No Evidence for Light-Induced Embolism Repair in Cut Stems of Drought-Resistant Mediterranean Species under Soaking
Abstract
:1. Introduction
2. Results
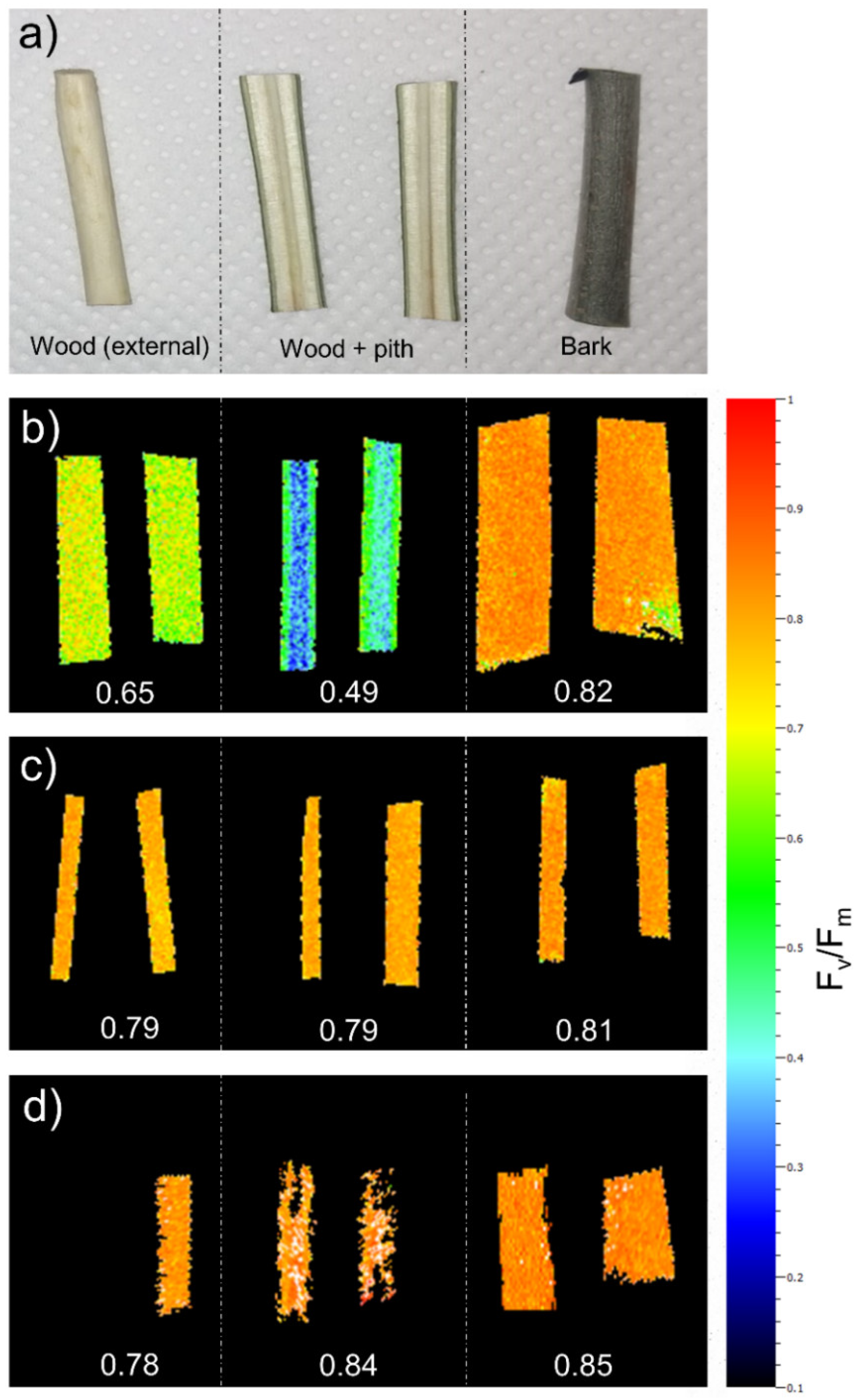
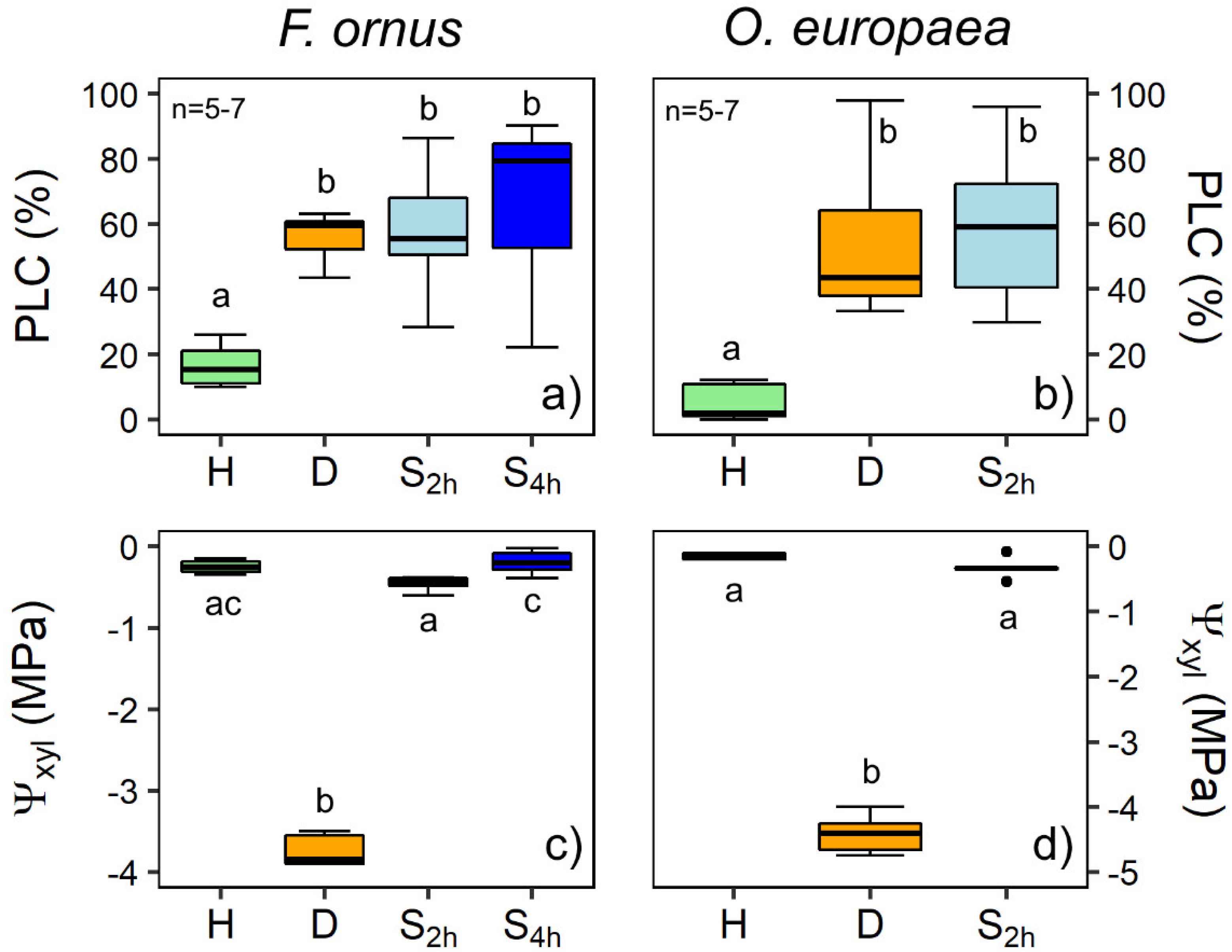
2.1. Hydraulic Measurements
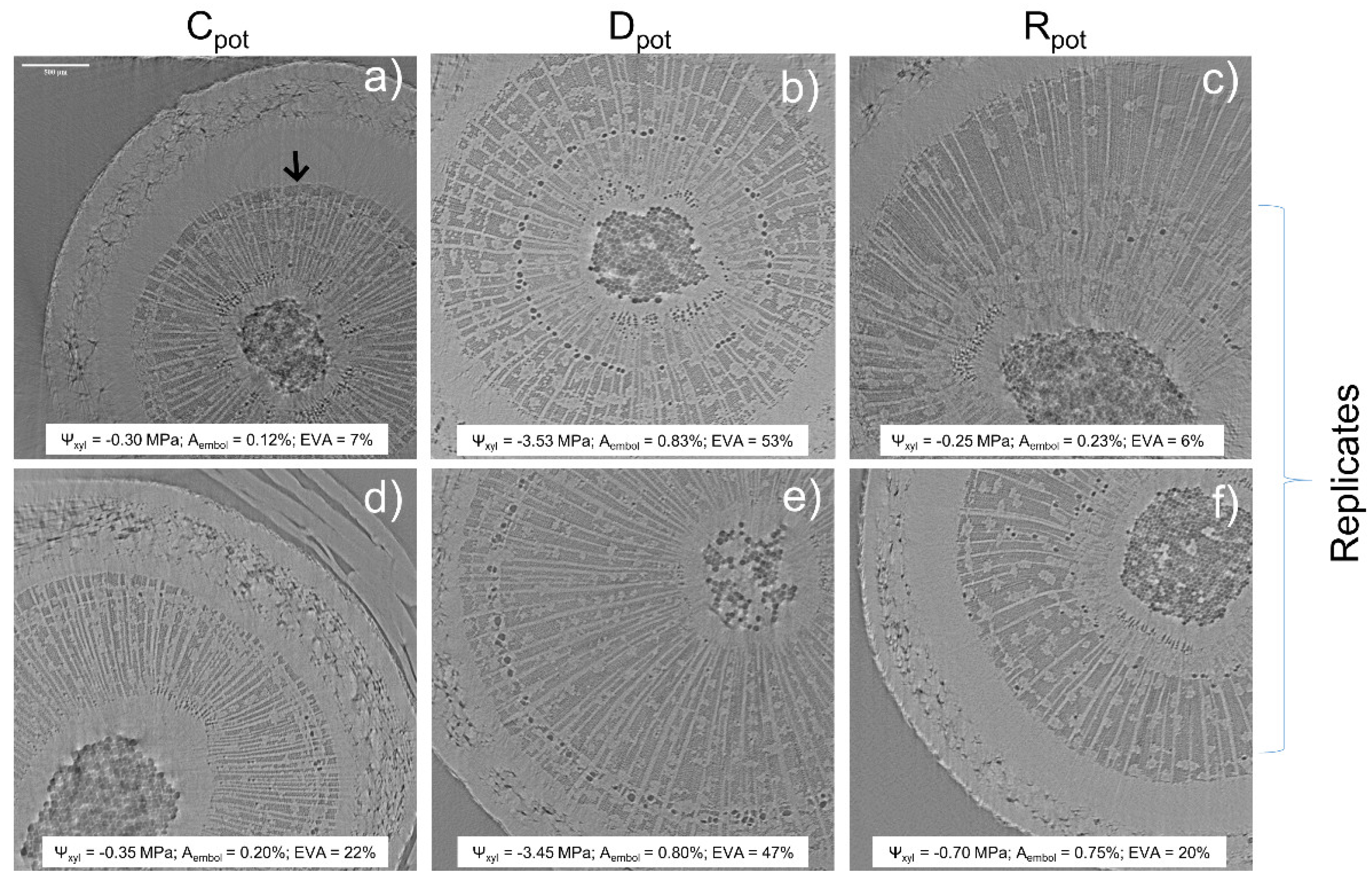
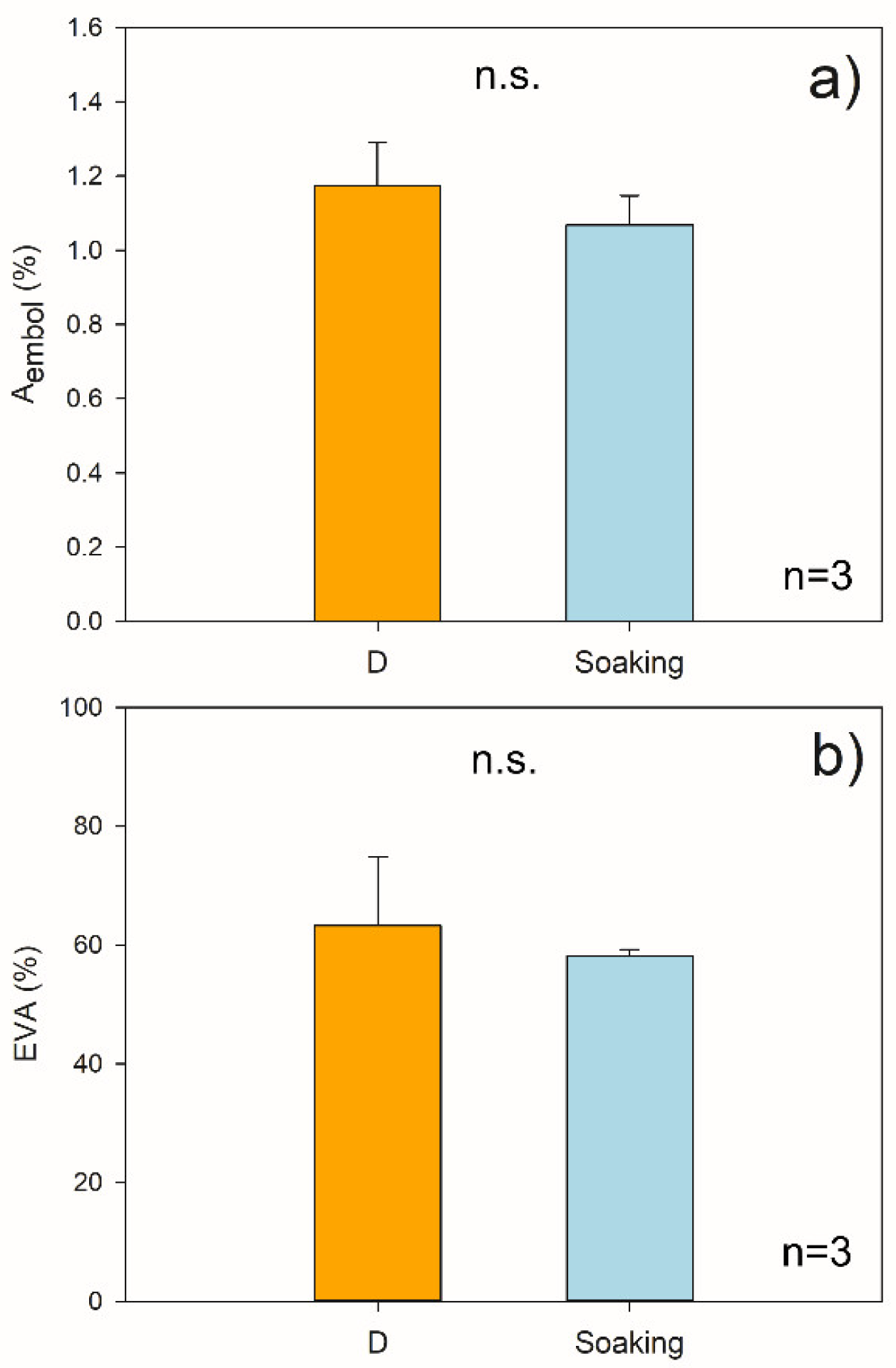
2.2. Micro-CT Analyses
3. Discussion
No Evidence for Hydraulic Recovery in Stem Segments upon Soaking
4. Materials and Methods
4.1. Plant Material
4.2. Hydraulic Measurements
4.3. Micro-CT Scans and Image Processing
4.4. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berry, Z.C.; Emery, N.C.; Gotsch, S.G.; Goldsmith, G.R. Foliar Water Uptake: Processes, Pathways, and Integration into Plant Water Budgets. Plant Cell Environ. 2019, 42, 410–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breshears, D.D.; McDowell, N.G.; Goddard, K.L.; Dayem, K.E.; Martens, S.N.; Meyer, C.W.; Brown, K.M. Foliar absorption of intercepted rainfall improves woody plant water status most during drought. Ecology 2008, 89, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.E.; Goldsmith, G.R. The Value of Wet Leaves. New Phytol. 2018, 219, 1156–1169. [Google Scholar] [CrossRef] [Green Version]
- Carmichael, M.J.; White, J.C.; Cory, S.T.; Berry, Z.C.; Smith, W.K. Foliar Water Uptake of Fog Confers Ecophysiological Benefits to Four Common Tree Species of Southeastern Freshwater Forested Wetlands. Ecohydrology 2020, 13, e2240. [Google Scholar] [CrossRef]
- Fernández, V.; Sancho-Knapik, D.; Guzmán, P.; Peguero-Pina, J.J.; Gil, L.; Karabourniotis, G.; Khayet, M.; Fasseas, C.; Heredia-Guerrero, J.A.; Heredia, A.; et al. Wettability, Polarity, and Water Absorption of Holm Oak Leaves: Effect of Leaf Side and Age. Plant Physiol. 2014, 166, 168–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreel, J.D.M.; Leroux, O.; Goossens, W.; Brodersen, C.; Rubinstein, A.; Steppe, K. Identifying the Pathways for Foliar Water Uptake in Beech (Fagus Sylvatica L.): A Major Role for Trichomes. Plant J. 2020, 103, 769–780. [Google Scholar] [CrossRef]
- Guzmán-Delgado, P.; Laca, E.; Zwieniecki, M.A. Unravelling Foliar Water Uptake Pathways: The Contribution of Stomata and the Cuticle. Plant Cell Environ. 2021, 44, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- Fuenzalida, T.I.; Bryant, C.J.; Ovington, L.I.; Yoon, H.; Oliveira, R.S.; Sack, L.; Ball, M.C. Shoot Surface Water Uptake Enables Leaf Hydraulic Recovery in Avicennia Marina. New Phytol. 2019, 224, 1504–1511. [Google Scholar] [CrossRef]
- Mayr, S.; Schmid, P.; Laur, J.; Rosner, S.; Charra-Vaskou, K.; Dämon, B.; Hacke, U.G. Uptake of Water via Branches Helps Timberline Conifers Refill Embolized Xylem in Late Winter. Plant Physiol. 2014, 164, 1731–1740. [Google Scholar] [CrossRef] [Green Version]
- Laur, J.; Hacke, U.G. Exploring Picea Glauca Aquaporins in the Context of Needle Water Uptake and Xylem Refilling. New Phytol. 2014, 203, 388–400. [Google Scholar] [CrossRef]
- Katz, C.; Oren, R.; Schulze, E.-D.; Milburn, J.A. Uptake of Water and Solutes through Twigs of Picea Abies (L.) Karst. Trees 1989, 3, 33–37. [Google Scholar] [CrossRef]
- Mason Earles, J.; Sperling, O.; Silva, L.C.; McElrone, A.J.; Brodersen, C.R.; North, M.P.; Zwieniecki, M.A. Bark Water Uptake Promotes Localized Hydraulic Recovery in Coastal Redwood Crown. Plant Cell Environ. 2016, 39, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gu, L.; Yu, Y.; Huang, P.; Wu, Z.; Zhang, Q.; Qian, Y.; Wan, X.; Sun, Z. Corticular Photosynthesis Drives Bark Water Uptake to Refill Embolized Vessels in Dehydrated Branches of Salix Matsudana. Plant Cell Environ. 2019, 42, 2584–2596. [Google Scholar] [CrossRef]
- Salleo, S.; Lo Gullo, M.A.; Trifilo, P.; Nardini, A. New Evidence for a Role of Vessel-Associated Cells and Phloem in the Rapid Xylem Refilling of Cavitated Stems of Laurus Nobilis L. Plant Cell Environ. 2004, 27, 1065–1076. [Google Scholar] [CrossRef]
- Zwieniecki, M.A.; Holbrook, N.M. Confronting Maxwell’s Demon: Biophysics of Xylem Embolism Repair. Trends Plant Sci. 2009, 14, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Secchi, F.; Pagliarani, C.; Zwieniecki, M.A. The Functional Role of Xylem Parenchyma Cells and Aquaporins during Recovery from Severe Water Stress: Response of Xylem Parenchyma Cells to Embolism. Plant Cell Environ. 2017, 40, 858–871. [Google Scholar] [CrossRef]
- Wheeler, J.K.; Huggett, B.A.; Tofte, A.N.; Rockwell, F.E.; Holbrook, N.M. Cutting Xylem under Tension or Supersaturated with Gas Can Generate PLC and the Appearance of Rapid Recovery from Embolism. Plant Cell Environ. 2013, 36, 1938–1949. [Google Scholar] [CrossRef]
- Trifilò, P.; Raimondo, F.; Lo Gullo, M.A.; Barbera, P.M.; Salleo, S.; Nardini, A. Relax and Refill: Xylem Rehydration Prior to Hydraulic Measurements Favours Embolism Repair in Stems and Generates Artificially Low PLC Values. Plant Cell Environ. 2014, 37, 2491–2499. [Google Scholar] [CrossRef]
- Sperry, J.S.; Donnelly, J.R.; Tyree, M.T. A Method for Measuring Hydraulic Conductivity and Embolism in Xylem. Plant Cell Environ 1988, 11, 35–40. [Google Scholar] [CrossRef]
- Brodersen, C.R.; McElrone, A.J.; Choat, B.; Matthews, M.A.; Shackel, K.A. The Dynamics of Embolism Repair in Xylem: In Vivo Visualizations Using High-Resolution Computed Tomography. Plant Physiol. 2010, 154, 1088–1095. [Google Scholar] [CrossRef] [Green Version]
- Secchi, F.; Pagliarani, C.; Cavalletto, S.; Petruzzellis, F.; Tonel, G.; Savi, T.; Tromba, G.; Obertino, M.M.; Lovisolo, C.; Nardini, A.; et al. Chemical Inhibition of Xylem Cellular Activity Impedes the Removal of Drought-induced Embolisms in Poplar Stems—New Insights from Micro-CT Analysis. New Phytol. 2020, 229, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Savi, T.; Miotto, A.; Petruzzellis, F.; Losso, A.; Pacilè, S.; Tromba, G.; Mayr, S.; Nardini, A. Drought-Induced Embolism in Stems of Sunflower: A Comparison of in Vivo Micro-CT Observations and Destructive Hydraulic Measurements. Plant Physiol. Biochem. 2017, 120, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Venturas, M.D.; Pratt, R.B.; Jacobsen, A.L.; Castro, V.; Fickle, J.C.; Hacke, U.G. Direct Comparison of Four Methods to Construct Xylem Vulnerability Curves: Differences among Techniques Are Linked to Vessel Network Characteristics. Plant Cell Environ. 2019, 42, 2422–2436. [Google Scholar] [CrossRef] [PubMed]
- Torres-Ruiz, J.M.; Jansen, S.; Choat, B.; McElrone, A.J.; Cochard, H.; Brodribb, T.J.; Badel, E.; Burlett, R.; Bouche, P.S.; Brodersen, C.R.; et al. Direct X-Ray Microtomography Observation Confirms the Induction of Embolism upon Xylem Cutting under Tension. Plant Physiol. 2015, 167, 40–43. [Google Scholar] [CrossRef] [Green Version]
- Tomasella, M.; Casolo, V.; Aichner, N.; Petruzzellis, F.; Savi, T.; Trifilò, P.; Nardini, A. Non-Structural Carbohydrate and Hydraulic Dynamics during Drought and Recovery in Fraxinus Ornus and Ostrya Carpinifolia Saplings. Plant Physiol. Biochem. 2019, 145, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Torres-Ruiz, J.M.; Cochard, H.; Mayr, S.; Beikircher, B.; Diaz-Espejo, A.; Rodriguez-Dominguez, C.M.; Badel, E.; Fernández, J.E. Vulnerability to Cavitation in Olea Europaea Current-Year Shoots: Further Evidence of an Open-Vessel Artifact Associated with Centrifuge and Air-Injection Techniques. Physiol. Plant. 2014, 152, 465–474. [Google Scholar] [CrossRef] [Green Version]
- Torres-Ruiz, J.M.; Cochard, H.; Choat, B.; Jansen, S.; López, R.; Tomášková, I.; Padilla-Díaz, C.M.; Badel, E.; Burlett, R.; King, A.; et al. Xylem Resistance to Embolism: Presenting a Simple Diagnostic Test for the Open Vessel Artefact. New Phytol. 2017, 215, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Zwieniecki, M.A.; Holbrook, N.M. Bordered Pit Structure and Vessel Wall Surface Properties. Implications for Embolism Repair. Plant Physiol. 2000, 123, 1015–1020. [Google Scholar] [CrossRef] [Green Version]
- Secchi, F.; Zwieniecki, M.A. Accumulation of Sugars in the Xylem Apoplast Observed under Water Stress Conditions Is Controlled by Xylem PH. Plant Cell Environ. 2016, 39, 2350–2360. [Google Scholar] [CrossRef]
- Burgess, S.S.O.; Dawson, T.E. The Contribution of Fog to the Water Relations of Sequoia Sempervirens (D. Don): Foliar Uptake and Prevention of Dehydration. Plant Cell Environ. 2004, 27, 1023–1034. [Google Scholar] [CrossRef]
- Losso, A.; Bär, A.; Unterholzner, L.; Bahn, M.; Mayr, S. Branch Water Uptake and Redistribution in Two Conifers at the Alpine Treeline. Sci. Rep. 2021, 11, 22560. [Google Scholar] [CrossRef] [PubMed]
- Ilek, A.; Siegert, C.M.; Wade, A. Hygroscopic Contributions to Bark Water Storage and Controls Exerted by Internal Bark Structure over Water Vapor Absorption. Trees 2021, 35, 831–843. [Google Scholar] [CrossRef]
- Schönherr, J.; Ziegler, H. Water Permeability of Betula Periderm. Planta 1980, 147, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Groh, B.; Hübner, C.; Lendzian, K. Water and Oxygen Permeance of Phellems Isolated from Trees: The Role of Waxes and Lenticels. Planta 2002, 215, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Nardini, A.; Lo Gullo, M.A.; Salleo, S. Refilling Embolized Xylem Conduits: Is It a Matter of Phloem Unloading? Plant Sci. 2011, 180, 604–611. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, L.; Zhang, S.; Cai, J.; Tyree, M.T. Water Relations of Robinia Pseudoacacia L.: Do Vessels Cavitate and Refill Diurnally or Are R-shaped Curves Invalid in Robinia? Plant Cell Environ. 2014, 37, 2667–2678. [Google Scholar] [CrossRef]
- Nardini, A.; Gasco, A.; Trifilo, P.; Lo Gullo, M.A.; Salleo, S. Ion-Mediated Enhancement of Xylem Hydraulic Conductivity Is Not Always Suppressed by the Presence of Ca2+ in the Sap. J. Exp. Bot. 2007, 58, 2609–2615. [Google Scholar] [CrossRef] [Green Version]
- Petruzzellis, F.; Pagliarani, C.; Savi, T.; Losso, A.; Cavalletto, S.; Tromba, G.; Dullin, C.; Bär, A.; Ganthaler, A.; Miotto, A.; et al. The Pitfalls of in Vivo Imaging Techniques: Evidence for Cellular Damage Caused by Synchrotron X-ray Computed Micro-Tomography. New Phytol. 2018, 220, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Brun, F.; Pacilè, S.; Accardo, A.; Kourousias, G.; Dreossi, D.; Mancini, L.; Tromba, G.; Pugliese, R. Enhanced and Flexible Software Tools for X-ray Computed Tomography at the Italian Synchrotron Radiation Facility Elettra. Fundam. Informaticae 2015, 141, 233–243. [Google Scholar] [CrossRef]
- Paganin, D.; Mayo, S.C.; Gureyev, T.E.; Miller, P.R.; Wilkins, S.W. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J. Microsc. 2002, 206, 33–40. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models. 2019. Available online: https://CRAN.R-project.org/package=nlme (accessed on 30 November 2021).
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.5.0. 2020. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 30 November 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomasella, M.; Natale, S.; Petruzzellis, F.; Di Bert, S.; D’Amico, L.; Tromba, G.; Nardini, A. No Evidence for Light-Induced Embolism Repair in Cut Stems of Drought-Resistant Mediterranean Species under Soaking. Plants 2022, 11, 307. https://doi.org/10.3390/plants11030307
Tomasella M, Natale S, Petruzzellis F, Di Bert S, D’Amico L, Tromba G, Nardini A. No Evidence for Light-Induced Embolism Repair in Cut Stems of Drought-Resistant Mediterranean Species under Soaking. Plants. 2022; 11(3):307. https://doi.org/10.3390/plants11030307
Chicago/Turabian StyleTomasella, Martina, Sara Natale, Francesco Petruzzellis, Sara Di Bert, Lorenzo D’Amico, Giuliana Tromba, and Andrea Nardini. 2022. "No Evidence for Light-Induced Embolism Repair in Cut Stems of Drought-Resistant Mediterranean Species under Soaking" Plants 11, no. 3: 307. https://doi.org/10.3390/plants11030307
APA StyleTomasella, M., Natale, S., Petruzzellis, F., Di Bert, S., D’Amico, L., Tromba, G., & Nardini, A. (2022). No Evidence for Light-Induced Embolism Repair in Cut Stems of Drought-Resistant Mediterranean Species under Soaking. Plants, 11(3), 307. https://doi.org/10.3390/plants11030307





