Evaluation of Genetic Diversity Based on Microsatellites and Phytochemical Markers of Core Collection of Cymbopogon winterianus Jowitt Germplasm
Abstract
1. Introduction
2. Results and Discussions
2.1. SSR Primer Competency
2.2. Intra- and Inter-Population Genetic Variation
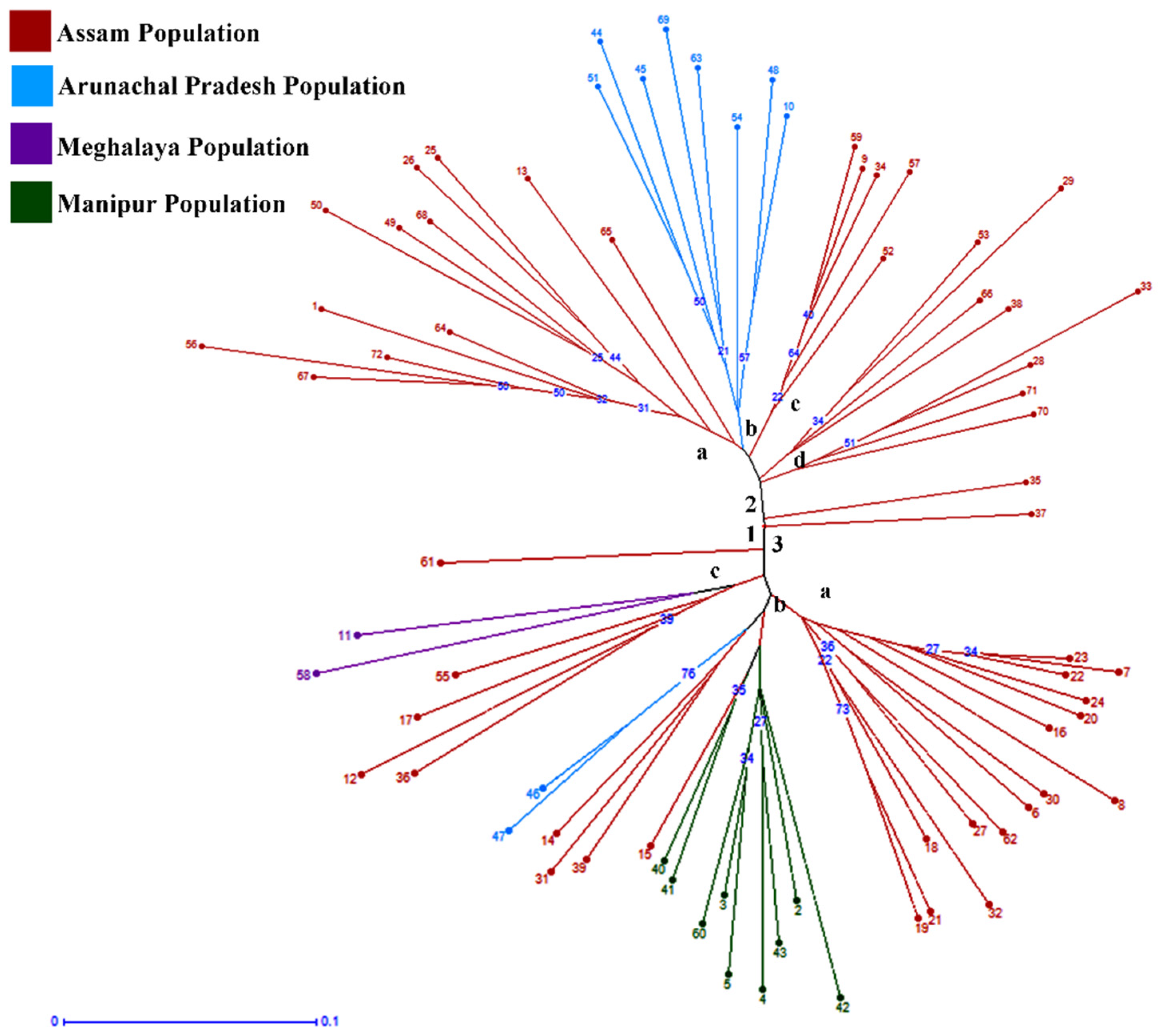
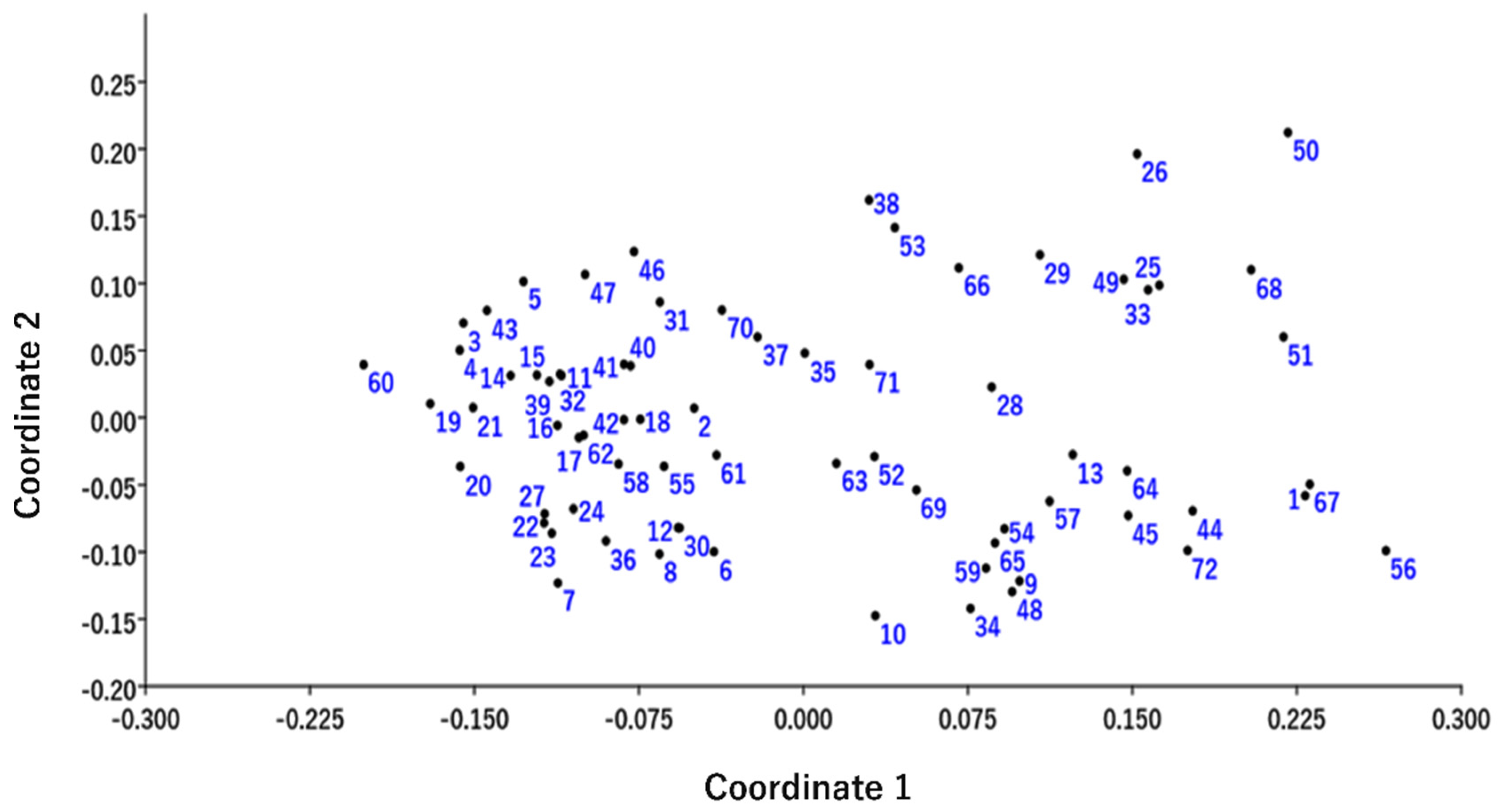
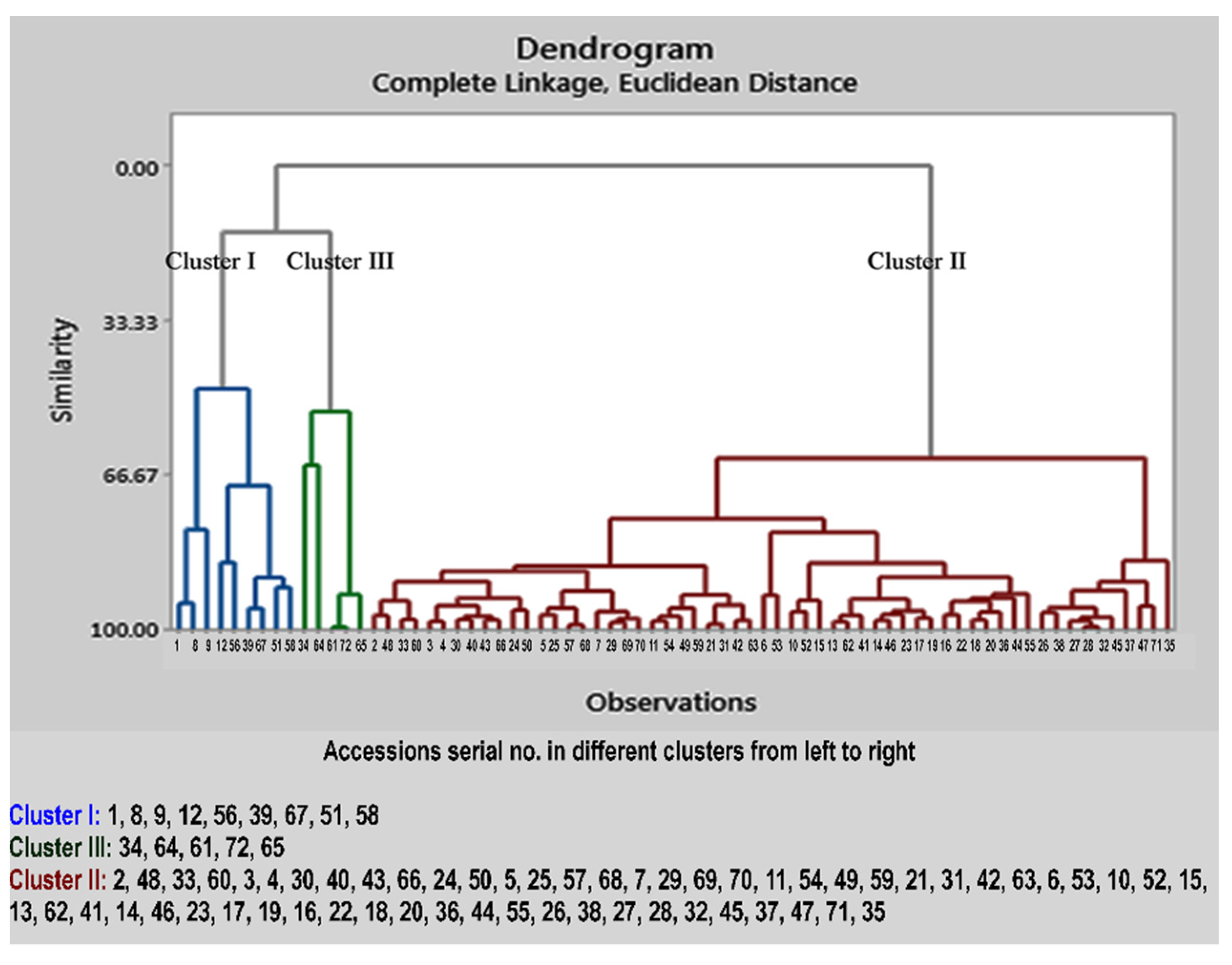
2.3. Cluster Analysis
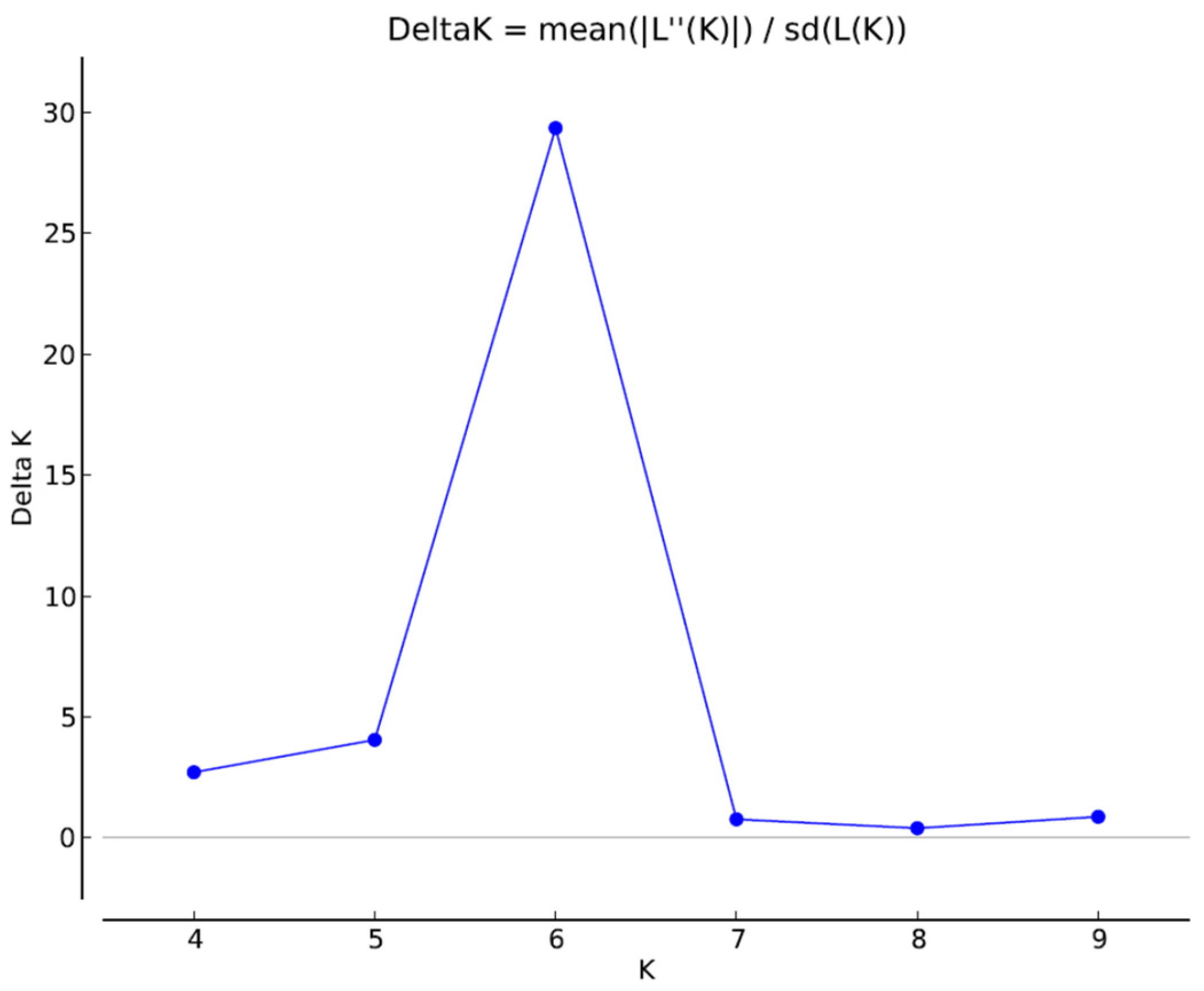
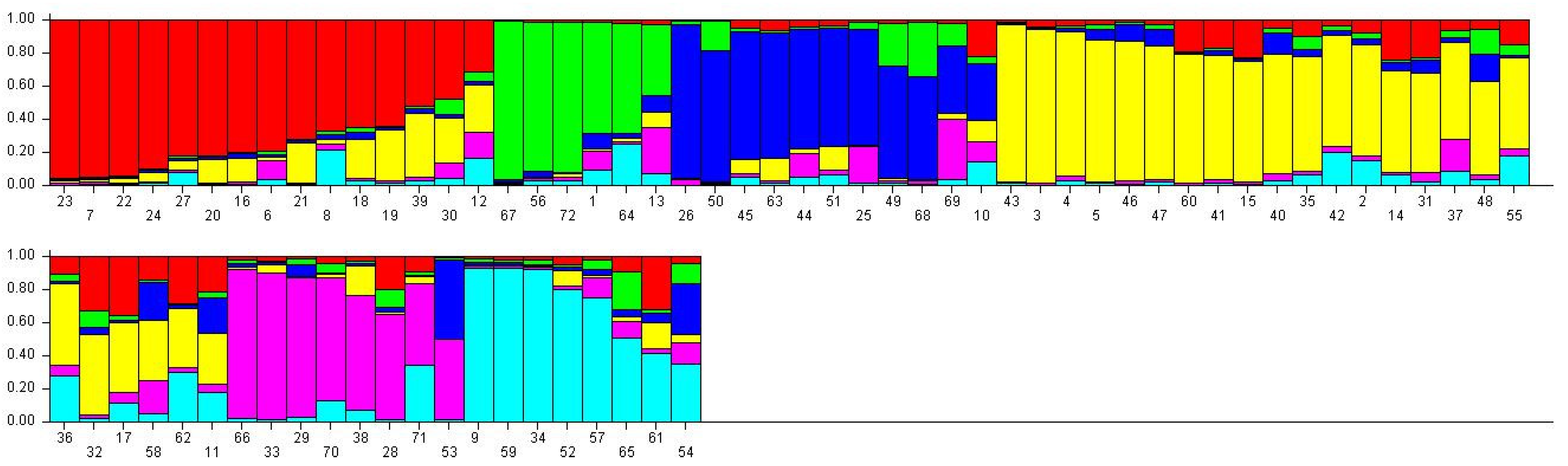
2.4. Population Structure
2.5. Analysis of Molecular Variance (AMOVA) of C. winterianus Accessions
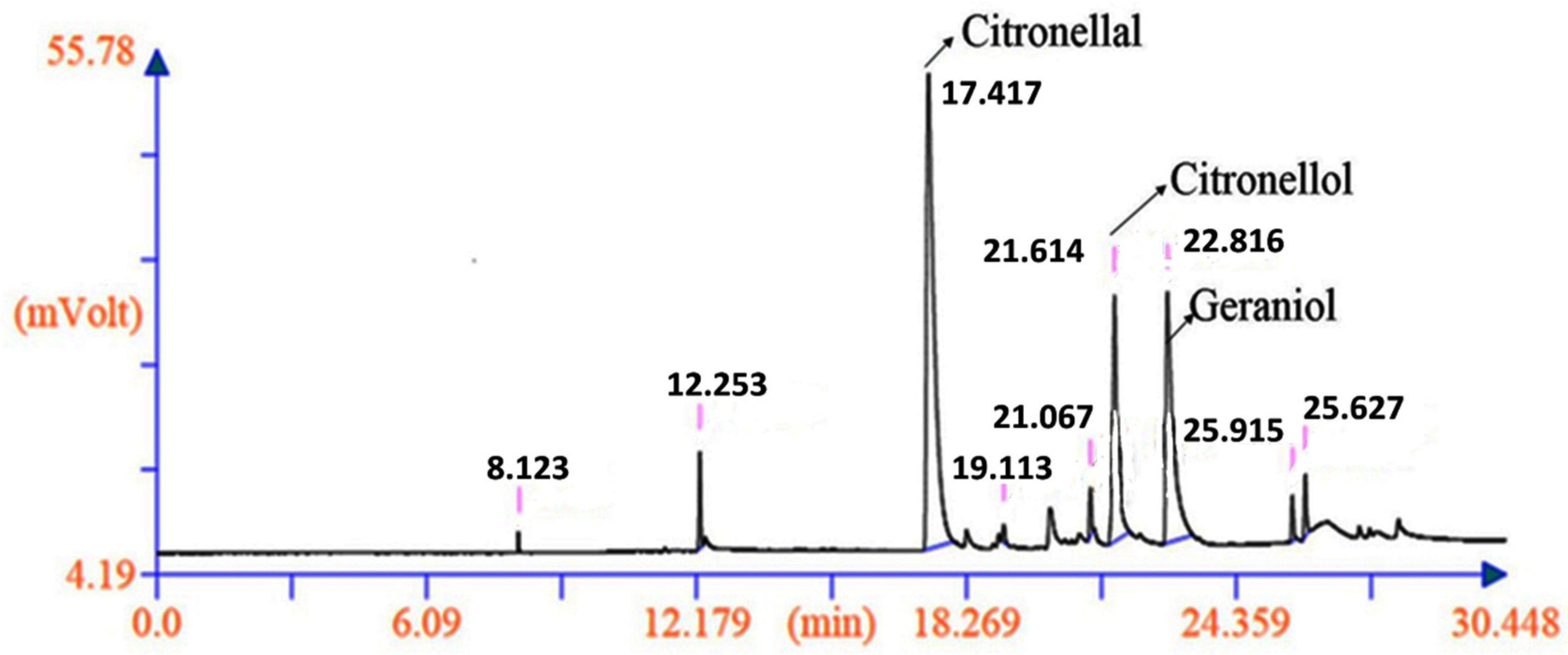
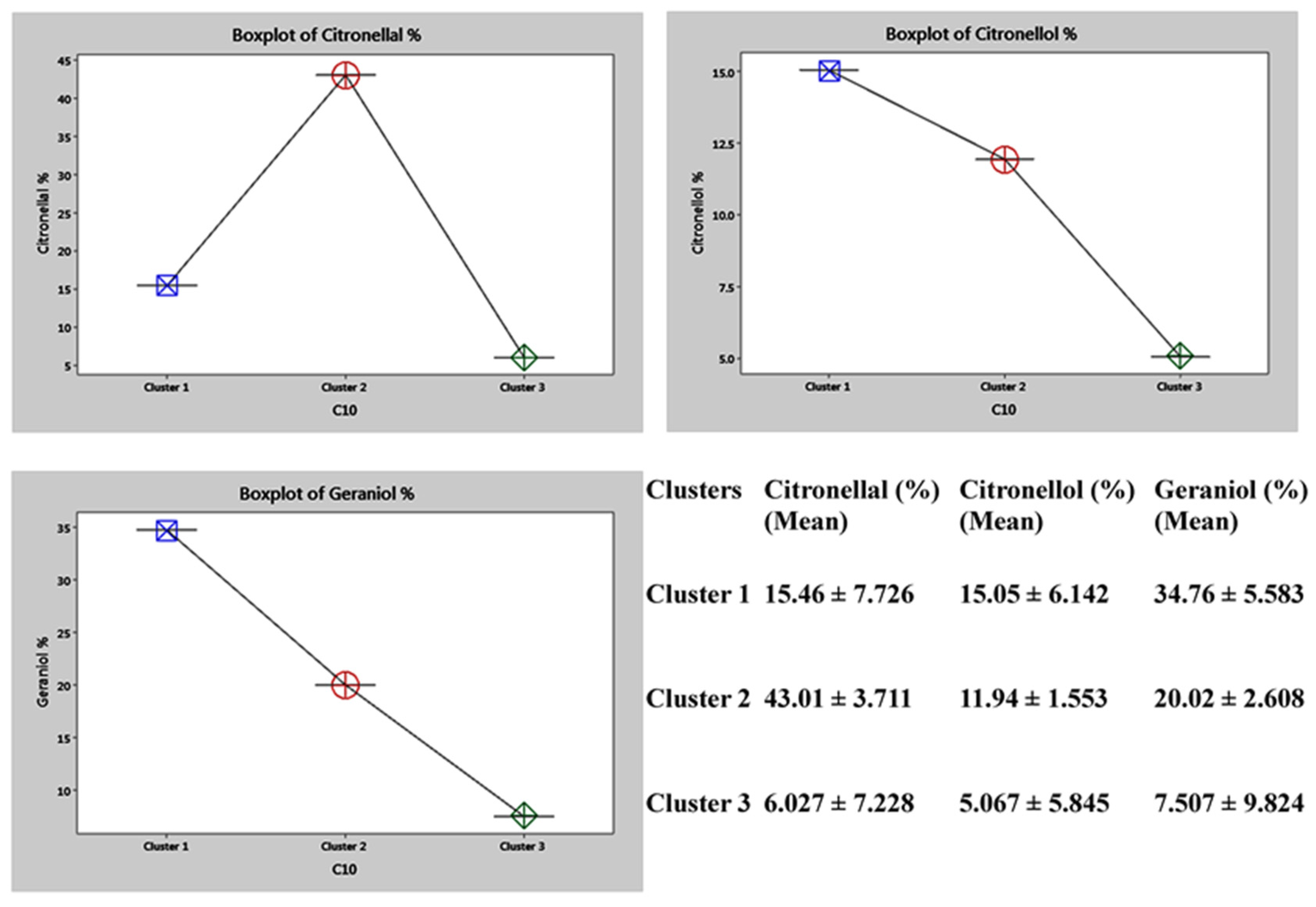
2.6. Genetic Diversity Based on Phytochemical Analysis of the Biomarkers
3. Materials and Methods
3.1. Plant Materials
3.2. Genetic Diversity Analysis Based on Molecular Marker
3.2.1. Genomic DNA Extraction
3.2.2. SSR Primers–PCR Analysis
3.2.3. Statistical Analysis of Data
3.3. Quantitative and Qualitative Analysis of the Citronellal, Citronellol and Geraniol (Biomarkers) by GC-FID and GC-MS
3.3.1. Extraction of Essential Oil
3.3.2. Essential Oil Analysis
3.3.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Pop I | Pop II | Pop III | Pop IV | Pop V | Pop VI | |
|---|---|---|---|---|---|---|
| Pop I | 0.1215 | 0.1589 | 0.1377 | 0.0620 | 0.1144 | 0.0999 |
| Pop II | 0.1774 | 0.0980 | 0.1793 | 0.0977 | 0.0804 | |
| Pop III | 0.2395 | 0.1171 | 0.0842 | 0.0913 | ||
| Pop IV | 0.1386 | 0.1240 | 0.1124 | |||
| Pop V | 0.2296 | 0.0707 | ||||
| Pop VI | 0.1666 |

References
- Bhattacharya, S.; Bandopadhyay, T.K.; Ghosh, P.D. Efficiency of RAPD and ISSR markers in assessment of molecular diversity in elite germplasms of Cymbopogon winterianus across West Bengal, India. Emir. J. Food Agric. 2010, 1, 13–24. [Google Scholar] [CrossRef]
- Paterson, A.H.; Tanksley, S.D.; Sorrells, M.E. DNA markers in plant improvement. Adv. Agron. 1991, 46, 39–90. [Google Scholar]
- Perrino, E.V.; Perrino, P. Crop wild relatives: Know how past and present to improve future research, conservation and utilization strategies, especially in Italy: A review. Resour. Crop Evol. 2020, 67, 1067–1105. [Google Scholar] [CrossRef]
- Adhikari, S.; Saha, S.; Bandyopadhyay, T.K.; Ghosh, P. Efficiency of ISSR marker for characterization of Cymbopogon germplasms and their suitability in molecular barcoding. Plant Syst. Evol. 2015, 301, 439–450. [Google Scholar] [CrossRef]
- Lal, M.; Munda, S.; Dutta, S.; Pandey, S.K. Identification of a novel germplasm (Jor Lab L-9) of lemon grass (Cymbopogon khasianus) rich in methyl eugenol. Crop Breed. Appl. Biotechnol. 2020, 3, e320720315. [Google Scholar] [CrossRef]
- Dutta, S.; Munda, S.; Lal, M.; Bhattacharyya, P.R. A short review on Chemical composition, therapeutic use and enzyme inhibition activities of Cymbopogon species. Indian J. Sci. Technol. 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Lal, M.; Dutta, S.; Munda, S.; Pandey, S.K. Novel high value elemicin-rich germplasm of Lemon grass (Cymbopogn khasianus (hack) Staf (ex Bor) from North East India. Ind. Crops Prod. 2018, 115, 98–103. [Google Scholar] [CrossRef]
- Deka, H.; Deka, S.; Baruah, C.K.; Das, J.; Hoque, S.; Sarma, N.S. Vermicomposting of distillation waste of citronella plant (Cymbopogon winterianus Jowitt.) employing Eudrilus eugeniae. Bioresour. Technol. 2011, 102, 6944–6950. [Google Scholar]
- Dey, T.; Saha, S.; Ghosh, P.D. Somaclonal variation among somatic embryo derived plants-Evaluation of agronomically important somaclones and detection of genetic changes by RAPD in Cymbopogon winterianus. S. Afr. J. Bot. 2015, 96, 112–121. [Google Scholar] [CrossRef]
- Lal, M.; Dutta, S.; Bhattacharyya, P.R. Development of a new superior variety (Jor Lab C-5) of Java Citronella with characteristics of stable and high oil yield. Ann. Biol. 2016, 32, 22–23. [Google Scholar]
- Lal, M. Jor Lab C-5 (IC0619027; INGR16021), a Java citronella (Cymbopogon winterianus Jowitt) germplasm with high herbage yield with high essential oil content. Indian J. Plant Genet. Resour. 2018, 31, 118. [Google Scholar]
- Simic, A.; Rancic, A.; Sokovic, M.D.; Ristic, M.; Grujic-Jovanovic, S.; Vukojevic, J.; Marin, P.D. Essential oil composition of Cymbopogon winterianus and Carum carvi and their antimicrobial activities. Pharm. Biol. 2008, 46, 437–441. [Google Scholar] [CrossRef]
- Lawless, J. The Encyclopaedia of Essential Oils; Thorsons: London, UK, 2002; pp. 70–83. [Google Scholar]
- Quintans-Junior, L.J.; Souza, T.T.; Leite, B.S.; Lessa, N.M.; Bonjardim, L.R.; Santos, M.R.; Alves, P.B.; Blank, A.F.; Antoniolli, A.R. Phytochemical screening and anticonvulsant activity of Cymbopogon winterianus Jowitt (Poaceae) leaf essential oil in rodents. Phytomedicine 2008, 15, 619–624. [Google Scholar] [CrossRef]
- Munda, S.; Dutta, S.; Pandey, S.K.; Sarma, N.; Lal, M. Antimicrobial activity of essential oils of medicinal and aromatic plants of the North east India: A biodiversity hot spot. J. Essent. Oil Bear. Pl. 2019, 2, 105–119. [Google Scholar] [CrossRef]
- Ganjewala, D. Cymbopogon essential oils: Chemical compositions and bioactivities. Int. J. Essent. Oil Ther. 2009, 3, 56–65. [Google Scholar]
- Ganjewala, D.; Gupta, A.K. Lemongrass (Cymbopogon flexuosus Steud.) wats essential oil: Overview and biological activities. Essent. Oils II RPMP 2016, 37, 232–274. [Google Scholar]
- Kumar, V.; Roy, B.K. Population authentication of the traditional medicinal plant Cassia tora L. based on ISSR markers and FTIR analysis. Sci. Rep. 2018, 8, 10714. [Google Scholar] [CrossRef] [PubMed]
- Perrino, E.V.; Valerio, F.; Jallali, S.; Trani, A.; Mezzapesa, G.N. Ecological and biological properties of Satureja cuneifolia and Thymus spinulosus Ten.: Two wild officinal species of conservation concern in Apulia (Italy). A preliminary survey. Plants 2021, 10, 1952. [Google Scholar] [CrossRef]
- Cassel, E.; Vargas, R.M.F. Experiments and modeling of the Cymbopogon winterianus essential oil extraction by steam distillation. J. Mex. Chem. Soc. 2006, 50, 126–129. [Google Scholar]
- Sousa, D.P.; Gonclaves, J.C.R.; Quintans, J.L.; Cruz, J.S.; Araujo, D.A.M.; Almeida, R.N. Study of anticonvulsant effect of citronellol, a monoterpene alcohol, in rodents. Neurosci. Lett. 2006, 401, 231–235. [Google Scholar] [CrossRef]
- Gogoi, R.; Loying, R.; Sarma, N.; Munda, S.; Pandey, S.; Lal, M. A comparative study on antioxidant, anti-inflammatory, genotoxicity, anti-microbial activities and chemical composition of fruit and leaf essential oils of Litsea cubeba Pers. from North-east India. Ind. Crops Prod. 2018, 125, 131–139. [Google Scholar] [CrossRef]
- Sultanbawa, Y. Essential oils in food application: Australian aspects. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 155–159. [Google Scholar]
- Silva, Y.M.S.; Silva, M.T.A.; Sampaio, P.A.; Quintans, J.S.S.; Qunitans-Junior, L.J.; Ribeiro, L.A.A. Relaxant effect of carvacrol, citronellal and p-cymene, monoterpenes present in Thymus and Cymbopogon species, in guinea-pig trachea: A comparative study. J. Med. Plant Res. 2014, 8, 881–888. [Google Scholar]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press Taylor and Francis Group: London, UK; New York, NY, USA, 2010; pp. 733–734. [Google Scholar]
- Chen, W.; Viljoen, A.M. Geraniol-A review of a commercially important fragrance material. S. Afr. J. Bot. 2010, 76, 643–651. [Google Scholar] [CrossRef]
- Lal, M.; Borah, A.; Pandey, S.K. Identification of a new high geraniol rich variety “Jor Lab L-15” of Lemongrass [Cymbopogon khasianus (Hack) Stapf (ex Bor)]. J. Essent. Oil Bear. Pl. 2019, 22, 972–978. [Google Scholar] [CrossRef]
- Surburg, H.; Panten, J. Common Fragrance and Flavor Materials: Preparation, Properties and Uses, 5th ed.; Wiley: Weinheim, Germany, 2006. [Google Scholar]
- Heiba, H.I.; Rizk, A.M. Constituents of Cymbopogon species. Qatar Univ. Sci. Bull. 1986, 6, 53–75. [Google Scholar]
- Munda, S.; Lal, M. Cymbopogon winterianus Jowitt ex Bor, a Hub for various industrial and pharmaceutical applications. In Botanical Leads for Drug Discovery; Singh, B., Ed.; Springer: Singapore, 2020; pp. 405–419. [Google Scholar] [CrossRef]
- Shasany, A.K.; Lal, R.K.; Patra, N.K.; Darokar, M.P.; Garg, A.; Kumar, S.; Khanuja, S.P.S. Phenotypic and RAPD diversity among Cymbopogon winterianus Jowitt accessions in relation to Cymbopogon nardus Rendle. Genet. Resour. Crop Evol. 2000, 47, 553–559. [Google Scholar] [CrossRef]
- Kumar, J.; Verma, V.; Goyal, A.; Shahi, A.K.; Sparoo, R.; Sangwan, R.S.; Qazi1, G.N. Genetic diversity analysis in Cymbopogon species using DNA markers. Plant Omics 2009, 2, 20–29. [Google Scholar]
- Munda, S.; Dutta, S.; Lal, M. Variability estimation and genetic divergence in Cymbopogon winterianus for development of superior genotype. Agron. J. 2020, 113, 993–1007. [Google Scholar] [CrossRef]
- Gupta, A.K.; Ganjewala, D. A study on developmental changes in essential oil content and composition in Cymbopogon flexuosus cultivar Suvarna. Acta Biol. Szeged. 2015, 59, 119–125. [Google Scholar]
- Baruah, J.; Gogoi, B.; Das, K.; Ahmed, N.M.; Sarmah, D.K.; Lal, M.; Bhau, B.S. Genetic diversity study amongst Cymbopogon species from NE-India using RAPD and ISSR markers. Ind. Crops Prod. 2016, 95, 235–243. [Google Scholar] [CrossRef]
- Sangwan, N.S.; Yadav, U.; Sangwan, R.S. Molecular analysis of genetic diversity in elite Indian cultivars of essential oil trade types of aromatic grasses (Cymbopogon species). Plant Cell Rep. 2001, 20, 437–444. [Google Scholar] [CrossRef]
- Herrera, M.R.; Ghislain, M. Robust and inexpensive SSR markers analyses using LI-COR DNA analyzer. Microsatellites 2013, 1006, 197–205. [Google Scholar]
- Naghavi, M.R.; Mardi, M.; Pirseyedi, S.M.; Kazemi, M.; Potki, P.; Ghaffari, M.R. Comparison of genetic variation among accessions of Aegilops tauschii using AFLP and SSR markers. Genet. Resour. Crop. Evol. 2007, 54, 237–240. [Google Scholar] [CrossRef]
- Henkrar, F.; El-Haddoury, J.; Ouabbou, H.; Nsarellah, N.; Iraqi, D.; Bendaou, N.; Udupa, S.M. Genetic diversity reduction in improved durum wheat cultivars of Morocco as revealed by microsatellite markers. Sci. Agric. 2016, 73, 134–141. [Google Scholar] [CrossRef]
- Abbasov, M.; Akparov, Z.; Gross, T.; Babayeva, S.; Izzatullayeva, V.; Hajiye, E.; Rustamov, K.; Gross, P.; Tekin, M.; Akar, T.; et al. Genetic relationship of diploid wheat (Triticum spp.) species assessed by SSR markers. Genet. Resour. Crop Evol. 2018, 65, 1441–1453. [Google Scholar] [CrossRef]
- Song, Q.J.; Shi, J.R.; Singh, S.; Fickus, E.W.; Costa, J.M.; Lewis, J.; Gill, B.S.; Ward, R.; Cregan, P.B. Development and mapping of microsatellite (STMS) markers in wheat. Theor. Appl. Genet. 2005, 110, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Baruah, J.; Pandey, S.K.; Sarmah, N.; Lal, M. Assessing molecular diversity among high capsaicin content lines of Capsicum chinense Jacq. using simple sequence repeat marker. Ind. Crops Prod. 2019, 141, 111769. [Google Scholar] [CrossRef]
- Hajiyev, E.S.; Akparov, Z.I.; Aliyev, R.T.; Saidova, S.V.; Izzatullayeva, V.I.; Babayeva, S.M.; Abbasov, M.A. Genetic polymorphism of durum wheat (Triticum durum Desf.) accessions of Azerbaijan. Russ. J. Genet. 2015, 51, 863–870. [Google Scholar] [CrossRef]
- Abbasov, M.; Brueggeman, R.; Raupp, J.; Akparov, Z.; Aminov, N.; Bedoshvili, D.; Gross, T.; Gross, P.; Babayeva, S.; Izzatullayeva, V.; et al. Genetic diversity of Aegilops L. species from Azerbaijan and Georgia using SSR markers. Genet. Resour. Crop Evol. 2019, 66, 453–463. [Google Scholar] [CrossRef]
- Kaur, P.; Pandeya, D.K.; Gupta, R.C.; Dey, A. Assessment of genetic diversity among different population of five Swertia species by using molecular and phytochemical markers. Ind. Crops Prod. 2019, 138, 111569. [Google Scholar] [CrossRef]
- Hyun, D.Y.; Gi, G.Y.; Sebastin, R.; Cho, G.T.; Kim, S.H.; Yoo, E.; Lee, S.; Son, D.M.; Lee, K.J. Utilization of phytochemical and molecular diversity to develop a target-oriented core collection in tea germplasm. Agronomy 2020, 10, 1667. [Google Scholar] [CrossRef]
- Qaderi, A.; Omidi, M.; Pour-Aboughadareh, A.; Poczai, P.; Shaghaghi, J.; Mehrafarin, A.; Nohooji, M.G.; Etminan, A. Molecular diversity and phytochemical variability in the Iranian poppy (Papaver bracteatum Lindl.): A baseline for conservation and utilization in future breeding programmes. Ind. Crops Prod. 2019, 130, 237–247. [Google Scholar] [CrossRef]
- Ray, A.; Jena, S.; Haldar, T.; Sahoo, A.; Kar, B.; Patnaik, J.; Ghosh, B.; Panda, P.C.; Mahapatra, N.; Nayak, S. Population genetic structure and diversity analysis in Hedychium coronarium populations using morphological, phytochemical and molecular markers. Ind. Crops Prod. 2019, 132, 118–133. [Google Scholar] [CrossRef]
- Saikia, D.; Dutta, S.; Ghosh, S.; Lal, M.; Bhau, B.S. RAPD and ISSR based intra-specific molecular genetic diversity analysis of Cymbopogon flexuosus L. Stapf with a distinct correlation of morpho-chemical observations. Res. J. Biotechol. 2015, 10, 105–113. [Google Scholar]
- Lal, N.; Awasthi, S.K. A comparative assessment of molecular marker assays (AFLP and RAPD) for Cymbopogon germplasm characterization. World J. Pharm. Res. 2015, 4, 1019–1041. [Google Scholar]
- Bishoyi, A.K.; Sharma, A.; Kavane, A.; Geetha, K.A. Varietal discrimination and genetic variability analysis of Cymbopogon using RAPD and ISSR markers analysis. Appl. Biochem. Biotechnol. 2016, 179, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Paw, M.; Borah, A.; Pandey, S.K.; Baruah, J.; Begum, T.; Lal, M. Simple sequence repeat marker based genetic diversity assessment amongst high essential oil yielding lines of Curcuma caesia Roxb. Genet. Resour. Crop Evol. 2021, 6, 1345–1358. [Google Scholar] [CrossRef]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar]
- Slatkin, M. Gene flow and geographic structure of natural populations. Science 1987, 236, 787–792. [Google Scholar] [CrossRef]
- Zheng, W.H.; Zhuo, Y.; Liang, L.; Ding, W.Y.; Liang, L.Y.; Wang, X.F. Conservation and population genetic diversity of Curcuma wenyujin (Zingiberaceae), a multifunctional medicinal herb. Genet. Mol. Res. 2015, 14, 10422–10432. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbour joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Harsono, T.; Pasaribu, N.; Fitmawati, F.; Sobir, S.; Prasetya, E. Genetic variability and classification of Gandaria (Bouea) in Indonesia based on inter simple sequence repeat (ISSR) markers. SABRAO J. Bred. Genet. 2018, 50, 129–144. [Google Scholar]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemometr. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Luo, C.; Chen, D.; Cheng, X.; Liu, H.; Li, Y.; Huang, C. SSR analysis of genetic relationship and classification in Chrysanthemum germplasm Collection. Hortic. Pl. J. 2018, 4, 73–82. [Google Scholar] [CrossRef]
- Rajwade, A.V.; Arora, R.S.; Kadoo, N.Y.; Harsulkar, A.M.; Ghorpade, P.B.; Gupta, V.S. Relatedness of Indian flax genotypes (Linum usitatissimum L.): An inter-simple sequence repeat (ISSR) primer assay. Mol. Biotechnol. 2010, 45, 161–170. [Google Scholar] [CrossRef]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef]
- Grunwald, N.J.; Everhart, S.E.; Knaus, B.J.; Kamvar, Z.N. Best practices for population genetic analyses. Phytopathology 2017, 107, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Hogbin, P.M.; Peakall, R. Evaluation of the conservation of genetic research to the management of endangered plant. Zieriaprostrata Conserv. Biol. 1999, 13, 514–522. [Google Scholar]
- Khanlou, K.M.; Vandepitte, K.; Asl, L.K.; Bockstaele, E.V. Towards an optimal sampling strategy for assessing genetic variation within and among white clover (Trifolium repens L.) cultivars using AFLP. Genet. Mol. Biol. 2011, 34, 252–258. [Google Scholar] [CrossRef]
- Young, A.; Merriam, H.; Warwick, S. The effects of forest fragmentation on genetic variation in Acer saccharum Marsh. (sugar maple) populations. Heredity 1993, 71, 277–289. [Google Scholar] [CrossRef][Green Version]
- Flather, C.H.; Bevers, M. Patchy reaction-diffusion and population abundance: The relative importance of habitat amount and arrangement. Am. Nat. 2002, 159, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.S.; Singha, S.; Padalia, R.C.; Tandon, S.; Venkatesh, K.T.; Chauhan, A. Essential oil composition of the sub-aerial parts of eight species of Cymbopogon (Poaceae). Ind. Crops Prod. 2019, 142, 111839. [Google Scholar] [CrossRef]
- Sangwan, R.S.; Sangwan, N.S. Metabolic analysis of oil-chemotypic diversity in Cymbopogons. In Cymbopogons: The Aromatic Grass Monograph; Kumar, S., Dwivedi, S., Kukreja, A.K., Sharma, J.R., Bagchi, G.D., Eds.; CIMAP: Lucknow, India, 2000; pp. 223–247. [Google Scholar]
- Munda, S.; Sarma, N.; Lal, M. G × E interaction of 72 accessions with three year evaluation of Cymbopogon winterianus Jowitt. using regression coefficient and Additive Main effects and Multiplicative Interaction model (AMMI). Ind. Crops Prod. 2020, 146, 112169. [Google Scholar] [CrossRef]
- Milbourne, D.; Meyer, R.; Bradshaw, J.E.; Baird, E.; Bonar, N.; Provan, J.; Powell, W.; Robbie, W. Comparison of PCR-based marker systems for the analysis of genetic relationships in cultivated potato. Mol. Breed. 1997, 3, 127–136. [Google Scholar] [CrossRef]
- Prevost, A.; Wilkinson, M.J. A new system of comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theor. Appl. Genet. 1999, 98, 107–112. [Google Scholar] [CrossRef]
- Yeh, F.C.; Yang, R.C.; Boyle, T. POPGENE 1.32: A Free Program for the Analysis of Genetic Variation among and within Populations Using Co-Dominant and Dominant Markers. 2000. Available online: https://agris.fao.org/agris-search/search.do?recordID=GB2012106241 (accessed on 7 January 2022).
- Peakall, R.; Smouse, P.E. GenAlEx 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]


| S. No | IC. No. | Collection Site | Citronellal % | Citronellol % | Geraniol % |
|---|---|---|---|---|---|
| 1 | IC-0627004 | Tinsukia/Tinsukia/Assam | 3.745 ± 0.098 | 20.433 ± 0.021 | 41.146 ± 0.058 |
| 2 | IC-0627019 | Senapati/Senapati/Manipur | 43.404 ± 0.067 | 14.935 ± 0.055 | 20.188 ± 0.041 |
| 3 | IC-0627018 | Senapati/Senapati/Manipur | 42.934 ± 0.015 | 11.264 ± 0.071 | 22.437 ± 0.034 |
| 4 | IC-0627017 | Churachandpur/Churachandpur/Manipur | 42.641 ± 0.053 | 11.374 ± 0.017 | 23.270 ± 0.062 |
| 5 | IC-0627016 | Chandel/Chandel/Manipur | 41.843 ± 0.067 | 9.862 ± 0.048 | 22.323 ± 0.056 |
| 6 | IC-0627006 | Tinsukia/Tinsukia/Assam | 43.333 ± 0.019 | 15.018 ± 0.003 | 13.339 ± 0.005 |
| 7 | IC-0627005 | Dibrugarh/Dibrugarh/Assam | 42.196 ± 0.055 | 11.234 ± 0.022 | 18.851 ± 0.037 |
| 8 | IC-0627003 | Titabar/Jorhat/Assam | 4.485 ± 0.083 | 23.000 ± 0.041 | 39.842 ± 0.075 |
| 9 | IC-0627002 | Titabar/Jorhat/Assam | 5.723 ± 0.080 | 26.389 ± 0.052 | 31.448 ± 0.005 |
| 10 | IC-0626993 | Tippi/West Kameng/AP | 51.450 ± 0.048 | 10.064 ± 0.087 | 19.320 ± 0.075 |
| 11 | IC-0626991 | Nongpoh/Ri Bhoi/Meghalaya | 42.501 ± 0.063 | 14.311 ± 0.014 | 17.301 ± 0.042 |
| 12 | IC-0627064 | Birjhora T.E/Bongaigaon/Assam | 19.975 ± 0.051 | 11.143 ± 0.018 | 29.912 ± 0.037 |
| 13 | IC-0627065 | Moijonga/Goalpara/Assam | 46.511 ± 0.063 | 13.747 ± 0.027 | 19.304 ± 0.079 |
| 14 | IC-0627063 | Birjhora T.E/Bongaigaon/Assam | 46.022 ± 0.018 | 11.919 ± 0.046 | 18.807 ± 0.031 |
| 15 | IC-0627062 | Dejoo T.E/North Lakhimpur/Assam | 48.477 ± 0.057 | 9.245 ± 0.061 | 20.480 ± 0.028 |
| 16 | IC-0627058 | Siajuli T.E/North Lakhimpur/Assam | 47.383 ± 0.063 | 11.282 ± 0.074 | 14.979 ± 0.052 |
| 17 | IC-0627057 | Dejoo T.E/North Lakhimpur/Assam | 46.658 ± 0.038 | 10.329 ± 0.140 | 20.040 ± 0.097 |
| 18 | IC-0627056 | Siajuli T.E/North Lakhimpur/Assam | 46.606 ± 0.071 | 11.425 ± 0.044 | 17.554 ± 0.086 |
| 19 | IC-0627052 | Tingkhong T.E/Dibrugarh/Assam | 46.287 ± 0.083 | 10.728 ± 0.052 | 18.898 ± 0.029 |
| 20 | IC-0627051 | Sarojini T.E/Dibrugarh/Assam | 46.411 ± 0.046 | 11.971 ± 0.061 | 16.671 ± 0.018 |
| 21 | IC-0627050 | Birjhora/Bongaigaon/Assam | 42.738 ± 0.022 | 12.880 ± 0.071 | 20.242 ± 0.034 |
| 22 | IC-0627049 | Manjha/Karbianglong/Assam | 48.744 ± 0.011 | 12.182 ± 0.058 | 15.282 ± 0.029 |
| 23 | IC-0627048 | Manjha/Karbianglong/Assam | 45.785 ± 0.042 | 11.419 ± 0.030 | 20.443 ± 0.057 |
| 24 | IC-0627047 | Manjha/Karbianglong/Assam | 40.192 ± 0.057 | 12.722 ± 0.092 | 24.037 ± 0.023 |
| 25 | IC-0627046 | Manjha/Karbianglong/Assam | 41.200 ± 0.014 | 8.999 ± 0.052 | 21.093 ± 0.075 |
| 26 | IC-0627045 | Manjha/Karbianglong/Assam | 38.411 ± 0.052 | 11.272 ± 0.037 | 24.342 ± 0.049 |
| 27 | IC-0627044 | Manjha/Karbianglong/Assam | 37.405 ± 0.043 | 11.925 ± 0.062 | 22.823 ± 0.057 |
| 28 | IC-0627042 | Manjha/Karbianglong/Assam | 38.166 ± 0.051 | 11.753 ± 0.009 | 23.059 ± 0.036 |
| 29 | IC-0627041 | Manjha/Karbianglong/Assam | 40.901 ± 0.034 | 11.049 ± 0.057 | 18.885 ± 0.044 |
| 30 | IC-0627040 | Manjha/Karbianglong/Assam | 40.860 ± 0.052 | 11.993 ± 0.043 | 21.321 ± 0.071 |
| 31 | IC-0627039 | Manjha/Karbianglong/Assam | 42.306 ± 0.049 | 12.591 ± 0.027 | 20.396 ± 0.052 |
| 32 | IC-0627038 | Powai/Tinsukia/Assam | 38.345 ± 0.061 | 11.878 ± 0.033 | 22.924 ± 0.038 |
| 33 | IC-0627037 | Raidang/Tinsukia/Assam | 43.995 ± 0.057 | 13.729 ± 0.061 | 22.272 ± 0.041 |
| 34 | IC-0627036 | Sokriting T.E./Tinsukia/Assam | 18.938 ± 0.043 | 12.122 ± 0.025 | 11.734 ± 0.076 |
| 35 | IC-0627033 | Moranhat/Dibrugarh/Assam | 31.997 ± 0.062 | 12.288 ± 0.059 | 22.196 ± 0.071 |
| 36 | IC-0627035 | Panitola/Tinsukia/Assam | 47.569 ± 0.055 | 11.116 ± 0.049 | 18.029 ± 0.038 |
| 37 | IC-0627034 | Bogapani/Tinsukia/Assam | 35.657 ± 0.037 | 10.261 ± 0.082 | 24.533 ± 0.051 |
| 38 | IC-0627032 | Sepon T.E/Dibrugarh/Assam | 39.754 ± 0.021 | 11.103 ± 0.076 | 22.976 ± 0.039 |
| 39 | IC-0627031 | Gangabari T.E./Tinsukia/Assam | 19.057 ± 0.069 | 9.474 ± 0.048 | 38.826 ± 0.082 |
| 40 | IC-0627028 | Angkailongdi/Senapati/Manipur | 40.541 ± 0.075 | 11.978 ± 0.024 | 22.376 ± 0.061 |
| 41 | IC-0627030 | Tuithapi/Churachandpur/Manipur | 46.781 ± 0.043 | 13.714 ± 0.037 | 20.406 ± 0.050 |
| 42 | IC-0627026 | Japhou/Chandel/Manipur | 44.400 ± 0.019 | 12.677 ± 0.095 | 19.164 ± 0.039 |
| 43 | IC-0627027 | Kalika/Imphal East/Manipur | 41.479 ± 0.038 | 11.134 ± 0.042 | 21.566 ± 0.017 |
| 44 | IC-0627024 | Gumte/East Kameng/AP | 49.090 ± 0.013 | 13.578 ± 0.074 | 17.144 ± 0.042 |
| 45 | IC-0627025 | Silluk/East Siang/AP | 38.012 ± 0.032 | 10.726 ± 0.056 | 22.645 ± 0.013 |
| 46 | IC-0627023 | Jangda/Tawang/AP | 45.200 ± 0.068 | 11.486 ± 0.057 | 19.139 ± 0.065 |
| 47 | IC-0627022 | Boleng/East Siang/AP | 36.825 ± 0.024 | 9.661 ± 0.049 | 20.483 ± 0.039 |
| 48 | IC-0627021 | Picha/Kurungkumey/AP | 42.863 ± 0.055 | 15.486 ± 008 | 21.574 ± 0.027 |
| 49 | IC-0627054 | Sarojini T.E/Dibrugarh/Assam | 43.105 ± 0.037 | 14.106 ± 0.029 | 18.136 ± 0.034 |
| 50 | IC-0627043 | Manjha/Karbianglong/Assam | 41.864 ± 0.022 | 13.929 ± 0.045 | 23.171 ± 0.017 |
| 51 | IC-0626995 | Itanagar/Itanagar/AP | 20.119 ± 0.039 | 14.112 ± 0.017 | 35.834 ± 0.045 |
| 52 | IC-0627060 | Ananda T.E/North Lakhimpur/Assam | 49.543 ± 0.046 | 10.153 ± 0.013 | 18.670 ± 0.082 |
| 53 | IC-0627055 | Birjhora T.E/Bongaigaon/Assam | 44.487 ± 0.061 | 11.503 ± 0.072 | 12.079 ± 0.042 |
| 54 | IC-0626994 | Jang/Tawang/AP | 42.225 ± 0.046 | 13.442 ± 0.095 | 17.160 ± 0.052 |
| 55 | IC-0627059 | Dolohat T.E/North Lakhimpur/ Assam | 45.059 ± 0.074 | 13.200 ± 0.024 | 16.611 ± 0.059 |
| 56 | IC-0627012 | Karbianglong/Karbianglong/Assam | 22.730 ± 0.043 | 11.733 ± 0.027 | 22.741 ± 0.038 |
| 57 | IC-0626998 | Golaghat/Golaghat/Assam | 43.068 ± 0.052 | 9.637 ± 0.078 | 21.123 ± 0.014 |
| 58 | IC-0627013 | Umiam/ Ri Bhoi/Meghalaya | 21.914 ± 0.024 | 9.986 ± 0.132 | 34.194 ± 0.154 |
| 59 | IC-0627001 | Mariani/Jorhat/Assam | 42.346 ± 0.092 | 15.290 ± 0.014 | 18.762 ± 0.043 |
| 60 | IC-0627029 | Saikot/Churachandpur/Manipur | 44.850 ± 0.089 | 13.011 ± 0.046 | 22.343 ± 0.057 |
| 61 | IC-0627009 | Nagaon/Nagaon/Assam | 2.356 ± 0.048 | 0.000 | 0.000 |
| 62 | IC-0627014 | Barpeta/Barpeta/Assam | 45.857 ± 0.074 | 13.245 ± 0.016 | 19.208 ± 0.061 |
| 63 | IC-0626996 | Rupa/West Kameng/AP | 45.015 ± 0.062 | 12.879 ± 0.047 | 19.924 ± 0.035 |
| 64 | IC-0626999 | Golaghat/Golaghat/Assam | 3.559 ± 0.071 | 10.532 ± 0.063 | 22.834 ± 0.054 |
| 65 | IC-0626997 | Golaghat/Golaghat/Assam | 2.703 ± 0.083 | 2.682 ± 0.075 | 2.967 ± 0.073 |
| 66 | IC-0627000 | Mariani/Jorhat/Assam | 40.551 ± 0.069 | 10.816 ± 0.021 | 21.960 ± 0.056 |
| 67 | IC-0627011 | Karbianglong/Karbianglong/Assam | 21.393 ± 0.007 | 9.147 ± 0.084 | 38.883 ± 0.037 |
| 68 | IC-0627061 | Harmoti T.E/North Lakhimpur/Assam | 43.561 ± 0.045 | 9.567 ± 0.063 | 21.127 ± 0.050 |
| 69 | IC-0626992 | Tippi/West Kameng/AP | 40.386 ± 0.037 | 11.145 ± 0.029 | 19.420 ± 0.010 |
| 70 | IC-0627053 | Tingkhong T.E/Dibrugarh/Assam | 40.309 ± 0.023 | 10.628 ± 0.031 | 18.320 ± 0.081 |
| 71 | IC-0627007 | Moran/Dibrugarh/Assam | 38.334 ± 0.045 | 11.773 ± 0.018 | 20.003 ± 0.063 |
| 72 | IC-0627008 | Dibrugarh/Dibrugarh/Assam | 2.578 ± 0.012 | 0.000 | 0.000 |
| S. No. | Primer Code | Primer Sequence (5ʹ–3ʹ and 3ʹ–5ʹ) | Total Alleles | Polymorphic Alleles | % Polymorphism | PIC | MI | Rp |
|---|---|---|---|---|---|---|---|---|
| 1 | 1CM0102 | TGCACGGGGAAGAGGAGAGA AACCGAAGCGCAAGAACCCA | 4 | 3 | 75 | 0.47 | 0.35 | 2.14 |
| 2 | 2CM0304 | ACCCCAGCGCGATCCGAGGT AGCGGCGTCTCCAGCCCGAA | 3 | 2 | 67 | 0.55 | 0.37 | 0.70 |
| 3 | 3CM0506 | CGCCGAAGTGGTAGAGGCAA TGCAGGAGGCAGGAGGAGAA | 5 | 5 | 100 | 0.69 | 0.69 | 3.12 |
| 4 | 4CM0910 | CGCCGCCGTACTGCTCCATC GCGGAGGAGACCTGCGGGT | 5 | 4 | 80 | 0.58 | 0.47 | 2.16 |
| 5 | 5CM1112 | GAATCCGATCCATCCATTGG CACGAACACGCGACGCACGA | 3 | 1 | 33 | 0.33 | 0.11 | 0.01 |
| 6 | 6CM1314 | ACTTTGCGTGCGAGGCGAGG CGAGGTCGAGTAGAAGGCGT | 3 | 2 | 67 | 0.42 | 0.28 | 0.63 |
| 7 | 7CM1516 | ACTTTGCGTGCGAGGCGAGG CGAGGTCGAGTAGAAGGCGT | 2 | 1 | 50 | 0.50 | 0.25 | 0.02 |
| 8 | 8CM1718 | CTCGCCGTCGAATCCGCCAT CACTCTCCTCTCCTGGCCCG | 2 | 1 | 50 | 0.49 | 0.25 | 0.03 |
| 9 | 10CM2122 | CTCCCCCTCCTCCTCCTCCC CGACCGGCCGGAATGGATGC | 3 | 2 | 67 | 0.66 | 0.44 | 0.05 |
| 10 | 12CM2728 | ATCTCTTCGCAGATCCACCT GCTGAGACGCGCGGGTCGGA | 3 | 2 | 67 | 0.58 | 0.39 | 0.51 |
| 11 | 13CM2930 | TTTATCCGCGTCCCTAGCTT GCCGCCGGGGTCACAGGTCA | 5 | 3 | 60 | 0.57 | 0.34 | 0.31 |
| 12 | 14CM3132 | GAGAGGATTCCGATACCCTT TCGGCCTCTCGCCCCCCGA | 3 | 3 | 100 | 0.50 | 0.50 | 2.84 |
| 13 | 15CM3334 | CGTGCTCGTGGATCCCCATC CACCGTCGAATCGAATCCAA | 5 | 4 | 80 | 0.65 | 0.52 | 1.47 |
| 14 | 17CM3738 | ATATATCCAGCCAGCCGCAT GGGCCGTGCCGTGCCTCACC | 3 | 2 | 67 | 0.16 | 0.11 | 0.95 |
| 15 | 18CM3940 | CTCCTCGATCTTCCTCTACT TCGACCCCATCACAAATCCA | 4 | 3 | 75 | 0.65 | 0.48 | 0.84 |
| 16 | 20CM4344 | GGGATGATCATCTCCGATGC CCCTTCTCCCACTCTTCTCC | 4 | 3 | 75 | 0.67 | 0.50 | 0.63 |
| 17 | 21CM038 | GGGAATGGGAAGGCAGTTG AGCGCAGCACCACGAGAA | 5 | 3 | 60 | 0.39 | 0.23 | 1.31 |
| 18 | 22CM075 | TGGCTAAGGAAGAATGCTC CAGCGACGTCAATGCCACC | 3 | 2 | 67 | 0.54 | 0.36 | 0.74 |
| 19 | 25CM003 | TGATGCAGTCGCCAAAAAGGATGA ACGCGGCCTTTTTACGGTTCCTG | 2 | 1 | 50 | 0.29 | 0.14 | 0.85 |
| 20 | 30CM056 | GGACACCATTGCTTTTTAGTAGAG CGGAGAAGGACGACAGAGACG | 3 | 3 | 100 | 0.52 | 0.52 | 1.26 |
| 21 | 31CM154 | TGAATAATCGCGGAATGAAGTGGA GCTCAGCACCCTTCACATCTTTAG | 4 | 3 | 75 | 0.41 | 0.31 | 2.36 |
| 22 | 33CM163 | ACGCGGAGCCGACAGAGGATCT GGCAGGGATGGGGGCGTCTGG | 2 | 1 | 50 | 0.49 | 0.25 | 0.04 |
| 23 | 34CM014 | GTTGCATATGCCGGCGACTGTG GCCGGGCGCCATCATTAGTTC | 3 | 3 | 100 | 0.34 | 0.34 | 1.62 |
| 24 | 35CM007 | CCGGGGACATTTGGGACTCG GGCGCCGTCCACAAAGAATC | 4 | 3 | 75 | 0.40 | 0.30 | 1.32 |
| 25 | 36CM126 | ACGAAGCACAGCGGGAAGGTTC CCTGCCGTTGTTCTCCATCCAC | 3 | 2 | 67 | 0.41 | 0.27 | 0.67 |
| 26 | 38CM168 | AACATGTTCAGAAAAGCGCAG CGCCGCTTGCGCTTATGCTCG | 2 | 1 | 50 | 0.49 | 0.25 | 0.03 |
| 27 | 39CM125 | CGATGCAGCGCGAGAGTAGAGA AACCTTCCCGCTGCTTCGTGCA | 2 | 1 | 50 | 0.49 | 0.24 | 0.05 |
| 28 | 42CM128 | CGTTTGCGCGAAGAGCCCTACCC CCGCACGCCATCCCCAACCA | 2 | 1 | 50 | 0.47 | 0.24 | 0.11 |
| Total | 92 | 65 | 1907 | 13.71 | 9.5 | 26.77 | ||
| Average | 3.29 | 2.32 | 68.10 | 0.49 | 0.34 | 0.96 | ||
| Standard deviation | 1.05 | 1.09 | 17.49 | 0.12 | 0.14 | 0.90 | ||
| S. No. | C. winterianus | Mean | Polymorphic % | Ht | Hs | Gst | Nm | |||
|---|---|---|---|---|---|---|---|---|---|---|
| na | ne | h | I | |||||||
| 1. | Pop 1 (Assam) | 1.71 ± 0.46 | 1.42 ± 0.39 | 0.24 ± 0.20 | 0.36 ± 0.28 | 70.65 | 0.22 ± 0.03 | 0.16 ± 0.02 | 0.25 | 1.48 |
| 2. | Pop 2 (Manipur) | 1.40 ± 0.49 | 1.23 ± 0.34 | 0.13 ± 0.19 | 0.20 ± 0.27 | 40.22 | ||||
| 3. | Pop 3 (Meghalaya) | 1.18 ± 0.39 | 1.13 ± 0.28 | 0.07 ± 0.16 | 0.11 ± 0.24 | 18.48 | ||||
| 4. | Pop 4 (Arunachal Pradesh) | 1.58 ± 0.50 | 1.34 ± 0.36 | 0.20 ± 0.20 | 0.31 ± 0.28 | 57.61 | ||||
| Mean | 1.71 | 1.41 | 0.24 | 0.36 | 70.65 | |||||
| Standard deviation | 0.46 | 0.38 | 0.20 | 0.27 | ||||||
| PC | Eigen Value | % Variance | Cumulative Variance |
|---|---|---|---|
| 1 | 1.91 | 16.09 | 16.09 |
| 2 | 0.99 | 8.36 | 24.45 |
| 3 | 0.69 | 5.77 | 30.22 |
| 4 | 0.67 | 5.61 | 35.83 |
| 5 | 0.54 | 4.56 | 40.31 |
| 6 | 0.53 | 4.42 | 44.81 |
| 7 | 0.48 | 4.07 | 48.88 |
| 8 | 0.46 | 3.86 | 52.74 |
| 9 | 0.40 | 3.38 | 56.12 |
| 10 | 0.35 | 2.96 | 59.08 |
| 11 | 0.34 | 2.88 | 61.96 |
| 12 | 0.30 | 2.54 | 64.50 |
| 13 | 0.30 | 2.49 | 66.99 |
| 14 | 0.28 | 2.34 | 69.33 |
| Source of Variation | Degrees of Freedom (df) | Sum of Squares (SS) | Mean Squares (MS) | Variance Component | Percentage of Total Variance | p Value |
|---|---|---|---|---|---|---|
| Among Pops | 3 | 10.223 | 3.408 | 0.243 | 25% | 0.255 |
| Within Pops | 68 | 48.374 | 0.711 | 0.711 | 75% | 0.045 |
| Total | 71 | 58.597 | 0.954 | 100% |
| GC-MS | GC-FID | |
|---|---|---|
| Instrument | TRACE Ultra Gas Chromatograph coupled with an ISQ Mass Spectrometer | Thermo Scientific TRACE 1110 |
| Column | TG WAX/MS (60 m × 0.25mm i.d) | TG WAX/MSA (60 m × 0.25mm i.d) |
| Film thickness | 0.25 μm | 0.25 μm |
| Running conditions | 40 °C for 2 min 250 °C at 5 °C/min 300 °C at 30 °C/min for 10 min | Initial temperature: 50 °C Oven temperature: 300 °C Injector temperature: 280 °C FID temperature: 310 °C |
| Sample injection | 1 μL | 1 μL |
| Carrier gas | Helium (1mL min−1) | Nitrogen (0.30 mL min−1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munda, S.; Saikia, R.J.; Begum, T.; Bhandari, S.; Gogoi, A.; Sarma, N.; Tamang, R.; Lal, M. Evaluation of Genetic Diversity Based on Microsatellites and Phytochemical Markers of Core Collection of Cymbopogon winterianus Jowitt Germplasm. Plants 2022, 11, 528. https://doi.org/10.3390/plants11040528
Munda S, Saikia RJ, Begum T, Bhandari S, Gogoi A, Sarma N, Tamang R, Lal M. Evaluation of Genetic Diversity Based on Microsatellites and Phytochemical Markers of Core Collection of Cymbopogon winterianus Jowitt Germplasm. Plants. 2022; 11(4):528. https://doi.org/10.3390/plants11040528
Chicago/Turabian StyleMunda, Sunita, Raktim Jyoti Saikia, Twahira Begum, Sangeeta Bhandari, Ankita Gogoi, Neelav Sarma, Raghu Tamang, and Mohan Lal. 2022. "Evaluation of Genetic Diversity Based on Microsatellites and Phytochemical Markers of Core Collection of Cymbopogon winterianus Jowitt Germplasm" Plants 11, no. 4: 528. https://doi.org/10.3390/plants11040528
APA StyleMunda, S., Saikia, R. J., Begum, T., Bhandari, S., Gogoi, A., Sarma, N., Tamang, R., & Lal, M. (2022). Evaluation of Genetic Diversity Based on Microsatellites and Phytochemical Markers of Core Collection of Cymbopogon winterianus Jowitt Germplasm. Plants, 11(4), 528. https://doi.org/10.3390/plants11040528







