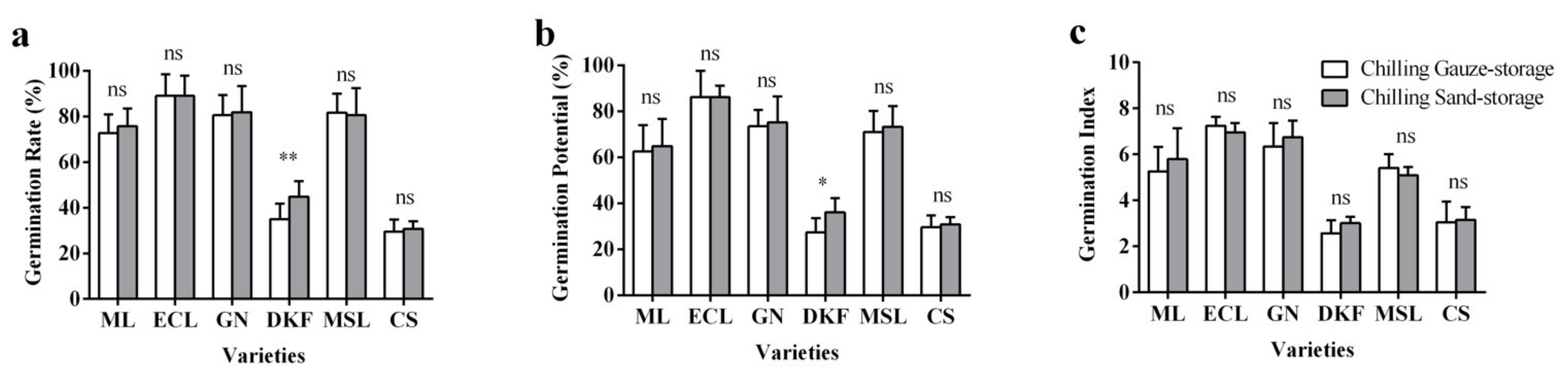
Figure 1.
Effect of stratification treatments on seed germination characteristics of six tested varieties: (a) influence of stratification treatments on seed germination rate of tested varieties, (b) influence of stratification treatments on seed germination potential of tested varieties, and (c) influence of stratification treatments on seed germination index of tested varieties. Data in this figure were tested by Student’s t test; * p < 0.05 and ** p < 0.01 represent significant differences between treatments, and ns indicates not significant. ML, ECL, GN, DKF, MSL, and CS represent varieties of Meili, Ecolly, Garanior, Dunkelfelder, Marselan, and Cabernet Sauvignon.
Figure 1.
Effect of stratification treatments on seed germination characteristics of six tested varieties: (a) influence of stratification treatments on seed germination rate of tested varieties, (b) influence of stratification treatments on seed germination potential of tested varieties, and (c) influence of stratification treatments on seed germination index of tested varieties. Data in this figure were tested by Student’s t test; * p < 0.05 and ** p < 0.01 represent significant differences between treatments, and ns indicates not significant. ML, ECL, GN, DKF, MSL, and CS represent varieties of Meili, Ecolly, Garanior, Dunkelfelder, Marselan, and Cabernet Sauvignon.
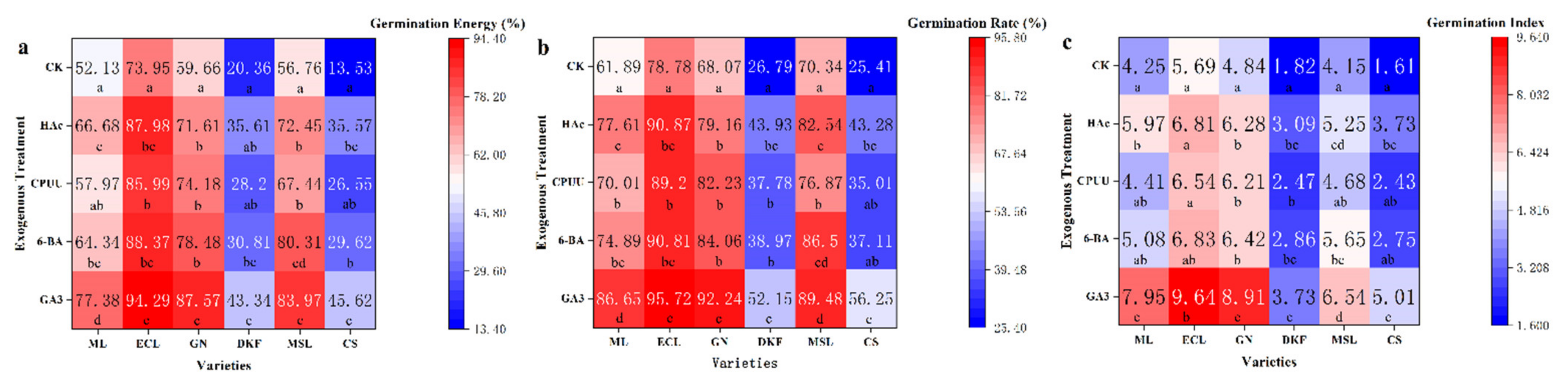
Figure 2.
Effect of chemical treatments on seed germination characteristics of six tested varieties: (a) influence of chemical treatments on seed germination rate of tested varieties, (b) influence of chemical treatments on seed germination potential of tested varieties, and (c) influence of chemical treatments on seed germination index of tested varieties. Data in this figure were tested by One-way ANOVA; means followed by the same letter in a column do not differ according to Tuker’s test (p ≤ 0.05). ML, ECL, GN, DKF, MSL, and CS represent varieties of Meili, Ecolly, Garanior, Dunkelfelder, Marselan, and Cabernet Sauvignon.
Figure 2.
Effect of chemical treatments on seed germination characteristics of six tested varieties: (a) influence of chemical treatments on seed germination rate of tested varieties, (b) influence of chemical treatments on seed germination potential of tested varieties, and (c) influence of chemical treatments on seed germination index of tested varieties. Data in this figure were tested by One-way ANOVA; means followed by the same letter in a column do not differ according to Tuker’s test (p ≤ 0.05). ML, ECL, GN, DKF, MSL, and CS represent varieties of Meili, Ecolly, Garanior, Dunkelfelder, Marselan, and Cabernet Sauvignon.
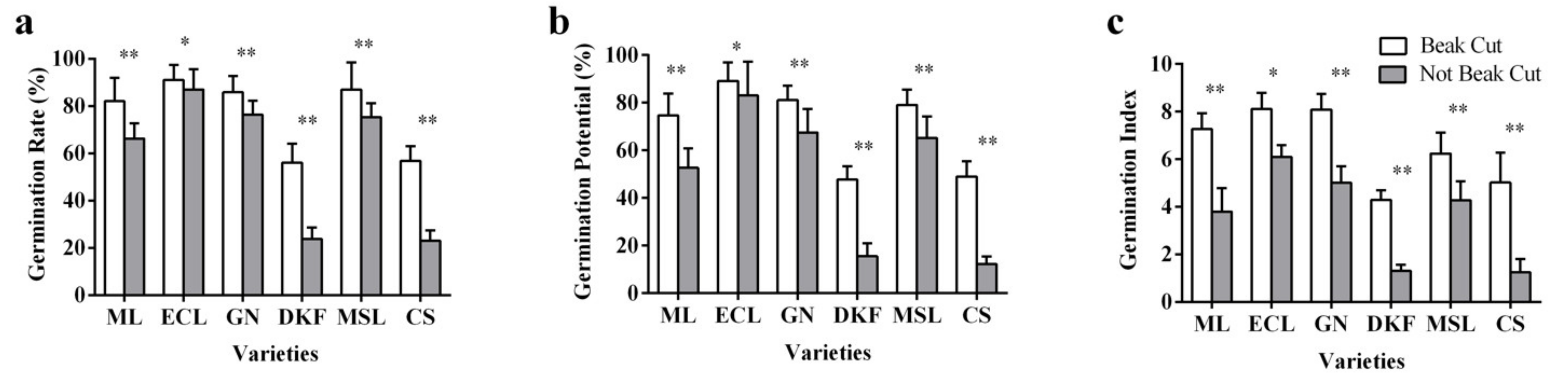
Figure 3.
Effect of beak cutting treatment on seed germination characteristics of six tested varieties: (a) influence of beak cutting on seed germination rate of tested varieties, (b) influence of beak cutting on the germination potential of the tested varieties, and (c) influence of beak cutting on the germination index of the tested varieties. Data in this figure were tested by Student’s t test; * p < 0.05 and ** p < 0.01 represent significant differences between treatments, and ns indicates not significant. ML, ECL, GN, DKF, MSL, and CS represent varieties of Meili, Ecolly, Garanior, Dunkelfelder, Marselan, and Cabernet Sauvignon.
Figure 3.
Effect of beak cutting treatment on seed germination characteristics of six tested varieties: (a) influence of beak cutting on seed germination rate of tested varieties, (b) influence of beak cutting on the germination potential of the tested varieties, and (c) influence of beak cutting on the germination index of the tested varieties. Data in this figure were tested by Student’s t test; * p < 0.05 and ** p < 0.01 represent significant differences between treatments, and ns indicates not significant. ML, ECL, GN, DKF, MSL, and CS represent varieties of Meili, Ecolly, Garanior, Dunkelfelder, Marselan, and Cabernet Sauvignon.
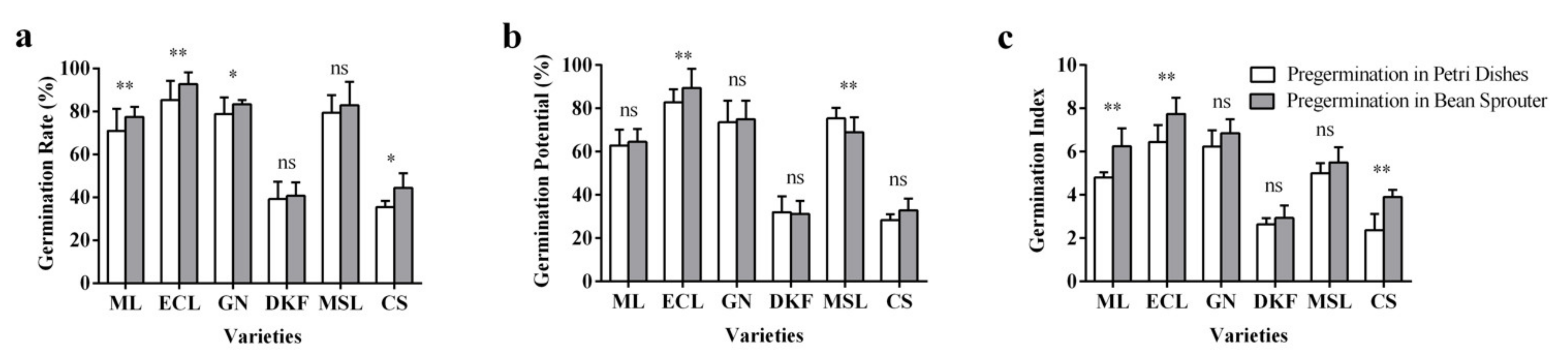
Figure 4.
Effect of pre-germination treatments on seed germination characteristics of six tested varieties: (a) influence of pre-germination treatments on seed germination rate of tested varieties, (b) influence of seed germination treatments on seed germination potential of tested varieties, and (c) influence of pre-germination treatments on the germination index of tested varieties. Data in this figure were tested by Student’s t test; * p < 0.05 and ** p < 0.01 represent significant differences between treatments, and ns indicates not significant. ML, ECL, GN, DKF, MSL, and CS represent varieties of Meili, Ecolly, Garanior, Dunkelfelder, Marselan, and Cabernet Sauvignon.
Figure 4.
Effect of pre-germination treatments on seed germination characteristics of six tested varieties: (a) influence of pre-germination treatments on seed germination rate of tested varieties, (b) influence of seed germination treatments on seed germination potential of tested varieties, and (c) influence of pre-germination treatments on the germination index of tested varieties. Data in this figure were tested by Student’s t test; * p < 0.05 and ** p < 0.01 represent significant differences between treatments, and ns indicates not significant. ML, ECL, GN, DKF, MSL, and CS represent varieties of Meili, Ecolly, Garanior, Dunkelfelder, Marselan, and Cabernet Sauvignon.
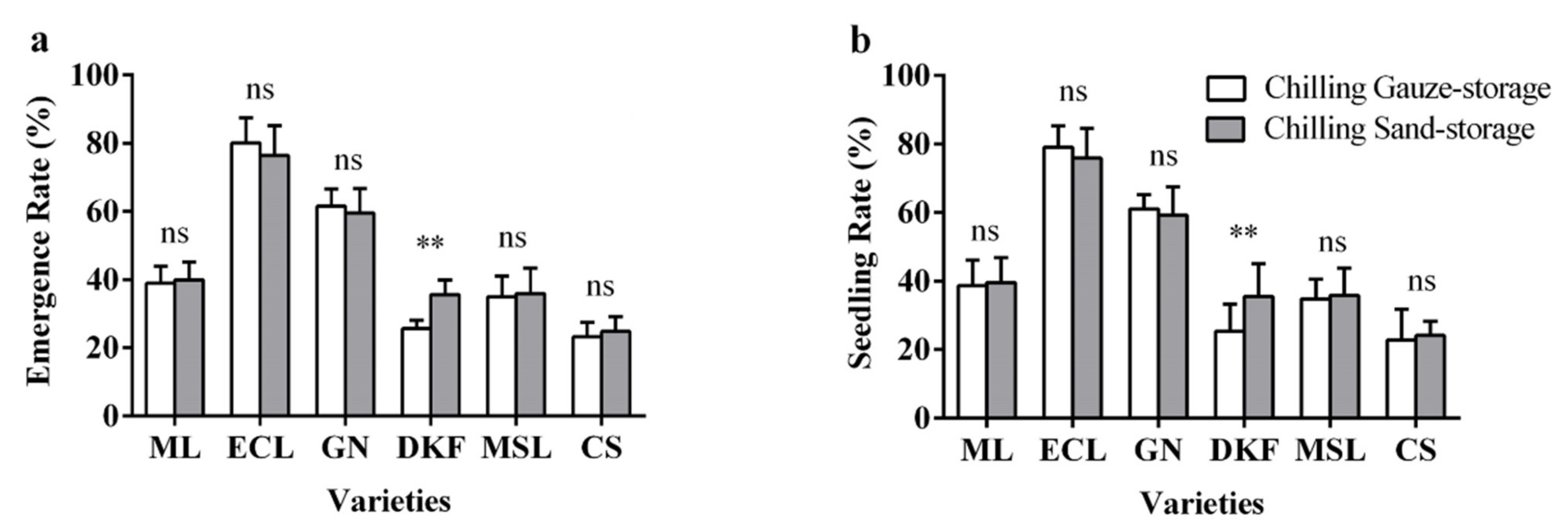
Figure 5.
Effect of stratification treatments on seed seedling characteristics of six tested varieties: (a) influence of stratification treatments on seed seedling rate of tested varieties, and (b) influence of stratification treatments on seed seedling rate of tested varieties. Data in this figure were tested by Student’s t test; ** p < 0.01 represent significant differences between treatments, and ns indicates not significant. ML, ECL, GN, DKF, MSL, and CS represent varieties of Meili, Ecolly, Garanior, Dunkelfelder, Marselan, and Cabernet Sauvignon.
Figure 5.
Effect of stratification treatments on seed seedling characteristics of six tested varieties: (a) influence of stratification treatments on seed seedling rate of tested varieties, and (b) influence of stratification treatments on seed seedling rate of tested varieties. Data in this figure were tested by Student’s t test; ** p < 0.01 represent significant differences between treatments, and ns indicates not significant. ML, ECL, GN, DKF, MSL, and CS represent varieties of Meili, Ecolly, Garanior, Dunkelfelder, Marselan, and Cabernet Sauvignon.
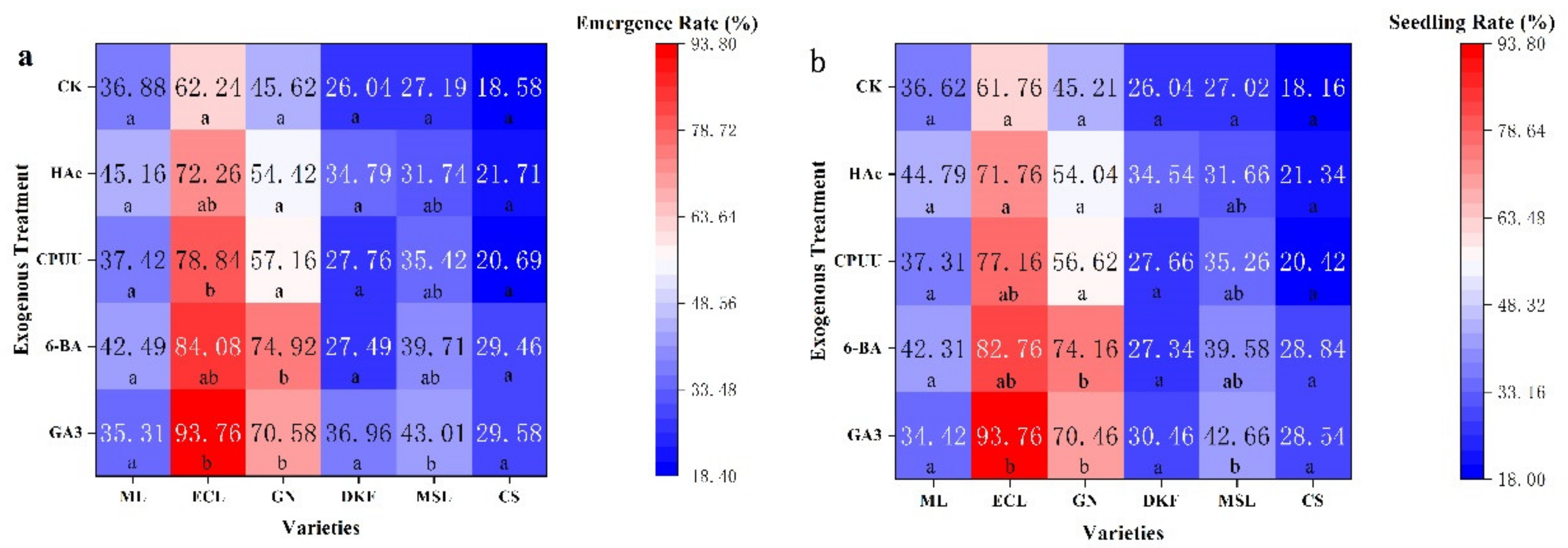
Figure 6.
Effect of chemical treatments on seed seedling characteristics of six tested varieties: (a) effect of chemical treatments on seedling rate of tested varieties, and (b) effect of chemical treatments on seed seedling rate in tested varieties. Data in this figure were tested by One-way ANOVA; means followed by the same letter in column do not differ according to Tuker’s test (p ≤ 0.05). ML, ECL, GN, DKF, MSL, and CS represent varieties of Meili, Ecolly, Garanior, Dunkelfelder, Marselan, and Cabernet Sauvignon.
Figure 6.
Effect of chemical treatments on seed seedling characteristics of six tested varieties: (a) effect of chemical treatments on seedling rate of tested varieties, and (b) effect of chemical treatments on seed seedling rate in tested varieties. Data in this figure were tested by One-way ANOVA; means followed by the same letter in column do not differ according to Tuker’s test (p ≤ 0.05). ML, ECL, GN, DKF, MSL, and CS represent varieties of Meili, Ecolly, Garanior, Dunkelfelder, Marselan, and Cabernet Sauvignon.
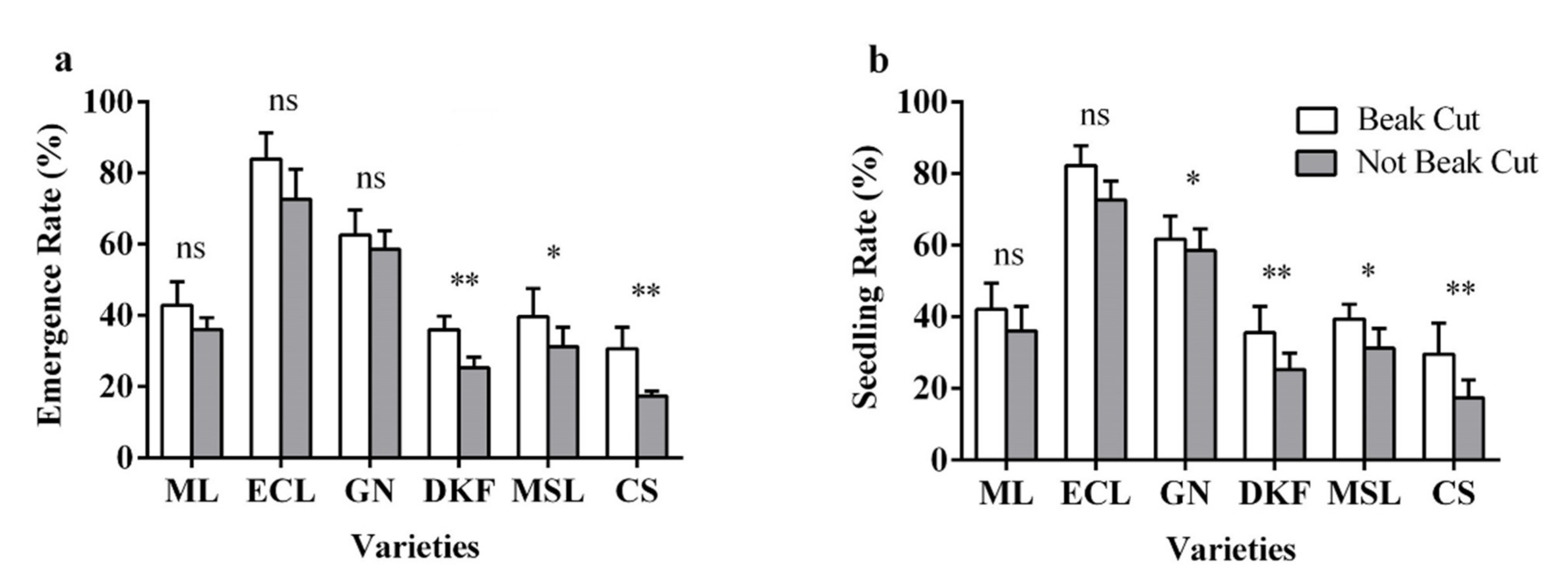
Figure 7.
Effect of beak cutting treatment on seedling characteristics of six tested varieties: (a) influence of beak cutting treatment on emergence rate of tested varieties, and (b) influence of beak cutting treatment on seedling rate of tested varieties. Data in this figure were tested by Student’s t test; * p < 0.05 and ** p < 0.01 represent significant differences between treatments, and ns indicates not significant. ML, ECL, GN, DKF, MSL, and CS represent varieties of Meili, Ecolly, Garanior, Dunkelfelder, Marselan, and Cabernet Sauvignon.
Figure 7.
Effect of beak cutting treatment on seedling characteristics of six tested varieties: (a) influence of beak cutting treatment on emergence rate of tested varieties, and (b) influence of beak cutting treatment on seedling rate of tested varieties. Data in this figure were tested by Student’s t test; * p < 0.05 and ** p < 0.01 represent significant differences between treatments, and ns indicates not significant. ML, ECL, GN, DKF, MSL, and CS represent varieties of Meili, Ecolly, Garanior, Dunkelfelder, Marselan, and Cabernet Sauvignon.
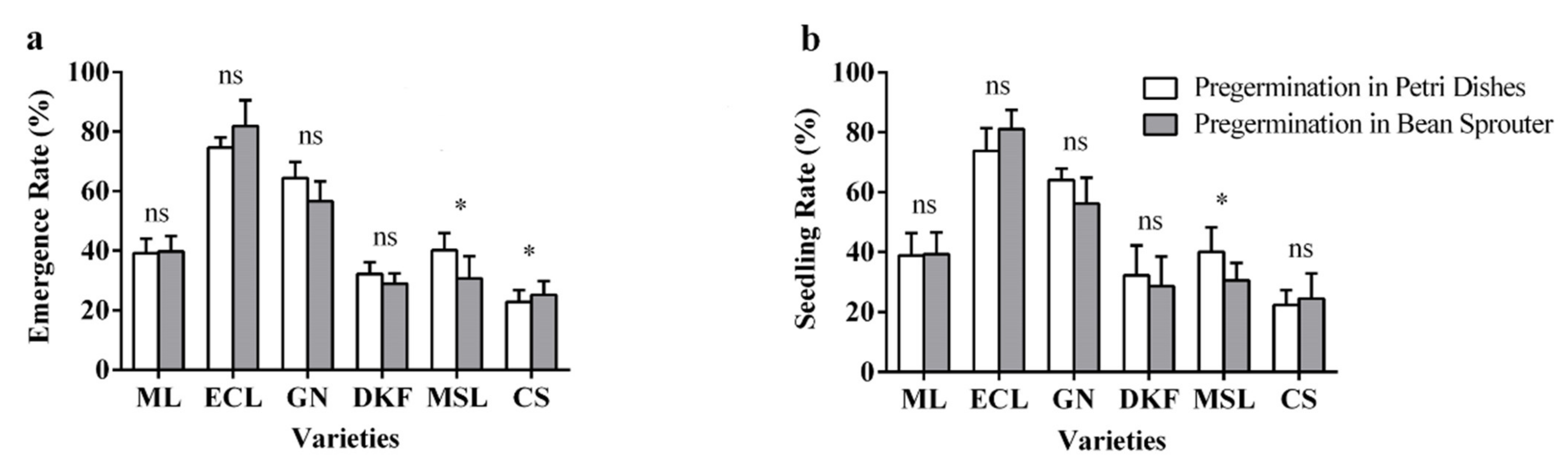
Figure 8.
Effect of pre-germination treatments on seed seedling characteristics of tested varieties: (a) influence of pre-germination treatments on seed emergence rate of tested varieties, and (b) influence of pre-germination treatments on seed seedling rate of tested varieties. Data in this figure were tested by Student’s t test; * p < 0.05 represent significant differences between treatments, and ns indicates not significant. ML, ECL, GN, DKF, MSL, and CS represent varieties of Meili, Ecolly, Garanior, Dunkelfelder, Marselan, and Cabernet Sauvignon.
Figure 8.
Effect of pre-germination treatments on seed seedling characteristics of tested varieties: (a) influence of pre-germination treatments on seed emergence rate of tested varieties, and (b) influence of pre-germination treatments on seed seedling rate of tested varieties. Data in this figure were tested by Student’s t test; * p < 0.05 represent significant differences between treatments, and ns indicates not significant. ML, ECL, GN, DKF, MSL, and CS represent varieties of Meili, Ecolly, Garanior, Dunkelfelder, Marselan, and Cabernet Sauvignon.
Table 1.
Orthogonal experimental design and optimum results of Meili grape seeds.
Table 1.
Orthogonal experimental design and optimum results of Meili grape seeds.
| Experiment Number | Experimental Factor | Experimental Result |
|---|
| A | B | C | D | Germination Rate (%) | Emergence Rate (%) |
|---|
| 1 | 1 | 3 | 1 | 2 | 84.33 | 47.52 |
| 2 | 1 | 1 | 1 | 1 | 98.67 | 48.00 |
| 3 | 1 | 3 | 1 | 1 | 78.61 | 38.90 |
| 4 | 2 | 3 | 2 | 1 | 68.18 | 44.43 |
| 5 | 2 | 5 | 1 | 2 | 85.41 | 63.89 |
| 6 | 2 | 2 | 1 | 1 | 91.33 | 49.55 |
| 7 | 2 | 1 | 2 | 1 | 88.08 | 42.36 |
| 8 | 2 | 1 | 2 | 2 | 89.75 | 50.00 |
| 9 | 1 | 2 | 2 | 2 | 74.00 | 38.18 |
| 10 | 2 | 3 | 2 | 2 | 72.95 | 34.00 |
| 11 | 1 | 1 | 1 | 2 | 99.00 | 59.33 |
| 12 | 1 | 5 | 2 | 1 | 56.33 | 34.67 |
| 13 | 1 | 4 | 2 | 1 | 80.27 | 73.03 |
| 14 | 2 | 4 | 1 | 2 | 96.00 | 51.67 |
| Germination rate (%) | k1 | 81.6 | 93.87 | 90.48 | 80.21 | | |
| k2 | 84.53 | 82.67 | 75.65 | 85.92 | | |
| k3 | | 76.02 | | | | |
| k4 | | 88.13 | | | | |
| k5 | | 70.87 | | | | |
| Range (R) | 2.93 | 23 | 14.83 | 5.71 | | |
| Primary and secondary order | B > C > D > A | | | | | |
| Superior level | A2 | B1 | C1 | D2 | | |
| Excellent combination | A2B1C1D2 | | | | | |
| Emergence rate (%) | k1 | 48.52 | 49.92 | 51.27 | 47.28 | | |
| k2 | 47.99 | 43.86 | 45.24 | 49.23 | | |
| k3 | | 41.21 | | | | |
| k4 | | 62.35 | | | | |
| k5 | | 49.28 | | | | |
| Range (R) | 0.53 | 21.14 | 6.03 | 1.95 | | |
| Primary and secondary order | B > C > D > A | | | | | |
| Superior level | A1 | B4 | C1 | D2 | | |
| Excellent combination | A1B4C1D2 | | | | | |
| Comprehensive optimum combination | A2B4C1D2 | | | | | |
Table 2.
Orthogonal experimental design and optimum results of Ecolly grape seeds.
Table 2.
Orthogonal experimental design and optimum results of Ecolly grape seeds.
| Experiment Number | Experimental Factor | Experimental Result |
|---|
| A | B | C | D | Germination Rate (%) | Emergence Rate (%) |
|---|
| 1 | 1 | 3 | 1 | 2 | 91.33 | 21.00 |
| 2 | 1 | 1 | 1 | 1 | 95.33 | 48.03 |
| 3 | 1 | 3 | 1 | 1 | 84.00 | 43.00 |
| 4 | 2 | 3 | 2 | 1 | 87.88 | 40.50 |
| 5 | 2 | 5 | 1 | 2 | 88.51 | 38.11 |
| 6 | 2 | 2 | 1 | 1 | 90.82 | 35.60 |
| 7 | 2 | 1 | 2 | 1 | 92.49 | 65.93 |
| 8 | 2 | 1 | 2 | 2 | 97.67 | 37.33 |
| 9 | 1 | 2 | 2 | 2 | 91.67 | 34.83 |
| 10 | 2 | 3 | 2 | 2 | 91.87 | 20.33 |
| 11 | 1 | 1 | 1 | 2 | 97.00 | 39.00 |
| 12 | 1 | 5 | 2 | 1 | 76.81 | 30.71 |
| 13 | 1 | 4 | 2 | 1 | 86.26 | 36.48 |
| 14 | 2 | 4 | 1 | 2 | 96.33 | 35.01 |
| Germination rate (%) | k1 | 88.91 | 95.62 | 91.9 | 87.66 | | |
| k2 | 92.22 | 91.25 | 89.24 | 93.48 | | |
| k3 | | 88.77 | | | | |
| k4 | | 91.3 | | | | |
| k5 | | 82.66 | | | | |
| Range (R) | 3.31 | 12.96 | 2.67 | 5.83 | | |
| Primary and secondary order | B > D > A > C | | | | | |
| Superior level | A2 | B1 | C1 | D2 | | |
| Excellent combination | A2B1C1D2 | | | | | |
| Emergence rate (%) | k1 | 36.15 | 47.57 | 37.11 | 42.89 | | |
| k2 | 38.97 | 35.22 | 38.02 | 32.23 | | |
| k3 | | 31.21 | | | | |
| k4 | | 35.75 | | | | |
| k5 | | 34.41 | | | | |
| Range (R) | 2.82 | 16.37 | 0.91 | 10.66 | | |
| Primary and secondary order | B > D > A > C | | | | | |
| Superior level | A2 | B1 | C2 | D1 | | |
| Excellent combination | A2B1C2D1 | | | | | |
| Comprehensive optimum combination | A2B1C1D1 | | | | | |
Table 3.
Orthogonal experimental design and optimum results of Garanior grape seeds.
Table 3.
Orthogonal experimental design and optimum results of Garanior grape seeds.
| Experiment Number | Experimental Factor | Experimental Result |
|---|
| A | B | C | D | Germination Rate (%) | Emergence Rate (%) |
|---|
| 1 | 1 | 3 | 1 | 2 | 85.24 | 66.74 |
| 2 | 1 | 1 | 1 | 1 | 96.49 | 48.20 |
| 3 | 1 | 3 | 1 | 1 | 84.71 | 51.33 |
| 4 | 2 | 3 | 2 | 1 | 83.08 | 59.77 |
| 5 | 2 | 5 | 1 | 2 | 87.92 | 54.27 |
| 6 | 2 | 2 | 1 | 1 | 90.77 | 51.59 |
| 7 | 2 | 1 | 2 | 1 | 86.33 | 72.00 |
| 8 | 2 | 1 | 2 | 2 | 91.19 | 72.00 |
| 9 | 1 | 2 | 2 | 2 | 81.00 | 52.15 |
| 10 | 2 | 3 | 2 | 2 | 78.69 | 51.69 |
| 11 | 1 | 1 | 1 | 2 | 97.62 | 49.33 |
| 12 | 1 | 5 | 2 | 1 | 55.46 | 41.37 |
| 13 | 1 | 4 | 2 | 1 | 81.68 | 81.78 |
| 14 | 2 | 4 | 1 | 2 | 95.22 | 46.90 |
| Germination rate (%) | k1 | 83.17 | 92.91 | 91.14 | 82.65 | | |
| k2 | 87.6 | 85.89 | 79.63 | 88.13 | | |
| k3 | | 82.93 | | | | |
| k4 | | 88.45 | | | | |
| k5 | | 71.69 | | | | |
| Range (R) | 4.43 | 21.22 | 11.51 | 5.48 | | |
| Primary and secondary order | B > C > D > A | | | | | |
| Superior level | A2 | B1 | C1 | D2 | | |
| Excellent combination | A2B1C1D2 | | | | | |
| Emergence rate (%) | k1 | 55.84 | 60.38 | 52.62 | 58.01 | | |
| k2 | 58.32 | 51.87 | 61.54 | 56.15 | | |
| k3 | | 57.38 | | | | |
| k4 | | 64.34 | | | | |
| k5 | | 47.82 | | | | |
| Range (R) | 2.47 | 16.52 | 8.91 | 1.85 | | |
| Primary and secondary order | B > C > A > D | | | | | |
| Superior level | A2 | B4 | C2 | D1 | | |
| Excellent combination | A2B4C2D1 | | | | | |
| Comprehensive optimum combination | A2B4C2D2 | | | | | |
Table 4.
Orthogonal experimental design and optimum results of Dunkelfelder grape seeds.
Table 4.
Orthogonal experimental design and optimum results of Dunkelfelder grape seeds.
| Experiment Number | Experimental Factor | Experimental Result |
|---|
| A | B | C | D | Germination Rate (%) | Emergence Rate (%) |
|---|
| 1 | 1 | 3 | 1 | 2 | 53.59 | 35.02 |
| 2 | 1 | 1 | 1 | 1 | 81.22 | 55.52 |
| 3 | 1 | 3 | 1 | 1 | 54.02 | 28.00 |
| 4 | 2 | 3 | 2 | 1 | 59.68 | 50.20 |
| 5 | 2 | 5 | 1 | 2 | 52.85 | 78.62 |
| 6 | 2 | 2 | 1 | 1 | 73.77 | 45.33 |
| 7 | 2 | 1 | 2 | 1 | 46.85 | 81.25 |
| 8 | 2 | 1 | 2 | 2 | 55.70 | 44.67 |
| 9 | 1 | 2 | 2 | 2 | 40.00 | 23.33 |
| 10 | 2 | 3 | 2 | 2 | 52.00 | 27.33 |
| 11 | 1 | 1 | 1 | 2 | 92.89 | 50.00 |
| 12 | 1 | 5 | 2 | 1 | 24.06 | 18.00 |
| 13 | 1 | 4 | 2 | 1 | 39.73 | 51.11 |
| 14 | 2 | 4 | 1 | 2 | 77.00 | 36.00 |
| Germination rate (%) | k1 | 55.07 | 69.16 | 69.33 | 54.19 | | |
| k2 | 59.69 | 56.88 | 45.43 | 60.58 | | |
| k3 | | 54.82 | | | | |
| k4 | | 58.37 | | | | |
| k5 | | 38.45 | | | | |
| Range (R) | 4.62 | 30.71 | 23.9 | 6.39 | | |
| Primary and secondary order | B > C > D > A | | | | | |
| Superior level | A2 | B1 | C1 | D2 | | |
| Excellent combination | A2B1C1D2 | | | | | |
| Emergence rate (%) | k1 | 37.28 | 57.86 | 46.93 | 47.06 | | |
| k2 | 51.91 | 34.33 | 42.27 | 42.14 | | |
| k3 | | 35.14 | | | | |
| k4 | | 43.56 | | | | |
| k5 | | 48.31 | | | | |
| Range (R) | 14.63 | 23.52 | 4.66 | 4.92 | | |
| Primary and secondary order | B > A > D > C | | | | | |
| Superior level | A2 | B1 | C1 | D1 | | |
| Excellent combination | A2B1C1D1 | | | | | |
| Comprehensive optimum combination | A2B1C1D2 | | | | | |
Table 5.
Orthogonal experimental design and optimum results of Marselan grape seeds.
Table 5.
Orthogonal experimental design and optimum results of Marselan grape seeds.
| Experiment Number | Experimental Factor | Experimental Result |
|---|
| A | B | C | D | Germination Rate (%) | Emergence Rate (%) |
|---|
| 1 | 1 | 3 | 1 | 2 | 91.41 | 48.50 |
| 2 | 1 | 1 | 1 | 1 | 98.67 | 72.00 |
| 3 | 1 | 3 | 1 | 1 | 90.61 | 48.00 |
| 4 | 2 | 3 | 2 | 1 | 84.17 | 56.02 |
| 5 | 2 | 5 | 1 | 2 | 66.49 | 22.00 |
| 6 | 2 | 2 | 1 | 1 | 94.00 | 79.43 |
| 7 | 2 | 1 | 2 | 1 | 94.33 | 49.50 |
| 8 | 2 | 1 | 2 | 2 | 98.00 | 72.00 |
| 9 | 1 | 2 | 2 | 2 | 91.47 | 43.44 |
| 10 | 2 | 3 | 2 | 2 | 69.14 | 33.33 |
| 11 | 1 | 1 | 1 | 2 | 99.33 | 51.33 |
| 12 | 1 | 5 | 2 | 1 | 74.39 | 48.70 |
| 13 | 1 | 4 | 2 | 1 | 84.09 | 45.62 |
| 14 | 2 | 4 | 1 | 2 | 87.09 | 54.48 |
| Germination rate (%) | k1 | 90 | 97.58 | 89.66 | 88.61 | | |
| k2 | 84.75 | 92.74 | 85.09 | 86.13 | | |
| k3 | | 83.83 | | | | |
| k4 | | 85.59 | | | | |
| k5 | | 70.44 | | | | |
| Range (R) | 5.25 | 27.14 | 4.57 | 2.47 | | |
| Primary and secondary order | B > A > C > D | | | | | |
| Superior level | A1 | B1 | C1 | D1 | | |
| Excellent combination | A1B1C1D1 | | | | | |
| Emergence rate (%) | k1 | 51.09 | 61.21 | 53.68 | 57.04 | | |
| k2 | 52.39 | 61.44 | 49.8 | 46.44 | | |
| k3 | | 46.46 | | | | |
| k4 | | 50.05 | | | | |
| k5 | | 35.35 | | | | |
| Range (R) | 1.31 | 26.09 | 3.88 | 10.59 | | |
| Primary and secondary order | B > D > C > A | | | | | |
| Superior level | A2 | B2 | C1 | D1 | | |
| Excellent combination | A1B1C1D1 | | | | | |
| Comprehensive optimum combination | A1B1C1D1 | | | | | |
Table 6.
Orthogonal experimental design and optimum results of Cabernet Sauvignon grape seeds.
Table 6.
Orthogonal experimental design and optimum results of Cabernet Sauvignon grape seeds.
| Experiment Number | Experimental Factor | Experimental Result |
|---|
| A | B | C | D | Germination Rate (%) | Emergence Rate (%) |
|---|
| 1 | 1 | 3 | 1 | 2 | 89.67 | 56.56 |
| 2 | 1 | 1 | 1 | 1 | 97.33 | 54.33 |
| 3 | 1 | 3 | 1 | 1 | 91.35 | 40.67 |
| 4 | 2 | 3 | 2 | 1 | 41.18 | 28.60 |
| 5 | 2 | 5 | 1 | 2 | 81.18 | 47.55 |
| 6 | 2 | 2 | 1 | 1 | 85.28 | 86.44 |
| 7 | 2 | 1 | 2 | 1 | 66.01 | 32.00 |
| 8 | 2 | 1 | 2 | 2 | 72.67 | 34.00 |
| 9 | 1 | 2 | 2 | 2 | 30.41 | 28.89 |
| 10 | 2 | 3 | 2 | 2 | 48.89 | 32.06 |
| 11 | 1 | 1 | 1 | 2 | 98.00 | 51.93 |
| 12 | 1 | 5 | 2 | 1 | 9.63 | 13.33 |
| 13 | 1 | 4 | 2 | 1 | 38.17 | 22.67 |
| 14 | 2 | 4 | 1 | 2 | 93.11 | 46.00 |
| Germination rate (%) | k1 | 64.94 | 83.5 | 90.85 | 61.28 | | |
| k2 | 69.76 | 57.84 | 43.85 | 73.42 | | |
| k3 | | 67.77 | | | | |
| k4 | | 65.64 | | | | |
| k5 | | 45.41 | | | | |
| Range (R) | 4.82 | 38.09 | 47 | 12.14 | | |
| Primary and secondary order | C > B > D > A | | | | | |
| Superior level | A2 | B1 | C1 | D2 | | |
| Excellent combination | A2B1C1D2 | | | | | |
| Emergence rate (%) | k1 | 38.34 | 43.06 | 54.78 | 39.72 | | |
| k2 | 43.81 | 57.67 | 27.36 | 42.43 | | |
| k3 | | 39.47 | | | | |
| k4 | | 34.33 | | | | |
| k5 | | 30.44 | | | | |
| Range (R) | 5.47 | 27.23 | 27.42 | 2.71 | | |
| Primary and secondary order | C > B > A > D | | | | | |
| Superior level | A2 | B2 | C1 | D2 | | |
| Excellent combination | A2B2C1D2 | | | | | |
| Comprehensive optimum combination | A2B1C1D2 | | | | | |
Table 7.
Results of repeated experiment.
Table 7.
Results of repeated experiment.
| Varieties | Optimal
Combination | Germination Rate | Emergence Rate |
|---|
| Ecolly | A2B1C1D1 | 94.21 ± 4.33 | 53.24 ± 4.19 |
| Garanior | A2B4C2D2 | 92.18 ± 4.56 | 67.65 ± 5.34 |
| Dunkelfelder | A2B1C1D2 | 79.99 ± 5.72 | 51.52 ± 4.24 |
| Cabernet Sauvignon | A2B1C1D2 | 82.49 ± 7.01 | 56.17 ± 3.55 |
Table 8.
Characteristics of the six test V. vinifera varieties.
Table 8.
Characteristics of the six test V. vinifera varieties.
| Variety | Breeding Method | Seed-Parent | Variety Type | Seed
Characteristics |
|---|
| Meili [46] | Intraspecific current selection | [Muscat Hamburg × (Merlot × Riesling)] × (Muscat Hamburg, Merlot, Riesling) | Red mid-variety | Large seed, seed coat is medium thickness. |
| Ecolly [47] | Intraspecific current selection | [Chenin Blanc × (Chardonnay × Riesling)] × (Chenin Blanc, Chardonnay, Riesling) | White mid-variety | Small seed, seed coat is relatively thin. |
| Garanior [48] | Intraspecific hybridization | Gamay × Reichensteiner | Red early-maturing variety | Small seed, seed coat is relatively thin. |
| Dunkelfelder [47] | Intraspecific hybridization | Madeleine Angevine × Teinturier | Red mid-variety | Medium-sized seed, seed coat is relatively thin. |
| Marselan [47] | Intraspecific hybridization | Cabernet Sauvignon × Grenache | Red mid-late maturity variety | Small seed, seed coat is relatively thin. |
| Cabernet Sauvignon [47] | Intraspecific hybridization | Cabernet Franc × Sauvignon Blanc | Red late-maturing variety | Medium-sized seed, seed coat is medium thickness. |
Table 9.
Level factors.
| Level | Experimental Factor |
|---|
| Stratification Method (A) | Chemical Substance (B) | Beak Cutting (C) | Pre-Germination Method (D) |
|---|
| 1 | chilling gauze-storage | GA3 | beak cutting | pre-germination in bean sprouter |
| 2 | chilling sand-storage | 6-BA | no beak cutting | pre-germination in petri dishes |
| 3 | | CPUU | | |
| 4 | | HAc | | |
| 5 | | CK | | |