Classical Morphometrics in V. arvensis and V. kitaibeliana (Viola sect. Melanium) Reveals Intraspecific Variation with Implications for Species Delimitation: Inferences from a Case Study in Central Italy
Abstract
1. Introduction
2. Results
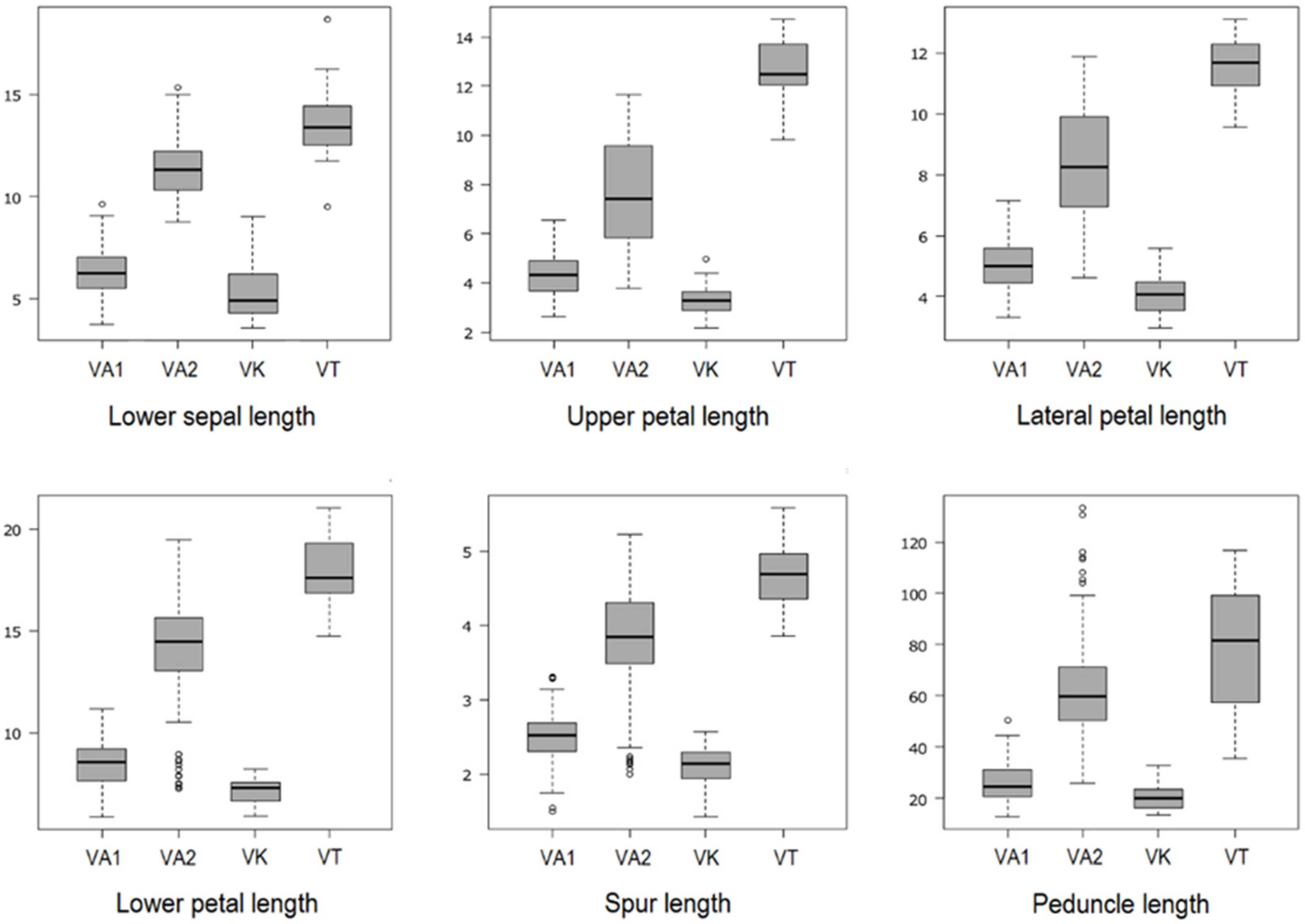
2.1. Descriptive Statistics and One-Way ANOVA
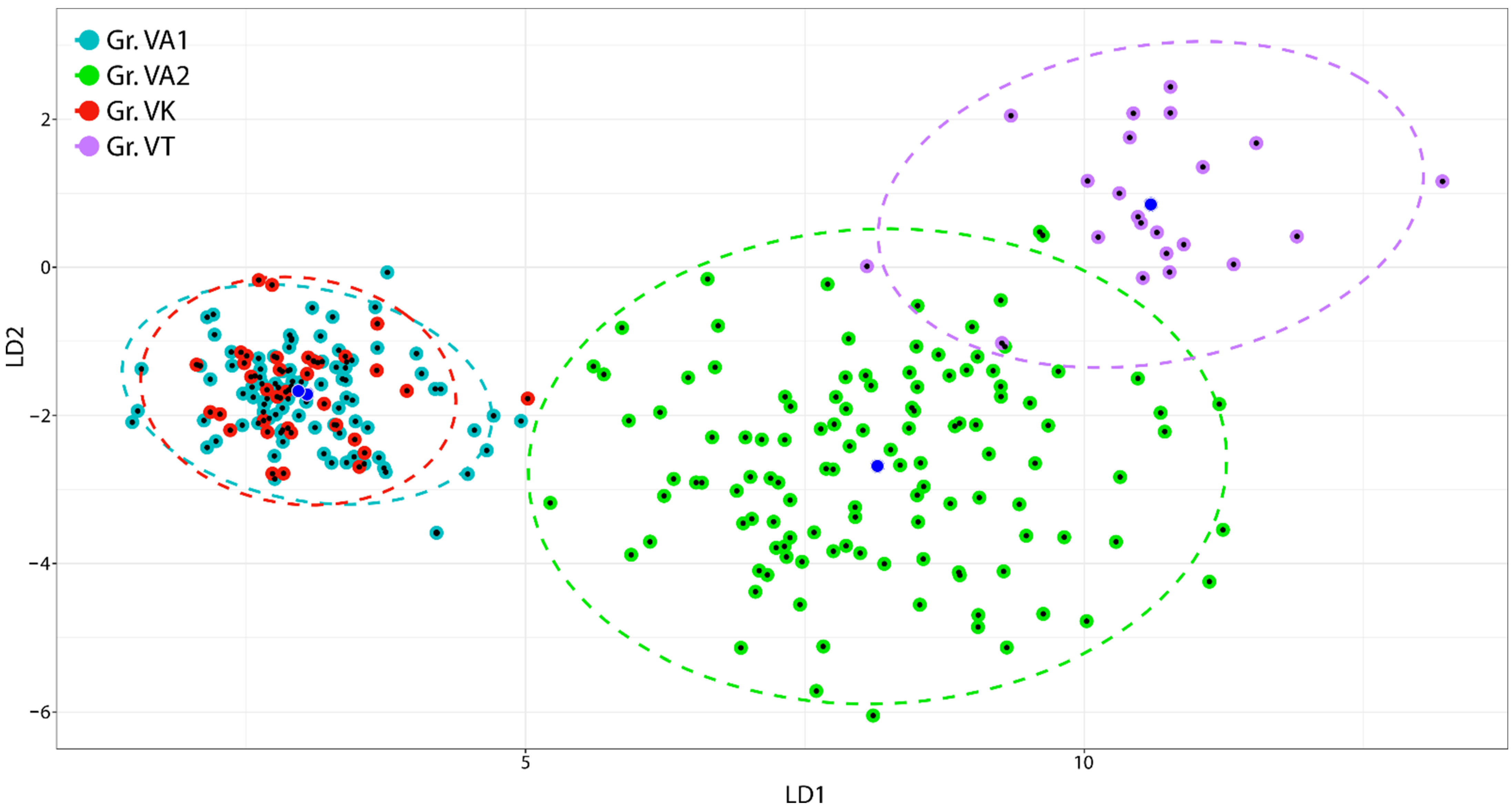
2.2. Multivariate Analysis
3. Discussion
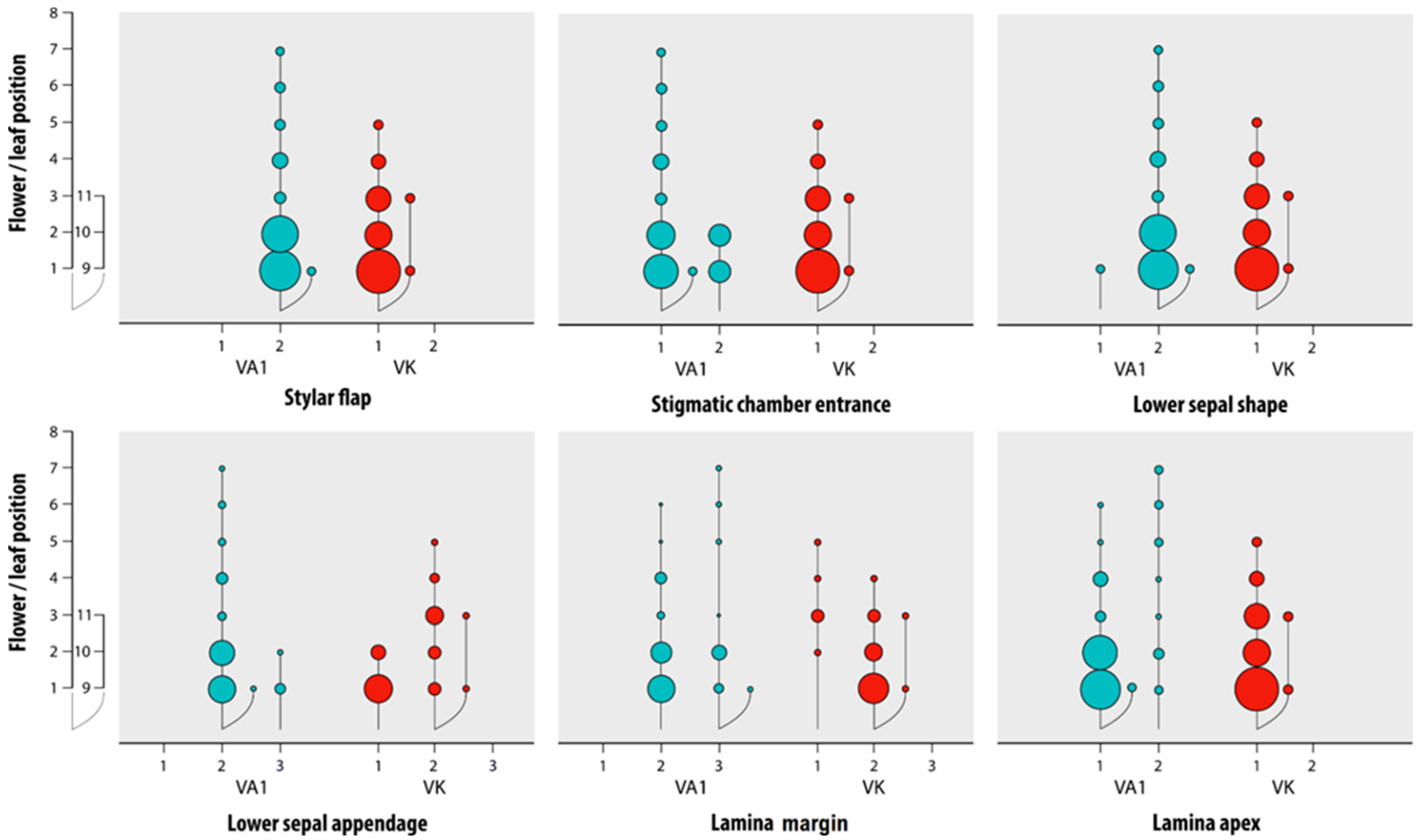
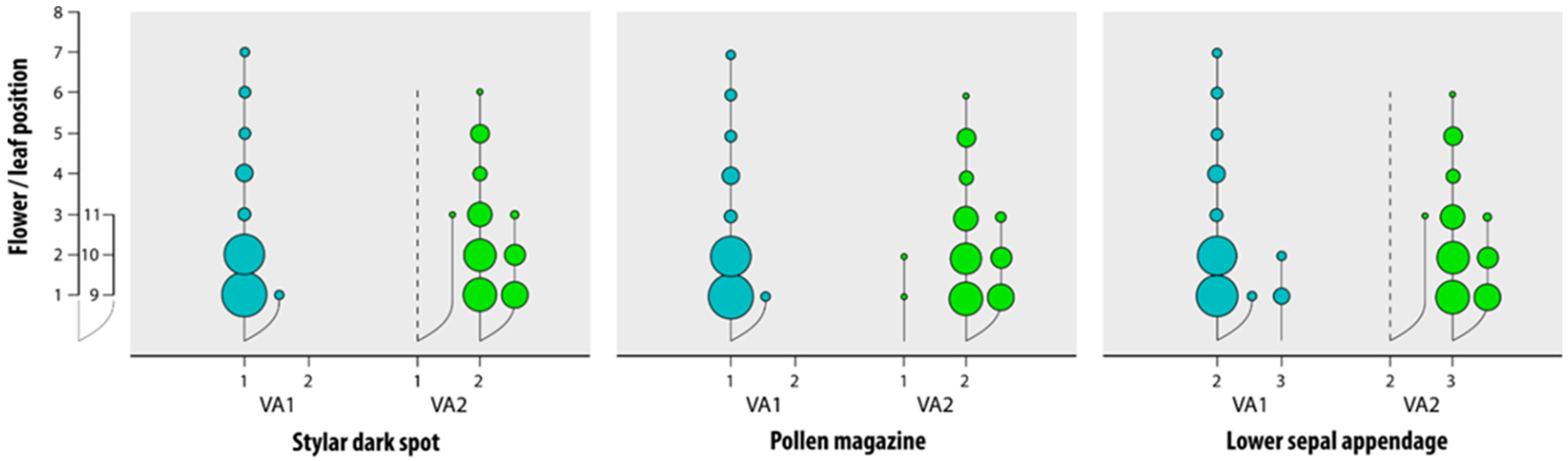
3.1. Reliability of the Morphological Characters
3.2. Explaining Variations in V. arvensis and Distinction of an Eco-Phenotype
4. Materials and Methods
4.1. Sampling Sites and Field Collection
4.2. Morphometry and Numerical Analyses
4.2.1. Characters Scored for Classical Morphometric Analyses
4.2.2. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaplan, D.R. The science of plant morphology: Definition, history, and role in modern biology. Am. J. Bot. 2001, 88, 1711–1741. [Google Scholar] [CrossRef]
- Henderson, A. Traditional morphometrics in plant systematics and its role in palm systematics. Bot. J. Linn. Soc. 2006, 151, 103–111. [Google Scholar] [CrossRef]
- Salmeri, C. Plant morphology: Outdated or advanced discipline in modern plant sciences? Flora Mediterr. 2019, 29, 163–180. [Google Scholar] [CrossRef]
- Scoppola, A.; Cancellieri, L. A comparative morphometric study of genus Gastridium P. Beauv. (Poaceae) and its implica-tions for species delimitation. Nord. J. Bot. 2019, 37, 1–10. [Google Scholar] [CrossRef]
- Kuta, E.; Bohdanowicz, J.; Slomka, A.; Pilarska, M.; Bothe, H. Floral structure and pollen morphology of two zinc violets (Viola lutea ssp. calaminaria and V. lutea ssp. westfalica) indicate their taxonomic affinity to Viola lutea. Plant Syst. Evol. 2012, 298, 445–455. [Google Scholar] [CrossRef]
- Kuta, E.; Jezdrzejczyk-Korycinska, M.; Cieslak, E.; Rostanski, A.; Szczepaniak, M.; Migdalek, G.; Wasowicz, P.; Suda, J.; Combik, M.; Slomka, A. Morphological versus genetic diversity of Viola reichenbachiana and V. riviniana (sect. Viola, Violace-ae) from soils differing in heavy metal content. Plant Biol. 2014, 16, 924–934. [Google Scholar] [CrossRef]
- Scoppola, A.; Lattanzi, E. Viola section Melanium (Violaceae) in Italy. New data on morphology of Viola tricolor-Group. Webbia 2012, 67, 47–64. [Google Scholar] [CrossRef]
- Slomka, A.; Godzik, B.; Szarek-Lukaszewska, G.; Shuka, L.; Hoef-Emden, K.; Bothe, H. Albanian violets of the section Mela-nium, their morphological variability, genetic similarity and their adaptations to serpentine or chalk soils. J. Plant Physiol. 2015, 174, 110–123. [Google Scholar] [CrossRef]
- Tomovic, G.; Niketic, M.; Lazarevic, M.; Melovski, L. Taxonomic reassessment of Viola aetolica and Viola elegantula (V. sect. Melanium, Violaceae), with descriptions of two new species from the Balkan Peninsula. Phytotaxa 2016, 253, 237–265. [Google Scholar] [CrossRef]
- Scoppola, A.; Magrini, S. Comparative palynology and seed morphology in annual pansies (Viola Sect. Melanium, Violaceae): Implications for species delimitation. Plant Biosyst. 2019, 153, 883–899. [Google Scholar] [CrossRef]
- Cennamo, P.E.; Del Guacchio, E.; Jury, S.L.; Caputo, P. Molecular markers in Viola L. subsect. Viola: Application and taxo-nomic implications for the identification of dubious herbarium specimens. Plant Biosyst. 2011, 145, 306–323. [Google Scholar] [CrossRef]
- Mereda, P., Jr.; Hodálová, I.; Mártonfi, P.; Kucera, J.; Lihová, J. Intraspecific Variation in Viola suavis in Europe: Parallel Evo-lution of White-flowered Morphotypes. Ann. Bot. 2008, 102, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Marcussen, T.; Heier, L.; Brysting, A.K.; Oxelman, B.; Jakobsen, K.S. From gene trees to a dated allopolyploid network: In-sights from the angiosperm genus Viola (Violaceae). Syst. Biol. 2015, 64, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Erben, M. Cytotaxonomische Untersuchungen an südosteuropäischen Viola-Arten der Sektion Melanium. Mitt. Bot. Staats München 1985, 21, 339–740. [Google Scholar]
- Clausen, J. Study on the Collective Species Viola tricolor L. (Preliminary Notes). Bot. Tidsskr. 1921, 37, 205–220. [Google Scholar]
- Sell, P.; Murrell, G. Flora of Great Britain and Ireland; Cambridge-University-Press: Cambridge, UK, 2018; Volume 1, pp. 595–610. [Google Scholar]
- Pignatti, S. Flora D’Italia; Edagricole-New Business Media: Bologna, Italy, 2017; Volume 2, pp. 366–391. [Google Scholar]
- Valentine, D.H.; Merxmüller, H.; Schmidt, A. Viola. In Flora Europaea; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1968; Volume 2, pp. 270–282. [Google Scholar]
- Clausen, J.; Channell, R.; Nur, U. Viola rafinesquii, the Only Melanium Violet Native to North America. Rhodora 1964, 66, 32–46. Available online: www.jstor.org/stable/23306571 (accessed on 22 April 2020).
- Shinners, L.H. Viola rafinesquii: Nomenclature and native status. Rhodora 1961, 63, 327–335. [Google Scholar]
- Plants of the World Online (POWO). Facilitated by the Royal Botanic Gardens, Kew. Available online: https://www.plantsoftheworldonline.org (accessed on 26 May 2020).
- Slomka, A.; Zabicka, J.; Shuka, L.; Bohdanowicz, J.; Kuta, E. Lack of correlation between pollen aperture number and envi-ronmental factors in pansies (Viola L., sect. Melanium Ging.)—Pollen heteromorphism re-examined. Plant Biol. 2018, 20, 555–562. [Google Scholar] [CrossRef]
- Erben, M. The Significance of Hybridization on the Forming of Species in the Genus Viola. Bocconea 1996, 5, 3–118. Available online: https://www.herbmedit.org/bocconea/5-113.pdf (accessed on 22 April 2020).
- Yousefi, N.; Saeidi Mehrvarz, S.; Marcussen, T. Taxonomy of Viola sect. Melanium (Violaceae) in Iran. Ot Sist. Bot. Dergisi 2012, 19, 35–43. [Google Scholar]
- Yousefi, N.; Saeidi Mehrvarz, S.; Marcussen, T. Anatomical studies on selected species of Viola (Violaceae). Nord. J. Bot. 2012, 30, 461–469. [Google Scholar] [CrossRef]
- Magrini, S.; Scoppola, A. Further studies in Viola Sect. Melanium (Violaceae). Identity and typification of Viola nana and V. henriquesii, two neglected European Atlantic taxa. Phytotaxa 2015, 230, 259–266. [Google Scholar] [CrossRef]
- Shahrestani, M.M.; Marcussen, T.; Mehrvarz, S.S.; Yousefi, N. Morphological and phylogenetic relationships within Viola sect. Sclerosium (Violaceae) in Iran. Flora 2015, 215, 67–74. [Google Scholar] [CrossRef]
- Düsen, O.; Süleyman Göktürk, R.; Kaya, E.; Sarpkaya, U.; Gürcan, B. Morphological and molecular determination of a new Viola species (Violaceae) from Turkey. Phytotaxa 2018, 369, 37–46. [Google Scholar] [CrossRef]
- Krause, S.; Kadereit, J.W. Identity of the Calcarata species complex in Viola sect. Melanium (Violaceae). Willdenowia 2020, 50, 195–206. [Google Scholar] [CrossRef]
- Clausen, J. Genetical and cytological investigations on Viola tricolor L. and V. arvensis Murr. Hereditas 1926, 8, 1–156. [Google Scholar] [CrossRef]
- Pettet, A. Studies on British pansies. II. The Status of Some Intermediates between Viola tricolor L. and V. arvensis Murr. Wat-sonia 1964, 6, 51–69. Available online: http://archive.bsbi.org.uk/Wats6p51.pdf (accessed on 22 April 2020).
- Coode, M.J.E.; Cullen, J. Viola L. In Flora of Turkey and the East Aegean Islands; Davis, P.H., Ed.; University Press: Edinburgh, UK, 1965; pp. 525–529. [Google Scholar]
- Krahulcová, A.; Krahulec, F.; Kirschner, J. Introgressive hybridization between a native and an introduced species: Viola lutea subsp. sudetica versus V. tricolor. Folia Geobot. 1996, 31, 219–244. [Google Scholar] [CrossRef]
- Meyer, F.K. Beiträge zur Flora von Albanien. Haussknechtia Beih. 2011, 15, 220. [Google Scholar]
- Porter, M.; Foley, M. Violas of Britain and Ireland; Handbook 17; Botanical Society of Britain & Ireland: Bristol, UK, 2017; pp. 1–157. [Google Scholar]
- Nauenburg, J.D. Taxonomie und Korrekturen zur Nomenklaturvon Viola tricolor s.l. (Violaceae) in Mitteleuropa. Willdenowia 1991, 21, 51–56. [Google Scholar]
- GBIF Secretariat. GBIF Backbone Taxonomy. Checklist Dataset. Available online: https://doi.org/10.15468/39omei (accessed on 27 July 2021).
- Vollrath, K.H. Viola in Nordostbayern. RegnitzFlora; Verein zur Erforschung der Flora des Regnitzgebietes e. V.: Eckental, Germany, 2011; Volume 4, pp. 65–75. [Google Scholar]
- Gams, H. Viola L. In Illustrierte Flora von Mitteleuropa; Hegi, G., Ed.; Lehmanns: Munchen, Germany, 1926; Volume 1, pp. 586–656. [Google Scholar]
- Marcussen, T.; Karlsson, T. Violaceae. In Flora Nordica; Jonsell, B., Karlsson, T., Eds.; Royal Swedish Academy Sciences: Stock-holm, Sweden, 2010; Volume 6, pp. 12–52. [Google Scholar]
- Schmidt, A. Chromasomenzahlen sudeuropaischer Viola-Arten der Sektion Melanium. Flora Allg. bot. Ztg. 1964, 154, 158–162. [Google Scholar] [CrossRef]
- Magrini, S.; Scoppola, A. Cytological status of Viola kitaibeliana (Section Melanium, Violaceae) in Europe. Phytotaxa 2015, 238, 288–292. [Google Scholar] [CrossRef]
- Muñoz Garmendia, F.; Montserrat, P.; Laínz, M.; Aldasoro, J.J. Violaceae. In Flora Iberica, 2nd ed.; Castroviejo, S., Aedo, C., Cirujano, S., Laínz, M., Montserrat, P., Morales, R., Muñoz Garmendia, F., Navarro, C., Paiva, J., Soriano, C., Eds.; Real Jardín Botánico: Madrid, Spain, 1993; Volume 3, pp. 276–317. [Google Scholar]
- von Raab-Straube, E.; Henning, T. Violaceae. In Euro + Med Plantbase—The Information Resource for Euro-Mediterranean Plant Diversity; 2018; Available online: https://www.emplantbase.org/home.html (accessed on 22 April 2020).
- de Halácsy, E. Conspectus Florae Graecae; G. Engelmann: Lipsia, Germany, 1900; Volume 1, p. 145. [Google Scholar]
- Becker, W. Systematische Behandlung der Viola arvensis s.l. auf Grundlage unserer phylogenetischen Kenntnisse. Mitth. Thüring. Bot. Vereins 1904, 19, 42. [Google Scholar]
- Degenhardt, R.F.; Spaner, D.; Harker, K.N.; Raatz, L.L.; Hall, L.M. Plasticity, life cycle and interference potential of field vi-olet (Viola arvensis Murr.) in direct-seeded wheat and canola in central Alberta. Can. J. Plant Sci. 2005, 85, 271–284. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D.; Fotiadis, S. Occurrence of European field pansy (Viola arvensis) in Orestiada, Greece. Hell. Plant Prot. J. 2014, 7, 25–29. [Google Scholar]
- The Plant List 2013. Version 1.1. Available online: http://www.theplantlist.org (accessed on 1 January 2020).
- IPNI 2020. International Plant Names Index. The Royal Botanic Gardens, Kew, Harvard University Herbaria & Libraries and Australian National Botanic Gardens. Available online: http://www.ipni.org (accessed on 4 May 2020).
- Tropicos.org, Missouri Botanical Garden. Available online: http://www.tropicos.org (accessed on 10 April 2019).
- Peruzzi, L.; Viciani, D.; Bedini, G. Contributi per una flora vascolare di Toscana. 1 (1–85). Atti. Soc. Tosc. Sci. Nat. Mem. 2009, 116, 33–44. [Google Scholar]
- Raus, T. Viola L. In Mountain Flora of Greece; Strid, A., Ed.; Cambridge University Press: Cambridge, UK, 1986; Volume 1, pp. 608–640. [Google Scholar]
- Blaxland, K. A new species of Viola (Violaceae) from southwest Turkey. Bot. J. Linnean Soc. 2004, 145, 505–509. [Google Scholar] [CrossRef][Green Version]
- Stace, C. New Flora of the British Isles; Cambridge University Press: Suffolk, UK, 1991; pp. 268–273. [Google Scholar]
- Clausen, J. Study on the Collective Species Viola tricolor L. II. Bot. Tidsskr. 1922, 37, 363–416. [Google Scholar]
- Kristofferson, K.B. Crossings in Melanium-Violets. Hereditas 1923, 4, 251–289. [Google Scholar] [CrossRef]
- Wittrock, V.B.; Viola-Studier, I. De Viola tricolore (L.) aliisque speciebus sectionis Melänii bservationes morphologicae, biologicae, systematicae. Acta Horti Berg. 1897, 2, 3–128. [Google Scholar]
- Nauenburg, J.D. Eine neue Viola arvensis-Sippe aus Mitteleuropa (mit einem Bestimmungs-Schlüssel für die Artengruppen Viola tricolor/V. lutea). Bauhinia 1990, 9, 233–244. Available online: https://www.zobodat.at/pdf/Bauhinia_9_0233-0244.pdf (accessed on 4 January 2020).
- Davis, P.H.; Heywood, V.H. Principles of Angiosperm Taxonomy; Van Nostrand: Princeton, NJ, USA, 1963. [Google Scholar]
- Lauber, K.; Wagner, G. Flora Helvetica, Flore Illustrée de Suisse, 2nd ed.; Belin: Paris, France, 2007; pp. 318–319. [Google Scholar]
- Tison, J.M.; de Foucault, B. Flora Gallica. Flore de France; Tison, J.M., de Foucault, B., Eds.; Editions Biotope: Mèze, France, 2014; pp. 1071–1077. [Google Scholar]
- Doohan, D.J.; Monaco, T.J. The biology of Canadian weeds. 99. Viola arvensis Murr. Can. J. Plant Sci. 1992, 72, 187–201. [Google Scholar] [CrossRef]
- Thiers, B.; Continuously Updated. Continuously Updated. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. Available online: http://sweetgum.nybg.org.ih (accessed on 10 April 2019).
- Portal to the Flora of Italy. Available online: http://dryades.units.it/floritaly/index.php (accessed on 26 May 2020).
- Loos, G.H. Taxonomische Neukombinationen zur Flora Mittel- und Osteuropas, Insbesondere Nordrhein-Westfalens. Jahrb. Bochumer Bot. Ver. 2010, 1, 114–133. Available online: https://www.zobodat.at/pdf/Jahrb-Bochumer-Bot-Ver_1_0114-0133.pdf (accessed on 10 April 2019).
- Scoppola, A.; Ceoloni, C.; Gennaro, A.; Magrini, S. Violaceae. In IAPT/IOPB Chromosome Data 17: E26-E27; Marhold, K., Ed.; Wiley: New York, NY, USA, 2014; Volume 63, p. 1155. Available online: https://doi.org/10.12705/635.34 (accessed on 22 April 2020).
- Werner, K. Viola kitaibeliana Schultes auf der Schwellenburg bei Erfurt—Ein Neufund für die DDR. Hercynia 1988, 25, 142–143. [Google Scholar]
- Amat, R. À propos de Viola kitaibeliana Schultes en Haute-Provence. Monde Plantes 1998, 462, 24–26. [Google Scholar]
- Stimper, R.; Korsch, H. Chromosomenzahlen von Farn-und Samenpflanzen aus Deutschland 5. Viola kitaibeliana. Kochia 2011, 5, 37. [Google Scholar]
- Orsenigo, S.; Fenu, G.; Gargano, D.; Montagnani, C.; Abeli, T.; Alessandrini, A.; Bacchetta, G.; Bartolucci, F.; Carta, A.; Ca-stello, M.; et al. Red list of threatened vascular plants in Italy. Plant Biosyst. 2021, 155, 310–335. [Google Scholar] [CrossRef]
- IUCN. IUCN Red List Categories and Criteria: Version 3.1, 2nd ed.; IUCN Species Survival Commission: Gland, Switzerland; Cambridge, UK, 2012; pp. 1–32. [Google Scholar]
- Clapham, A.R.; Tutin, T.G.; Moore, D.M. Flora of the British Isles, 3rd ed.; Cambridge University Press: Cambridge, UK, 1987; pp. 107–112. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org (accessed on 13 November 2019).
- Mardia, K.V.; Kent, J.T.; Bibby, J.M. Multivariate Analysis; Academic Press: London, UK, 1979; p. 567. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; pp. 622–623. [Google Scholar]
- Johnson, N.L.; Kotz, S.; Balakrishnan, N. Continuous Univariate Distributions. 2, 2nd ed.; Wiley: New York, NY, USA, 1995; p. 549. [Google Scholar]
- Spearman, C. The Proof and Measurement of Association between Two Things. Am. J. Psychol. 1904, 15, 72–101. [Google Scholar] [CrossRef]
- Schweizer, B.; Wolff, E.F. On Nonparametric Measures of Dependence for Random Variables. Ann. Statist. 1981, 9, 879–885. [Google Scholar] [CrossRef]


| (a) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | SE_LE * | UPP_LE * | UPP_WI | LAP_LE * | LAP_WI | LAP_UPHA | LAP_LOHA | LOP_LE * | LOP_WI | SP_LE * | SP_WI |
| VA1 | 6.31 ± 1.18 a (3.73–9.60) | 4.34 ± 0.79 a (2.63–6.55) | 2.35 ± 0.48 a (1.33–3.97) | 5.01 ± 0.77 a (3.32–7.15) | 2.48 ± 0.42 a (1.67–3.55) | 2.67 ± 0.49 a (1.70–4.10) | 2.33 ± 0.38 a (1.47–3.05) | 8.48 ± 1.07 a (5.89–11.18) | 3.45 ± 0.51 a (2.43–4.87) | 2.51 ± 0.35 a (1.50–3.31) | 1.53 ± 0.22 a (0.93–1.97) |
| VA2 | 11.31 ± 1.40 b (8.74–15.34) | 7.61 ± 2.12 b (3.79–11.64) | 4.93 ± 1.79 b (2.17–8.70) | 8.29 ± 1.82 b (4.63–11.87) | 4.38 ± 1.37 b (2.00–6.93) | 5.36 ± 1.54 b (2.74–8.45) | 2.93 ± 0.40 b (1.80–3.75) | 13.95 ± 2.61 b (7.26–19.47) | 7.04 ± 2.18 b (2.78–11.66) | 3.79 ± 0.70 b (2.00–5.23) | 1.65 ± 0.35 b (0.86–2.29) |
| VK | 5.30 ± 1.34 c (3.57–9.03) | 3.35 ± 0.63 c (2.18–4.98) | 2.00 ± 0.39 a (1.44–2.85) | 4.03 ± 0.61 c (2.98–5.57) | 2.17 ± 0.38 a (1.54–3.27) | 2.13 ± 0.35 c (1.56–3.17) | 1.90 ± 0.32 c (1.29–2.69) | 7.16 ± 0.58 c (5.90–8.23) | 3.10 ± 0.35 a (2.44–3.78) | 2.11 ± 0.26 c (1.43–2.57) | 1.14 ± 0.19 c (0.67–1.48) |
| VT | 13.57 ± 1.85 d (9.49–18.67) | 12.56 ± 1.31 d (9.81–14.70) | 8.30 ± 1.43 c (6.28–11.11) | 11.55 ± 1.04 d (9.55–13.09) | 6.87 ± 1.07 c (5.20–8.60) | 8.36 ± 0.90 d (6.46–9.56) | 3.19 ± 0.32 d (2.45–3.91) | 17.87 ± 1.58 d (14.71–21.02) | 10.76 ± 1.32 c (8.41–12.98) | 4.70 ± 0.47 d (3.86–5.58) | 1.70 ± 0.33 d (1.21–2.46) |
| R2 | 0.82 | 0.73 | 0.67 | 0.73 | 0.65 | 0.73 | 0.53 | 0.76 | 0.70 | 0.70 | 0.76 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| (b) | |||||||||||
| Code | PED_LE * | LA_LE | LA_WI | PET_LE | ST_LE | HALA_TE | ST_EXLO | ST_INLO | |||
| VA1 | 26.60 ± 7.15 a (12.91- 50.40) | 6.52 ± 2.79 a (2.65–15.65) | 3.71 ± 1.28 a (1.80–7.82) | 4.70 ± 2.20 a (1.52–13.65) | 9.61 ± 3.93 a (3.88–24.91) | 2 ± 1 a (1–4) | 4 ± 1 a (2–8) | 2 ± 1 a (1–3) | |||
| VA2 | 63.80 ± 20.65 b (25.92–133.25) | 21.67 ± 6.77 b (9.88–49.42) | 10.02 ± 4.23 b (3.95–25.76) | 9.56 ± 4.15 b (2.60–22.04) | 23.89 ± 7.36 b (7.84–54.50) | 4 ± 1 b (2–7) | 5 ± 1 b (3–9) | 2 ± 1 a (0–4) | |||
| VK | 20.15 ± 4.44 c (13.60–32.78) | 5.57 ± 2.59 a (2.62–12.21) | 2.99 ± 0.85 a (1.62–5.14) | 4.01 ± 1.47 a (2.13–7.54) | 7.46 ± 2.95 a (3.82–14.88) | 1 ± 1 c (0–3) | 3 ± 1 c (2–5) | 2 ± 1 a (1–3) | |||
| VT | 79.09 ± 24.17 d (35.59–116.85) | 28.44 ± 5.10 c (18.08–36.43) | 12.43 ± 2.58 c (8.37–18.76) | 11.04 ± 3.62 b (4.25–17.70) | 24.23 ± 5.24 b (13.33–34.78) | 5 ± 1 d (4–7) | 5 ± 1 b (3–8) | 2 ± 1 a (1–3) | |||
| R2 | 0.64 | 0.73 | 0.57 | 0.40 | 0.63 | 0.72 | 0.29 | 0.24 | |||
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| LD1 | LD2 | |||||
|---|---|---|---|---|---|---|
| Variable | Cor | Fisher Transf | Signif | Cor | Fisher Transf | Signif |
| (a) | ||||||
| SE_LE | 0.96 | 30.96 | *** | −0.03 | −0.48 | n.s. |
| UPP_LE | 0.86 | 21.08 | *** | 0.38 | 6.48 | *** |
| UPP_WI | 0.83 | 19.73 | *** | 0.32 | 5.45 | *** |
| LAP_LE | 0.89 | 23.22 | *** | 0.21 | 3.42 | *** |
| LAP_WI | 0.82 | 19.07 | *** | 0.32 | 5.37 | *** |
| LAP_UPHA | 0.88 | 22.93 | *** | 0.26 | 4.40 | *** |
| LAP_LOHA | 0.74 | 15.42 | *** | −0.05 | −0.85 | n.s. |
| LOP_LE | 0.92 | 25.79 | *** | 0.10 | 1.63 | n.s. |
| LOP_WI | 0.87 | 21.96 | *** | 0.24 | 4.01 | *** |
| SP_LE | 0.88 | 22.34 | *** | 0.09 | 1.42 | n.s. |
| SP_WI | 0.36 | 6.26 | *** | −0.07 | −1.07 | n.s. |
| (b) | ||||||
| PED_LE | 0.86 | 21.08 | *** | 0.10 | 1.57 | n.s. |
| LA_LE | 0.92 | 26.00 | *** | 0.04 | 0.70 | n.s. |
| LA_WI | 0.81 | 18.71 | *** | 0.09 | 1.53 | n.s. |
| PET_LE | 0.68 | 13.61 | *** | 0.13 | 2.06 | * |
| ST_LE | 0.83 | 19.58 | *** | 0.36 | 6.14 | *** |
| HALA_TE | 0.91 | 24.94 | *** | 0.13 | 2.15 | * |
| ST_INLO | 0.45 | 8.04 | *** | 0.07 | 1.18 | n.s. |
| ST_EXLO | 0.54 | 9.95 | *** | 0.38 | 6.52 | *** |
| VA1 | VA2 | VK | VT | |||||
|---|---|---|---|---|---|---|---|---|
| Variables | ρs | Signif | ρs | Signif | ρs | Signif | ρs | Signif |
| (a) | ||||||||
| SE_LE | 0.46 | *** | 0.05 | n.s. | 0.62 | *** | −0.17 | n.s. |
| UPP_LE | 0.31 | ** | 0.00 | n.s. | 0.59 | *** | −0.47 | * |
| UPP_WI | 0.40 | *** | −0.04 | n.s. | 0.38 | * | −0.48 | * |
| LAP_LE | 0.36 | *** | −0.05 | n.s. | 0.50 | ** | −0.40 | n.s. |
| LAP_WI | 0.40 | *** | −0.04 | n.s. | 0.44 | ** | 0.66 | ** |
| LAP_UPHA | 0.36 | *** | −0.05 | n.s. | 0.65 | *** | −0.47 | * |
| LAP_LOHA | 0.25 | * | −0.09 | n.s. | 0.27 | n.s. | 0.01 | n.s. |
| LOP_LE | 0.22 | * | −0.03 | n.s. | 0.10 | n.s. | −0.66 | *** |
| LOP_WI | 0.25 | * | 0.02 | n.s. | 0.00 | n.s. | −0.51 | * |
| SP_LE | 0.21 | * | −0.01 | n.s. | 0.01 | n.s. | 0.02 | n.s. |
| CO_LE | 0.29 | ** | 0.01 | n.s. | 0.36 | * | −0.70 | *** |
| CO_WI | 0.24 | * | 0.05 | n.s. | 0.44 | ** | −0.64 | ** |
| (b) | ||||||||
| PED_LE | 0.18 | n.s. | −0.31 | ** | 0.23 | n.s. | −0.84 | *** |
| LA_LE | 0.36 | *** | 0.02 | n.s. | 0.75 | *** | 0.13 | n.s. |
| LA_WI | −0.29 | ** | −0.47 | *** | −0.18 | n.s. | −0.58 | ** |
| PET_LE | 0.10 | n.s. | −0.53 | *** | 0.29 | n.s. | −0.54 | ** |
| ST_LE | 0.27 | ** | −0.23 | * | 0.68 | *** | −0.68 | *** |
| HALA_TE | −0.38 | *** | −0.35 | *** | −0.26 | n.s. | −0.45 | * |
| ST_INLO | 0.58 | *** | −0.09 | n.s. | 0.18 | n.s. | 0.12 | n.s. |
| ST_EXLO | 0.52 | *** | −0.33 | *** | 0.54 | ** | −0.52 | * |
| Characters | Grassland-Grown V. arvensis | Field and Fallow Land-Grown V. arvensis | Viola kitaibeliana |
|---|---|---|---|
| Lower sepal | narrowly lanceolate-acuminate | narrowly lanceolate-acuminate | ovate-lanceolate |
| Lower sepal appendage | irregularly sinuate, rarely coarsely dentate | coarsely dentate | entire to irregularly sinuate |
| Pollen magazine | open | almost always closed | open |
| Stylar flap | small and scarcely protruding | almost always small and scarcely protruding | absent |
| Stylar dark spot | absent | present | absent |
| Lamina margin | crenate to dentate | crenate to dentate | entire to crenate |
| Lamina apex | almost always rounded | acute to rounded | apex rounded |
| Bracts in the fruit-bearing peduncle | below bend 1 | far below bend 1 | just below fruit or at the bend 1 |
| ID | Sampling Site | Habitat | Elevation | Coordinates | Voucher Code |
|---|---|---|---|---|---|
| Viola arvensis subsp. arvensis (Va) | |||||
| VA-ST | Ex Polverificio Stacchini, Tivoli Terme (Roma) | Open stony grassland on travertine | 45 | 41°56′29″ N, 12°43′24″ E | UTV 37188 |
| VA-LA | Zona Laghi, Tivoli Terme (Roma) | Shrubby grassland on travertine | 66 | 41°57′44″ N, 12°43′11″ E | UTV 37190 |
| VA-SCI | Loc. Le Sparagine (SCI), Tivoli Terme (Roma) | Waste ground on travertine | 66 | 41°57′46″ N, 12°42′50″ E | UTV 37859 |
| VA-BS | Bassano Scalo, Orte (Viterbo) | Olive grove | 73 | 42°27′50″ N, 12°21′51″ E | UTV 36771 |
| VA-SG | San Gregorio da Sassola (Roma) | Resting field | 143 | 41°54′02″ N, 12°48′45″ E | UTV 38286 |
| VA-AZ | Azienda Agr. Riello, Viterbo (Viterbo) | Resting field | 303 | 42°25′35″ N, 12°04′50″ E | UTV 30441 |
| VA-B/1 | Loc. Bagnaccio, Viterbo (Viterbo) | Cereal field | 320 | 42°28′59″ N, 12°03′52″ E | UTV 36783 |
| VA-B/2 | Loc. Bagnaccio, Viterbo (Viterbo) | Resting field | 319 | 42°27′42″ N, 12°03′54″ E | UTV 36780 |
| VA-CE | Monte Cetona, Sarteano (Siena) | Stony grassland on calcareous soil | 1100 | 42°55′52″ N, 11°52′32″ E | UTV 30445 |
| VA-TA | Monte Tancia, Monte San Giovanni in Sabina (Rieti) | Stony grassland on calcareous soil | 1180 | 42°19′04″ N, 12°44′41″ E | UTV 32125 |
| Viola kitaibeliana subsp. kitaibeliana (Vk) | |||||
| VK-BS | Bassano Scalo, Orte (Viterbo) | Arid and stony grassland on calcareous soil | 70 | 42°29′12″ N, 12°19′28″ E | UTV 30152 |
| VK-NE | Loc. Cerreta, Nespolo (Rieti) | Open grassland in rural environment on calcareous soil | 1040 | 42°09′46″ N, 13°04′48″ E | UTV 29512 |
| VK-NA | Monte Navegna, Varco Sabino (Rieti) | Stony grassland on calcareous soil | 1184 | 42°14′03″ N, 12°59′34″ E | UTV 32126 |
| Viola tricolor subsp. tricolor (Vt) | |||||
| VT-PG | Poggio Nibbio, Viterbo (Viterbo) | Grassy edge in semi-natural land on volcanic soil | 885 | 42°21′42″ N, 12°10′17″ E | UTV 36752 |
| Quantitative Variables | ||||
|---|---|---|---|---|
| Continuous (mm) | ||||
| CO_LE | Corolla length * | SP_LE | Spur length | |
| CO_WI | Corolla width * | SP_WI | Spur width | |
| LAP_LE | Lateral petal length | UPP_LE | Upper petal length | |
| LAP_LOHA | Lateral petal lower half | UPP_WI | Upper petal width | |
| LAP_UPHA | Lateral petal upper half | LA_LE | Lamina length | |
| LAP_WI | Lateral petal width | LA_WI | Lamina width | |
| LOP_LE | Lower petal length | PED_LE | Peduncle length | |
| LOP_WI | Lower petal width | PET_LE | Petiole length | |
| SE_LE | Lower sepal length | ST_LE | Stipule length | |
| Discrete | ||||
| HALA_TE | Half lamina teeth number | ST_INLO | Stipule internal lobes number | |
| ST_EXLO | Stipule external lobes number | |||
| Categorical variables | ||||
| PO_MA | Pollen magazine: 1 open, 2 closed | |||
| SE_AP | Lower sepal appendage: 1 entire, 2 irregularly sinuate, 3 coarsely dentate | |||
| SE_SH | Lower sepal shape:1 ovate-lanceolate, 2 narrowly lanceolate-acuminate | |||
| ST_CH | Stigmatic chamber entrance (front view):1 in front, 2 lightly oblique (intermediate), 3 upward | |||
| ST_DS | Stylar dark spot: 1 absent, 2 present | |||
| STY | Stylar flap:1 absent, 2 small and scarcely protruding, 3 conspicuous | |||
| LA_AP | Lamina apex: 1 rounded, 2 acute | |||
| LA_ED | Lamina margin: 1 entire, 2 crenate, 3 dentate | |||
| ST_ED | Stipule midlobe margin: 1 entire, 2 crenate-dentate | |||
| ST_SH | Stipule shape: 1 palmately lobed, 2 pinnately lobed | |||
| FL-LE_PO | Flower/leaf position: on main stems 1 to 8, on lateral branchs 9 to11 | |||
| Indices | ||||
| CO_LE/WI | Corolla length/width | LOHA/UPHA | Lateral petal lower half/upper half | |
| LAP_LE/WI | Lateral petal length/width | UPP_LE/WI | Upper petal length/width | |
| LOP/SE_LE | Lower petal/lower sepal length | LA/PET_LE | Lamina/petiole length | |
| LOP_LE/WI | Lower petal length/width | LA_LE/WI | Lamina length/width | |
| SP_LE/WI | Spur length/width | PED/LE_LE | Peduncle/leaf length | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scoppola, A.; Angeloni, D.; Franceschini, C. Classical Morphometrics in V. arvensis and V. kitaibeliana (Viola sect. Melanium) Reveals Intraspecific Variation with Implications for Species Delimitation: Inferences from a Case Study in Central Italy. Plants 2022, 11, 379. https://doi.org/10.3390/plants11030379
Scoppola A, Angeloni D, Franceschini C. Classical Morphometrics in V. arvensis and V. kitaibeliana (Viola sect. Melanium) Reveals Intraspecific Variation with Implications for Species Delimitation: Inferences from a Case Study in Central Italy. Plants. 2022; 11(3):379. https://doi.org/10.3390/plants11030379
Chicago/Turabian StyleScoppola, Anna, Daniele Angeloni, and Cinzia Franceschini. 2022. "Classical Morphometrics in V. arvensis and V. kitaibeliana (Viola sect. Melanium) Reveals Intraspecific Variation with Implications for Species Delimitation: Inferences from a Case Study in Central Italy" Plants 11, no. 3: 379. https://doi.org/10.3390/plants11030379
APA StyleScoppola, A., Angeloni, D., & Franceschini, C. (2022). Classical Morphometrics in V. arvensis and V. kitaibeliana (Viola sect. Melanium) Reveals Intraspecific Variation with Implications for Species Delimitation: Inferences from a Case Study in Central Italy. Plants, 11(3), 379. https://doi.org/10.3390/plants11030379







