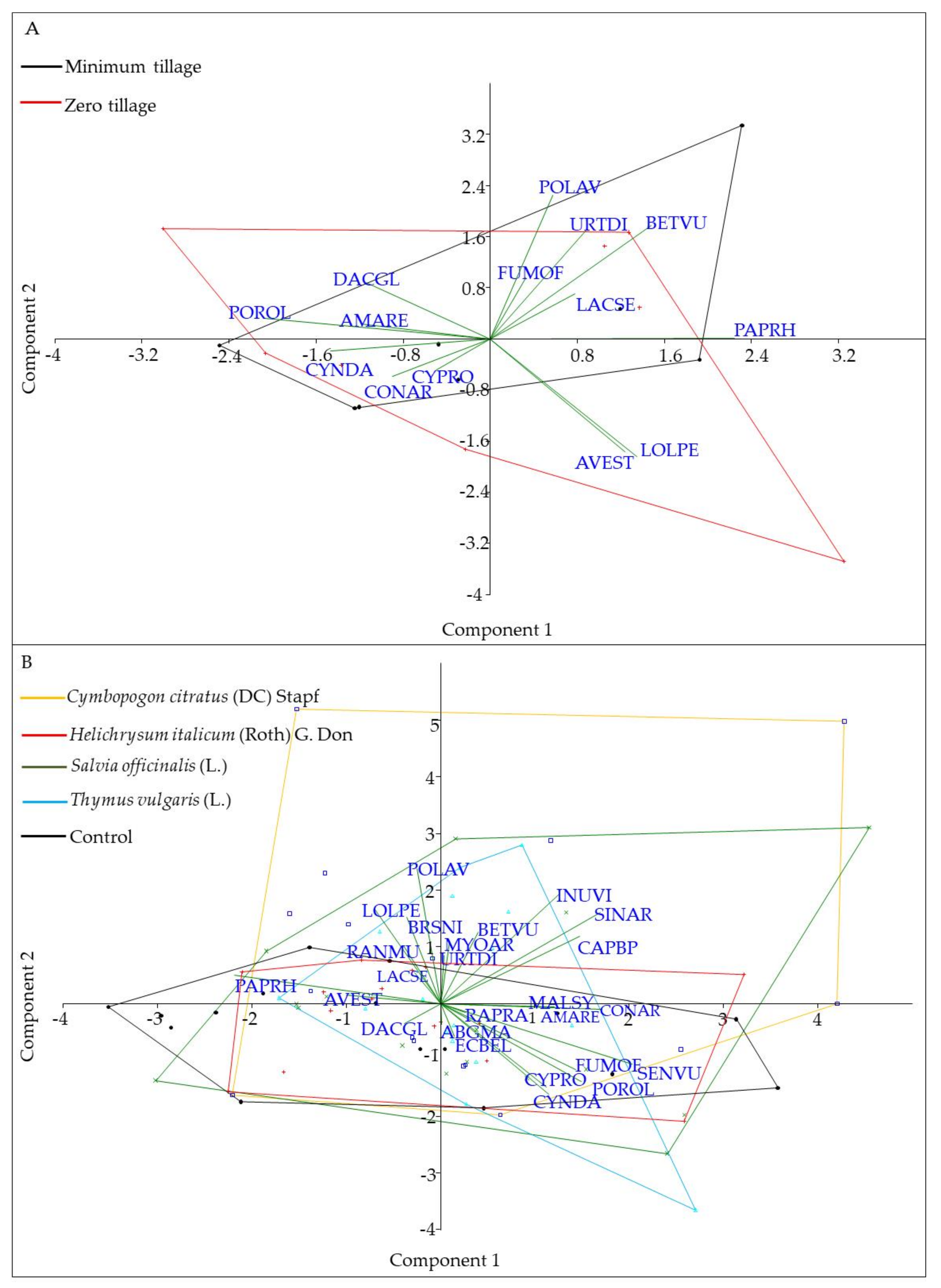
2.2. Spontaneous Flora Distribution and Diversity
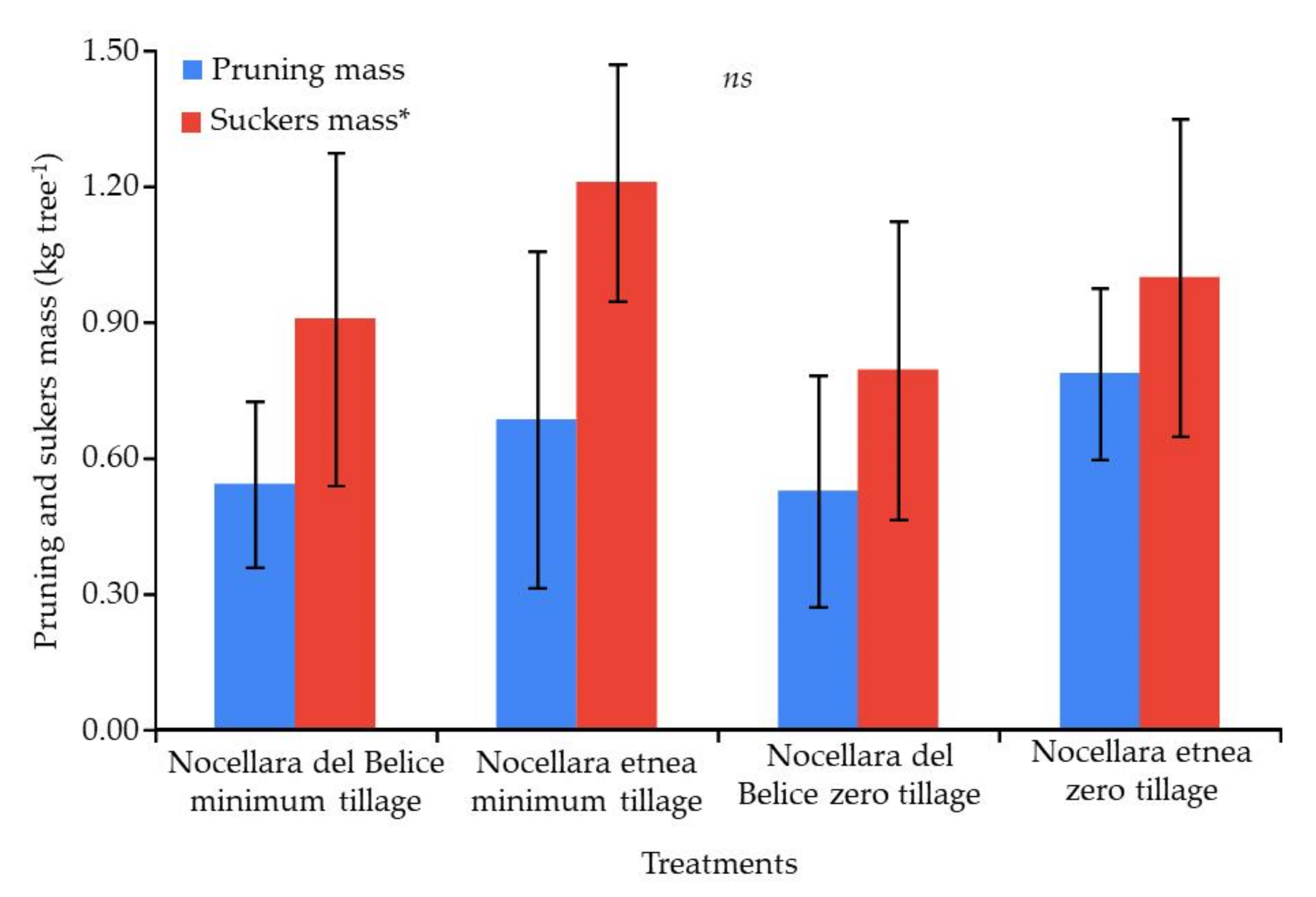
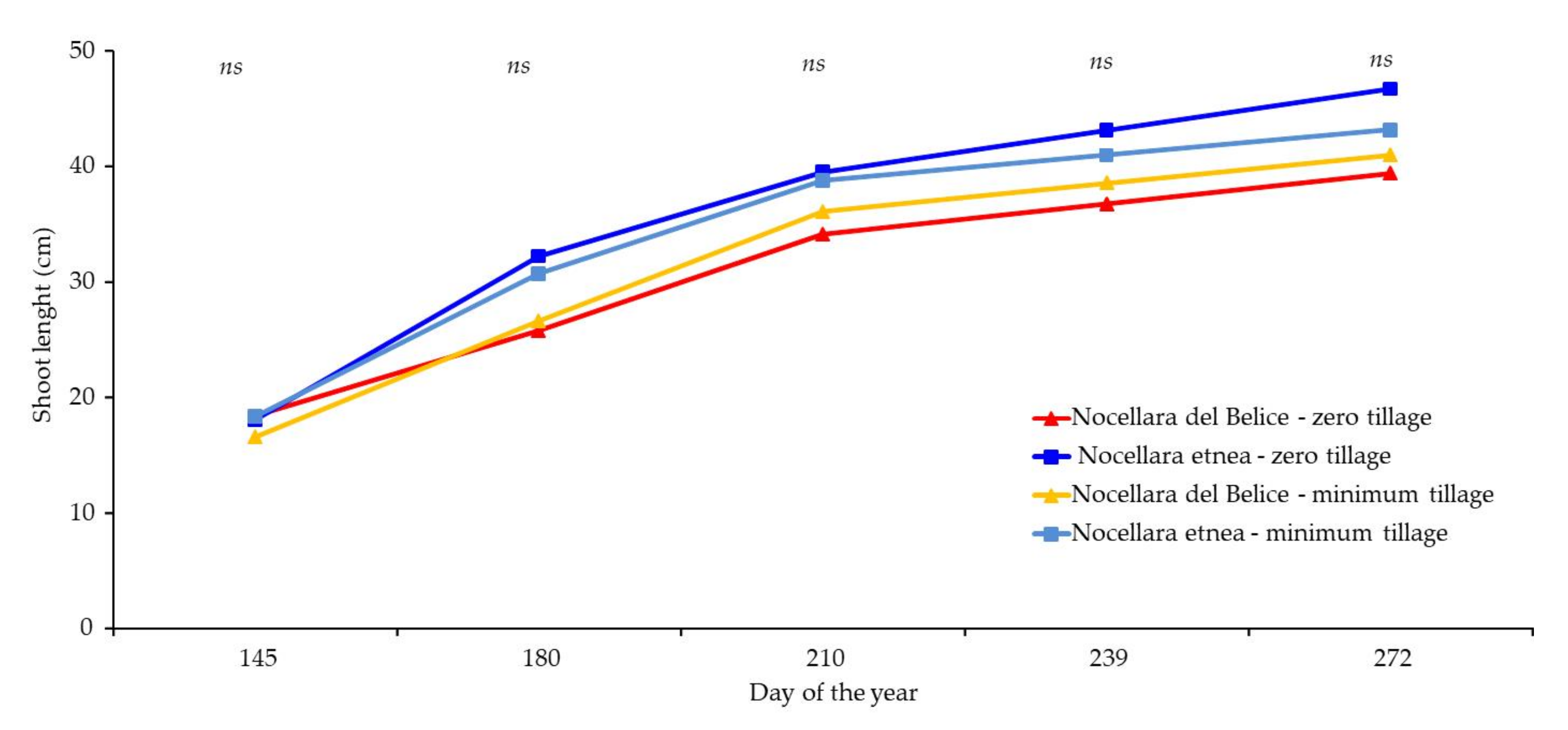
2.3. Plant Growth Analysis
3. Discussion
4. Materials and Methods
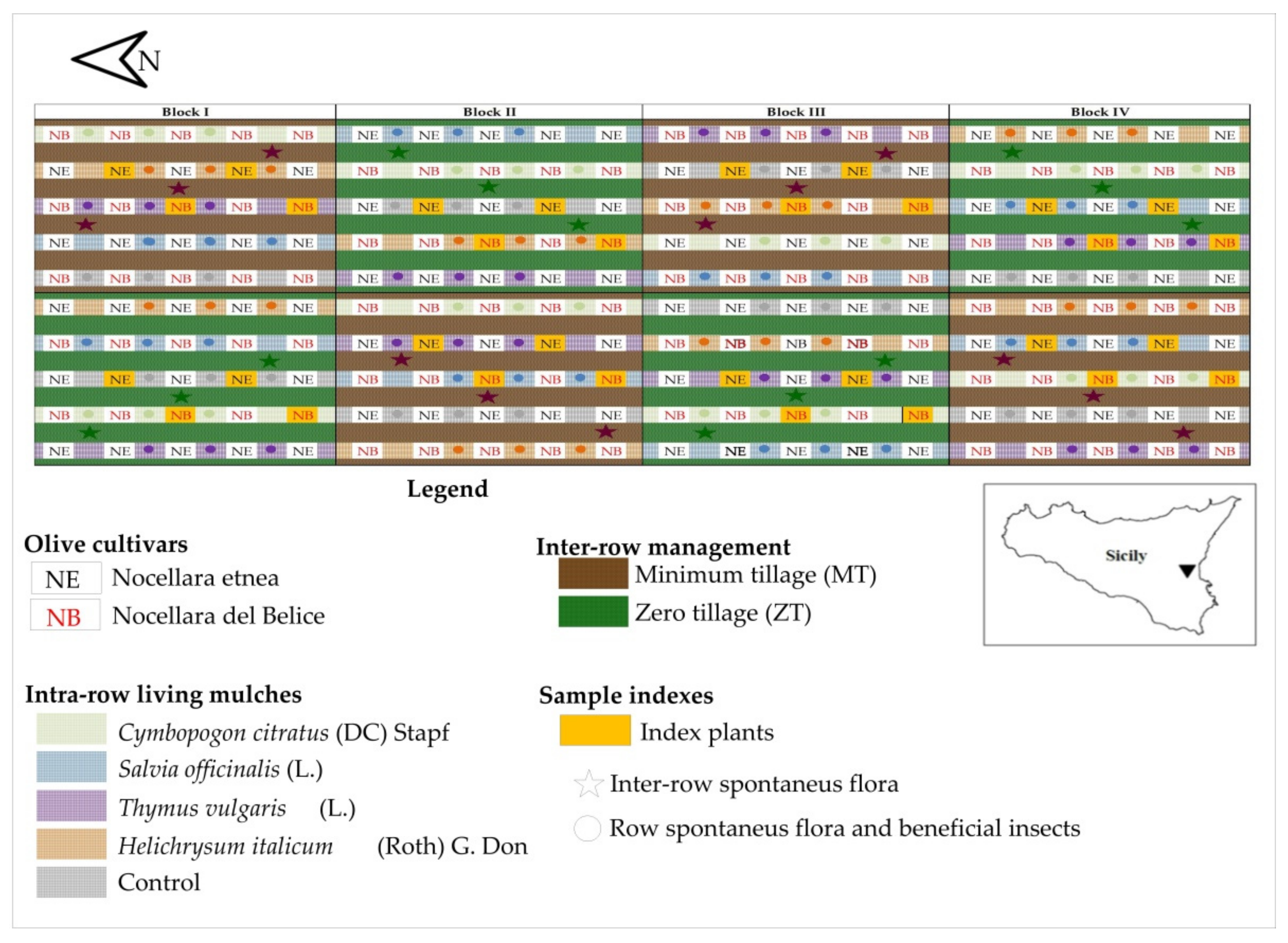
4.1. Site Description, Experimental Design, and Treatments
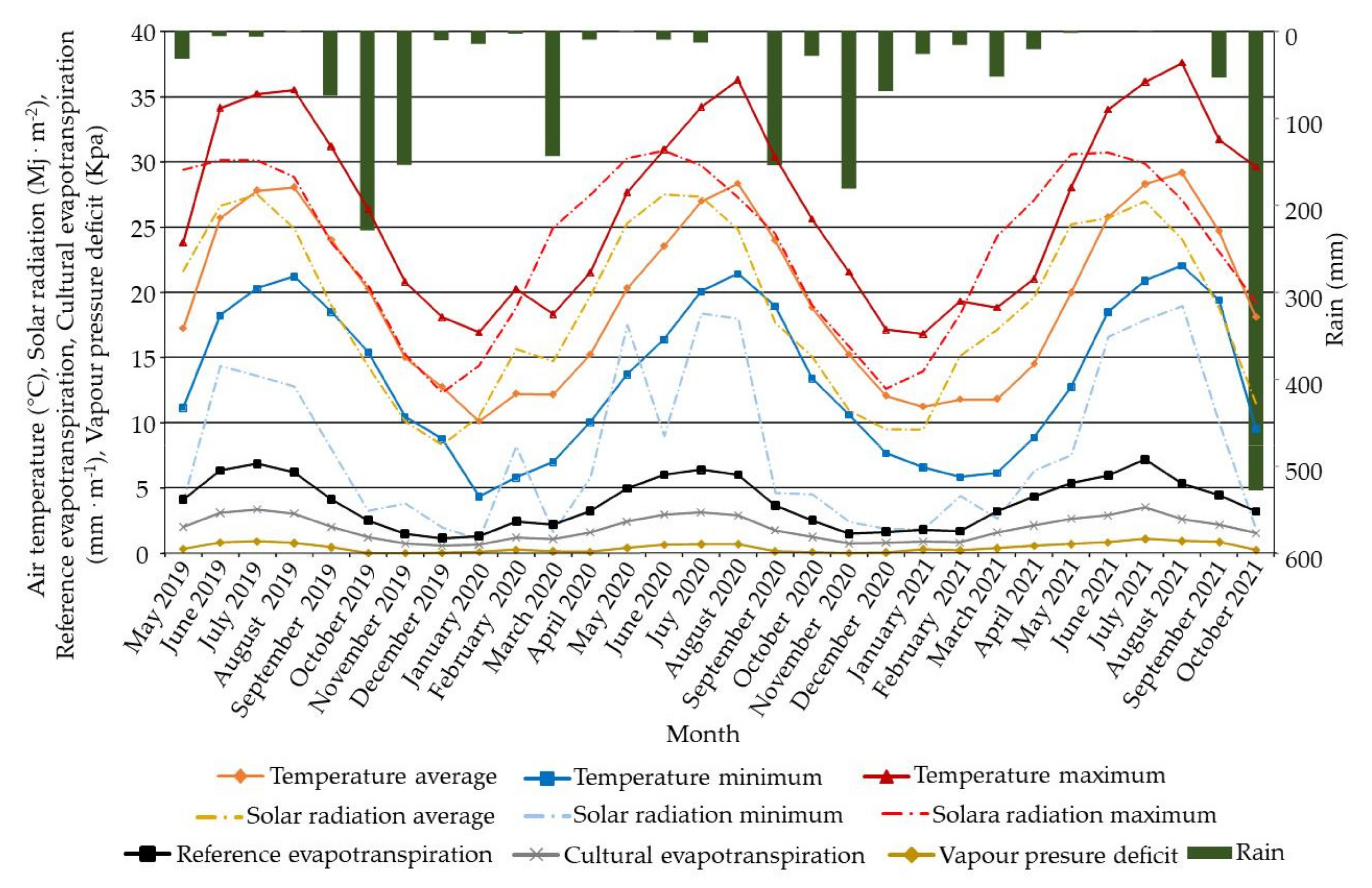
4.2. Soil Analysis and Climatic Data
4.3. Entomological Samplings and Analysis
4.4. Spontaneous Flora Assessment and Analysis
4.5. Tree Growth Monitoring
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montanarella, L.; Panagos, P. The relevance of sustainable soil management within the European Green Deal. Land Use Policy 2021, 100, 104950. [Google Scholar] [CrossRef]
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; ESAWorking Paper No. 12-03; FAO: Rome, Italy, 2012. [Google Scholar]
- Food and Agriculture Organization. 2050: A Third More Mouths to Feed. 2009. Available online: http://www.fao.org/news/story/en/item/35571/icode/ (accessed on 1 September 2021).
- Ciaccia, C.; Testani, E.; Fiore, A.; Iocola, I.; Di Pierro, M.; Mele, G.; Ferlito, F.; Cutuli, M.; Montemurro, F.; Farina, R.; et al. Organic Agroforestry Long-Term Field Experiment Designing Trough Actors’ Knowledge towards Food System Sustainability. Sustainability 2021, 13, 5532. [Google Scholar] [CrossRef]
- Michalopoulos, G.; Kasapi, K.A.; Koubouris, G.; Psarras, G.; Arampatzis, G.; Hatzigiannakis, E.; Kavvadias, V.; Xiloyannis, C.; Montanaro, G.; Malliaraki, S.; et al. Adaptation of Mediterranean Olive Groves to Climate Change through Sustainable Cultivation Practices. Climate 2020, 8, 54. [Google Scholar] [CrossRef]
- Gómez, J.A.; Amato, M.; Celano, G.; Koubouris, G.C. Organic olive orchards on sloping land: More than a specialty niche production system? J. Environ. Manag. 2008, 89, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Stroosnijder, L.; Mansinho, M.I.; Palese, A.M. OLIVERO: The project analysing the future of olive production systems on sloping land in the Mediterranean basin. J. Environ. Manag. 2008, 89, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Beaufoy, G. EU Policies for Olive Farming: Unsustainable on All Counts; WWF Europe/Birdlife International: Brussels, Belgium, 2001; p. 16. [Google Scholar]
- Connor, D.J.; Gomez-del-Campo, M.; Rousseaux, M.C.; Searles, P.S. Structure, management and productivity of hedgerow olive orchards: A review. Sci. Hortic. 2014, 169, 71–93. [Google Scholar] [CrossRef]
- Tous, J. Olive production systems and mechanization. Acta Hortic. 2011, 924, 169–184. [Google Scholar] [CrossRef]
- Caruso, G.; Palai, G.; Marra, F.P.; Caruso, T. High-Resolution UAV Imagery for Field Olive (Olea europaea L.) Phenotyping. Horticulturae 2021, 7, 258. [Google Scholar] [CrossRef]
- Assirelli, A.; Romano, E.; Bisaglia, C.; Lodolini, E.M.; Neri, D.; Brambilla, M. Canopy Index Evaluation for Precision Management in an Intensive Olive Orchard. Sustainability 2021, 13, 8266. [Google Scholar] [CrossRef]
- Neri, D.; Cioccolanti, T.; Zuccherelli, G.; Navacchi, O.; Giorgi, V.; Lodolini, E.M. Micropropagation effects on juvenile traits, flower differentiation, and tree architecture in young olive trees. Agronomy 2020, 10, 1742. [Google Scholar] [CrossRef]
- The International Olive Oil Council. 2016. Available online: http://www.internationaloliveoil.org (accessed on 15 September 2021).
- Eurostat. Agriculture, Forestry and Fishery Statistics, 2014 ed.; Eurostat: Luxembourg, 2015. [Google Scholar]
- De Graaff, J.; Eppink, L.A.A.J. Olive oil production and soil conservation in southern Spain, in relation to EU subsidy policies. Land Use Policy 1999, 16, 259–267. [Google Scholar] [CrossRef]
- Potts, S.G.; Petanidou, T.; Roberts, S.; O’Toole, C.; Hulbert, A.; Willmer, P. Plant-pollinator biodiversity and pollination services in a complex Mediterranean landscape. Biol. Conserv. 2006, 129, 519–529. [Google Scholar] [CrossRef]
- Palese, A.M.; Vignozzi, N.; Celano, G.; Agnelli, A.E.; Pagliai, M.; Xiloyannis, C. Influence of soil management on soil physical characteristics and water storage in a mature rainfed olive orchard. Soil Tillage Res. 2014, 144, 96–109. [Google Scholar] [CrossRef]
- Wagner, D.L. Insect Declines in the Anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Roberts, S.P.M.; Kemp, J.; Rasmont, P.; Kuhlmann, M.; García Criado, M.; Biesmeijer, J.C.; Bogusch, P.; Dathe, H.H.; De la Rúa, P.; et al. European Red List of Bees; Publication Office of the European Union: Luxembourg, 2014; 84p.
- Palm, C.; Blanco-Canqui, H.; DeClerck, F.; Gatere, L.; Grace, P. Conservation agriculture and ecosystem services: An overview. Agric. Ecosyst. Environ. 2014, 187, 87–105. [Google Scholar] [CrossRef]
- Ciaccia, C.; Testani, E.; Roccuzzo, G.; Canali, S. The role of agrobiodiversity in sustainable food systems design and management. In Genetic Diversity in Horticultural Plants; Springer: Cham, Switzerland, 2019; pp. 245–271. [Google Scholar]
- Landis, D.A. Designing agricultural landscapes for biodiversity-based ecosystem services. Basic Appl. Ecol. 2017, 18, 1–12. [Google Scholar] [CrossRef]
- Wood, S.A.; Karp, D.S.; DeClerck, F.; Kremen, C.; Naeem, S.; Palm, C.A. Functional traits in agriculture: Agrobiodiversity and ecosystem services. Trends Ecol. Evol. 2015, 30, 531–539. [Google Scholar] [CrossRef]
- Canali, S.; Diacono, M.; Campanelli, G.; Montemurro, F. Organic No-Till with Roller Crimpers: Agro-ecosystem Services and Applications in Organic Mediterranean Vegetable Productions. Sustain. Agric. Res. 2015, 4, 70–79. [Google Scholar] [CrossRef]
- Bommarco, R.; Kleijn, D.; Potts, S.G. Ecological intensification: Harnessing ecosystem services for food security. Trends Ecol. Evol. 2013, 28, 230–238. [Google Scholar] [CrossRef]
- Ramos, M.E.; Benítez, E.; García, P.A.; Robles, A.B. Cover crops under different managements vs. frequent tillage in almond orchards in semiarid conditions: Effects on soil quality. Appl. Soil. Ecol. 2010, 44, 6–14. [Google Scholar] [CrossRef]
- Nieto, O.M.; Castro, J.; Fernandez-Ondono, E. Conventional tillage versus cover crops in relation to carbon fixation in Mediterranean olive cultivation. Plant Soil 2013, 365, 321–335. [Google Scholar] [CrossRef]
- Mourugàn-Coronado, A.; Linares, C.; Gòmez-Lòpeez, M.D.; Faz, A.; Zornova, R. The impact of intercropping, tillage and fertilizer type on soil and crop yield in fruit orchards under Mediterranean conditions: A meta-analysis of field studies. Agric. Syst. 2020, 178, 102736. [Google Scholar] [CrossRef]
- Sestini, A. Il Paesaggio; Club Italiano: Milano, Italy, 1963. [Google Scholar]
- Paris, P.; Camilli, F.; Rosati, A.; Mantino, A.; Mezzalira, G.; Dalla Valle, C.; Franca, A.; Seddaiu, G.; Pisanelli, A.; Lauteri, M.; et al. What is the future for agroforestry in Italy? Agroforest Syst. 2019, 93, 2243–2256. [Google Scholar] [CrossRef]
- Nicolosi, E.; Ferlito, F.; Allegra, M.; Cicala, A.; Trovato, F.; La Malfa, S. Influences of aspect and tillage on two winegrape cultivars on Mount Etna. N. Z. J. Crop Hortic. Sci. 2016, 44, 83–102. [Google Scholar] [CrossRef][Green Version]
- Ferlito, F.; Allegra, M.; Torrisi, B.; Pappalardo, H.; Gentile, A.; La Malfa, S.; Continella, A.; Stagno, F.; Nicolosi, E. Early defoliation effect on water status, fruit yield and must quality of ‘Nerello mascalese’ grapes. Sci. Agric. 2020, 77, 1–10. [Google Scholar] [CrossRef]
- Nicolosi, E.; Iovino, V.; Distefano, G.; Di Guardo, M.; La Malfa, S.; Gentile, A.; Palliotti, A.; Las Casas, G.; Ferlito, F. Mid-Term Effects of Conservative Soil Management and Fruit-Zone Early Leaf Removal Treatments on the Performance of Nerello Mascalese (Vitis vinifera L.) Grapes on Mount Etna (Southern Italy). Agronomy 2021, 11, 1070. [Google Scholar] [CrossRef]
- Bennici, S.; Las Casas, G.; Distefano, G.; Di Guardo, M.; Continella, A.; Ferlito, F.; Gentile, A.; La Malfa, S. Elucidating the contribution of wild related species on autochthonous pear germplasm: A case study from Mount Etna. PLoS ONE 2018, 13, e0198512. [Google Scholar] [CrossRef]
- Russo, C.; Cappelletti, G.M.; Nicoletti, G.M.; Di Noia, A.E.; Michalopoulos, G. Comparison of European Olive Production Systems. Sustainability 2016, 8, 825. [Google Scholar] [CrossRef]
- Magdoff, F. Ecological agriculture: Principles, practices, and constraints. Renew. Agric. Food Syst. 2007, 22, 109–117. [Google Scholar] [CrossRef]
- Burgio, G.; Sommaggio, D.; Birtele, D. I Sirfidi (Ditteri): Biodiversità e conservazione. In Manuali e Linee Guida; ISPRA: Roma, Italy, 2015; Volume 128, p. 182. [Google Scholar]
- Comba, M. Hymenoptera: Apoidea: Anthophila of Italy. 2021. Available online: http://digilander.libero.it/mario.comba (accessed on 27 July 2021).
- Mazzeo, G.; Bella, S.; Seminara, A.R.; Longo, S. Bumblebees in natural and agro-ecosystems at different altitudes from Mount Etna, Sicily (Hymenoptera Apidae Bombinae): Long-term faunistic and ecological observations. Redia 2015, XCVIII, 123–131. [Google Scholar]
- Mazzeo, G.; Longo, S.; Seminara, A.R.; Bella, S. Faunistic and ecological studies on Apidae (Hymenoptera, Apoidea) in natural and cultivated ecosystems in Sicily. Redia 2019, 102, 153–162. [Google Scholar] [CrossRef]
- Bella, S.; Catania, R.; Nobile, V.; Mazzeo, G. New or little known bees (Hymenoptera, Apoidea) from Sicily. Fragm. Entomol. 2020, 52, 113–117. [Google Scholar] [CrossRef]
- Incalcaterra, G.; Iapichino, G.; D’Anna, F.; Sinacori, A. Influences of different Pollinators on Winter Melon Grown under Polyethylene Tunnel. Acta Hort. 2003, 614, 297–299. [Google Scholar] [CrossRef]
- Lo Verde, G.; La Mantia, T. The role of native flower visitors in pollinating Opuntia ficus-indica (L.) Mill. naturalized in Sicily. Acta Oecol. 2011, 37, 413–417. [Google Scholar] [CrossRef]
- Mazzeo, G.; Scavo, A.; Lo Monaco, A.; Longo, S.; Mauromicale, G. Insect pollinators improve seed production in globe artichoke (Cynara cardunculus var. scolymus). Ann. Appl. Biol. 2020, 176, 241–248. [Google Scholar] [CrossRef]
- Bella, S.; Catania, R.; Baviera, C. First record of the genus Serangium Blackburn, 1889 (Coleoptera Coccinellidae) in Italy. Redia 2021, 104, 185–191. [Google Scholar] [CrossRef]
- Michener, C.D. The Bees of the World, 2nd ed.; John Hopkins University Press: Baltimore, MD, USA, 2007; p. 953. [Google Scholar]
- Holzschuh, A.; Steffan-Dewenter, I.; Kleijn, D.; Tscharntke, T. Diversity of flower-visiting bees in cereal fields: Effects of farming system, landscape composition and regional context. J. Appl. Ecol. 2007, 44, 41–49. [Google Scholar] [CrossRef]
- Scheper, J.A. Promoting wild bees in European agricultural landscapes. The Role of Floral Resources in Driving and Mitigating Wild Bee Decline; Alterra, Wageningen University & Research Centre: Wageningen, The Netherlands, 2015; 175p. [Google Scholar]
- Wezel, A.; Casagrande, M.; Celette, F.; Vian, J.-F.; Ferrer, A.; Peigné, J. Agroecological practices for sustainable agriculture. A review. Agron. Sustain. Dev. 2014, 34, 1–20. [Google Scholar] [CrossRef]
- Baviera, C.; Bellavista, M.; Altadonna, G.; Turrisi, G.F.; Bella, S.; Muscarella, C.; Sparacio, I. The Cerambycidae (Coleoptera: Chrysomeloidea) of Sicily: Recent records and updated checklist. AAPP Atti Accad. Peloritana dei Pericolanti Cl. Sci. Fis. Mat. Nat. 2017, 95, 2. [Google Scholar]
- Ramos, M.E.; Robles, A.B.; Sánchez-Navarro, A.; González-Rebollar, J.L. Soil responses to different management practices in rainfed orchards in semiarid environments. Soil Tillage Res. 2011, 112, 85–91. [Google Scholar] [CrossRef]
- Koch, J.B.; Lozier, J.; Strange, J.P.; Ikerd, H.; Griswold, T.; Cordes, N.; Solter, L.; Stewart, I.; Cameron, S.A. USBombus, a database of contemporary survey data for North American Bumble Bees (Hymenoptera, Apidae, Bombus) distributed in the United States. Biodivers. Data J. 2015, 3, e6833. [Google Scholar] [CrossRef] [PubMed]
- Taguas, E.V.; Gómez, J.A. Vulnerability of olive orchards under the current CAP (Common Agricultural Policy) regulations on soil erosion: A study case in Southern Spain. Land Use Policy 2015, 42, 683–694. [Google Scholar] [CrossRef]
- Almagro, M.; de Vente, J.; Boix-Fayós, C.; García-Franco, N.; de Aguilar, J.M.; González, D.; Solé-Benet, A.; Martínez-Mena, M. Sustainable land management practices as providers of several ecosystem services under rainfed Mediterranean agroecosystems. Mitig. Adapt. Strateg. Glob. Chang. 2016, 21, 1029–1043. [Google Scholar] [CrossRef]
- Chamizo, S.; Serrano-Ortiz, P.; López-Ballesteros, A.; Sánchez-Cañete, E.P.; Vicente-Vicente, J.L.; Kowalski, A.S. Net ecosystem CO2 exchange in an irrigated olive orchard of SE Spain: Influence of weed cover. Agric. Ecosyst. Environ. 2017, 239, 51–64. [Google Scholar] [CrossRef]
- Armengot, L.; Berner, A.; Blanco-Moreno, J.M.; Mäder, P.; Sans, F.X. Long-term feasibility of reduced tillage in organic farming. Agron. Sustain. Develop. 2015, 35, 339–346. [Google Scholar] [CrossRef]
- Nichols, V.; Verhulst, N.; Cox, R.; Govaerts, B. Weed dynamics and conservation agriculture principles: A review. Field Crops Res. 2015, 183, 56–68. [Google Scholar] [CrossRef]
- Mia, M.J.; Massetani, F.; Murri, G.; Neri, D. Sustainable alternatives to chemicals for weed control in the orchard a review. Hortic. Sci. 2020, 47, 1–12. [Google Scholar] [CrossRef]
- Bateni, S.; Vosoughifar, H.; Ek, M.; Xu, T. Estimation of Daily Reference Evapotranspiration from Limited Climatic Variables in Coastal Regions. In Proceedings of the AGU Fall Meeting, San Francisco, CA, USA, 9–13 December 2019; American Geophysical Union: Washington, DC, USA, 2019. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration. In Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper; FAO: Rome, Italy, 1998; Volume 56, p. 300. [Google Scholar]
- Saitta, D.; Vanella, D.; Ramírez-Cuesta, J.M.; Longo-Minnolo, G.; Ferlito, F.; Consoli, S. Comparison of Orange Orchard Evapotranspiration by Eddy Covariance, Sap Flow, and FAO-56 Methods under Different Irrigation Strategies. J. Irrig. Drain. 2020, 146, 05020002. [Google Scholar] [CrossRef]
- Lo Cicero, L.; Puglisi, I.; Nicolosi, E.; Gentile, A.; Ferlito, F.; Continella, A.; Lo Piero, A.R. Anthocyanin levels and expression analysis of biosynthesis-related genes during ripening of sicilian and international grape berries subjected to leaf removal and water deficit. J. Agric. Sci. 2016, 18, 1333–1344. [Google Scholar]
- Miller, W.P.; Miller, D.M. A micro-pipette method for soil mechanical analysis. Commun. Soil Sci. Plant Anal. 1987, 18, 1–15. [Google Scholar] [CrossRef]
- Ferlito, F.; Torrisi, B.; Allegra, M.; Stagno, F.; Caruso, P.; Fascella, G. Evaluation of Conifer Wood Biochar as Growing Media Component for Citrus Nursery. Appl. Sci. 2020, 10, 1618. [Google Scholar] [CrossRef]
- Torrisi, B.; Allegra, M.; Amenta, M.; Gentile, F.; Rapisarda, P.; Fabroni, S.; Ferlito, F. Physico-chemical and multielemental traits of anaerobic digestate from Mediterranean agro-industrial wastes and assessment as fertiliser for citrus nurseries. Waste Manag. 2021, 131, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Ferlito, F.; Distefano, G.; Gentile, A.; Allegra, M.; Lakso, A.N.; Nicolosi, E. Scion–rootstock interactions influence the growth and behaviour of the grapevine root system in a heavy clay soil. Aust. J. Grape Wine Res. 2020, 26, 68–78. [Google Scholar] [CrossRef]
- Olsen, S.; Cole, C.; Watanabe, F.; Dean, L. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate (Circular No. 939); United States Department of Agriculture: Washington, DC, USA, 1954.
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. In Methods of Soil Analysis; Sparks, D.L., Ed.; Part 3, Chemical Methods; Book Series No. 5; Soil Science Society of America: Madison, WI, USA, 1996. [Google Scholar]
- Springer, U.; Klee, J. Prufung der Leistungsfahigkeit von einigen wichtigeren Verfahren zur Bestimming des Kohlemstoffs mittels Chromschwefelsaure sowie Vorschlag einer neuen Schnellmethode. Z. Pflanz. Düngung Bodenkd. 1954, 64, 1–8. [Google Scholar] [CrossRef]
- Leonardi, M.; Caruso, G.M.; Carroccio, S.C.; Boninelli, S.; Curcuruto, G.; Zimbone, M.; Allegra, M.; Torrisi, B.; Ferlito, F.; Miritello, M. Smart nanocomposites of chitosan/alginate nanoparticles loaded with copper oxide as alternative nanofertilizers. Environ. Sci. Nano 2021, 8, 174–187. [Google Scholar] [CrossRef]
- Klingebiel, A.A.; Montgomery, P.H. Land Capability Classification; Agriculture USDA: Washington, DC, USA, 1961. [Google Scholar]
- Saitta, D.; Consoli, S.; Ferlito, F.; Torrisi, B.; Allegra, M.; Longo-Minnolo, G.; Ramírez-Cuesta, J.M.; Vanella, D. Adaptation of citrus orchards to deficit irrigation strategies. Agric. Water Manag. 2021, 247, 106734. [Google Scholar] [CrossRef]
- Ciaccia, C.; La Torre, A.; Ferlito, F.; Testani, E.; Battaglia, V.; Salvati, L.; Roccuzzo, G. Agroecological Practices and Agrobiodiversity: A Case Study on Organic Orange in Southern Italy. Agronomy 2019, 9, 85. [Google Scholar] [CrossRef]
- Schmid-Egger, C.; Scheuchl, E. Illustrierte Bestimmungstabellen der Wildbienen Deutschlands und Österreichs Unter Berücksichtigung der Arten der Schweiz. In Band III, Andrenidae; Eigenverlag: Velden, Austria, 1997; p. 180. [Google Scholar]
- Scheuchl, E. Illustrierte Bestimmungstabellen der Wildbienen Deutschlands und Österreichs. In Band I: Anthophoridae. 2. Erweiterte Auflage; Eigenverlag: Berlin, Germany, 2006; 158p. [Google Scholar]
- Scheuchl, E. Illustrierte Bestimmungstabellen der Wildbienen Deutschlands und Österreichs Für Osmia s.1. Unter Berücksichtigung der Arten der Schweiz, Norditaliens, Ungarns, Sloweniens und der Slowakei. In Band II, Schlüssel der Arten der Familien Megachilidae und Melittidae, 2nd ed.; Apollo Books: Tsim Sha Tsui, Japan, 2006; p. 192. [Google Scholar]
- Mazzei, P.; Morel, D.; Panfili, R. Moths and Buttes of Europe and North Africa. 2021. Available online: http://www.leps.eu (accessed on 2 November 2021).
- Fürsch, H. Übersicht über die Genera und Subgenera der Scymnini mit besonderer Berücksichtigung der Westpalaearktis (Insecta, Coleoptera, Coccinellidae). Entomol. Abh. (Dresd.) 1987, 51, 57–74. [Google Scholar]
- El-Saeady, A.A.; Hafez, S.F.; Abied, M.K.; Bedewy, M.M.M. Taxonomy of the Tribe Scymnini (Coleoptera: Coccinellidae: Scymninae). Egypt. Bull. Ent. Soc. 2016, 93, 117–150. [Google Scholar]
- Brooks, S.J. A taxonomic review of the common green lacewing genus Chrysoperla (Neuroptera: Chrysopidae). Bull. Br. Nat. Hist. (Entomol.) 1994, 63, 137–210. [Google Scholar]
- Polaszek, A. Fauna Europaea: Apidae. In Fauna Europaea: Hymenoptera—Apocryta. Fauna Europaea Version 2.6; Mitroiu, M.D., Noyes, J., Cetkovic, A., Nonveiller, G., Radchenko, A., Polaszek, A., Ronquist, F., Forshage, M., Pagliano, G., Gusenleitner, J., et al., Eds.; Fauna Europaea Secretariat: Berlin, Germany, 2013; Available online: http://www.fauna-eu.org (accessed on 27 July 2021).
- Turrisi, G.F.; Bella, S.; Catania, R.; La Greca, P.; Nobile, V.; D’urso, V. Bee diversity in fragmented areas of Volcano Etna (Sicily, Italy) at different degrees of anthropic disturbance (Hymenoptera: Apoidea, Anthophila). J. Entomol. Acarol. Res. 2021, 53, 10362. [Google Scholar] [CrossRef]
- Lodolini, E.M.; Paoletti, A.; Nolasco, A.; Ferlito, F.; Cutuli, M.; Torrisi, B.F.; Santilli, E.; Zaffina, F.; Desando, M.; Rosti, A.; et al. Biomass recovery from olive rejuvenation pruning in different varieties. In Proceedings of the 29th European Biomass Conference and Exhibition, Marseille, France, 26–29 April 2021; pp. 273–278. [Google Scholar] [CrossRef]

| Order | Family | Species | Wild Plants in the Inter-Rows | Consociated Plants in the Row |
|---|---|---|---|---|
| Pollinators | ||||
| Hymenoptera | Colletidae | Hylaeus cornutus Curtis, 1831 | Foeniculum vulgare | Helichrysum italicum |
| Andrenidae | Andrena aerinifrons Dours, 1873 | Sinapis arvensis | ||
| Ranunculus muricatus | ||||
| Andrena bicolorata (Rossi, 1790) | Sinapis arvensis | |||
| Andrena pilipes Fabricius, 1781 | Senecio vulgaris | |||
| Andrena brumanensis Friese, 1899 | Ranunculus muricatus | Salvia officinalis | ||
| Andrena distinguenda Schenck, 1871 | Glebionis coronaria | |||
| Andrena labialis (Kirby, 1802) | Ecballium elaterium | |||
| Andrena nigroaenea (Kirby, 1802) | Sinapis arvensis | |||
| Halictidae | Halictus fulvipes (Klug, 1817) | Galactites tomentosa | Thymus vulgaris | |
| Halictus quadricinctus (Fabricius, 1776) | Senecio vulgaris | |||
| Halictus scabiosae (Rossi, 1790) | Senecio vulgaris Dittrichia viscosa | Thymus vulgaris | ||
| Lasioglossum malachurum (Kirby, 1802) | Ecballium elaterium | Helichrysum italicum | ||
| Megachilidae | Heriades rubicola Pérez, 1890 | Dittrichia viscosa | Helichrysum italicum | |
| Osmia latreillei (Spinola, 1806) | Glebionis coronaria | Salvia officinalis | ||
| Osmia signata Erichson, 1835 | Glebionis coronaria | |||
| Rhodanthidium siculum (Spinola, 1838) | Oxalis pes-caprae | Salvia officinalis | ||
| Megachile sicula (Rossi, 1792) | Galactites tomentosa | |||
| Apidae | Xylocopa violacea (Linnaeus, 1758) | - | Salvia officinalis Thymus vulgaris | |
| Ceratina cyanea Kirby, 1802 | Ecballium elaterium | Helichrysum italicum | ||
| Nomada discrepans Schmiedeknecht, 1882 | Sinapis arvensis | |||
| Nomada distinguenda Morawitz, 1874 | Raphanus raphanistrum | |||
| Eucera algira Brullé, 1840 | Raphanus raphanistrum | |||
| Eucera eucnemidea Dours, 1873 | Galactites tomentosa | |||
| Eucera nigrescens Pérez, 1879 | Glebionis coronaria; Vicia sp. | |||
| Eucera nigrilabris Lepeletier, 1841 | Raphanus raphanistrum | |||
| Eucera numida Lepeletier, 1841 | Vicia sativa | |||
| Eucera oraniensis Lepeletier, 1841 | Glebionis coronaria | Salvia officinalis | ||
| Galactites tomentosa | ||||
| Amegilla garrula (Rossi, 1790) | - | Salvia officinalis Thymus vulgaris | ||
| Amegilla quadrifasciata (de Villers, 1789) | - | Salvia officinalis Thymus vulgaris | ||
| Anthophora dispar Lepeletier, 1841 | Fumaria officinalis | Salvia officinalis | ||
| Anthophora plumipes squalens Dours, 1869 | Fumaria officinalis Papaver rhoeas | |||
| Bombus pascuorum siciliensis Tkalcu, 1977 | Vicia sativa | Salvia officinalis Thymus vulgaris | ||
| Bombus terrestris (Linnaeus, 1758) | Vicia sativa | Salvia officinalisThymus vulgaris | ||
| Lepidoptera | Sphingidae | Macroglossum stellatarum (Linnaeus, 1758) | Convolvulus arvensis | Thymus vulgaris |
| Hyles euphorbiae (Linnaeus, 1758) | - | |||
| Hyles livornica (Esper, 1780) | Convolvulus arvensis | Helichrysum italicum | ||
| Sesiidae | Tinthia tineiformis (Esper, 1789) | Convolvulus arvensis | ||
| Geometridae | Rhodometra sacraria (Linnaeus, 1767) | - | Thymus vulgaris | |
| Menophra abruptaria (Thunberg, 1792) | Dittrichia viscosa | Thymus vulgaris | ||
| Noctuidae | Heliothis peltigera (Denis & Schiffermüller, 1775) | Senecio vulgaris | Helichrysum italicum Thymus vulgaris | |
| Autographa gamma (Linnaeus, 1758) | Salvia officinalis | |||
| Hesperiidae | Carcharodus alceae (Esper, 1780) | Lysimachia arvensis | Thymus vulgaris | |
| Lycaenidae | Lycaena alciphron (Rottemburg, 1775) | Althaea officinalis | Thymus vulgaris | |
| Lycaena phlaeas (Linnaeus, 1761) | Polygonum aviculare | Thymus vulgaris | ||
| Portulaca oleracea | ||||
| Ranunculus muricatus | ||||
| Nymphalidae | Aglais urticae (Linnaeus, 1758) | Althaea officinalis | Helichrysum italicum | |
| Vanessa atalanta (Linnaeus, 1758) | Althaea officinalis Convolvulus arvensis Ecballium elaterium | Salvia officinalis Thymus vulgaris | ||
| Vanessa cardui (Linnaeus, 1758) | Ranunculus muricatus | Thymus vulgaris | ||
| Lasiommata megera (Linnaeus, 1767) | Polygonum aviculare | Salvia officinalis Thymus vulgaris | ||
| Pararge aegeria (Linnaeus, 1758) | - | Thymus vulgaris | ||
| Papilionidae | Iphiclides podalirius (Linnaeus, 1758) | - | Helichrysum italicum | |
| Papilio machaon Linnaeus, 1758 | Dittrichia viscosa | |||
| Pieridae | Colias croceus (Geoffroy, 1785) | Ecballium elaterium | Thymus vulgaris | |
| Gonepteryx cleopatra (Linnaeus, 1767) | - | Thymus vulgaris | ||
| Pieris brassicae (Linnaeus, 1758) | Capsella bursa-pastoris | Thymus vulgaris | ||
| Raphanus raphanistrum | ||||
| Sinapis arvensis | ||||
| Pieris mannii (Mayer, 1851) | Beta vulgaris | |||
| Pieris rapae (Linnaeus, 1758) | Portulaca oleracea Sinapis arvensis | Thymus vulgaris | ||
| Diptera | Syrphidae | Episyrphus balteatus (DeGeer, 1776) * | - | Thymus vulgaris |
| Eupeodes luniger (Meigen, 1822) * | Ranunculus muricatus | |||
| Eristalinus taeniops (Wiedemann, 1818) | Beta vulgaris Polygonum aviculare | Thymus vulgaris | ||
| Eristalis tenax (Linnaeus, 1758) | - | Helichrysum italicum | ||
| Thymus vulgaris | ||||
| Syritta pipiens (Linnaeus, 1758) | Portulaca oleracea | |||
| Predators | ||||
| Neuroptera | Chrysopidae | Chrysopa viridana Schneider, 1845 | - | Thymus vulgaris |
| Chrysoperla carnea (Stephens, 1836) | Ranunculus muricatus | Salvia officinalis | ||
| Beta vulgaris | Helichrysum italicum | |||
| Coleoptera | Coccinellidae | Chilocorus bipustulatus (Linnaeus, 1758) | Ecballium elaterium | Thymus vulgaris |
| Coccinella septempunctata Linnaeus, 1758 | Amaranthus retroflexus | Helichrysum italicum | ||
| Diplotaxis erucoides | Salvia officinalis | |||
| Hippodamia variegata (Goeze, 1777) | Cerinthe major | - | ||
| Propylea quatuordecimpunctata (Linnaeus, 1758) | Althaea officinalis | - | ||
| Scymnus interruptus (Goeze, 1777) | Salvia officinalis | Senecio vulgaris | ||
| Scymnus subvillosus (Goeze, 1777) | Diplotaxis erucoides | |||
| Hymenoptera | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | Years | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | ||||||||||||||
| Colletidae | |||||||||||||||
| 1 | Hylaeus cornutus | √ | X | ||||||||||||
| Andrenidae | |||||||||||||||
| 2 | Andrena aerinifrons | √ | X | ||||||||||||
| 3 | Andrena bicolorata | √ | X | ||||||||||||
| 4 | Andrena brumanensis | √ | X | ||||||||||||
| 5 | Andrena distinguenda | √ | √ | X | X | ||||||||||
| 6 | Andrena labialis | √ | √ | X | X | ||||||||||
| 7 | Andrena nigroaenea | √ | √ | X | X | ||||||||||
| 8 | Andrena pilipes | √ | √ | X | X | ||||||||||
| Halictidae | |||||||||||||||
| 9 | Halictus fulvipes | √ | √ | √ | √ | X | X | ||||||||
| 10 | Halictus quadricinctus | √ | √ | √ | X | X | |||||||||
| 11 | Halictus scabiosae | √ | √ | √ | √ | X | X | ||||||||
| 12 | Lasioglossum malachurum | √ | √ | X | |||||||||||
| Megachilidae | |||||||||||||||
| 13 | Heriades rubicola | √ | √ | √ | √ | X | X | ||||||||
| 14 | Osmia latreillei | √ | √ | X | X | ||||||||||
| 15 | Osmia signata | √ | X | ||||||||||||
| 16 | Rhodanthidium siculum | √ | √ | X | X | ||||||||||
| 17 | Megachile sicula | √ | √ | X | X | ||||||||||
| Apidae | |||||||||||||||
| 18 | Xylocopa violacea | √ | √ | √ | √ | √ | √ | √ | X | X | |||||
| 19 | Ceratina cyanea | √ | √ | X | X | ||||||||||
| 20 | Nomada discrepans | √ | X | ||||||||||||
| 21 | Nomada distinguenda | √ | X | ||||||||||||
| 22 | Eucera algira | √ | √ | X | |||||||||||
| 23 | Eucera eucnemidea | √ | √ | √ | X | X | |||||||||
| 24 | Eucera nigrescens | √ | √ | √ | X | X | |||||||||
| 25 | Eucera nigrilabris | √ | X | ||||||||||||
| 26 | Eucera numida | √ | √ | X | X | ||||||||||
| 27 | Eucera oraniensis | √ | √ | √ | X | X | |||||||||
| 28 | Amegilla garrula | √ | √ | X | X | ||||||||||
| 29 | Amegilla quadrifasciata | √ | √ | √ | X | X | |||||||||
| 30 | Anthophora dispar | √ | √ | √ | √ | X | X | ||||||||
| 31 | Anthophora plumipes squalens | √ | √ | √ | √ | X | X | ||||||||
| 32 | Bombus pascuorum siciliensis | √ | √ | √ | √ | √ | X | X | |||||||
| 33 | Bombus terrestris | √ | √ | √ | √ | √ | √ | √ | √ | X | X | ||||
| Spontaneous Flora Species | Family | EPPO Code | Spring | Autumn | |||||
|---|---|---|---|---|---|---|---|---|---|
| Inter-Row | Intra-Row | Inter-Row | Intra-Row | ||||||
| Zero Tillage | Minimum Tillage | Zero Tillage | Minimum Tillage | ||||||
| Amaranthus retroflexus L. | Amaranthaceae | AMARE | + | + | + | + | + | + | |
| Arum maculatum L. | Araceae | ABGMA | - | - | + | - | - | - | |
| Avena sterilis L. | Poaceae | AVEST | + | - | + | - | - | - | |
| Beta vulgaris L. | Chenopodiaceae | BEAVX | + | + | + | + | + | + | |
| Brassica nigra (L.) W.D.J. Koch | Brassicaceae | BRSNI | - | - | + | + | + | - | |
| Capsella bursa-pastoris (L.) Medik. | Brassicaceae | CAPBP | - | - | + | + | + | - | |
| Convolvolus arvensis L. | Convolvulaceae | CONAR | - | + | + | + | + | - | |
| Cynodon dactylon (L.) Pers. | Poaceae | CYNDA | + | + | + | + | + | + | |
| Cyperus rotundus L. | Cyperaceae | CYPRO | + | + | + | + | + | + | |
| Dactylis glomerata L. | Poaceae | DACGL | + | - | + | - | - | - | |
| Digitaria sanguinalis (L.) Scop. | Poaceae | DIGSA | - | - | + | - | - | + | |
| Dittrichia viscosa (L.) Greuter | Asteraceae | INUVI | - | - | + | - | - | - | |
| Ecballium elaterium (L.) A. Rich. | Cucurbitaceae | ECBEL | - | - | + | - | - | + | |
| Elymus repens (L.) Gould | Poaceae | AGGRE | - | - | - | + | + | - | |
| Erigeron canadensis L. | Asteraceae | ERICA | - | - | - | - | - | + | |
| Euphorbia prostrata Aiton | Euphorbiaceae | EPHPT | - | - | - | - | - | + | |
| Fumaria officinalis L. | Papaveraceae | FUMOF | + | + | + | + | - | - | |
| Lactuca sativa subsp. serriola (L.) Galasso, Banfi, Bartolucci & Ardenghi | Asteraceae | LACSE | + | + | + | - | - | - | |
| Lamium amplexicaule L. | Lamiaceae | LAMAM | - | - | - | - | - | + | |
| Lolium perenne L. | Poaceae | LOLPE | + | + | + | - | - | - | |
| Lysimachia arvensis (L.) U. Manns & Anderb. | Primulaceae | LYSAR | - | - | + | - | - | - | |
| Malva sylvestris L. | Malvaceae | MALSY | - | - | + | + | + | - | |
| Myosotis arvensis (L.) Hill | Boraginaceae | MYOAR | - | - | + | - | - | - | |
| Oxalis pes-caprae L. | Oxalidaceae | OXAPC | - | - | - | - | - | + | |
| Papaver rhoeas L. | Papaveraceae | PAPRH | + | + | + | - | - | - | |
| Polygonum aviculare L. | Polygonaceae | POLAV | + | + | + | + | + | + | |
| Portulaca oleracea L. | Portulacaceae | POROL | + | + | + | + | + | + | |
| Ranunculus muricatus L. | Ranunculaceae | RANMU | - | - | + | - | - | - | |
| Raphanus raphanistrum L. | Brassicaceae | RAPRA | - | - | + | + | + | + | |
| Senecio vulgaris L. | Asteraceae | SENVU | - | - | + | - | - | + | |
| Setaria verticillata (L.) P. Beauv. | Poaceae | SETVE | - | - | - | + | + | + | |
| Sinapis arvensis L. | Brassicaceae | SINAR | - | - | + | - | - | - | |
| Solanum nigrum L. | Solanaceae | SOLNI | - | - | - | - | - | + | |
| Sonchus asper subsp. glaucescens (Jord.) Ball | Asteraceae | SONAR | - | - | - | - | - | + | |
| Sonchus oleraceus L. | Asteraceae | SONOL | - | - | - | - | - | + | |
| Stellaria media (L.) Vill. | Caryophyllaceae | STEME | - | - | - | - | - | + | |
| Triticum spp. | Poaceae | - | - | - | + | - | - | - | |
| Urtica dioica L. | Urticaceae | URTDI | - | + | + | - | - | - | |
| Veronica peregrina L. | Plantaginaceae | VERPG | - | - | - | - | - | + | |
| Total richness (No. species) | 12 | 12 | 28 | 14 | 13 | 20 | |||
| A | B | ||||
|---|---|---|---|---|---|
| PC | Eigenvalue | % Variance | PC | Eigenvalue | % Variance |
| 1 | 3.36 | 20.97 | 1 | 3.02 | 11.17 |
| 2 | 2.51 | 15.71 | 2 | 2.38 | 8.80 |
| 3 | 2.41 | 15.08 | 3 | 2.15 | 7.95 |
| 4 | 1.78 | 11.13 | 4 | 1.87 | 6.93 |
| 5 | 1.30 | 8.15 | 5 | 1.53 | 5.67 |
| 6 | 1.25 | 7.80 | 6 | 1.49 | 5.50 |
| 7 | 1.19 | 7.41 | 7 | 1.39 | 5.17 |
| 8 | 0.68 | 4.24 | 8 | 1.26 | 4.67 |
| 9 | 0.55 | 3.42 | 9 | 1.13 | 4.17 |
| 10 | 0.44 | 2.77 | 10 | 1.12 | 4.14 |
| 11 | 0.26 | 1.60 | 11 | 1.04 | 3.86 |
| 12 | 0.20 | 1.23 | 12 | 1.01 | 3.76 |
| 13 | 0.05 | 0.29 | 13 | 0.94 | 3.49 |
| 14 | 0.02 | 0.13 | 14 | 0.89 | 3.30 |
| 15 | 0.01 | 0.07 | 15 | 0.85 | 3.16 |
| 16 | 0.77 | 2.86 | |||
| 17 | 0.71 | 2.62 | |||
| 18 | 0.65 | 2.39 | |||
| 19 | 0.58 | 2.14 | |||
| 20 | 0.52 | 1.93 | |||
| 21 | 0.38 | 1.42 | |||
| 22 | 0.35 | 1.30 | |||
| 23 | 0.26 | 0.97 | |||
| 24 | 0.23 | 0.84 | |||
| 25 | 0.19 | 0.69 | |||
| 26 | 0.16 | 0.58 | |||
| 27 | 0.13 | 0.50 |
| 15 December 2020 | 15 October 2021 | Percentage Increase (Δ%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Trunk Cross-Sectional Area (cm2) | Canopy Height (cm) | Canopy Volume (m3) | Trunk Cross-Sectional Area (cm2) | Canopy Height (cm) | Canopy Volume (m3) | Trunk Cross-Sectional Area (Δ%) | Canopy Height (Δ%) | Canopy Volume (Δ%) |
| Nocellara etnea—minimum tillage | 6.32 ± 2.4 ab | 103.9 ± 22.06 a | 0.29 ± 0.11 ns | 13.7 ± 2.69 b | 152.6 ± 23.43 ns | 1.55 ± 0.38 a | 117 | 146 | 542 |
| Nocellara del Belice—minimum tillage | 4.99 ± 2.09 b | 72.5 ± 18.65 b | 0.21 ± 0.09 ns | 12.4 ± 2.74 b | 105.8 ± 22.31 ns | 0.73 ± 0.19 b | 148 | 145 | 339 |
| Nocellara etnea—zero tillage | 8.87 ± 2.03 a | 82.6 ± 31.68 ab | 0.32 ± 0.12 ns | 18.2 ± 2.23 a | 143.1 ± 29.29 ns | 1.23 ± 0.22 a | 205 | 173 | 387 |
| Nocellara del Belice—zero tillage | 4.13 ± 2.09 ab | 82.13 ± 21.65 ab | 0.34 ± 0.10 ns | 8.1 ± 3.87 b | 120.5 ± 28.20 ns | 1.05 ± 0.19 b | 196 | 146 | 438 |
| Parameter | Unit Measure | Value |
|---|---|---|
| Sand | % | 60 |
| Silt | % | 21 |
| Clay | % | 19 |
| pH | 7.8 | |
| Electrical conductivity (1:2.5) | dS/m | 0.26 |
| Organic matter | % | 2.69 |
| Total nitrogen (N) | ‰ | 0.140 |
| Exchangeable phosphorus (P) | ppm P | 53 |
| Exchangeable potassium (K) | ppm K | 3628 |
| Cation exchange capacity (CEC) | meq/100 g | 64.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Las Casas, G.; Ciaccia, C.; Iovino, V.; Ferlito, F.; Torrisi, B.; Lodolini, E.M.; Giuffrida, A.; Catania, R.; Nicolosi, E.; Bella, S. Effects of Different Inter-Row Soil Management and Intra-Row Living Mulch on Spontaneous Flora, Beneficial Insects, and Growth of Young Olive Trees in Southern Italy. Plants 2022, 11, 545. https://doi.org/10.3390/plants11040545
Las Casas G, Ciaccia C, Iovino V, Ferlito F, Torrisi B, Lodolini EM, Giuffrida A, Catania R, Nicolosi E, Bella S. Effects of Different Inter-Row Soil Management and Intra-Row Living Mulch on Spontaneous Flora, Beneficial Insects, and Growth of Young Olive Trees in Southern Italy. Plants. 2022; 11(4):545. https://doi.org/10.3390/plants11040545
Chicago/Turabian StyleLas Casas, Giuseppina, Corrado Ciaccia, Valeria Iovino, Filippo Ferlito, Biagio Torrisi, Enrico Maria Lodolini, Alessio Giuffrida, Roberto Catania, Elisabetta Nicolosi, and Salvatore Bella. 2022. "Effects of Different Inter-Row Soil Management and Intra-Row Living Mulch on Spontaneous Flora, Beneficial Insects, and Growth of Young Olive Trees in Southern Italy" Plants 11, no. 4: 545. https://doi.org/10.3390/plants11040545
APA StyleLas Casas, G., Ciaccia, C., Iovino, V., Ferlito, F., Torrisi, B., Lodolini, E. M., Giuffrida, A., Catania, R., Nicolosi, E., & Bella, S. (2022). Effects of Different Inter-Row Soil Management and Intra-Row Living Mulch on Spontaneous Flora, Beneficial Insects, and Growth of Young Olive Trees in Southern Italy. Plants, 11(4), 545. https://doi.org/10.3390/plants11040545









