Effect of Processing on Bioactive Compounds, Antioxidant Activity, Physicochemical, and Sensory Properties of Orange Sweet Potato, Red Rice, and Their Application for Flake Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Chemicals
2.2. Sample Preparation
2.2.1. Raw Samples
2.2.2. Boiled Samples
2.2.3. Flakes Production
2.3. Bioactive Compounds and Antioxidant Activity Analysis
2.3.1. Methanolic Extract of Samples
2.3.2. Phenolic Content
2.3.3. Anthocyanin Content
2.3.4. Carotenoid and Tocopherol Analysis
2.3.5. DPPH Radical Scavenging Activity
2.3.6. FRAP Assay
2.3.7. Superoxide Radical Scavenging Capacity
2.4. Physicochemical Properties of Flake Products
2.4.1. Moisture and Dietary Fiber Contents
2.4.2. Water Absorption Index
2.4.3. Fracturability, Crispness, and Color Profiles
2.5. Sensory Analysis
2.6. Statistical Analysis
3. Results
3.1. Bioactive Compounds of OSP, RR, and Their Flake Products
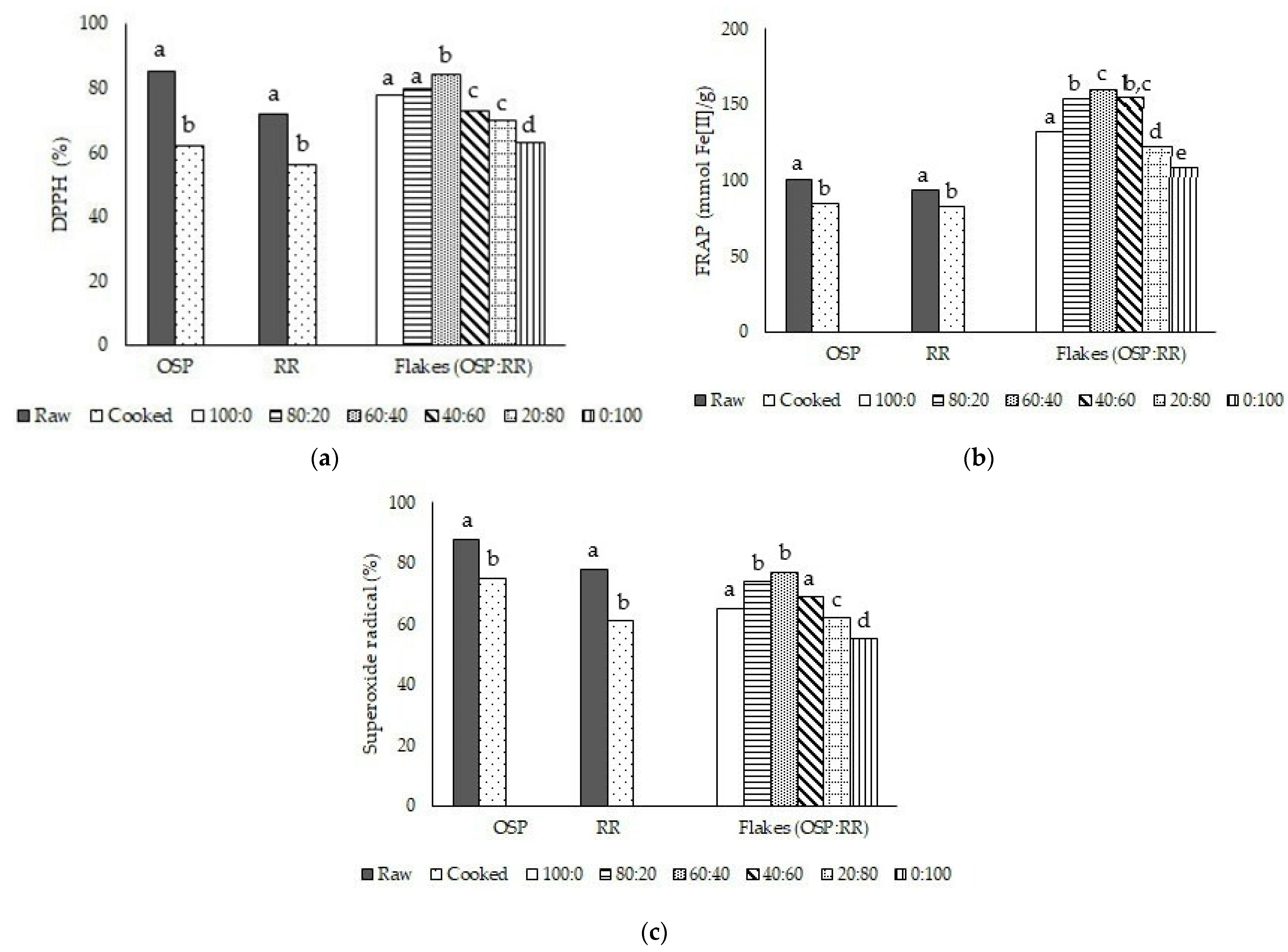
3.2. Antioxidant Activity of Raw and Cooked OSP and RR and Their Flake Products
3.3. Physicochemical Properties of OSP- and RR-Based Flake Products
3.4. Sensory Characteristics of Flakes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahidi, F.; Yeo, J. Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Y.; Xu, F.; Sun, X.; Bao, J.; Beta, T. Identification and Quantification of Phenolic Acids and Anthocyanins as Antioxidants in Bran, Embryo and Endosperm of White, Red and Black Rice Kernels (Oryza Sativa L.). J. Cereal Sci. 2014, 59, 211–218. [Google Scholar] [CrossRef]
- Bhat, F.M.; Sommano, S.R.; Riar, C.S.; Seesuriyachan, P.; Chaiyaso, T.; Prom-u-Thai, C. Status of Bioactive Compounds from Bran of Pigmented Traditional Rice Varieties and Their Scope in Production of Medicinal Food with Nutraceutical Importance. Agronomy 2020, 10, 1817. [Google Scholar] [CrossRef]
- Rathna Priya, T.S.; Eliazer Nelson, A.R.L.; Ravichandran, K.; Antony, U. Nutritional and Functional Properties of Coloured Rice Varieties of South India: A Review. J. Ethn. Food 2019, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Upanan, S.; Yodkeeree, S.; Thippraphan, P.; Punfa, W.; Wongpoomchai, R.; Limtrakul, P. The Proanthocyanidin-Rich Fraction Obtained from Red Rice Germ and Bran Extract Induces HepG2 Hepatocellular Carcinoma Cell Apoptosis. Molecules 2019, 24, 813. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.J.; Kim, S.Y.; Park, Y.J.; Lim, S.-H.; Ha, S.-H.; Park, S.U.; Lee, B.; Kim, J.K. Metabolite Profiling Reveals Distinct Modulation of Complex Metabolic Networks in Non-Pigmented, Black, and Red Rice (Oryza Sativa L.) Cultivars. Metabolites 2021, 11, 367. [Google Scholar] [CrossRef]
- Samyor, D.; Deka, S.C.; Das, A.B. Effect of Extrusion Conditions on the Physicochemical and Phytochemical Properties of Red Rice and Passion Fruit Powder Based Extrudates. J. Food Sci. Technol. 2018, 55, 5003–5013. [Google Scholar] [CrossRef]
- Müller, C.P.; Hoffmann, J.F.; Ferreira, C.D.; Diehl, G.W.; Rossi, R.C.; Ziegler, V. Effect of Germination on Nutritional and Bioactive Properties of Red Rice Grains and Its Application in Cupcake Production. Int. J. Gastron. Food Sci. 2021, 25, 100379. [Google Scholar] [CrossRef]
- Mbanjo, E.G.N.; Kretzschmar, T.; Jones, H.; Ereful, N.; Blanchard, C.; Boyd, L.A.; Sreenivasulu, N. The Genetic Basis and Nutritional Benefits of Pigmented Rice Grain. Front. Genet. 2020, 11, 229. [Google Scholar] [CrossRef] [Green Version]
- Cappa, C.; Laureati, M.; Casiraghi, M.C.; Erba, D.; Vezzani, M.; Lucisano, M.; Alamprese, C. Effects of Red Rice or Buckwheat Addition on Nutritional, Technological, and Sensory Quality of Potato-Based Pasta. Foods 2021, 10, 91. [Google Scholar] [CrossRef]
- Kraithong, S.; Lee, S.; Rawdkuen, S. Effect of Red Jasmine Rice Replacement on Rice Flour Properties and Noodle Qualities. Food Sci. Biotechnol. 2019, 28, 25–34. [Google Scholar] [CrossRef]
- Meza, S.L.R.; Sinnecker, P.; Schmiele, M.; Massaretto, I.L.; Chang, Y.K.; Marquez, U.M.L. Production of Innovative Gluten-Free Breakfast Cereals Based on Red and Black Rice by Extrusion Processing Technology. J. Food Sci. Technol. 2019, 56, 4855–4866. [Google Scholar] [CrossRef] [PubMed]
- Deeseenthum, S.; Luang-In, V.; Chunchom, S. Characteristics of Thai Pigmented Rice Milk Kefirs with Potential as Antioxidant and Anti-Inflammatory Foods. Pharmacogn. J. 2017, 10, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Sulistyaningtyas, A.R.; Lunggani, A.T.; Kusdiyantini, E. Kefir Produced from Red Rice Milk by Lactobacillus Bulgaricus and Candida Kefir Starter. IOP Conf. Ser. Earth Environ. Sci. 2019, 292, 012038. [Google Scholar] [CrossRef] [Green Version]
- Kourouma, V.; Mu, T.-H.; Zhang, M.; Sun, H.-N. Effects of Cooking Process on Carotenoids and Antioxidant Activity of Orange-Fleshed Sweet Potato. LWT 2019, 104, 134–141. [Google Scholar] [CrossRef]
- Sebben, J.A.; da Silveira Espindola, J.; Ranzan, L.; Fernandes de Moura, N.; Trierweiler, L.F.; Trierweiler, J.O. Development of a Quantitative Approach Using Raman Spectroscopy for Carotenoids Determination in Processed Sweet Potato. Food Chem. 2018, 245, 1224–1231. [Google Scholar] [CrossRef]
- Alam, M.K.; Sams, S.; Rana, Z.H.; Akhtaruzzaman, M.; Islam, S.N. Minerals, Vitamin C, and Effect of Thermal Processing on Carotenoids Composition in Nine Varieties Orange-Fleshed Sweet Potato (Ipomoea Batatas L.). J. Food Compos. Anal. 2020, 92, 103582. [Google Scholar] [CrossRef]
- Low, J.W.; Mwanga, R.O.M.; Andrade, M.; Carey, E.; Ball, A.-M. Tackling Vitamin A Deficiency with Biofortified Sweetpotato in Sub-Saharan Africa. Glob. Food Secur. 2017, 14, 23–30. [Google Scholar] [CrossRef]
- Laryea, D.; Wireko-Manu, F.D.; Oduro, I. Formulation and Characterization of Sweetpotato-Based Complementary Food. Cogent Food Agric. 2018, 4, 1517426. [Google Scholar] [CrossRef]
- Neela, S.; Fanta, S.W. Review on Nutritional Composition of Orange-fleshed Sweet Potato and Its Role in Management of Vitamin A Deficiency. Food Sci. Nutr. 2019, 7, 1920–1945. [Google Scholar] [CrossRef] [Green Version]
- Olagunju, A.I.; Omoba, O.S.; Awolu, O.O.; Rotowa, K.O.; Oloniyo, R.O.; Ogunowo, O.C. Physiochemical, Antioxidant Properties and Carotenoid Retention/Loss of Culinary Processed Orange Fleshed Sweet Potato. J. Culin. Sci. Technol. 2020, 19, 1–20. [Google Scholar] [CrossRef]
- Pan, Z.; Sun, Y.; Zhang, F.; Guo, X.; Liao, Z. Effect of Thermal Processing on Carotenoids and Folate Changes in Six Varieties of Sweet Potato (Ipomoes Batata L.). Foods 2019, 8, 215. [Google Scholar] [CrossRef] [Green Version]
- Klepacka, J.; Najda, A. Effect of Commercial Processing on Polyphenols and Antioxidant Activity of Buckwheat Seeds. Int. J. Food Sci. Technol. 2021, 56, 661–670. [Google Scholar] [CrossRef]
- Santos, D.I.; Saraiva, J.M.A.; Vicente, A.A.; Moldão-Martins, M. Methods for Determining Bioavailability and Bioaccessibility of Bioactive Compounds and Nutrients. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 23–54. ISBN 978-0-12-814174-8. [Google Scholar]
- Radix, A.P.; Jati, I.; Nohr, D.; Biesalski, H.K. Nutrients and Antioxidant Properties of Indonesian Underutilized Colored Rice. Nutr. Food Sci. 2014, 44, 193–203. [Google Scholar] [CrossRef]
- Astadi, I.R.; Astuti, M.; Santoso, U.; Nugraheni, P.S. In Vitro Antioxidant Activity of Anthocyanins of Black Soybean Seed Coat in Human Low Density Lipoprotein (LDL). Food Chem. 2009, 112, 659–663. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 00, F1–F2. [Google Scholar] [CrossRef]
- Gautam, B.; Vadivel, V.; Stuetz, W.; Biesalski, H.K. Bioactive Compounds Extracted from Indian Wild Legume Seeds: Antioxidant and Type II Diabetes-Related Enzyme Inhibition Properties. Int. J. Food Sci. Nutr. 2012, 63, 242–245. [Google Scholar] [CrossRef]
- Vadivel, V.; Biesalski, H.K. Contribution of Phenolic Compounds to the Antioxidant Potential and Type II Diabetes Related Enzyme Inhibition Properties of Pongamia Pinnata L. Pierre Seeds. Process Biochem. 2011, 46, 1973–1980. [Google Scholar] [CrossRef]
- Obi, O.F.; Ezeoha, S.L.; Egwu, C.O. Evaluation of Air Oven Moisture Content Determination Procedures for Pearl Millet (Pennisetum Glaucum L.). Int. J. Food Prop. 2016, 19, 454–466. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.-Z.; Zheng, J.-M.; Li, X.; Xu, C.; Zhao, Q. Chemical Composition and Sensory Characteristics of Oat Flakes: A Comparative Study of Naked Oat Flakes from China and Hulled Oat Flakes from Western Countries. J. Cereal Sci. 2014, 60, 297–301. [Google Scholar] [CrossRef]
- Salawi, A.; Nazzal, S. The Rheological and Textural Characterization of Soluplus®/Vitamin E Composites. Int. J. Pharm. 2018, 546, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Karatas, N.; Sengul, M. Some Important Physicochemical and Bioactive Characteristics of the Main Apricot Cultivars from Turkey. Turk. J. Agric. For. 2020, 44, 651–661. [Google Scholar] [CrossRef]
- Luna-Guevara, M.L.; Luna-Guevara, J.J.; Hernández-Carranza, P.; Ruíz-Espinosa, H.; Ochoa-Velasco, C.E. Phenolic Compounds: A Good Choice Against Chronic Degenerative Diseases. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 59, pp. 79–108. ISBN 978-0-444-64179-3. [Google Scholar]
- Horvat, D.; Šimić, G.; Drezner, G.; Lalić, A.; Ledenčan, T.; Tucak, M.; Plavšić, H.; Andrić, L.; Zdunić, Z. Phenolic Acid Profiles and Antioxidant Activity of Major Cereal Crops. Antioxidants 2020, 9, 527. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Karbalaii, M.T.; Jaafar, H.Z.E.; Rahmat, A. Phytochemical Constituents, Antioxidant Activity, and Antiproliferative Properties of Black, Red, and Brown Rice Bran. Chem. Cent. J. 2018, 12, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, Y.; Ahmed, S.; Xu, Y.; Beta, T.; Zhu, Z.; Shao, Y.; Bao, J. Bound Phenolic Compounds and Antioxidant Properties of Whole Grain and Bran of White, Red and Black Rice. Food Chem. 2018, 240, 212–221. [Google Scholar] [CrossRef]
- Fracassetti, D.; Pozzoli, C.; Vitalini, S.; Tirelli, A.; Iriti, M. Impact of Cooking on Bioactive Compounds and Antioxidant Activity of Pigmented Rice Cultivars. Foods 2020, 9, 967. [Google Scholar] [CrossRef]
- Azeem, M.; Mu, T.-H.; Zhang, M. Influence of Particle Size Distribution of Orange-Fleshed Sweet Potato Flour on Dough Rheology and Simulated Gastrointestinal Digestion of Sweet Potato-Wheat Bread. LWT 2020, 131, 109690. [Google Scholar] [CrossRef]
- Ruttarattanamongkol, K.; Chittrakorn, S.; Weerawatanakorn, M.; Dangpium, N. Effect of Drying Conditions on Properties, Pigments and Antioxidant Activity Retentions of Pretreated Orange and Purple-Fleshed Sweet Potato Flours. J. Food Sci. Technol. 2016, 53, 1811–1822. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, V.; Ferreira, C.D.; Hoffmann, J.F.; Chaves, F.C.; Vanier, N.L.; de Oliveira, M.; Elias, M.C. Cooking Quality Properties and Free and Bound Phenolics Content of Brown, Black, and Red Rice Grains Stored at Different Temperatures for Six Months. Food Chem. 2018, 242, 427–434. [Google Scholar] [CrossRef]
- Sasongko, S.B.; Djaeni, M.; Utari, F.D. Kinetic of Anthocyanin Degradation in Roselle Extract Dried with Foaming Agent at Different Temperatures. Bull. Chem. React. Eng. Catal. 2019, 14, 320. [Google Scholar] [CrossRef]
- Zhu, F. Anthocyanins in Cereals: Composition and Health Effects. Food Res. Int. 2018, 109, 232–249. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J. An Overview of Carotenoids, Apocarotenoids, and Vitamin A in Agro-Food, Nutrition, Health, and Disease. Mol. Nutr. Food Res. 2019, 63, 1801045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trono, D. Carotenoids in Cereal Food Crops: Composition and Retention throughout Grain Storage and Food Processing. Plants 2019, 8, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oduro-Obeng, H.; Fu, B.X.; Beta, T. Influence of Cooking Duration on Carotenoids, Physical Properties and in Vitro Antioxidant Capacity of Pasta Prepared from Three Canadian Durum Wheat Cultivars. Food Chem. 2021, 363, 130016. [Google Scholar] [CrossRef] [PubMed]
- Melini, V.; Panfili, G.; Fratianni, A.; Acquistucci, R. Bioactive Compounds in Rice on Italian Market: Pigmented Varieties as a Source of Carotenoids, Total Phenolic Compounds and Anthocyanins, before and after Cooking. Food Chem. 2019, 277, 119–127. [Google Scholar] [CrossRef]
- Nartea, A.; Fanesi, B.; Falcone, P.M.; Pacetti, D.; Frega, N.G.; Lucci, P. Impact of Mild Oven Cooking Treatments on Carotenoids and Tocopherols of Cheddar and Depurple Cauliflower (Brassica Oleracea L. Var. Botrytis). Antioxidants 2021, 10, 196. [Google Scholar] [CrossRef]
- Tang, Y.; Cai, W.; Xu, B. Profiles of Phenolics, Carotenoids and Antioxidative Capacities of Thermal Processed White, Yellow, Orange and Purple Sweet Potatoes Grown in Guilin, China. Food Sci. Hum. Wellness 2015, 4, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Da Silva Timm, N.; Ramos, A.H.; Ferreira, C.D.; de Oliveira Rios, A.; Zambiazi, R.C.; de Oliveira, M. Influence of Germ Storage from Different Corn Genotypes on Technological Properties and Fatty Acid, Tocopherol, and Carotenoid Profiles of Oil. Eur. Food Res. Technol. 2021, 247, 1449–1460. [Google Scholar] [CrossRef]
- Bagchi, T.B.; Chattopadhyay, K.; Sivashankari, M.; Roy, S.; Kumar, A.; Biswas, T.; Pal, S. Effect of Different Processing Technologies on Phenolic Acids, Flavonoids and Other Antioxidants Content in Pigmented Rice. J. Cereal Sci. 2021, 100, 103263. [Google Scholar] [CrossRef]
- Călinoiu, L.; Vodnar, D. Thermal Processing for the Release of Phenolic Compounds from Wheat and Oat Bran. Biomolecules 2019, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Sitarek, P.; Merecz-Sadowska, A.; Kowalczyk, T.; Wieczfinska, J.; Zajdel, R.; Śliwiński, T. Potential Synergistic Action of Bioactive Compounds from Plant Extracts against Skin Infecting Microorganisms. Int. J. Mol. Sci. 2020, 21, 5105. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, Q.; Ferdinand, U.; Gong, X.; Qu, Y.; Gao, W.; Ivanistau, A.; Feng, B.; Liu, M. Isolation and Characterization of Starch from Light Yellow, Orange, and Purple Sweet Potatoes. Int. J. Biol. Macromol. 2020, 160, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Markus, J.E.R.; Ndiwa, A.S.S.; Oematan, S.S.; Mau, Y.S. Variations of Grain Physical Properties, Amylose and Anthocyanin of Upland Red Rice Cultivars from East Nusa Tenggara, Indonesia. Biodiversitas 2021, 22, 1345–1354. [Google Scholar] [CrossRef]
- Gates, F. Role of Heat Treatment in the Processing and Quality of Oat Flakes; University of Helsinki: Helsinki, Finland, 2007; ISBN 978-952-10-3907-2. [Google Scholar]
- Zhou, Y.; Dhital, S.; Zhao, C.; Ye, F.; Chen, J.; Zhao, G. Dietary Fiber-Gluten Protein Interaction in Wheat Flour Dough: Analysis, Consequences and Proposed Mechanisms. Food Hydrocoll. 2021, 111, 106203. [Google Scholar] [CrossRef]
- Trancoso-Reyes, N.; Ochoa-Martínez, L.A.; Bello-Pérez, L.A.; Morales-Castro, J.; Estévez-Santiago, R.; Olmedilla-Alonso, B. Effect of Pre-Treatment on Physicochemical and Structural Properties, and the Bioaccessibility of β-Carotene in Sweet Potato Flour. Food Chem. 2016, 200, 199–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akintayo, O.A.; Obadu, J.M.; Karim, O.R.; Balogun, M.A.; Kolawole, F.L.; Oyeyinka, S.A. Effect of Replacement of Cassava Starch with Sweet Potato Starch on the Functional, Pasting and Sensory Properties of Tapioca Grits. LWT 2019, 111, 513–519. [Google Scholar] [CrossRef]
- Niu, S.; Li, X.-Q.; Tang, R.; Zhang, G.; Li, X.; Cui, B.; Mikitzel, L.; Haroon, M. Starch Granule Sizes and Degradation in Sweet Potatoes during Storage. Postharvest Biol. Technol. 2019, 150, 137–147. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; van Camp, J.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant Activity, Total Phenolics and Flavonoids Contents: Should We Ban in Vitro Screening Methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
| OSP | RR | Proportions of OSP and RR in Flakes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Raw | Cooked | Raw | Cooked | 100:0 | 80:20 | 60:40 | 40:60 | 20:80 | 0:100 | |
| Phenolic (mg GAE/100 g DW) | 110.68 ± 18.3 a | 65.21± 7.3 b | 301.89 ± 24.86 a | 152.91 ± 28.92 b | 77.46 ± 8,28 a | 97.34 ± 8.79 b | 102.03 ± 11.65 c | 131.79 ± 10.93 d | 146.09 ± 15.64 e | 162.40 ± 21.54 f |
| Anthocyanin (mg/100 g DW) | nd | nd | 8,81± 0.05 a | 8.64± 0.08 a | nd | nd | 1.74 ± 0.06 c | 2.09 ± 0.05 d | 3.73 ± 0.07 e | 5.81 ± 0.04 f |
| β-carotene (µg/g) | 278.58 ± 31.5 a | 134.17± 17.2 b | 13.17 ± 2.62 a | 7.37 ± 0.5 b | 48,83 ± 3,31 a | 36.27 ± 3.01 b | 25.77 ± 3.45 c | 27.23 ± 2.72 d | 15.69 ± 2.21 e | 3.12 ± 0.66 f |
| α-carotene (µg/g) | 19.57 ± 1.8 a | 23.83 ± 1.6 b | 5.53 ± 1.4 a | 11.66 ± 1.5 b | 15.61 ± 1.44 a | 11.82 ± 3.11 b | 5.31 ± 1.59 c | 2.53 ± 0.87 d | nd | nd |
| β-cryptoxanthin (µg/g) | 4.83 ± 0.2 a | 4.48± 0.8 a | 3.67 ± 2.15 a | 3.96 ± 1.9 a | 2.64 ± 0,05 a | 2.81 ± 0.13 b | 2.97 ± 0.08 | 2.78 ± 0.12 c | 2.77 ± 0.25 c | 2.81 ± 0.16 b,c |
| Lutein (µg/g) | 3.77 ± 0.8 a | 3.81 ± 0.7 a | 2.16 ± 0.8 a | 1.82 ± 0.5 b | nd | nd | nd | nd | nd | nd |
| α-tocopherol (µg/g) | 13,23 ± 1.1 a | 15.11 ± 0,5 b | 34.08 ± 2.2 a | 57.65 ± 2.1 b | 4.58 ± 0.73 a | 7.34 ± 1.49 b | 10.51 ± 1.27 c | 12.45 ± 1.21 c | 16.82 ± 0.52 e | 18.31 ± 0.77 f |
| γ-tocopherol (µg/g) | 2.40 ± 0,2 a | 5.38± 0.05 b | 29.27 ± 2.4 a | 40,11 ± 1.8 b | nd | nd | 3.38 ± 1.22 c | 6.71 ± 1.19 d | 8.06 ± 0.98 e | 12.15 ± 0.73 f |
| Proportions of OSP and RR in Flakes | ||||||
|---|---|---|---|---|---|---|
| 100:0 | 80:20 | 60:40 | 40:60 | 20:80 | 0:100 | |
| Moisture content (%) | 5.71 ± 0,07 a | 5.31 ± 0.10 b | 5.09 ± 0.06 c | 4.87 ± 0.01 d | 4.43 ± 0.03 e | 4.25 ± 0.03 f |
| Dietary fiber (%) | 9.47 ± 0,01 a | 9.9 ± 0.02 b | 10.9 ± 0.05 c | 11.63 ± 0.34 d | 12.73 ± 0.26 e | 13.86 ± 0.73 f |
| Water absorption index | 1.69 ± 0,03 a | 1.14 ± 0.02 b | 1.06 ± 0.03 c | 0.96 ± 0.03 d | 1.09 ± 0.03 b,c | 1.12 ± 0.02 b,c |
| Fracturability | 8.48 ± 0,09 a | 5.35 ± 0.85 b | 3.34 ± 0.34 c | 2.27 ± 0.04 d | 3.17 ± 0.09 e | 4.64 ± 0.12 f |
| Crispness | 3.9 ± 0,03 a | 2.4 ± 0.02 b | 1.5 ± 0.02 c | 1.9 ± 0.03 d | 3.21 ± 0.05 e | 3.7 ± 0.03 f |
| Proportions of OSP and RR in Flakes | ||||||
|---|---|---|---|---|---|---|
| 100:0 | 80:20 | 60:40 | 40:60 | 20:80 | 0:100 | |
| L* | 44.0 ± 0.1 | 47.3 ± 0.2 | 51.5 ± 0.1 | 52.7 ± 0.4 | 51.8 ± 0.2 | 51.8 ± 0.7 |
| a* | 8.2 ± 0.3 | 8,5 ± 0.3 | 8.9 ± 0.4 | 9.4 ± 0.4 | 10.2 ± 0.6 | 10.8 ± 0.4 |
| b* | 16.5 ± 0.3 | 14.7 ± 0.2 | 13.4 ± 0.2 | 10.3 ± 0.2 | 9.0 ± 0.4 | 5.9 ± 0.4 |
| oh | 63.574 | 59.9622 | 56.4087 | 47.6158 | 41.4237 | 28.6476 |
| C | 18.47 | 16.9685 | 16.0703 | 13.898 | 13.56 | 12.3145 |
| Proportions of OSP and RR in Flakes | ||||||
|---|---|---|---|---|---|---|
| 100:0 | 80:20 | 60:40 | 40:60 | 20:80 | 0:100 | |
| Color | 3.35 ± 1.42 a | 4.40 ± 1.22 b | 5.14 ± 1.12 c | 4.93 ± 1.21 c | 4.89 ± 1.30 c | 4.30 ± 1.12 b |
| Taste | 4.43 ± 1.34 a | 4.69 ± 1.28 ab | 5.08 ± 1.18 c | 5.09 ± 1.20 c | 5.05 ± 1.26 bc | 4.72 ± 1.29 abc |
| Crispness | 4.76 ± 1.16 b | 4.85 ± 1.04 b | 4.76 ± 1.40 b | 4.08 ± 1.17 a | 4.96 ± 1.07 b | 5.00 ± 1.04 b |
| Mouthfeel | 5.41 ± 0.91 d | 5.04 ± 1.18 c | 4.84 ± 1.31 bc | 3.91 ± 1.59 a | 4.66 ± 1.32 b | 5.05 ± 1.03 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jati, I.R.A.P.; Darmoatmodjo, L.M.Y.D.; Suseno, T.I.P.; Ristiarini, S.; Wibowo, C. Effect of Processing on Bioactive Compounds, Antioxidant Activity, Physicochemical, and Sensory Properties of Orange Sweet Potato, Red Rice, and Their Application for Flake Products. Plants 2022, 11, 440. https://doi.org/10.3390/plants11030440
Jati IRAP, Darmoatmodjo LMYD, Suseno TIP, Ristiarini S, Wibowo C. Effect of Processing on Bioactive Compounds, Antioxidant Activity, Physicochemical, and Sensory Properties of Orange Sweet Potato, Red Rice, and Their Application for Flake Products. Plants. 2022; 11(3):440. https://doi.org/10.3390/plants11030440
Chicago/Turabian StyleJati, Ignasius Radix A. P., Laurensia M. Y. D. Darmoatmodjo, Thomas I. P. Suseno, Susana Ristiarini, and Condro Wibowo. 2022. "Effect of Processing on Bioactive Compounds, Antioxidant Activity, Physicochemical, and Sensory Properties of Orange Sweet Potato, Red Rice, and Their Application for Flake Products" Plants 11, no. 3: 440. https://doi.org/10.3390/plants11030440
APA StyleJati, I. R. A. P., Darmoatmodjo, L. M. Y. D., Suseno, T. I. P., Ristiarini, S., & Wibowo, C. (2022). Effect of Processing on Bioactive Compounds, Antioxidant Activity, Physicochemical, and Sensory Properties of Orange Sweet Potato, Red Rice, and Their Application for Flake Products. Plants, 11(3), 440. https://doi.org/10.3390/plants11030440






