The Anti-Inflammatory Response of Lavandula luisieri and Lavandula pedunculata Essential Oils
Abstract
:1. Introduction
2. Results
2.1. Essential Oil Characterization
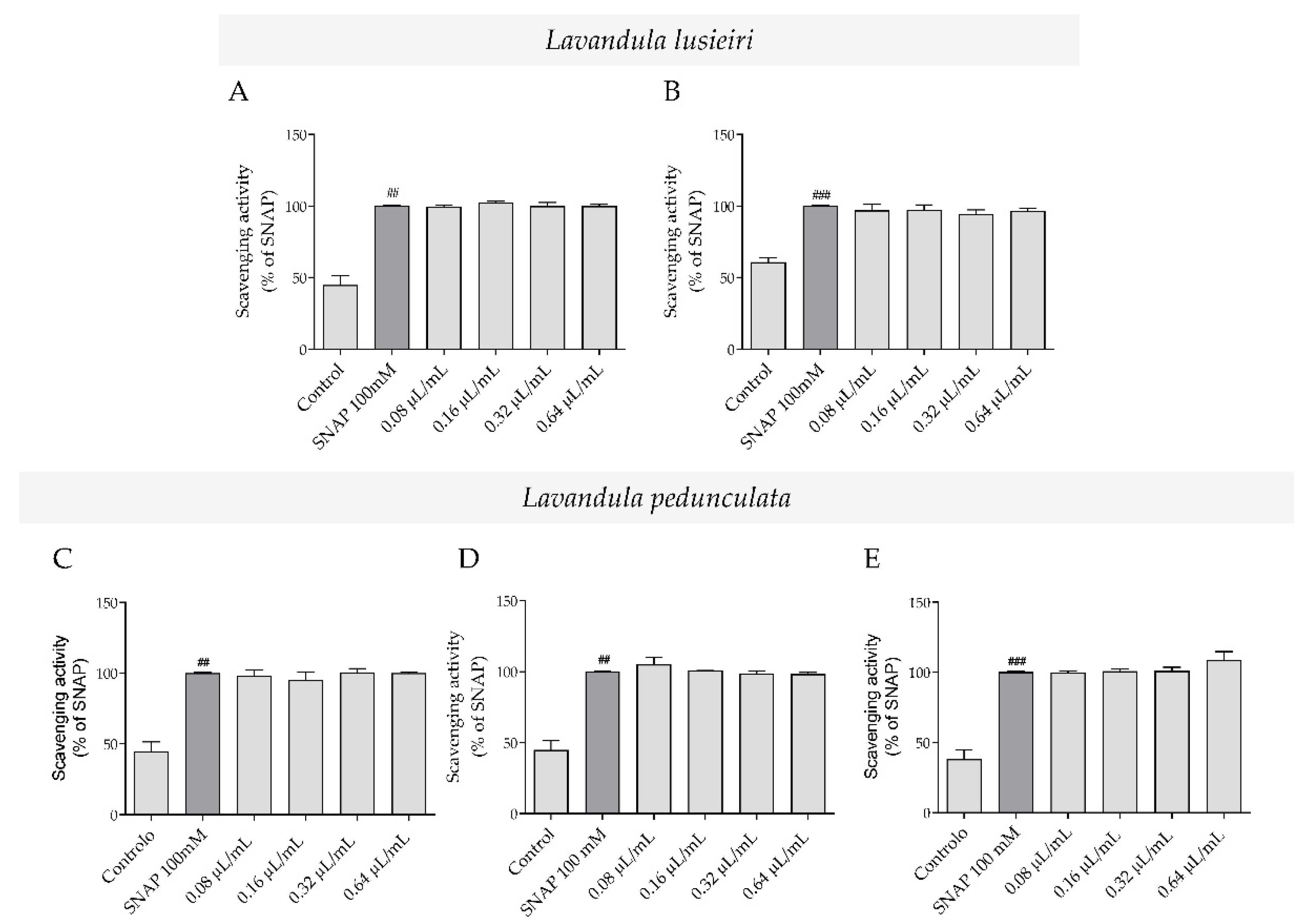
2.2. Nitric Oxide Scavenging Potential of Lavandula luisieri and Lavandula pedunculata Essential Oils
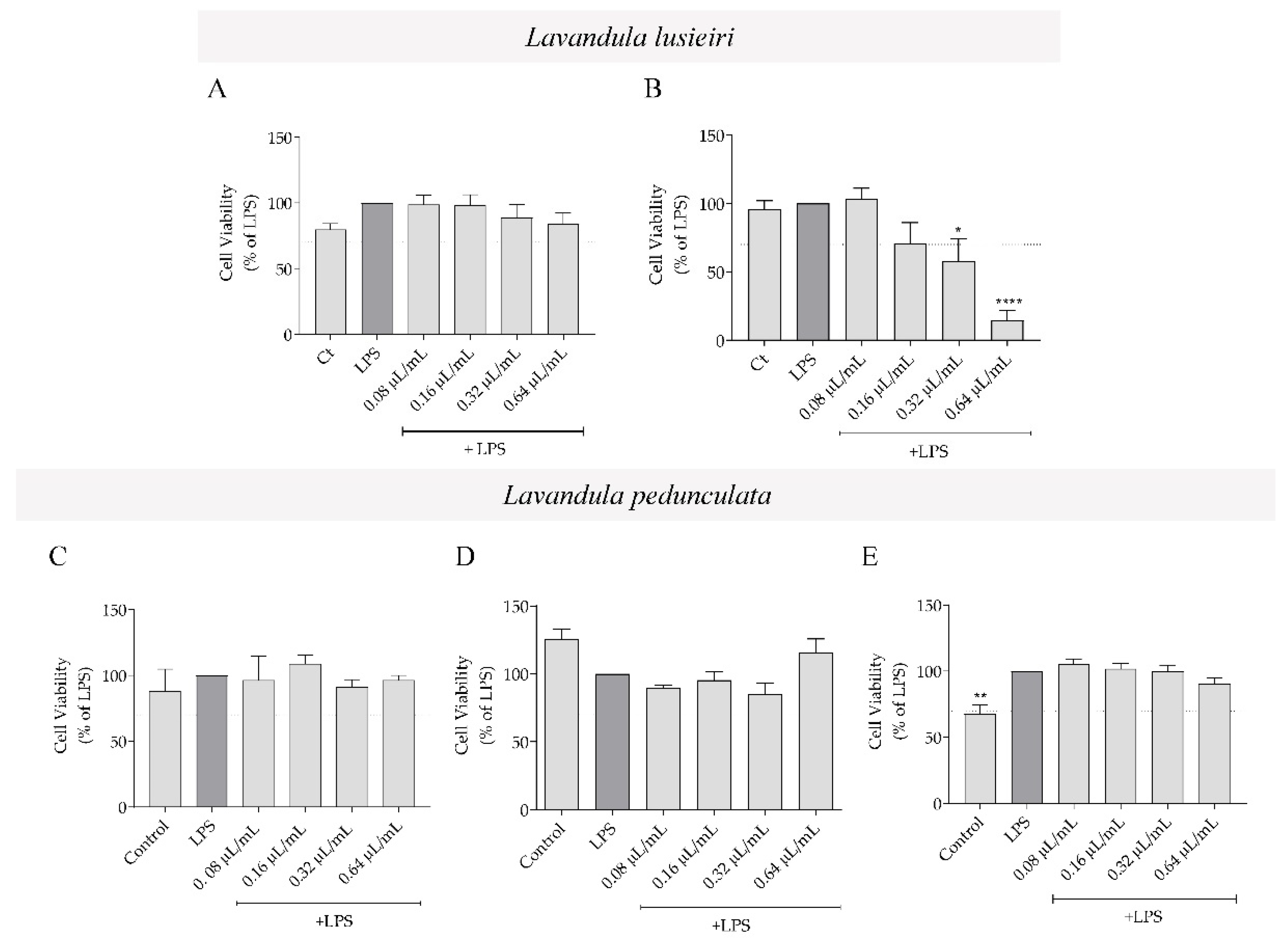
2.3. Effect of Lavandula luisieri and Lavandula pedunculata Essential Oils on Macrophages’ Viability
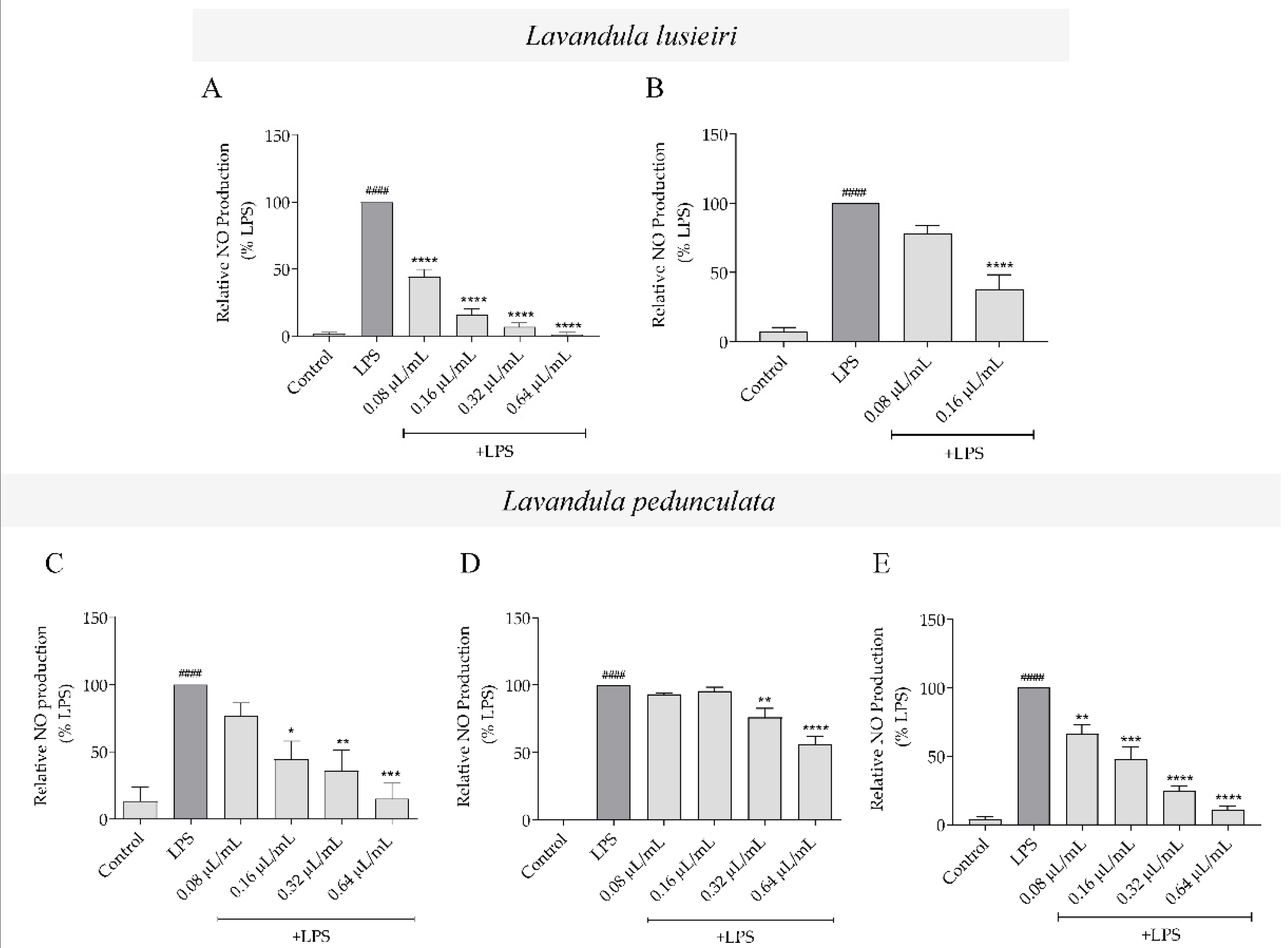
2.4. Effect of Lavandula luisieri and Lavandula pedunculata Essential Oils on NO Production
2.5. Effect of 1,8-Cineole and Fenchone on NO Production
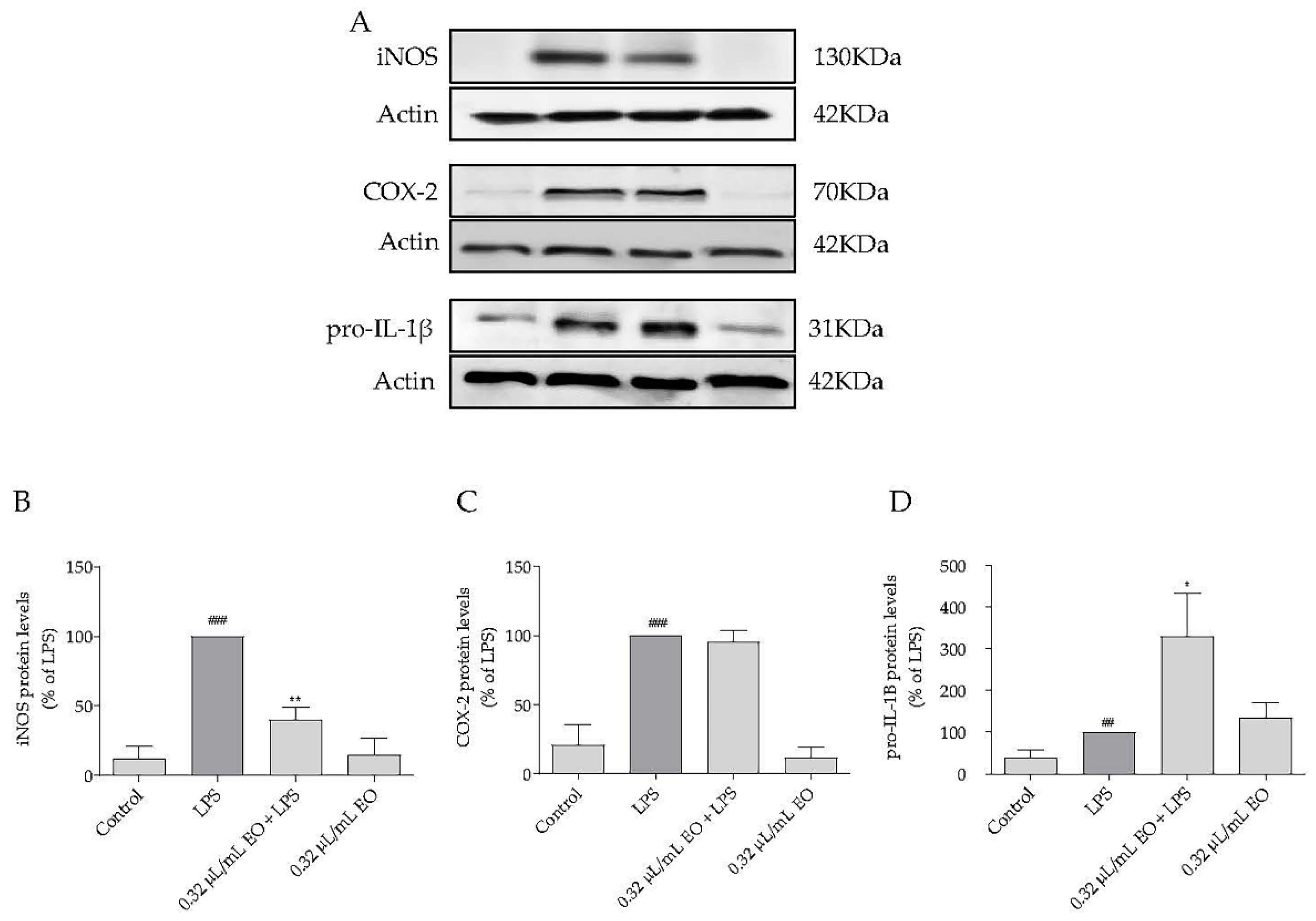
2.6. Effect of Lavandula luisieri Essential on the Expression of Inflammatory Mediators
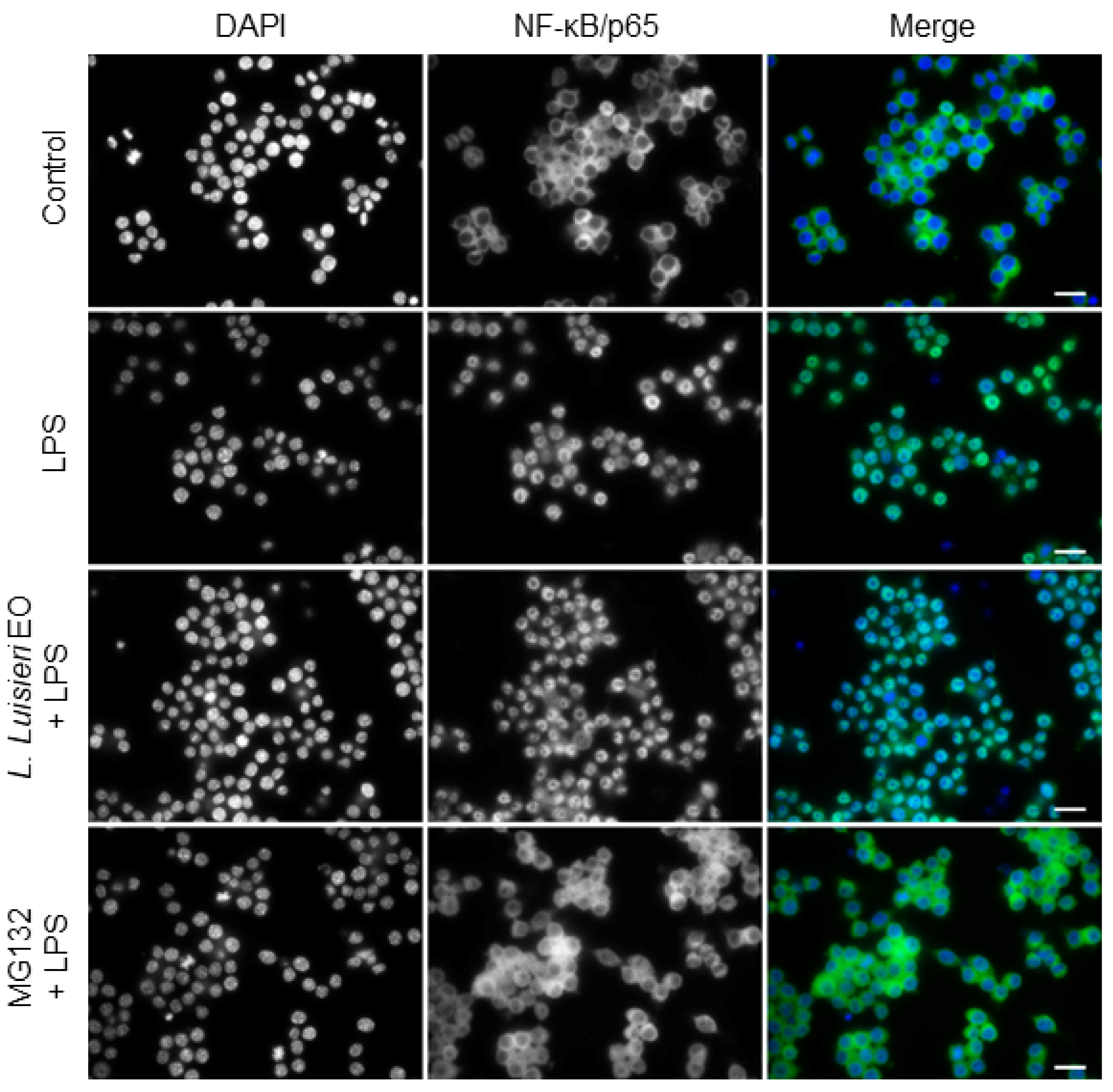
2.7. Effect of L. luisieri Essential Oil on NF-κB/p65 Nuclear Translocation
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Essential Oil Isolation and Analysis
4.3. Nitric Oxide Scavenging Potential
4.4. Cell Culture and Treatments
4.5. Cell Viability
4.6. Nitric Oxide Production
4.7. Western Blotting
4.8. Immunocytochemistry
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inoue, M.; Hayashi, S.; Craker, L.E. Role of Medicinal and Aromatic Plants: Past, Present, and Future. Pharmacogn. Med. Plants 2019. [Google Scholar] [CrossRef] [Green Version]
- Barreca, S.; La Bella, S.; Maggio, A.; Licata, M.; Buscemi, S.; Leto, C.; Pace, A.; Tuttolomondo, T. Flavouring extra-virgin olive oil with aromatic and medicinal plants essential oils stabilizes oleic acid composition during photo-oxidative stress. Agriculture 2021, 11, 266. [Google Scholar] [CrossRef]
- Upson, T.; Andrews, S. The Genus Lavandula. Kew: Royal Botanic Gardens; Kew Publishing: London, UK, 2004. [Google Scholar]
- ISO TC 54-ISO/CD 8902; Oil of Lavandin Grosso [Lavandula angustifolia Miller × Lavandula latifolia (L. f) Medikus]. French Type, ISO: Geneva, Switzerland, 2007.
- ISO TC 54 N-ISO/WD 4719; Oil of Spike Lavender [Lavandula latifolia (L.F.) Medikus]. Spanish Type, ISO: Geneva, Switzerland, 2009.
- Vairinhos, J.; Miguel, M.G. Essential oils of spontaneous species of the genus Lavandula from Portugal: A brief review. Zeitschrift Für Naturforsch. C 2020, 75, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Gonçalves, M.; Cruz, M.; Cavaleiro, C.; Canhoto, J.; Vaz, S.; Pinto, E.; Salgueiro, L. Lavandula luisieri essential oil as a source of antifungal drugs. Food Chem. 2012, 135, 1505–1510. [Google Scholar] [CrossRef]
- Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Dinis, A.M.; Canhoto, J.M.; Salgueiro, L.R. Chemical composition and antifungal activity of the essential oils of Lavandula pedunculata (Miller) Cav. Chem. Biodivers. 2009, 6, 1283–1292. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Gille, E.; Trifan, A.; Luca, V.S.; Miron, A. Essential oils of Lavandula genus: A systematic review of their chemistry. Phytochem. Rev. 2017, 16, 761–799. [Google Scholar] [CrossRef]
- Tavares, A.C.; Zuzarte, M.R.; Salgueiro, L.R. Plantas Aromáticas e Medicinais: Escola Médica do Jardim Botânico da Universidade de Coimbra; Imprensa da Universidade de Coimbra: Coimbra, Portugal, 2010. [Google Scholar] [CrossRef]
- Neves, J.; Matos, C.; Moutinho, C.; Queiroz, G.; Gomes, L.R. Ethnopharmacological notes about ancient uses of medicinal plants in Trás-os-Montes (northern of Portugal). J. Ethnopharmacol. 2009, 124, 270–283. [Google Scholar] [CrossRef]
- Rivera, D.; Obón, C. The ethnopharmacology of Madeira and Porto Santo Islands, a review. J. Ethnopharmacol. 1995, 46, 73–93. [Google Scholar] [CrossRef]
- Ferreira, A.; Proença, C.; Serralheiro, M.L.; Araújo, M. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J. Ethnopharmacol. 2006, 108, 31–37. [Google Scholar] [CrossRef]
- Amira, S.; Dade, M.; Schinella, G.; Ríos, J.-L. Anti-inflammatory, anti-oxidant, and apoptotic activities of four plant species used in folk medicine in the Mediterranean basin. Pak. J. Pharm. Sci. 2012, 25, 65–72. [Google Scholar]
- Hajhashemi, V.; Ghannadi, A.; Sharif, B. Anti-inflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J. Ethnopharmacol. 2003, 89, 67–71. [Google Scholar] [CrossRef]
- Wei, A.; Shibamoto, T. Antioxidant/lipoxygenase inhibitory activities and chemical compositions of selected essential oils. J. Agric. Food Chem. 2010, 58, 7218–7225. [Google Scholar] [CrossRef] [PubMed]
- Sosa, S.; Altinier, G.; Politi, M.; Braca, A.; Morelli, I.; Della Loggia, R. Extracts and constituents of Lavandula multifida with topical anti-inflammatory activity. Phytomedicine 2005, 12, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Francisco, V.; Neves, B.M.; Liberal, J.; Cavaleiro, C.; Canhoto, J.M.; Salgueiro, L.; Cruz, M.T. Lavandula viridis essential oil inhibits the inflammatory response in macrophages through blockade of NF-KB signaling cascade. Front. Pharmacol. 2022; 12, 695911. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ferreira, I.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Differential effects of the essential oils of Lavandula luisieri and Eryngium duriaei subsp. juresianum in cell models of two chronic inflammatory diseases. Pharm. Biol. 2015, 53, 1220–1230. [Google Scholar] [CrossRef] [Green Version]
- Miguel, M.G.; Da Silva, C.I.; Farah, L.; Braga, F.C.; Figueiredo, A.C. Effect of essential oils on the release of TNF-a and CCL2 by LPS-stimulated THP-1 cells. Plants 2020, 10, 50. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Hunter, J.D.; Doddi, M. Sepsis and the heart. Br. J. Anaesth. 2010, 104, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Standardization, I.O.f. Biological Evaluation of Medical Devices. Part 5 Tests Vitro Cytotoxicity; HIS: Geneva, Switzerland, 2009; pp. 1–34. [Google Scholar]
- Mulero, M.C.; Huxford, T.; Ghosh, G. NF-κB, IκB, and IKK: Integral components of immune system signaling. Struct. Immunol. 2019, 1172, 207–226. [Google Scholar] [CrossRef]
- Cavanagh, H.M.A.; Wilkinson, J.M. Lavender essential oil: A review. Aust. Infect. Control. 2005, 10, 35–37. [Google Scholar] [CrossRef] [Green Version]
- Garcia Vallejo, M.I. Aceites Esenciales de las Lavandulas Ibéricas-Ensayo de la Quimiotaxonomia; Universidad Complutense de Madrid: Madrid, Spain, 1992. [Google Scholar]
- Lavoine-Hanneguelle, S.; Casabianca, H. New Compounds from the essential oil and absolute of Lavandula luisieri L. J. Essent. Oil Res. 2004, 16, 445–448. [Google Scholar] [CrossRef]
- Sanz, J.; Soria, A.C.; García-Vallejo, M. Analysis of volatile components of Lavandula luisieri L. by direct thermal desorption–gas chromatography–mass spectrometry. J. Chromatogr. A 2004, 1024, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Baldovini, N.; Lavoine-Hanneguelle, S.; Ferrando, G.; Dusart, G.; Lizzani-Cuvelier, L. Necrodane monoterpenoids from Lavandula luisieri. Phytochemistry 2005, 66, 1651–1655. [Google Scholar] [CrossRef]
- González-Coloma, A.; Delgado, F.; Rodilla, J.M.; Silva, L.; Sanz, J.; Burillo, J. Chemical and biological profiles of Lavandula luisieri essential oils from western Iberia Peninsula populations. Biochem. Syst. Ecol. 2011, 39, 1–8. [Google Scholar] [CrossRef]
- Skoula, M.; Abidi, C.; Kokkalou, E. Essential oil variation of Lavandula stoechas L. ssp. stoechas growing wild in crete (Greece). Biochem. Syst. Ecol. 1996, 24, 255–260. [Google Scholar] [CrossRef]
- Dadalioǧlu, I.; Evrendilek, G.A. Chemical compositions and antibacterial effects of essential oils of Turkish oregano (Origanum minutiflorum), bay laurel (Laurus nobilis), Spanish lavender (Lavandula stoechas L.), and fennel (Foeniculum vulgare) on common foodborne pathogens. J. Agric. Food Chem. 2004, 52, 8255–8260. [Google Scholar] [CrossRef]
- Antonelli, M.; Kushner, I. It’s time to redefine inflammation. FASEB J. 2017, 31, 1787–1791. [Google Scholar] [CrossRef] [Green Version]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Cardia, G.F.E.; Silva-Filho, S.E.; Silva, E.L.; Uchida, N.S.; Cavalcante, H.A.O.; Cassarotti, L.L.; Salvadego, V.E.C.; Spironello, R.A.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of Lavender (Lavandula angustifolia) essential oil on acute inflammatory response. Evidence-Based Complement. Altern. Med. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Souri, F.; Rakhshan, K.; Erfani, S.; Azizi, Y.; Maleki, S.N.; Aboutaleb, N. Natural lavender oil (Lavandula angustifolia) exerts cardioprotective effects against myocardial infarction by targeting inflammation and oxidative stress. Inflammopharmacology 2019, 27, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. European Pharmacopoeia, 9th ed; Council of Europe: Strasbourg, France, 2016. [Google Scholar]
- Joulain, D.; Konig, W.A. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons; E.B.-Verlag: Hamburg, Germany, 1998. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2021; Volume 9. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.; Neves, B.; Leitão, A.; Mendes, A. Elucidation of the mechanism underlying the anti-inflammatory properties of (S)-(+)-carvone identifies a novel class of sirtuin-1 activators in a murine macrophage cell line. Biomedicines 2021, 9, 777. [Google Scholar] [CrossRef] [PubMed]

| Lavandula luisieri | Lavandula pedunculata | ||||||
| RI SPB-1 | RI SW 10 | Compound | A1 | A2 | B1 | B2 | B3 |
| 930 | 1030 | α-pinene | 1.1 | 3.2 | 2.5 | 3.9 | 3.8 |
| 942 | 1075 | camphene | 0.5 | - | 0.8 | 1.8 | 6.1 |
| 969 | 1116 | β-pinene | 0.7 | 1.6 | 9.0 | 0.2 | 1.4 |
| 1020 | 1215 | 1,8-cineole | 33.9 | 6.4 | 34.3 | 12 | 25.1 |
| 1065 | 1400 | fenchone | 18.2 | - | 7.6 | 49.5 | 6.2 |
| 1082 | 1542 | linalool | 3.0 | 6.2 | 3.8 | 2.4 | 1.2 |
| 1118 | 1514 | camphor | 2.2 | 2.5 | 9.9 | 15 | 34.0 |
| 1121 | 1645 | cis-verbenol | - | 0.2 | 2.8 | 0.3 | 0.2 |
| 1125 | 1669 | trans-verbenol | - | - | 1.1 | 0.4 | 2 |
| 1130 | 1657 | trans-α-necrodol | 4.5 | 7.1 | - | - | - |
| 1146 | 1692 | borneol | - | - | 3.4 | 0.3 | 0.6 |
| 1154 | 1712 | 1,1,2,3-tetramethyl-4-hidroximethyl-2-cyclopentane | 1.1 | 2.0 | - | - | - |
| 1159 | 1645 | 2,3,4,4-tetramethyl-5-methylene-cyclopent-2-enone | 0.3 | 2.8 | - | - | - |
| 1165 | 1621 | myrtenal | - | 0.3 | 2.4 | 0.4 | 0.8 |
| 1265 | 1590 | trans-α-necrodyl acetate | 3.2 | 17.4 | - | - | - |
| 1269 | 1602 | lavandulyl acetate | 2.2 | 7.6 | - | - | - |
| 1269 | 1657 | lyratyl acetate | 0.3 | 2.4 | - | - | - |
| 1301 | 1683 | myrtenyl acetate | 2.0 | - | - | - | - |
| 1571 | 2068 | viridiflorol | 1.4 | 2.1 | - | - | - |
| 1628 | 2218 | α-cadinol | - | 0.7 | 3.1 | 0.2 | 0.2 |
| Monoterpene hydrocarbons | 2.3 | 4.5 | 12.3 | 5.9 | 11.3 | ||
| Oxygen containing monoterpenes (including necrodane derivatives) | 72.3 | 54.9 | 65.3 | 80.3 | 70.1 | ||
| Oxygen containing sesquiterpenes | 1.4 | 2.8 | 3.1 | 0.2 | 0.2 | ||
| Species | Region | Site of Collection | Sample |
|---|---|---|---|
| L. luisieri | Coimbra | Piódão | A1 |
| Algarve | Cabo de São Vicente | A2 | |
| L. pedunculata | Guarda | Celorico da Beira | B1 |
| Bragança | Serra da Nogueira | B2 | |
| Coimbra | Foz de Arouce | B3 |
| Protein | Source | Clonality | Dilution | Supplier | Catalogue Number |
|---|---|---|---|---|---|
| COX-2 | rabbit | polyclonal | 1:10,000 | Abcam, Cambridge, UK | ab6665 |
| IL-1β | rabbit | polyclonal | 1:1000 | Abcam | ab9722 |
| iNOS | mouse | monoclonal | 1:1000 | R&D Systems, Minneapolis, MN, USA | MAB9502 |
| Actin | mouse | monoclonal | 1:20,000 | Sigma-Aldrich Co. | MAB1501 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuzarte, M.; Sousa, C.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. The Anti-Inflammatory Response of Lavandula luisieri and Lavandula pedunculata Essential Oils. Plants 2022, 11, 370. https://doi.org/10.3390/plants11030370
Zuzarte M, Sousa C, Cavaleiro C, Cruz MT, Salgueiro L. The Anti-Inflammatory Response of Lavandula luisieri and Lavandula pedunculata Essential Oils. Plants. 2022; 11(3):370. https://doi.org/10.3390/plants11030370
Chicago/Turabian StyleZuzarte, Monica, Cátia Sousa, Carlos Cavaleiro, Maria Teresa Cruz, and Lígia Salgueiro. 2022. "The Anti-Inflammatory Response of Lavandula luisieri and Lavandula pedunculata Essential Oils" Plants 11, no. 3: 370. https://doi.org/10.3390/plants11030370
APA StyleZuzarte, M., Sousa, C., Cavaleiro, C., Cruz, M. T., & Salgueiro, L. (2022). The Anti-Inflammatory Response of Lavandula luisieri and Lavandula pedunculata Essential Oils. Plants, 11(3), 370. https://doi.org/10.3390/plants11030370










