Biopotential of Underutilized Rosaceae Inflorescences: LC-DAD-MS Phytochemical Profiles Associated with Antioxidant, Antidiabetic, Anti-Inflammatory and Antiproliferative Activity In Vitro
Abstract
:1. Introduction
2. Results and Discussion
2.1. Spectrophotometric Analysis of the Phytochemical Content and Antioxidant Capacity of the Inflorescences
2.2. LC-DAD-MS Analysis of the Individual Phenolics in the Inflorescences
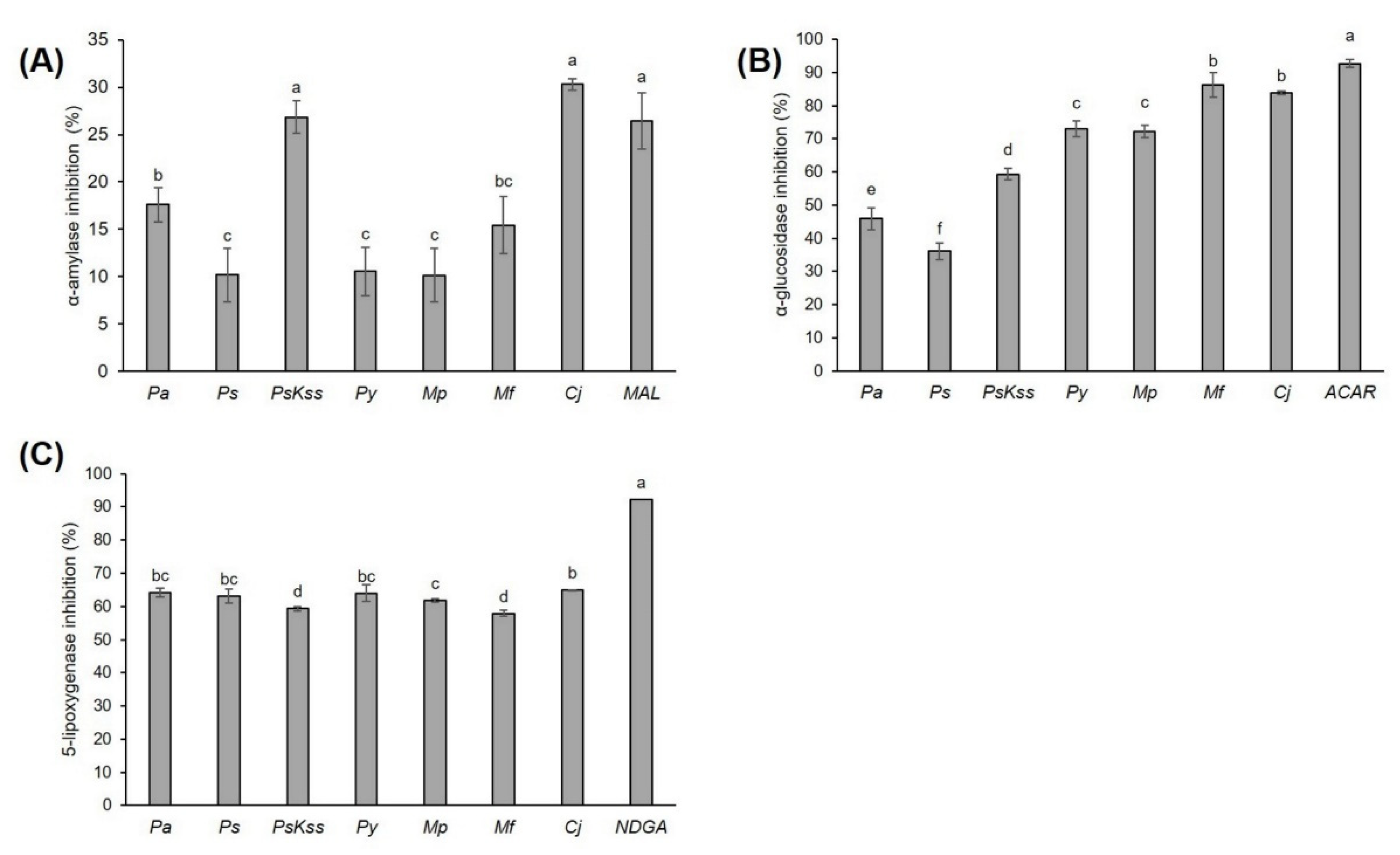
2.3. Antidiabetic Activity of the Inflorescences
2.4. Anti-Inflammatory Activity of Inflorescences
2.5. Cytotoxic Activity of the Inflorescences
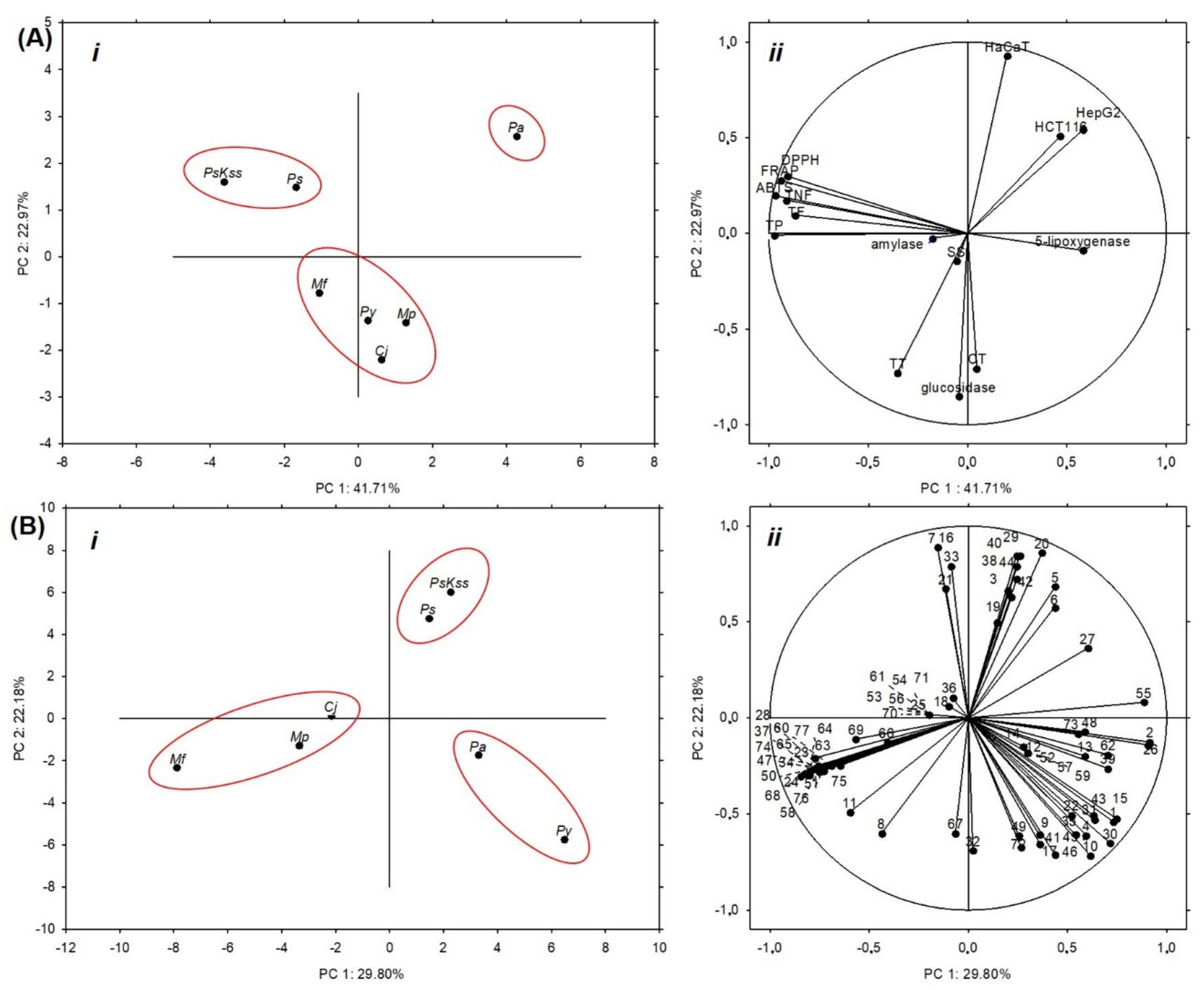
2.6. Statistical Analysis
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Extraction of the Phenolic Compounds
3.3. Spectrophotometric Determination of the Phytochemicals and Antioxidant Capacity
3.4. LC-DAD-MS Analysis
3.5. Effect of the Extracts on Antidiabetic (α-Amylase and α-Glucosidase) and Anti-Inflammatory (5-Lipoxygenase) Activity
3.6. In Vitro Antiproliferative Activity
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orlando, G.; Recinella, L.; Chiavaroli, A.; Brunetti, L.; Leone, S.; Carradori, S.; Di Simone, S.; Ciferri, M.C.; Zengin, G.; Ak, G.; et al. Water extract from inflorescences of industrial hemp futura 75 variety as a source of anti-inflammatory, anti-proliferative and antimycotic agents: Results from in silico, in vitro and ex vivo studies. Antioxidants 2020, 9, 437. [Google Scholar] [CrossRef]
- Lau, B.F.; Kong, K.W.; Leong, K.H.; Sun, J.; He, X.; Wang, Z.; Mustafa, M.R.; Ling, T.C.; Ismail, A. Banana inflorescence: Its bio-prospects as an ingredient for functional foods. Trends Food Sci. Technol. 2020, 97, 14–28. [Google Scholar] [CrossRef]
- Sarv, V.; Venskutonis, P.R.; Bhat, R. The Sorbus spp.—Underutilised plants for foods and nutraceuticals: Review on polyphenolic phytochemicals and antioxidant potential. Antioxidants 2020, 9, 813. [Google Scholar] [CrossRef]
- El Euch, S.K.; Bouajila, J.; Bouzouita, N. Chemical composition, biological and cytotoxic activities of Cistus salviifolius flower buds and leaves extracts. Ind. Crops Prod. 2015, 76, 1100–1105. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, J.S.; Kim, H.P.; Lee, L.-H.; Kang, S.S. Phenolic constituents from the flower buds of Lonicera japonica and their 5-lipoxygenase inhibitory activities. Food Chem. 2010, 120, 134–139. [Google Scholar] [CrossRef]
- Ferreres, F.; Andrade, C.; Gomes, N.G.; Andrade, P.B.; Gil-Izquierdo, A.; Pereira, D.M.; Suksungworn, R.; Duangsrisai, S.; Videira, R.A.; Valentão, P. Valorisation of kitul, an overlooked food plant: Phenolic profiling of fruits and inflorescences and assessment of their effects on diabetes-related targets. Food Chem. 2021, 342, 128323. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Michel, P. Antioxidant activity of inflorescences, leaves and fruits of three Sorbus species in relation to their polyphenolic composition. Nat. Prod. Res. 2009, 23, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- Jesus, F.; Gonçalves, A.C.; Alves, G.; Silva, L.R. Health benefits of Prunus avium plant parts: An unexplored source rich in phenolic compounds. Food Rev. Int. 2020. [Google Scholar] [CrossRef]
- Acero, N.; Gradillas, A.; Beltran, M.; García, A.; Muñoz Mingarro, D. Comparison of phenolic compounds profile and antioxidant properties of different sweet cherry (Prunus avium L.) varieties. Food Chem. 2019, 279, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Usenik, V.; Fabčič, J.; Štampar, F. Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.). Food Chem. 2008, 107, 185–192. [Google Scholar] [CrossRef]
- Kang, G.-J.; Lee, H.-J.; Yoon, W.-J.; Yang, E.-J.; Park, S.-S.; Kang, H.-K.; Park, M.-H.; Yoo, E.-S. Prunus yedoensis inhibits the inflammatory chemokines, MDC and TARC, by regulating the STAT1-signaling pathway in IFN-γ-stimulated HaCaT human keratinocytes. Biomol. Ther. 2008, 16, 394–402. [Google Scholar] [CrossRef] [Green Version]
- Teleszko, M.; Wojdyło, A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Zhang, X.; Su, M.; Du, J.; Zhou, H.; Li, X.; Li, X.; Ye, Z. Comparison of phytochemical differences of the pulp of different peach (Prunus persica (L.) Batsch) cultivars with alpha-glucosidase inhibitory activity variations in China using UPLC-Q-TOF/MS. Molecules 2019, 24, 1968. [Google Scholar] [CrossRef] [Green Version]
- Wojdyło, A.; Nowicka, P.; Grimalt, M.; Legua, P.; Almansa, M.S.; Amorós, A.; Carbonell-Barrachina, A.; Hernández, F. Polyphenol compounds and biological activity of caper (Capparis spinosa L.) flowers buds. Plants 2019, 8, 539. [Google Scholar] [CrossRef] [Green Version]
- Borneo, R.; León, A.E.; Aguirre, A.; Ribotta, P.; Cantero, J.J. Antioxidant capacity of medicinal plants from the Province of Córdoba (Argentina) and their in vitro testing in a model food system. Food Chem. 2009, 112, 664–670. [Google Scholar] [CrossRef]
- Afonso, S.; Oliveira, I.V.; Meyer, A.S.; Aires, A.; Saavedra, M.J.; Gonçalves, B. Phenolic profile and bioactive potential of stems and seed kernels of sweet cherry fruit. Antioxidants 2020, 9, 1295. [Google Scholar] [CrossRef]
- Yang, S.; Meng, Z.; Li, Y.; Chen, R.; Yang, Y.; Zhao, Z. Evaluation of physiological characteristics, soluble sugars, organic acids and volatile compounds in ‘Orin’ apples (Malus domestica) at different ripening stages. Molecules 2021, 26, 807. [Google Scholar] [CrossRef]
- Walker, R.P.; Battistelli, A.; Bonghi, C.; Drincovich, M.F.; Falchi, R.; Lara, M.V.; Moscatello, S.; Vizzotto, G.; Famiani, F. Non-structural carbohydrate metabolism in the flesh of stone fruits of the genus Prunus (Rosaceae)—A review. Front. Plant Sci. 2020, 11, 549921. [Google Scholar] [CrossRef]
- Bastos, C.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Queiroz, M.J.R.; Santos-Buelga, C.; Ferreira, I.C. Chemical characterisation and bioactive properties of Prunus avium L.: The widely studied fruits and the unexplored stems. Food Chem. 2015, 173, 1045–1053. [Google Scholar] [CrossRef] [Green Version]
- Turkiewicz, I.P.; Wojdyło, A.; Tkacz, K.; Nowicka, P.; Golis, T.; Bąbelewski, P. ABTS On-line antioxidant, α-amylase, α-glucosidase, pancreatic lipase, acetyl-and butyrylcholinesterase inhibition activity of Chaenomeles fruits determined by polyphenols and other chemical compounds. Antioxidants 2020, 9, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, L.; Meng, W.; Min, W. Phenolic compounds and antioxidant activities of flowers, leaves and fruits of five crabapple cultivars (Malus Mill. species). Sci. Hortic. 2018, 235, 460–467. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Bento, C.; Silva, B.M.; Silva, L.R. Sweet cherries from Fundão possess antidiabetic potential and protect human erythrocytes against oxidative damage. Food Res. Int. 2017, 95, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, B.; Landbo, A.K.; Knudsen, D.; Silva, A.P.; Moutinho-Pereira, J.; Rosa, E.; Meyer, A.S. Effect of ripeness and postharvest storage on the phenolic profiles of cherries (Prunus avium L.). J. Agric. Food Chem. 2004, 52, 523–530. [Google Scholar] [CrossRef]
- Olszewska, M. Quantitative HPLC analysis of flavonoids and chlorogenic acid in the leaves and inflorescences of Prunus serotina Ehrh. Acta Chromatogr. 2007, 19, 253–269. [Google Scholar]
- Olszewska, M.A.; Kwapisz, A. Metabolite profiling and antioxidant activity of Prunus padus L. flowers and leaves. Nat. Prod. Res. 2011, 25, 1115–1131. [Google Scholar] [CrossRef]
- Coklar, H.; Akbulut, M.; Alhassan, I.; Kirpitci, Ş.; Korkmaz, E. Organic acids, sugars, phenolic compounds and antioxidant activity of Malus floribunda coccinella fruit, peel and flesh. Acta Sci. Pol. Hortorum Cultus 2018, 17, 47–59. [Google Scholar] [CrossRef]
- Du, H.; Wu, J.; Li, H.; Zhong, P.-X.; Xu, Y.-J.; Li, C.-H.; Ji, K.-X.; Wang, L.-S. Polyphenols and triterpenes from Chaenomeles fruits: Chemical analysis and antioxidant activities assessment. Food Chem. 2013, 141, 4260–4268. [Google Scholar] [CrossRef] [PubMed]
- Valcheva-Kuzmanova, S.V.; Denev, P.N.; Ognyanov, M.H. Chemical composition and antioxidant activity of Chaenomeles maulei fruit juice. J. Biomed. Clin. Res. 2018, 11, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, S.M.; Koehnlein, E.A.; Bracht, A.; Castoldi, R.; de Morais, G.R.; Baesso, M.L.; Peralta, R.; de Souza, C.G.M.; de Sá-Nakanishi, A.B.; Peralta, R.M. Inhibition of salivary and pancreatic α-amylases by a pinhão coat (Araucaria angustifolia) extract rich in condensed tannin. Food Res. Int. 2014, 56, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mkandawire, N.L.; Kaufman, R.C.; Bean, S.R.; Weller, C.L.; Jackson, D.S.; Rose, D.J. Effects of sorghum (Sorghum bicolor (L.) Moench) tannins on alpha-amylase activity and in vitro digestibility of starch in raw and processed flours. J. Agric. Food Chem. 2013, 61, 4448–4454. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef]
- Assefa, S.T.; Yang, E.Y.; Chae, S.Y.; Song, M.; Lee, J.; Cho, M.C.; Jang, S. Alpha glucosidase inhibitory activities of plants with focus on common vegetables. Plants 2020, 9, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Sachan, R.; Rahman, M.; Sharma, K.; Al-Abassi, F.A.; Anwar, F. Prunus amygdalus extract exert antidiabetic effect via inhibition of DPP-IV: In-silico and in-vivo approaches. J. Biomol. Struct. Dyn. 2021, 39, 4160–4174. [Google Scholar] [CrossRef]
- Meier, J.J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2012, 8, 728–742. [Google Scholar] [CrossRef]
- Seino, Y.; Fukushima, M.; Yabe, D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J. Diabetes Investig. 2010, 1, 8–23. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.K.; Xu, S.; Li, S.; Zhang, Y. Proanthocyanidins should be a candidate in the treatment of cancer, cardiovascular diseases and lipid metabolic disorder. Molecules 2020, 25, 5971. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Q.; Gao, Y.; Granato, D. Effects of epigallocatechin gallate, epigallocatechin and epicatechin gallate on the chemical and cell-based antioxidant activity, sensory properties, and cytotoxicity of a catechin-free model beverage. Food Chem. 2021, 339, 128060. [Google Scholar] [CrossRef]
- Muccilli, V.; Cardullo, N.; Spatafora, C.; Cunsolo, V.; Tringali, C. α-Glucosidase inhibition and antioxidant activity of an oenological commercial tannin. Extraction, fractionation and analysis by HPLC/ESI-MS/MS and 1H NMR. Food Chem. 2017, 215, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Poljuha, D.; Šola, I.; Bilić, J.; Dudaš, S.; Bilušić, T.; Markić, J.; Rusak, G. Phenolic composition, antioxidant capacity, energy content and gastrointestinal stability of Croatian wild edible plants. Eur. Food Res. Technol. 2015, 241, 573–585. [Google Scholar] [CrossRef]
- Šola, I.; Vujčić Bok, V.; Dujmović, M.; Rusak, G. Developmentally-related changes in phenolic and L-ascorbic acid content, and antioxidant capacity of Chinese cabbage sprouts. J. Food Sci. Technol. 2020, 57, 702–712. [Google Scholar] [CrossRef]
- Šola, I.; Vujčić Bok, V.; Pinterić, M.; Auer, S.; Ludwig-Müller, J.; Rusak, G. Improving the phytochemical profile and bioactivity of Chinese cabbage sprouts by interspecific transfer of metabolites. Food Res. Int. 137, 109726. [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Samoticha, J.; Eler, K.; Stampar, F.; Veberic, R. Traditional elderflower beverages: A rich source of phenolic compounds with high antioxidant activity. J. Agric. Food Chem. 2015, 63, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, M.A.H.; Ismail, A.; Kassim, N.K.; Hamid, M.; Ali, M.S.M. Phenolic profiling and evaluation of in vitro antioxidant, α-glucosidase and α-amylase inhibitory activities of Lepisanthes fruticosa (Roxb) Leenh fruit extracts. Food Chem. 2020, 331, 127240. [Google Scholar] [CrossRef] [PubMed]
| Prunus avium | Prunus serrulata | Prunus serrulata ‘Kiku Shidare Zakura’ | Prunus yedoensis | Malus purpurea | Malus floribunda | Chaenomeles japonica | |
|---|---|---|---|---|---|---|---|
| TP (mg GAE/g DW) | 27.83 ± 0.69 f | 46.84 ± 0.75 b | 53.12 ± 0.79 a | 37.31 ± 0.48 e | 40.29 ± 0.64 c | 46.74 ± 0.93 b | 39.42 ± 0.97 d |
| TF (mg CE/g DW) | 13.57 ± 0.84 f | 32.35 ± 2.13 b | 38.89 ± 4.04 a | 25.78 ± 1.03 d | 13.43 ± 0.82 f | 23.83 ± 0.88 e | 29.45 ± 0.65 c |
| TNF (mg GAE/g DW) | 16.40 ± 0.91 g | 29.35 ± 0.83 b | 31.32 ± 0.71 a | 22.21 ± 0.86 e | 23.52 ± 0.56 d | 28.85 ± 0.58 c | 18.73 ± 0.48 f |
| TT (mg CE/g DW) | 27.26 ± 0.22 g | 59.44 ± 1.32 f | 71.59 ± 0.33 d | 83.55 ± 0.55 b | 64.32 ± 0.45 e | 107.85 ± 1.09 a | 80.27 ± 0.33 c |
| CT (mg CE/g DW) | 4.25 ± 0.33 e | 7.74 ± 1.02 d | 6.99 ± 0.17 d | 16.52 ± 0.01 b | 15.45 ± 0.10 b | 10.98 ± 0.24 c | 51.68 ± 0.38 a |
| SS (mg SE/g DW) | 3.37 ± 0.06 c | 3.04 ± 0.05 d | 2.41 ± 0.10 e | 8.61 ± 0.12 a | 1.56 ± 0.08 f | 3.58 ± 0.06 b | 3.27 ± 0.07 c |
| ABTS (mg TE/g DW) | 22.86 ± 4.61 e | 49.41 ± 7.23 b | 61.32 ± 5.84 a | 36.63 ± 4.32 c | 28.78 ± 2.52 d | 47.78 ± 6.26 b | 35.05 ± 4.25 c |
| FRAP (mg TE/g DW) | 27.89 ± 0.60 g | 51.68 ± 0.12 b | 58.06 ± 0.78 a | 40.28 ± 1.18 d | 29.12 ± 0.83 f | 44.36 ± 0.85 c | 36.36 ± 1.50 e |
| DPPH (mg TE/g DW) | 25.47 ± 2.57 d | 52.95 ± 4.22 b | 69.42 ± 3.27 a | 39.21 ± 4.86 c | 25.10 ± 3.25 d | 40.38 ± 3.4 c | 39.61 ± 3.95 c |
| Prunus avium | Prunus serrulata | Prunus serrulata ‘Kiku Shidare Zakura’ | Prunus yedoensis | Malus purpurea | Malus floribunda | Chaenomeles japonica | ||
|---|---|---|---|---|---|---|---|---|
| 1 | Gallic acid | 0.28 ± 0.06 b | 0.15 ± 0.04 c | 0.06 ± 0.01 d | 0.38 ± 0.03 a | 0.17 ± 0.04 c | 0.03 ± 0.01 d | nd |
| Total identified hydroxybenzoic acids | 0.28 ± 0.06 b | 0.15 ± 0.04 c | 0.06 ± 0.01 d | 0.38 ± 0.03 a | 0.17 ± 0.04 c | 0.03 ± 0.01 d | nd | |
| 2 | Caffeic acid | 1.95 ± 0.27 c | 1.25 ± 0.14 c | 2.50 ± 0.08 b | 3.90 ± 0.10 a | nd | nd | 0.53 ± 0.08 d |
| 3 | Caffeic acid hexoside 1 | 0.14 ± 0.03 b | 0.39 ± 0.06 b | 15.48 ± 2.23 a | 0.10 ± 0.01 b | 0.05 ± 0.01 b | 0.23 ± 0.02 b | 0.75 ± 0.04 b |
| 4 | Caffeic acid hexoside 2 | nd | nd | nd | 5.62 ± 0.25 a | nd | nd | 0.02 ± 0.00 b |
| 5 | Caffeic acid dihexoside | 0.29 ± 0.01 b | 0.19 ± 0.04 c | 0.41 ± 0.02 a | nd | nd | nd | nd |
| 6 | 3-caffeoylquinic acid | nd | 0.36 ± 0.04 b | 1.70 ± 0.25 a | 0.54 ± 0.03 b | nd | nd | 0.02 ± 0.00 c |
| 7 | 4-caffeoylquinic acid | nd | 0.27 ± 0.08 a | 0.35 ± 0.02 a | nd | 0.13 ± 0.06 b | 0.15 ± 0.02 b | 0.07 ± 0.01 b |
| 8 | 5-caffeoylquinic acid 1 | 1.59 ± 0.18 d | 0.59 ± 0.17 e | 0.47 ± 0.02 e | 5.75 ± 0.15 c | 1.41 ± 0.30 d | 8.41 ± 0.69 a | 7.04 ± 0.23 b |
| 9 | 5-caffeoylquinic acid 2 | 0.43 ± 0.36 a | nd | nd | 0.27 ± 0.01 a | 0.31 ± 0.13 a | nd | 0.14 ± 0.02 a |
| 10 | di-caffeoylquinic acid 1 | 3.12 ± 0.08 b | 0.15 ± 0.03 c | 0.31 ± 0.02 c | 7.06 ± 0.82 a | 0.65 ± 0.14 c | 0.50 ± 0.03 c | 2.83 ± 0.30 b |
| 11 | di-caffeoylquinic acid 2 | 0.13 ± 0.01 b | nd | nd | 0.29 ± 0.01 b | 0.16 ± 0.06 b | 1.02 ± 0.15 a | nd |
| 12 | di-caffeoylquinic acid 3 | 0.17 ± 0.03 a | nd | nd | nd | nd | nd | nd |
| 13 | 3-feruloylquinic acid | 0.24 ± 0.04 a | 0.03 ± 0.00 c | 0.05 ± 0.00 bc | 0.08 ± 0.00 b | nd | nd | 0.004 ± 0.001 c |
| 14 | 5-feruloylquinic acid | 0.26 ± 0.03 a | 0.06 ± 0.01 d | 0.11 ± 0.00 bc | 0.09 ± 0.01 cd | 0.01 ± 0.00 e | 0.13 ± 0.04 b | 0.01 ± 0.00 e |
| 15 | 3-p-coumaroylquinic acid | 0.52 ± 0.08 b | 0.14 ± 0.02 d | 0.21 ± 0.01 c | 0.73 ± 0.03 a | 0.03 ± 0.01 e | 0.15 ± 0.01 cd | 0.001 ± 0.000 e |
| 16 | 4-p-coumaroylquinic acid | nd | 0.27 ± 0.08 a | 0.35 ± 0.02 a | nd | 0.13 ± 0.06 b | 0.15 ± 0.02 b | 0.07 ± 0.02 b |
| 17 | 5-p-coumaroylquinic acid 1 | 0.39 ± 0.02 a | 0.09 ± 0.01 d | 0.07 ± 0.00 d | 0.33 ± 0.03 b | 0.10 ± 0.01 d | 0.19 ± 0.03 c | 0.12 ± 0.03 d |
| 18 | 5-p-coumaroylquinic acid 2 | 0.03 ± 0.00 c | 0.09 ± 0.01 b | 0.08 ± 0.01 bc | 0.10 ± 0.01 b | 0.07 ± 0.06 bc | 0.06 ± 0.02 bc | 0.25 ± 0.04 a |
| 19 | p-coumaric acid hexoside 1 | 0.23 ± 0.06 b | 0.21 ± 0.04 b | 0.31 ± 0.01 a | 0.09 ± 0.00 c | 0.04 ± 0.01 cd | 0.22 ± 0.04 b | 0.001 ± 0.000 d |
| 20 | p-coumaric acid hexoside 2 | 0.37 ± 0.06 b | 1.42 ± 0.15 a | 1.34 ± 0.05 a | 0.21 ± 0.01 c | 0.02 ± 0.00 d | 0.11 ± 0.01 cd | 0.13 ± 0.00 cd |
| Total identified hydroxycinnamic acids | 9.87 ± 0.08 b | 5.51 ± 0.06 c | 23.70 ± 0.18 a | 25.16 ± 0.10 a | 3.12 ± 0.06 d | 11.31 ± 0.09 b | 11.93 ± 0.05 b | |
| 21 | Catechin | 0.23 ± 0.04 e | 2.61 ± 0.27 a | 2.46 ± 0.09 a | 0.91 ± 0.02 d | nd | 1.99 ± 0.16 b | 1.67 ± 0.06 c |
| 22 | Epicatechin | 1.10 ± 0.15 d | 0.91 ± 0.10 d | 1.81 ± 0.06 b | 6.92 ± 0.18 a | 1.18 ± 0.18 d | 1.38 ± 0.22 c | 0.40 ± 0.06 e |
| Total identified flavanols | 1.33 ± 0.09 e | 3.52 ± 0.18 cd | 4.27 ± 0.07 b | 7.83 ± 0.10 a | 1.18 ± 0.18 e | 3.38 ± 0.19 bc | 2.08 ± 0.06 de | |
| 23 | Eriodictyol hexoside 1 | 0.03 ± 0.00 b | nd | nd | nd | 0.63 ± 0.04 b | 2.92 ± 0.86 a | nd |
| 24 | Eriodictyol hexoside 2 | nd | nd | nd | nd | 0.35 ± 0.09 b | 1.02 ± 0.15 a | nd |
| 25 | Naringenin hexoside | nd | nd | nd | nd | nd | nd | 0.62 ± 0.04 a |
| Total identified flavanones | 0.03 ± 0.00 b | nd | nd | nd | 0.98 ± 0.06 b | 3.93 ± 0.50 a | 0.62 ± 0.04 b | |
| 26 | Quercetin-glycoside | 0.18 ± 0.01 c | 0.13 ± 0.01 d | 0.21 ± 0.02 b | 0.36 ± 0.02 a | nd | nd | nd |
| 27 | Quercetin-3-rutinoside | 2.78 ± 0.28 b | 0.58 ± 0.10 d | 4.79 ± 0.13 a | 1.93 ± 0.03 c | 0.08 ± 0.03 e | 0.21 ± 0.03 e | 0.72 ± 0.04 d |
| 28 | Quercetin-3-rhamnoside hexoside | nd | nd | nd | nd | 0.58 ± 0.21 a | 0.41 ± 0.06 a | nd |
| 29 | Quercetin-hexoside pentoside | nd | 0.25 ± 0.08 b | 0.59 ± 0.01 a | nd | nd | nd | nd |
| 30 | Quercetin-rhamnoside dihexoside 1 | 0.15 ± 0.01 b | nd | nd | 0.20 ± 0.00 a | nd | nd | nd |
| 31 | Quercetin-rhamnoside dihexoside 2 | nd | 0.02 ± 0.01 c | 0.05 ± 0.00 b | 0.47 ± 0.01 a | nd | nd | nd |
| 32 | Quercetin-3-galactoside | 0.26 ± 0.02 c | nd | nd | 0.98 ± 0.01 b | 1.21 ± 0.27 a | 0.309 ± 0.070 c | 0.17 ± 0.01 c |
| 33 | Quercetin-3-glucoside | 0.03 ± 0.00 d | 0.19 ± 0.02 a | 0.12 ± 0.01 b | nd | 0.02 ± 0.00 d | 0.089 ± 0.004 c | 0.02 ± 0.00 d |
| 34 | Quercetin-3-rhamnoside | nd | nd | nd | nd | 2.26 ± 0.38 b | 4.31 ± 0.51 a | nd |
| 35 | Quercetin-3-xyloside | 0.01 ± 0.00 e | 0.02 ± 0.00 de | 0.06 ± 0.00 c | 0.56 ± 0.04 a | 0.09 ± 0.01 b | 0.05 ± 0.01 cd | 0.02 ± 0.00 de |
| 36 | Quercetin-arabinofuranoside | 0.13 ± 0.04 c | 0.15 ± 0.02 c | 0.25 ± 0.03 b | 0.17 ± 0.00 c | 0.03 ± 0.01 d | 0.31 ± 0.04 a | 0.004 ± 0.000 d |
| 37 | Quercetin-arabinopyranoside | 0.01 ± 0.00 c | nd | nd | nd | 2.07 ± 0.20 a | 1.61 ± 0.05 b | nd |
| 38 | Quercetin-acetyl hexoside 1 | nd | 4.00 ± 0.40 a | 1.53 ± 0.07 b | 0.22 ± 0.00 c | nd | nd | nd |
| 39 | Quercetin-acetyl hexoside 2 | nd | 0.14 ± 0.02 b | 0.08 ± 0.00 c | 0.35 ± 0.02 a | nd | nd | nd |
| 40 | Kaempferol trihexoside | nd | 1.27 ± 0.11 a | 0.81 ± 0.08 b | nd | nd | nd | nd |
| 41 | Kaempferol-3-rutinoside | 0.92 ± 0.02 a | 0.06 ± 0.00 f | 0.05 ± 0.01 f | 0.54 ± 0.01 b | 0.37 ± 0.08 c | 0.24 ± 0.03 d | 0.15 ± 0.01 e |
| 42 | Kaempferol acetyl hexoside 1 | nd | 1.50 ± 0.15 a | 0.32 ± 0.01 b | 0.09 ± 0.01 a | nd | nd | nd |
| 43 | Kaempferol acetyl hexoside 2 | nd | 0.07 ± 0.01 a | nd | 0.33 ± 0.04 a | nd | nd | nd |
| 44 | Kaempferol dihexoside | nd | 0.12 ± 0.01 b | 0.44 ± 0.03 a | nd | nd | nd | nd |
| 45 | Kaempferol pentoside 1 | nd | nd | nd | 1.34 ± 0.09 a | nd | nd | nd |
| 46 | Kaempferol pentoside 2 | nd | nd | nd | 0.12 ± 0.01 a | nd | nd | nd |
| 47 | Kaempferol rhamnoside | 0.06 ± 0.01 b | nd | nd | nd | 0.32 ± 0.08 b | 4.43 ± 0.63 a | nd |
| 48 | Kaempferol hexoside 1 | 0.01 ± 0.00 e | 0.20 ± 0.02 b | 0.08 ± 0.00 d | 0.27 ± 0.02 a | 0.03 ± 0.01 e | 0.02 ± 0.00 e | 0.14 ± 0.01 c |
| 49 | Kaempferol hexoside 2 | 0.69 ± 0.23 a | nd | nd | 0.38 ± 0.01 b | 0.01 ± 0.00 c | 0.34 ± 0.04 b | nd |
| 50 | Kaempferol rhamnosyl hexoside | nd | nd | nd | nd | 0.02 ± 0.01 b | 0.22 ± 0.03 a | nd |
| 51 | Isorhamnetin hexoside | nd | 0.01 ± 0.00 c | 0.04 ± 0.00 c | 0.04 ± 0.00 c | 0.28 ± 0.04 b | 3.12 ± 0.21 a | 0.22 ± 0.00 b |
| 52 | Isorhamnetin dihexoside | 0.31 ± 0.02 a | nd | nd | nd | nd | nd | nd |
| 53 | Isorhamnetin acetyl hexoside 1 | nd | nd | nd | nd | nd | nd | 0.98 ± 0.12 a |
| 54 | Isorhamnetin acetyl hexoside 2 | nd | nd | nd | nd | nd | nd | 0.04 ± 0.00 a |
| 55 | Isorhamnetin-3-rutinoside | 0.02 ± 0.00 a | 0.01 ± 0.00 c | 0.02 ± 0.00 b | 0.02 ± 0.00 b | nd | nd | nd |
| 56 | Myricetin rutinoside | nd | nd | nd | nd | nd | nd | 0.004 ± 0.000 a |
| 57 | Laricitrin glucuronide | 0.07 ± 0.01 a | nd | nd | nd | nd | nd | nd |
| 58 | Syringetin hexoside 1 | 0.02 ± 0.00 b | nd | nd | nd | 0.30 ± 0.03 b | 5.18 ± 0.62 a | nd |
| 59 | Syringetin hexoside 2 | 0.29 ± 0.10 a | nd | nd | nd | nd | nd | nd |
| 60 | Syringetin acetyl hexoside 1 | nd | nd | nd | nd | 1.61 ± 0.41 a | 0.78 ± 0.14 b | 0.30 ± 0.02 b |
| 61 | Syringetin acetyl hexoside 2 | nd | nd | nd | nd | nd | nd | 0.07 ± 0.00 a |
| Total identified flavonols | 5.93 ± 0.05 b | 8.71 ± 0.06 b | 9.42 ± 0.03 b | 8.37 ± 0.02 b | 9.29 ± 0.11 b | 21.60 ± 0.16 a | 2.84 ± 0.02 c | |
| 62 | Apigenin hexoside | nd | 0.02 ± 0.00 b | 0.01 ± 0.00 b | 0.04 ± 0.00 a | nd | nd | nd |
| Total identified flavones | nd | 0.02 ± 0.00 b | 0.01 ± 0.00 b | 0.04 ± 0.00 a | nd | nd | nd | |
| 63 | Phloretin xylosylglucoside | nd | nd | nd | nd | 0.18 ± 0.03 a | 0.09 ± 0.02 b | nd |
| 64 | Phloridzin | nd | nd | nd | nd | 5.14 ± 0.74 a | 5.23 ± 0.52 a | nd |
| 65 | Trilobatin | nd | nd | nd | nd | 0.30 ± 0.08 b | 1.47 ± 0.20 a | nd |
| Total identified chalcones | nd | nd | nd | nd | 5.61 ± 0.28 a | 6.80 ± 0.24 a | nd | |
| 66 | Procyanidin dimer 1 | nd | 0.33 ± 0.03 c | 0.52 ± 0.03 c | 1.24 ± 0.04 c | 1.45 ± 0.42 bc | 2.52 ± 0.36 b | 7.90 ± 1.48 a |
| 67 | Procyanidin dimer 2 | nd | nd | nd | 5.07 ± 0.19 a | 0.75 ± 0.31 c | 2.93 ± 0.47 b | 5.30 ± 0.27 a |
| 68 | Procyanidin dimer 3 | nd | nd | nd | nd | 0.02 ± 0.01 b | 3.43 ± 0.40 a | 0.36 ± 0.02 b |
| 69 | Procyanidin dimer 4 | nd | nd | nd | nd | 0.03 ± 0.01 c | 3.43 ± 0.39 b | 6.31 ± 0.25 a |
| 70 | Procyanidin dimer 5 | nd | nd | nd | nd | nd | nd | 1.21 ± 0.08 a |
| 71 | Procyanidin dimer 6 | nd | nd | nd | nd | nd | nd | 0.50 ± 0.23 a |
| 72 | Procyanidin trimer 1 | nd | 1.18 ± 0.09 d | 0.86 ± 0.05 d | 11.05 ± 0.30 a | 2.15 ± 0.31 c | 4.76 ± 0.39 b | 0.02 ± 0.00 e |
| 73 | Procyanidin trimer 2 | nd | 2.63 ± 0.18 c | 2.68 ± 0.16 c | 5.97 ± 0.55 a | 0.12 ± 0.05 d | 0.07 ± 0.01 d | 4.52 ± 0.16 b |
| 74 | Procyanidin trimer 3 | nd | nd | nd | nd | 0.42 ± 0.04 b | 8.66 ± 1.08 a | 0.02 ± 0.00 b |
| 75 | Procyanidin trimer 4 | nd | nd | nd | nd | 2.33 ± 0.58 a | 1.72 ± 0.28 a | nd |
| 76 | Procyanidin trimer 5 | nd | nd | nd | nd | nd | 4.10 ± 1.60 a | nd |
| 77 | Procyanidin tetramer | nd | nd | nd | nd | nd | 4.11 ± 1.60 a | 2.89 ± 0.18 a |
| Total identified condensed tannins | nd | 4.14 ± 0.10 c | 4.06 ± 0.08 c | 23.33 ± 0.27 b | 7.27 ± 0.22 c | 35.73 ± 0.66 a | 29.03 ± 0.27 b | |
| Total identified compounds | 16.44 ± 0.06 d | 22.05 ± 0.07 d | 41.54 ± 0.06 c | 65.10 ± 0.09 b | 27.62 ± 0.14 d | 82.77 ± 0.26 a | 46.49 ± 0.09 c |
| TP | TF | TNF | TT | CT | SS | ABTS | FRAP | DPPH | HepG2 | HCT116 | HaCaT | α-Amyl | α-Glucos | 5-Lipoxy | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | 1.000 | ||||||||||||||
| TF | 0.761 | 1.000 | |||||||||||||
| TNF | 0.938 | 0.629 | 1.000 | ||||||||||||
| TT | 0.471 | 0.014 | 0.333 | 1.000 | |||||||||||
| CT | −0.065 | 0.178 | −0.367 | 0.314 | 1.000 | ||||||||||
| SS | 0.194 | −0.210 | 0.251 | 0.262 | 0.045 | 1.000 | |||||||||
| ABTS | 0.937 | 0.875 | 0.908 | 0.160 | −0.140 | 0.115 | 1.000 | ||||||||
| FRAP | 0.873 | 0.913 | 0.862 | 0.016 | −0.176 | −0.022 | 0.979 | 1.000 | |||||||
| DPPH | 0.830 | 0.952 | 0.762 | −0.015 | −0.094 | −0.170 | 0.945 | 0.969 | 1.000 | ||||||
| HepG2 | −0.459 | −0.567 | −0.396 | −0.336 | −0.353 | 0.059 | −0.451 | −0.489 | −0.393 | 1.000 | |||||
| HCT116 | −0.416 | −0.353 | −0.327 | −0.602 | −0.171 | 0.454 | −0.250 | −0.274 | −0.265 | 0.689 | 1.000 | ||||
| HaCaT | −0.148 | −0.067 | −0.029 | −0.670 | −0.512 | −0.059 | −0.004 | 0.050 | 0.104 | 0.729 | 0.623 | 1.000 | |||
| α-amyl | 0.169 | 0.458 | −0.116 | −0.038 | 0.585 | −0.064 | 0.237 | 0.191 | 0.377 | 0.146 | 0.234 | 0.117 | 1.000 | ||
| α-glucos | 0.077 | −0.083 | −0.101 | 0.645 | 0.627 | 0.429 | −0.065 | −0.205 | −0.213 | −0.312 | −0.121 | −0.786 | 0.263 | 1.000 | |
| 5-lipoxy | −0.345 | 0.020 | −0.371 | −0.530 | 0.431 | 0.318 | −0.172 | −0.114 | −0.152 | −0.077 | 0.534 | 0.154 | 0.232 | 0.011 | 1.000 |
| Cell Type (IC50 μg/mL) | |||
|---|---|---|---|
| HepG2 | HCT 116 | HaCaT | |
| Prunus avium | 300.89 ± 0.21 c A | 261.97 ± 13.12 c A | 323.84 ± 46.61 c A |
| Prunus serrulata | 473.59 ± 35.69 ab A | 517.42 ± 37.10 a A | 377.66 ± 34.85 bc B |
| Prunus serrulata 2018Kiku Shidare Zakura’ | 409.71 ± 103.52 b A | 464.01 ± 57.31 a A | 385.20 ± 7.27 bc A |
| Prunus yedoensis | 508.09 ± 26.28 a A | 537.92 ± 43.0 a A | 521.64 ± 67.29 a A |
| Malus purpurea | 386.2 ± 19.92 b B | 539.66 ± 45.19 a A | 461.39 ± 71.56 ab AB |
| Malus floribunda | 445.78 ± 27.42 ab A | 361.83 ± 31.19 b B | 459.28 ± 43.69 ab A |
| Chaenomeles japonica | 452.48 ± 15.18 ab A | 470.66 ± 48.16 a A | 473.27 ± 92.54 ab A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šola, I.; Poljuha, D.; Mikulic-Petkovsek, M.; Davosir, D.; Pinterić, M.; Bilić, J.; Veberic, R.; Hudina, M.; Rusak, G. Biopotential of Underutilized Rosaceae Inflorescences: LC-DAD-MS Phytochemical Profiles Associated with Antioxidant, Antidiabetic, Anti-Inflammatory and Antiproliferative Activity In Vitro. Plants 2022, 11, 271. https://doi.org/10.3390/plants11030271
Šola I, Poljuha D, Mikulic-Petkovsek M, Davosir D, Pinterić M, Bilić J, Veberic R, Hudina M, Rusak G. Biopotential of Underutilized Rosaceae Inflorescences: LC-DAD-MS Phytochemical Profiles Associated with Antioxidant, Antidiabetic, Anti-Inflammatory and Antiproliferative Activity In Vitro. Plants. 2022; 11(3):271. https://doi.org/10.3390/plants11030271
Chicago/Turabian StyleŠola, Ivana, Danijela Poljuha, Maja Mikulic-Petkovsek, Dino Davosir, Marija Pinterić, Josipa Bilić, Robert Veberic, Metka Hudina, and Gordana Rusak. 2022. "Biopotential of Underutilized Rosaceae Inflorescences: LC-DAD-MS Phytochemical Profiles Associated with Antioxidant, Antidiabetic, Anti-Inflammatory and Antiproliferative Activity In Vitro" Plants 11, no. 3: 271. https://doi.org/10.3390/plants11030271
APA StyleŠola, I., Poljuha, D., Mikulic-Petkovsek, M., Davosir, D., Pinterić, M., Bilić, J., Veberic, R., Hudina, M., & Rusak, G. (2022). Biopotential of Underutilized Rosaceae Inflorescences: LC-DAD-MS Phytochemical Profiles Associated with Antioxidant, Antidiabetic, Anti-Inflammatory and Antiproliferative Activity In Vitro. Plants, 11(3), 271. https://doi.org/10.3390/plants11030271