Flip-Flap: A Simple Dual-View Imaging Method for 3D Reconstruction of Thick Plant Samples
Abstract
:1. Introduction
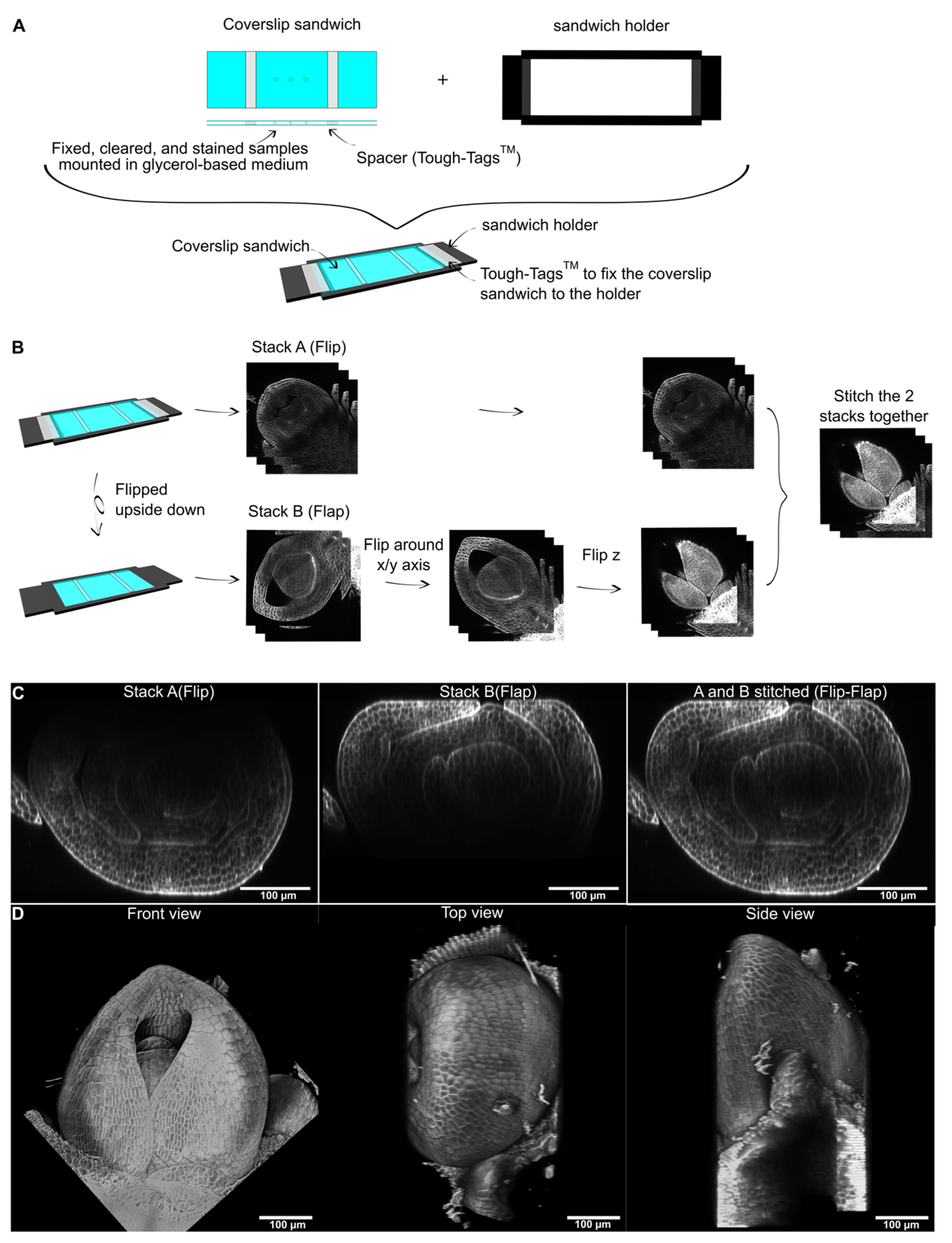
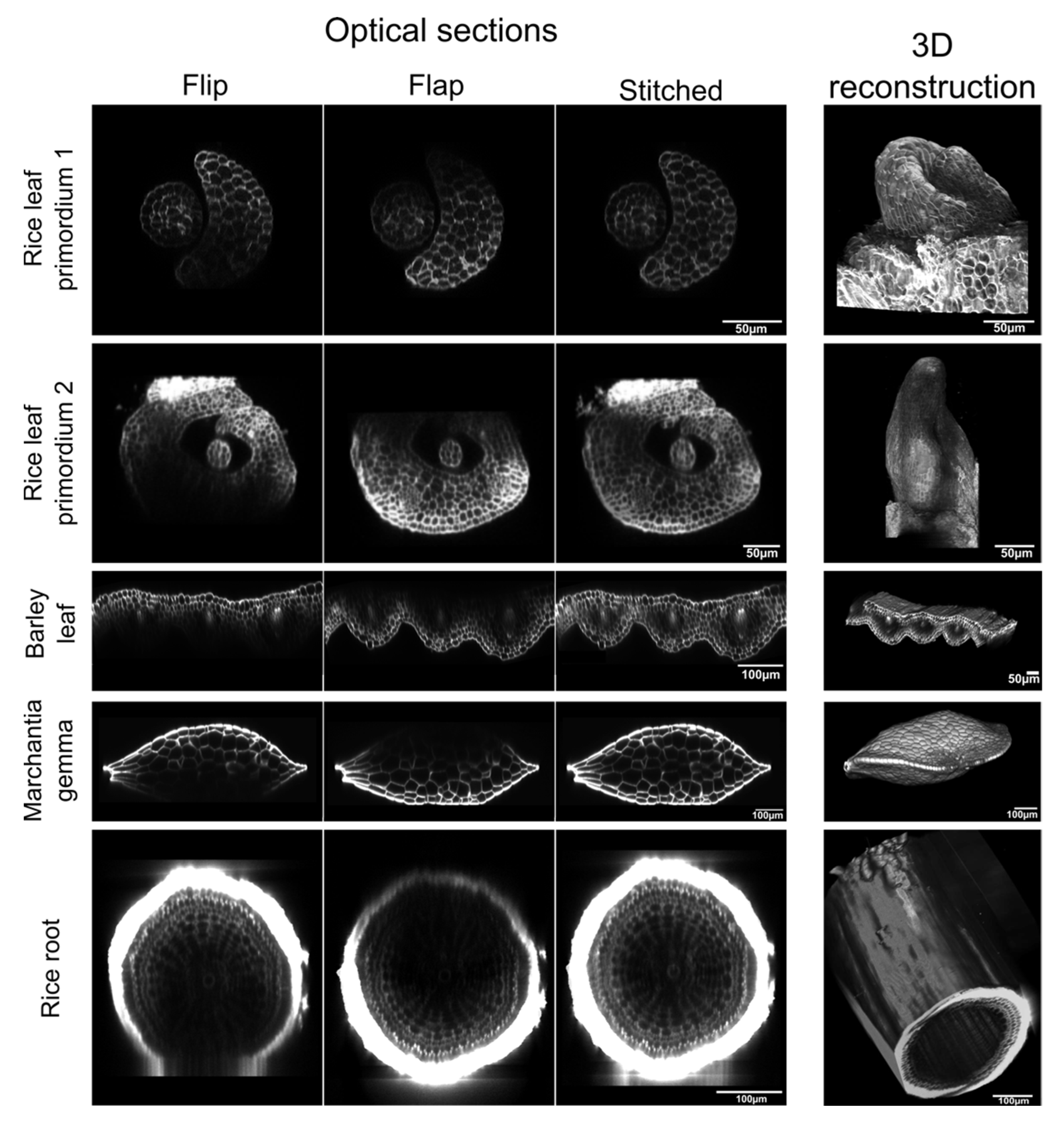
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ustin, S.L.; Jacquemoud, S. How the optical properties of leaves modify the absorption and scattering of energy and enhance leaf functionality. In Remote Sensing of Plant Biodiversity; Cavender-Bares, J., Gamon, J.A., Townsend, P.A., Eds.; Springer International Publishing: Cham, Germany, 2020; pp. 349–384. [Google Scholar]
- Clark, J.B.; Lister, G.R. Photosynthetic Action Spectra of Trees: II. The Relationship of Cuticle Structure to the Visible and Ultraviolet Spectral Properties of Needles from Four Coniferous Species. Plant Physiol. 1975, 55, 407–413. [Google Scholar] [CrossRef] [Green Version]
- Luginbuehl, L.H.; El-Sharnouby, S.; Wang, N.; Hibberd, J.M. Fluorescent reporters for functional analysis in rice leaves. Plant Direct 2020, 4, e00188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurihara, D.; Mizuta, Y.; Sato, Y.; Higashiyama, T. ClearSee: A rapid optical clearing reagent for whole-plant fluorescence imaging. Development 2015, 142, 4168–4179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurihara, D.; Mizuta, Y.; Nagahara, S.; Higashiyama, T. ClearSeeAlpha: Advanced optical clearing for whole-plant imaging. Plant Cell Physiol. 2021, 62, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Truernit, E.; Bauby, H.; Dubreucq, B.; Grandjean, O.; Runions, J.; Barthélémy, J.; Palauqui, J.-C. High-resolution whole-mount imaging of three-dimensional tissue organization and gene expression enables the study of Phloem development and structure in Arabidopsis. Plant Cell 2008, 20, 1494–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ursache, R.; Andersen, T.G.; Marhavý, P.; Geldner, N. A protocol for combining fluorescent proteins with histological stains for diverse cell wall components. Plant J. 2018, 93, 399–412. [Google Scholar] [CrossRef] [Green Version]
- Graeff, M.; Rana, S.; Wendrich, J.R.; Dorier, J.; Eekhout, T.; Aliaga Fandino, A.C.; Guex, N.; Bassel, G.W.; De Rybel, B.; Hardtke, C.S. A morpho-transcriptomic map of brassinosteroid action in the Arabidopsis root. BioRxiv 2021. preprint. [Google Scholar]
- Vijayan, A.; Tofanelli, R.; Strauss, S.; Cerrone, L.; Wolny, A.; Strohmeier, J.; Kreshuk, A.; Hamprecht, F.A.; Smith, R.S.; Schneitz, K. A digital 3D reference atlas reveals cellular growth patterns shaping the Arabidopsis ovule. eLife 2021, 10, e63262. [Google Scholar] [CrossRef] [PubMed]
- Mooney, S.J.; Pridmore, T.P.; Helliwell, J.; Bennett, M.J. Developing X-ray Computed Tomography to non-invasively image 3-D root systems architecture in soil. Plant Soil 2012, 352, 1–22. [Google Scholar] [CrossRef]
- Wang, Z.; Verboven, P.; Nicolai, B. Contrast-enhanced 3D micro-CT of plant tissues using different impregnation techniques. Plant Methods 2017, 13, 105. [Google Scholar] [CrossRef] [Green Version]
- Silvestri, L.; Bria, A.; Sacconi, L.; Iannello, G.; Pavone, F.S. Confocal light sheet microscopy: Micron-scale neuroanatomy of the entire mouse brain. Opt. Express 2012, 20, 20582–20598. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.; Das, P.; Mirabet, V.; Moscardi, E.; Traas, J.; Verdeil, J.-L.; Malandain, G.; Godin, C. Imaging plant growth in 4D: Robust tissue reconstruction and lineaging at cell resolution. Nat. Methods 2010, 7, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Preibisch, S.; Saalfeld, S.; Tomancak, P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 2009, 25, 1463–1465. [Google Scholar] [CrossRef]
- Barbier de Reuille, P.; Routier-Kierzkowska, A.-L.; Kierzkowski, D.; Bassel, G.W.; Schüpbach, T.; Tauriello, G.; Bajpai, N.; Strauss, S.; Weber, A.; Kiss, A.; et al. MorphoGraphX: A platform for quantifying morphogenesis in 4D. eLife 2015, 4, 05864. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Cai, W.; Chen, X.; Chen, M.; Chu, J.; Liang, W.; Persson, S.; Liu, Z.; Zhang, D. Bright fluorescent vacuolar marker lines allow vacuolar tracing across multiple tissues and stress conditions in rice. Int. J. Mol. Sci. 2020, 21, 4203. [Google Scholar] [CrossRef]
- Gao, R.; Asano, S.M.; Upadhyayula, S.; Pisarev, I.; Milkie, D.E.; Liu, T.-L.; Singh, V.; Graves, A.; Huynh, G.H.; Zhao, Y.; et al. Cortical column and whole-brain imaging with molecular contrast and nanoscale resolution. Science 2019, 363, eaau8302. [Google Scholar] [CrossRef]
- Strauss, S.; Runions, A.; Lane, B.; Eschweiler, D.; Bajpai, N.; Trozzi, N.; Routier-Kierzkowska, A.-L.; Yoshida, S.; Rodrigues da Silveira, S.; Vijayan, A.; et al. MorphoGraphX 2.0: Providing context for biological image analysis with positional information. BioRxiv 2021. [Google Scholar] [CrossRef]
- Palmer, W.M.; Martin, A.P.; Flynn, J.R.; Reed, S.L.; White, R.G.; Furbank, R.T.; Grof, C.P.L. PEA-CLARITY: 3D molecular imaging of whole plant organs. Sci. Rep. 2015, 5, 13492. [Google Scholar] [CrossRef] [Green Version]
- Vieites-Prado, A.; Renier, N. Tissue clearing and 3D imaging in developmental biology. Development 2021, 148, dev199369. [Google Scholar] [CrossRef] [PubMed]
- Pietzsch, T.; Saalfeld, S.; Preibisch, S.; Tomancak, P. BigDataViewer: Visualization and processing for large image data sets. Nat. Methods 2015, 12, 481–483. [Google Scholar] [CrossRef]
- Wolny, A.; Cerrone, L.; Vijayan, A.; Tofanelli, R.; Barro, A.V.; Louveaux, M.; Wenzl, C.; Strauss, S.; Wilson-Sánchez, D.; Lymbouridou, R.; et al. Accurate and versatile 3D segmentation of plant tissues at cellular resolution. eLife 2020, 9, e57613. [Google Scholar] [CrossRef]
- McKown, K.H.; Bergmann, D.C. Stomatal development in the grasses: Lessons from models and crops (and crop models). New Phytol. 2020, 227, 1636–1648. [Google Scholar] [CrossRef] [Green Version]
- Baillie, A.L.; Fleming, A.J. The developmental relationship between stomata and mesophyll airspace. New Phytol. 2020, 225, 1120–1126. [Google Scholar] [CrossRef]
- Chakrabortty, B.; Willemsen, V.; de Zeeuw, T.; Liao, C.-Y.; Weijers, D.; Mulder, B.; Scheres, B. A Plausible Microtubule-Based Mechanism for Cell Division Orientation in Plant Embryogenesis. Curr. Biol. 2018, 28, 3031–3043.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, P.; Allsman, L.A.; Brakke, K.A.; Hoyt, C.; Hayes, J.; Liang, H.; Neher, W.; Rui, Y.; Roberts, A.M.; Moradifam, A.; et al. Predicting Division Planes of Three-Dimensional Cells by Soap-Film Minimization. Plant Cell 2018, 30, 2255–2266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eng, R.C.; Schneider, R.; Matz, T.W.; Carter, R.; Ehrhardt, D.W.; Jönsson, H.; Nikoloski, Z.; Sampathkumar, A. KATANIN and CLASP function at different spatial scales to mediate microtubule response to mechanical stress in Arabidopsis cotyledons. Curr. Biol. 2021, 31, 3262–3274.e6. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, L.; Tan, S.; Robinson, S.; Langdale, J.A. Flip-Flap: A Simple Dual-View Imaging Method for 3D Reconstruction of Thick Plant Samples. Plants 2022, 11, 506. https://doi.org/10.3390/plants11040506
Serra L, Tan S, Robinson S, Langdale JA. Flip-Flap: A Simple Dual-View Imaging Method for 3D Reconstruction of Thick Plant Samples. Plants. 2022; 11(4):506. https://doi.org/10.3390/plants11040506
Chicago/Turabian StyleSerra, Leo, Sovanna Tan, Sarah Robinson, and Jane A. Langdale. 2022. "Flip-Flap: A Simple Dual-View Imaging Method for 3D Reconstruction of Thick Plant Samples" Plants 11, no. 4: 506. https://doi.org/10.3390/plants11040506
APA StyleSerra, L., Tan, S., Robinson, S., & Langdale, J. A. (2022). Flip-Flap: A Simple Dual-View Imaging Method for 3D Reconstruction of Thick Plant Samples. Plants, 11(4), 506. https://doi.org/10.3390/plants11040506





