Foliar Application of Salicylic Acid Improves Salt Tolerance of Sorghum (Sorghum bicolor (L.) Moench)
Abstract
1. Introduction
2. Results
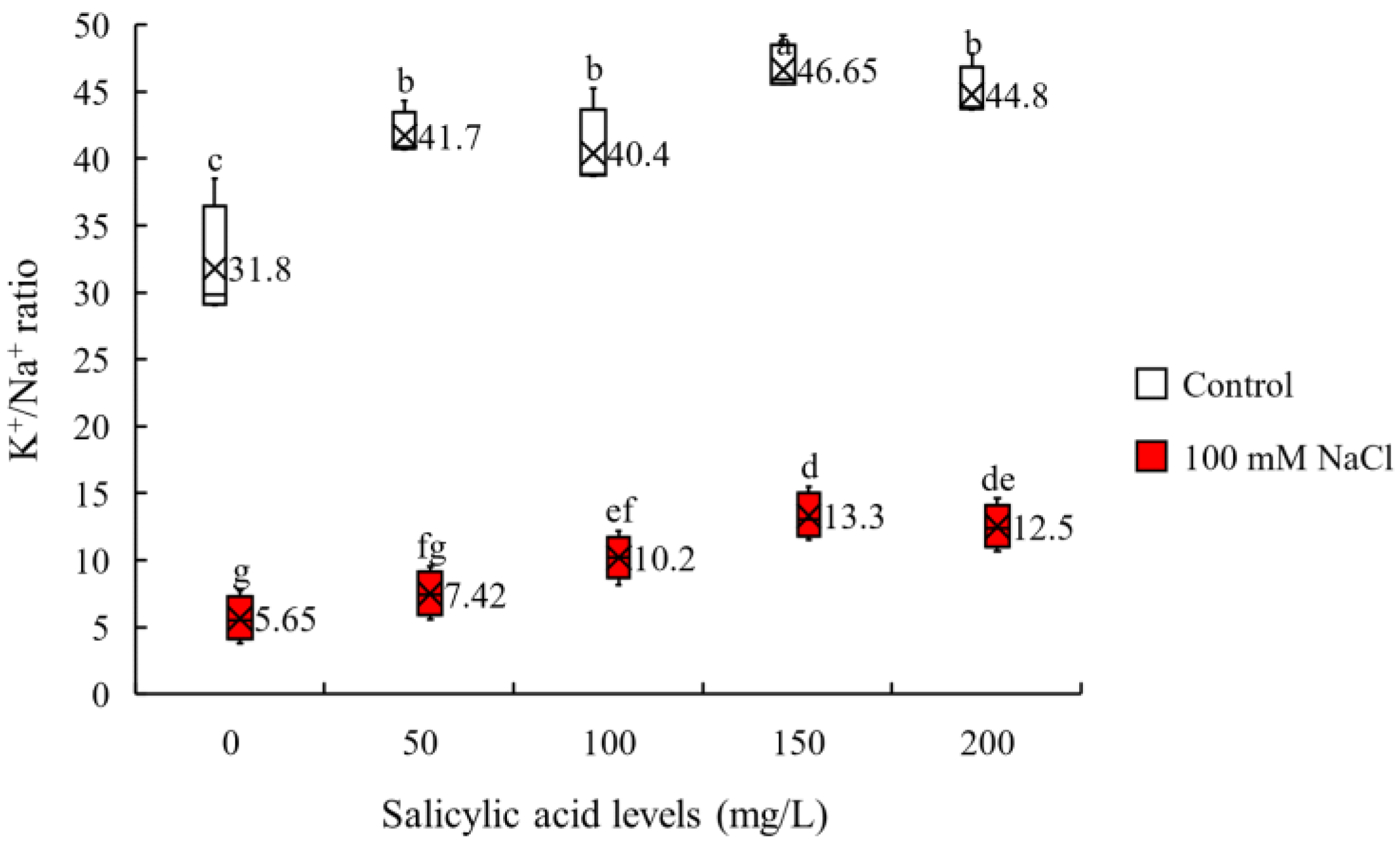
2.1. Ionic Balance
2.2. Photosynthetic Parameters
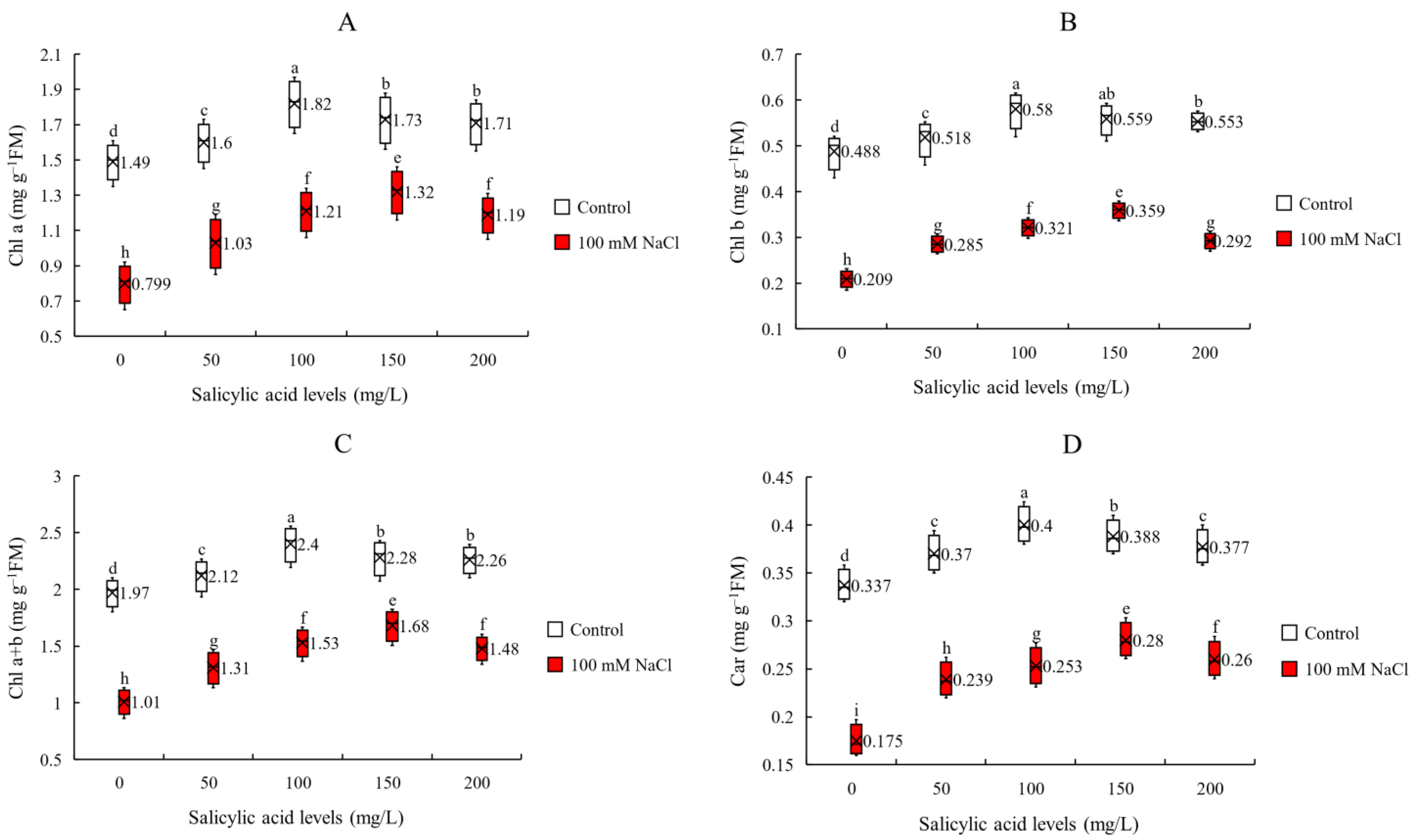
2.3. Photosynthetic Pigments
2.4. Antioxidant Enzymes Activities
2.5. Osmolyte
2.6. Growth Parameters
3. Discussion
4. Materials and Methods
4.1. Photosynthesis Measurement
4.2. Photosynthetic Pigments Content
4.3. Measurement of Antioxidant Enzymes Activities
4.4. Proline Content
4.5. Shoot and Root Dry Weight
4.6. K+/Na+ Ratio
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sytar, O.; Brestic, M.; Zivcak, M.; Olsovska, K.; Kovar, M.; Shao, H.; He, X. Applying hyperspectral imaging to explore natural plant diversity towards improving salt stress tolerance. Sci. Total Environ. 2017, 578, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: Aan overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.; Sun, J.; Shao, H. Negative interactive effects between biochar and phosphorus fertilization on phosphorus availability and plant yield in saline sodic soil. Sci. Total Environ. 2016, 568, 910–915. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Y.; Sun, S.; Mu, C.; Yan, X. Effects of arbuscular mycorrhizal fungi on the growth, photosynthesis and photosynthetic pigments of Leymus chinensis seedlings under salt-alkali stress and nitrogen deposition. Sci. Total Environ. 2017, 576, 234–241. [Google Scholar] [CrossRef]
- Foyer, C.H.; Descourvieres, P.; Kunert, K.J. Protection against oxygen radicals: An important defence mechanism studied in transgenic plants. Plant Cell Environ. 1994, 17, 507–523. [Google Scholar] [CrossRef]
- Rehman, A.U.; Bashir, F.; Ayaydin, F.; Kóta, Z.; Páli, T.; Vass, I. Proline is a quencher of singlet oxygen and superoxide both in in vitro systems and isolated thylakoids. Physiol. Plant. 2021, 172, 7–18. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Berwal, M.K.; Kumar, R.; Prakash, K.; Rai, G.K.; Hebbar, K. Antioxidant Defense System in Plants Against Abiotic Stress. In Abiotic Stress Tolerance Mechanisms in Plants; Rai, G.K., Kumar, R.R., Bagati, S., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 175–202. [Google Scholar]
- Spychalla, J.P.; Desborough, S.L. Superoxide dismutase, catalase, and α-tocopherol content of stored potato tubers. Plant Physiol. 1990, 94, 1214–1218. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, Y.S.; Lee, C.B. The inductive responses of the antioxidant enzymes by salt stress in the rice (Oryza sativa L.). J. Plant Physiol. 2001, 158, 737–745. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Bio/Technol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, H.J.; Shen, B. Transformation and compatible solutes. Sci. Hortic. 1998, 78, 237–260. [Google Scholar] [CrossRef]
- Koyro, H.-W.; Ahmad, P.; Geissler, N. Abiotic Stress Responses in Plants: An Overview. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 1–28. [Google Scholar]
- Turner, N.C. Turgor maintenance by osmotic adjustment: 40 years of progress. J. Exp. Bot. 2018, 69, 3223–3233. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Sairam, R.; Tyagi, A. Physiology and molecular biology of salinity stress tolerance in plants. Curr. Sci. 2004, 86, 407–421. [Google Scholar]
- Fayez, K.A.; Bazaid, S.A. Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate. J. Saudi Soc. Agric. Sci. 2014, 13, 45–55. [Google Scholar] [CrossRef]
- Qiu, Z.; Guo, J.; Zhu, A.; Zhang, L.; Zhang, M. Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotoxicol. Environ. Saf. 2014, 104, 202–208. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Emam, Y.; Rousta, M.J.; Ashraf, M. Salicylic acid induced salinity tolerance through manipulation of ion distribution rather than ion accumulation. J. Plant Growth Regul. 2017, 36, 227–239. [Google Scholar] [CrossRef]
- Quamruzzaman, M.; Manik, S.; Shabala, S.; Zhou, M. Improving Performance of Salt-Grown Crops by Exogenous Application of Plant Growth Regulators. Biomolecules 2021, 11, 788. [Google Scholar] [CrossRef]
- Singh, A.P.; Dixit, G.; Kumar, A.; Mishra, S.; Kumar, N.; Dixit, S.; Singh, P.K.; Dwivedi, S.; Trivedi, P.K.; Pandey, V.; et al. A protective role for nitric oxide and salicylic acid for arsenite phytotoxicity in rice (Oryza sativa L.). Plant Physiol. Biochem. 2017, 115, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Fariduddin, Q.; Castroverde, C.D.M. Salicylic acid: A key regulator of redox signalling and plant immunity. Plant Physiol. Biochem. 2021, 168, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Cutt, J.R.; Klessig, D.F. Pathogenesis-related proteins. In Genes Involved in Plant Defense; Boller, T., Meins, F., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 209–243. [Google Scholar]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.R.; Balke, N.E. Characterization of the inhibition of K+ absorption in oat roots by salicylic acid. Plant Physiol. 1981, 68, 1349–1353. [Google Scholar] [CrossRef]
- Barkosky, R.R.; Einhellig, F.A. Effects of salicylic acid on plant-water relationships. J. Chem. Ecol. 1993, 19, 237–247. [Google Scholar] [CrossRef]
- Singh, N.B.; Khare, S.; Singh, A.; Yadav, V.; Yadav, R.K. Salicylic acid and Indole acetic acid synergistically ameliorates Ferulic acid toxicity in Brassica juncea L. seedlings. Plant Physiol. Rep. 2021, 26, 729–740. [Google Scholar] [CrossRef]
- Khan, W.; Prithiviraj, B.; Smith, D.L. Photosynthetic responses of corn and soybean to foliar application of salicylates. J. Plant Physiol. 2003, 160, 485–492. [Google Scholar] [CrossRef]
- Janda, T.; Gondor, O.K.; Yordanova, R.; Szalai, G.; Pál, M. Salicylic acid and photosynthesis: Signalling and effects. Acta Physiol. Plant. 2014, 36, 2537–2546. [Google Scholar] [CrossRef]
- Mutlu, S.; Karadağoğlu, Ö.; Atici, Ö.; Tasgin, E.; Nalbantoğlu, B. Time-dependent effect of salicylic acid on alleviating cold damage in two barley cultivars differing in cold tolerance. Turk. J. Bot. 2013, 37, 343–349. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Noreen, S.; Shakeela, N.; Ahmad, S.; Fehmeeda, B.; Hasanuzzaman, M. Quantifying some physiological and productivity indices of canola (Brassica napus L.) crop under an arid environment. Not. Bot. Horti Agrobot. 2016, 44, 272–279. [Google Scholar] [CrossRef]
- Iqbal, M.A. Agronomic management strategies elevate forage sorghum yield: A Review. J. Adv. Bot. Zool. 2015, 3, 1–6. [Google Scholar]
- Arzani, A. Improving salinity tolerance in crop plants: A biotechnological view. Vitr. Cell. Dev. Biol.-Plant 2008, 44, 373–383. [Google Scholar] [CrossRef]
- Soltabayeva, A.; Ongaltay, A.; Omondi, J.O.; Srivastava, S. Morphological, Physiological and Molecular Markers for Salt-Stressed Plants. Plants 2021, 10, 243. [Google Scholar] [CrossRef]
- Osman, M.S.; Badawy, A.A.; Osman, A.I.; Abdel Latef, A.A.H. Ameliorative impact of an extract of the halophyte Arthrocnemum macrostachyum on growth and biochemical parameters of soybean under salinity stress. J. Plant Growth Regul. 2021, 40, 1245–1256. [Google Scholar] [CrossRef]
- Alam, H.; Khattak, J.Z.; Ksiksi, T.S.; Saleem, M.H.; Fahad, S.; Sohail, H.; Ali, Q.; Zamin, M.; El-Esawi, M.A.; Saud, S.; et al. Negative impact of long-term exposure of salinity and drought stress on native Tetraena mandavillei L. Physiol. Plant. 2021, 172, 1336–1351. [Google Scholar] [CrossRef] [PubMed]
- Dashti, A.; Khan, A.A.; Collins, J.C. Effects of salinity on growth, ionic relations and solute content of Sorghum bicolor (L.) Monench. J. Plant Nutr. 2009, 32, 1219–1236. [Google Scholar] [CrossRef]
- Kafi, M.; Nabati, J.; Masoumi, A.; Mehrgerdi, M.Z. Effect of salinity and silicon application on oxidative damage of sorghum [Sorghum bicolor (L.) Moench.]. Pak. J. Bot. 2011, 43, 2457–2462. [Google Scholar]
- Rajabi Dehnavi, A.; Zahedi, M.; Ludwiczak, A.; Cardenas Perez, S.; Piernik, A. Effect of salinity on seed germination and seedling development of sorghum (Sorghum bicolor (L.) Moench) genotypes. Agronomy 2020, 10, 859. [Google Scholar] [CrossRef]
- Leegood, R.C. Handbook of photosynthesis. In Annals of Botany, 2nd ed.; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2006; Volume 97, pp. 152–153. [Google Scholar]
- Pan, T.; Liu, M.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Nie, C.; Yu, M.; Kuznetsov, V.V.; Allakhverdiev, S.I.; Shabala, S. Non-stomatal limitation of photosynthesis by soil salinity. Crit. Rev. Environ. Sci. Technol. 2021, 51, 791–825. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In Ecophysiology and Responses of Plants under Salt Stress; Ahmad, P., Azooz, M., Prasad, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 25–87. [Google Scholar]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Chen, P.; Shao, H.; Zhao, S.; Zhang, L.; Zhang, L.; Xu, G.; Sun, J. Responses of photosynthesis and photosystem II to higher temperature and salt stress in Sorghum. J. Agron. Crop Sci. 2012, 198, 218–225. [Google Scholar] [CrossRef]
- Sadeghi, H.; Shourijeh, F.A. Salinity induced effects on growth parameters, chemical and biochemical characteristics of two forage sorghum (Sorghum bicolor L.) cultivars. Asian J. Plant Sci. 2012, 11, 19–27. [Google Scholar] [CrossRef][Green Version]
- Morales, F.; Ancín, M.; Fakhet, D.; González-Torralba, J.; Gámez, A.L.; Seminario, A.; Soba, D.; Ben Mariem, S.; Garriga, M.; Aranjuelo, I. Photosynthetic metabolism under stressful growth conditions as a bases for crop breeding and yield improvement. Plants 2020, 9, 88. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Stępień, P.; Kłbus, G. Water relations and photosynthesis in Cucumis sativus L. leaves under salt stress. Biol. Plant. 2006, 50, 610–616. [Google Scholar] [CrossRef]
- Rasouli, F.; Kiani-Pouya, A.; Tahir, A.; Shabala, L.; Chen, Z.; Shabala, S. A comparative analysis of stomatal traits and photosynthetic responses in closely related halophytic and glycophytic species under saline conditions. Environ. Exp. Bot. 2021, 181, 104300. [Google Scholar] [CrossRef]
- De La Rosa-Ibarra, M.; Maiti, R. Biochemical mechanism in glossy sorghum lines for resistance to salinity stress. J. Plant Physiol. 1995, 146, 515–519. [Google Scholar] [CrossRef]
- Ali, A.Y.A.; Ibrahim, M.E.H.; Zhou, G.; Nimir, N.E.A.; Elsiddig, A.M.I.; Jiao, X.; Zhu, G.; Salih, E.G.I.; Suliman, M.S.E.S.; Elradi, S.B.M. Gibberellic acid and nitrogen efficiently protect early seedlings growth stage from salt stress damage in Sorghum. Sci. Rep. 2021, 11, 6672. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M. The effect of NaCl on water relations, chlorophyll, and protein and proline contents of two cultivars of blackgram (Vigna mungo L.). Plant Soil 1989, 119, 205–210. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Arulbalachandran, D.; Ganesh, K.S.; Subramani, A. Changes in metabolites and antioxidant enzyme activity of three Vigna species induced by NaCl stress. Am.-Eurasian J. Agron. 2009, 2, 109–116. [Google Scholar]
- Mahmood, T.; Iqbal, N.; Raza, H.; Qasim, M.; Ashraf, M.Y. Growth modulation and ion partitioning in salt stressed sorghum (Sorghum bicolor L.) by exogenous supply of salicylic acid. Pak. J. Bot. 2010, 42, 3047–3054. [Google Scholar]
- Nimir, N.E.A.; Zhou, G.; Guo, W.; Ma, B.; Lu, S.; Wang, Y. Effect of foliar application of GA3, kinetin, and salicylic acid on ions content, membrane permeability, and photosynthesis under salt stress of sweet sorghum [Sorghum bicolor (L.) Moench]. Can. J. Plant Sci. 2016, 97, 525–535. [Google Scholar] [CrossRef]
- Nazar, R.; Iqbal, N.; Syeed, S.; Khan, N.A. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J. Plant Physiol. 2011, 168, 807–815. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Asgher, M.; Khan, N.A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol. Biochem. 2014, 80, 67–74. [Google Scholar] [CrossRef]
- Anosheh, H.P.; Emam, Y.; Ashraf, M.; Foolad, M. Exogenous application of salicylic acid and chlormequat chloride alleviates negative effects of drought stress in wheat. Adv. Stud. Biol. 2012, 4, 501–520. [Google Scholar]
- El-Tayeb, M. Response of barley grains to the interactive effect of salinity and salicylic acid. Plant Growth Regul. 2005, 45, 215–224. [Google Scholar] [CrossRef]
- Saxena, S.C.; Kaur, H.; Verma, P.; Petla, B.P.; Andugula, V.R.; Majee, M. Osmoprotectants: Potential for crop improvement under adverse conditions. In Plant Acclimation to Environmental Stress; Tuteja, N., Singh Gill, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 197–232. [Google Scholar]
- Matysik, J.A.; Bhalu, B.; Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002, 82, 525–532. [Google Scholar]
- Ben Ahmed, C.; Ben Rouina, B.; Sensoy, S.; Boukhriss, M.; Ben Abdullah, F. Exogenous proline effects on photosynthetic performance and antioxidant defense system of young olive tree. J. Agric. Food Chem. 2010, 58, 4216–4222. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.; Misra, R. Salicylic acid changes plant growth parameters and proline metabolism in Rauwolfia serpentina leaves grown under salinity stress. Am.-Eurasian J. Agric. Environ. Sci. 2012, 12, 1601–1609. [Google Scholar]
- Rejeb, K.B.; Abdelly, C.; Savouré, A. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 2014, 80, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Mundada, P.S.; Jadhav, S.V.; Salunkhe, S.S.; Gurme, S.T.; Umdale, S.D.; Nikam, T.D.; Ahire, M.L. Plant Performance and Defensive Role of Proline Under Environmental Stress. In Plant Performance Under Environmental Stress; Husen, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 201–223. [Google Scholar]
- Chai, Y.Y.; Jiang, C.D.; Shi, L.; Shi, T.S.; Gu, W.B. Effects of exogenous spermine on sweet sorghum during germination under salinity. Biol. Plant. 2010, 54, 145–148. [Google Scholar] [CrossRef]
- Eraslan, F.; Inal, A.; Gunes, A.; Alpaslan, M. Impact of exogenous salicylic acid on the growth, antioxidant activity and physiology of carrot plants subjected to combined salinity and boron toxicity. Sci. Hortic. 2007, 113, 120–128. [Google Scholar] [CrossRef]
- Durner, J.; Klessig, D.F. Salicylic acid is a modulator of tobacco and mammalian catalases. J. Biol. Chem. 1996, 271, 28492–28501. [Google Scholar] [CrossRef]
- Noreen, S.; Ashraf, M.; Akram, N.A. Does exogenous application of salicylic acid improve growth and some key physiological attributes in sunflower plants subjected to salt stress? J. Appl. Bot. Food Qual. 2012, 84, 169. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Alici, E.H.; Arabaci, G. Determination of SOD, POD, PPO and cat enzyme activities in Rumex obtusifolius L. Annu. Res. Rev. Biol. 2016, 11, 1–7. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Allen, S.E.; Grimshaw, H.M.; Rowland, A.P. Chemical Analysis. In Methods of Plant Ecology; Moore, P.D., Chapman, S.B., Eds.; Blackwell: Oxford, UK, 1986; pp. 285–344. [Google Scholar]



| Trait | Abbreviation | Sources of Variations | |||
|---|---|---|---|---|---|
| S | SA | S × SA | Error | ||
| df | 1 | 4 | 4 | 30 | |
| Ionic balance | K+/Na+ | 9768 * | 152 * | 20.3 * | 4.12 |
| Photosynthetic | Pn | 212 * | 131 * | 7.41 * | 0.686 |
| parameters | Ci | 397 * | 116 * | 1.12 n.s | 2.55 |
| Gs | 6184 * | 316 * | 49.9 * | 4.10 | |
| Photosynthetic | Chl a | 3.13 * | 0.217 * | 0.011 * | 0.002 |
| pigments | Chl b | 0.606 * | 0.016 * | 0.001 * | 0.003 |
| Chl a + b | 6.49 * | 0.353 * | 0.018 * | 0.003 | |
| Car | 0.194 * | 0.005 * | 0.003 * | 0.001 | |
| Antioxidant enzymes | CAT | 7.86 * | 0.462 * | 0.193 * | 0.002 |
| APX | 107 * | 9.08 * | 2.00 * | 0.106 | |
| SOD | 229 * | 13.8 * | 6.14 * | 0.012 | |
| Osmolytes | P | 2528 * | 259 * | 28.1 * | 3.17 |
| Growth | SDW | 1669 * | 211 * | 3.92 * | 0.511 |
| parameters | RDW | 5111 * | 264 * | 3.10 * | 0.631 |
| Trait | SDW | RDW | K+/Na+ | Chl a | Chl b | Chl a + b | P | CAT | APX | SOD | Pn | Gs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SDW | 1 | 0.984 * | 0.940 * | 0.993 * | 0.943 * | 0.988 * | 0.983 * | 0.987 * | 0.976 * | 0.981 * | 0.995 * | 0.969 * |
| RDW | 0.945 * | 1 | 0.951 * | 0.993 * | 0.943 * | 0.987 * | 0.992 * | 0.973 * | 0.990 * | 0.980 * | 0.975 * | 0.940 * |
| K+/Na+ | 0.871 n.s | 0.964 * | 1 | 0.936 * | 0.840 n.s | 0.922 * | 0.977 * | 0.890 * | 0.975 * | 0.891 * | 0.905 * | 0.840 n.s |
| Chl a | 0.994 * | 0.947 * | 0.875 n.s | 1 | 0.971 * | 0.998 * | 0.982 * | 0.991 * | 0.990 * | 0.993 * | 0.989 * | 0.955 * |
| Chl b | 0.998 * | 0.942 * | 0.866 n.s | 0.998 * | 1 | 0.979 * | 0.906 * | 0.978 * | 0.935 * | 0.985 * | 0.953 * | 0.919 * |
| Chl a + b | 0.994 * | 0.952 * | 0.885 * | 0.999 * | 0.997 * | 1 | 0.971 * | 0.994 * | 0.984 * | 0.996 * | 0.987 * | 0.952 * |
| P | 0.941 * | 0.870 n.s | 0.847 n.s | 0.920 * | 0.932 * | 0.920 * | 1 | 0.954 * | 0.993 * | 0.961 * | 0.970 * | 0.930 * |
| CAT | 0.993 * | 0.941 * | 0.866 n.s | 0.994 * | 0.998 * | 0.993 * | 0.946 * | 1 | 0.970 * | 0.997 * | 0.994 * | 0.975 * |
| APX | 0.951 * | 0.998 * | 0.963 * | 0.942 * | 0.941 * | 0.950 * | 0.887 * | 0.943 * | 1 | 0.970 * | 0.970 * | 0.921 * |
| SOD | 0.993 * | 0.942 * | 0.864 n.s | 0.986 * | 0.994 * | 0.986 * | 0.952 * | 0.998 * | 0.950 * | 1 | 0.987 * | 0.963 * |
| Pn | 0.785 n.s | 0.874 n.s | 0.820 n.s | 0.830 n.s | 0.802 n.s | 0.835 n.s | 0.576 n.s | 0.780 n.s | 0.844 n.s | 0.761 n.s | 1 | 0.985 * |
| Gs | 0.620 n.s | 0.590 n.s | 0.371 n.s | 0.590 n.s | 0.603 n.s | 0.581 n.s | 0.430 n.s | 0.610 n.s | 0.595 n.s | 0.630 n.s | 0.555 n.s | 1 |
| Month | Mean Temperature (°C) | Total Precipitation (mm) | Mean Relative Humidity (%) | ||
|---|---|---|---|---|---|
| Lowest | highest | Average | |||
| May | 11 | 29 | 20 | 16 | 31 |
| June | 17 | 35 | 26 | 6 | 23 |
| July | 20 | 38 | 29 | 3 | 22 |
| August | 18 | 37 | 27.5 | 0 | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajabi Dehnavi, A.; Zahedi, M.; Ludwiczak, A.; Piernik, A. Foliar Application of Salicylic Acid Improves Salt Tolerance of Sorghum (Sorghum bicolor (L.) Moench). Plants 2022, 11, 368. https://doi.org/10.3390/plants11030368
Rajabi Dehnavi A, Zahedi M, Ludwiczak A, Piernik A. Foliar Application of Salicylic Acid Improves Salt Tolerance of Sorghum (Sorghum bicolor (L.) Moench). Plants. 2022; 11(3):368. https://doi.org/10.3390/plants11030368
Chicago/Turabian StyleRajabi Dehnavi, Ahmad, Morteza Zahedi, Agnieszka Ludwiczak, and Agnieszka Piernik. 2022. "Foliar Application of Salicylic Acid Improves Salt Tolerance of Sorghum (Sorghum bicolor (L.) Moench)" Plants 11, no. 3: 368. https://doi.org/10.3390/plants11030368
APA StyleRajabi Dehnavi, A., Zahedi, M., Ludwiczak, A., & Piernik, A. (2022). Foliar Application of Salicylic Acid Improves Salt Tolerance of Sorghum (Sorghum bicolor (L.) Moench). Plants, 11(3), 368. https://doi.org/10.3390/plants11030368







