Potassium and Humic Acid Synergistically Increase Salt Tolerance and Nutrient Uptake in Contrasting Wheat Genotypes through Ionic Homeostasis and Activation of Antioxidant Enzymes
Abstract
1. Introduction
2. Results
2.1. Plant Growth
2.2. Stomatal Conductance and Chlorophyll Contents
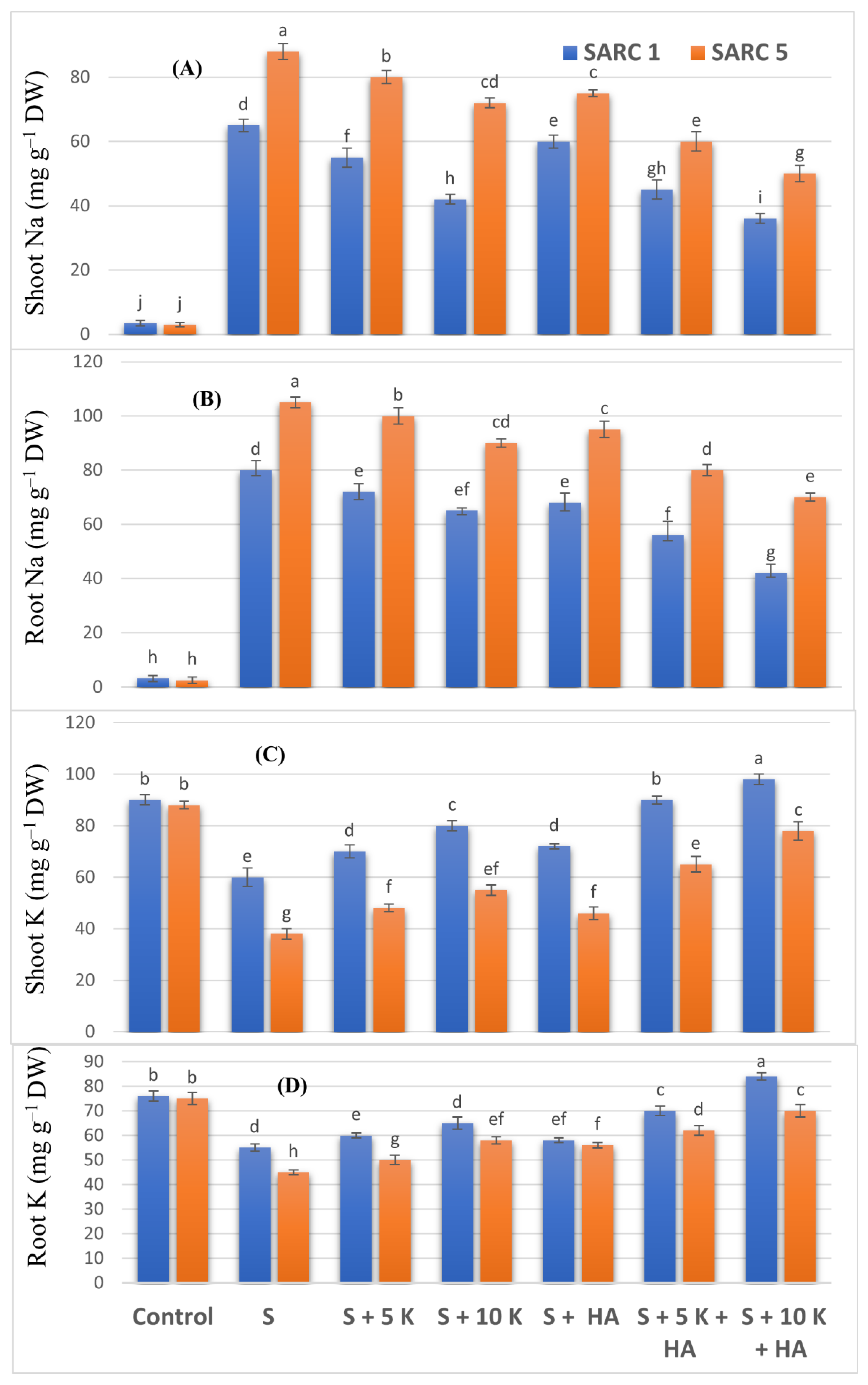
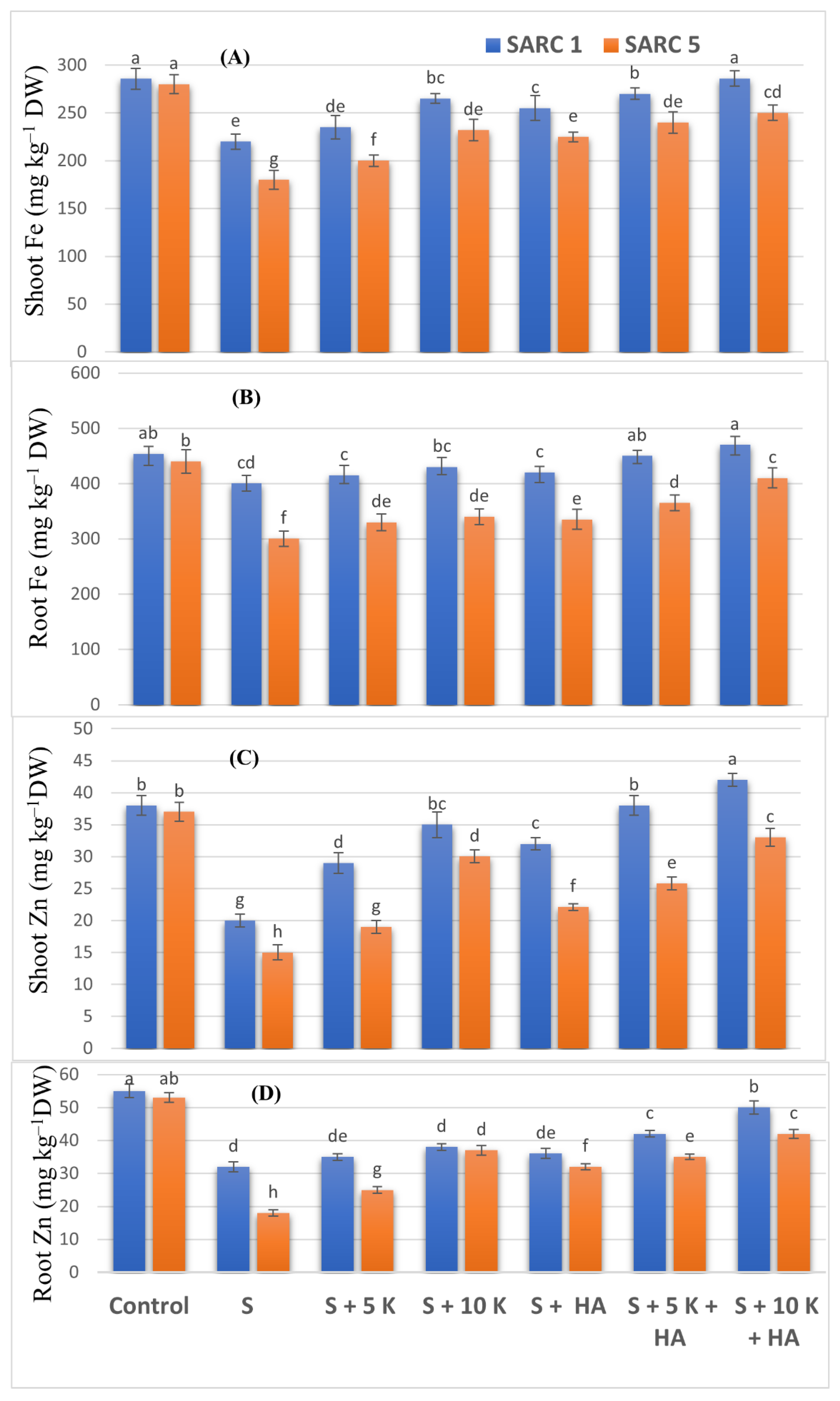
2.3. Nutrient Element Measurements
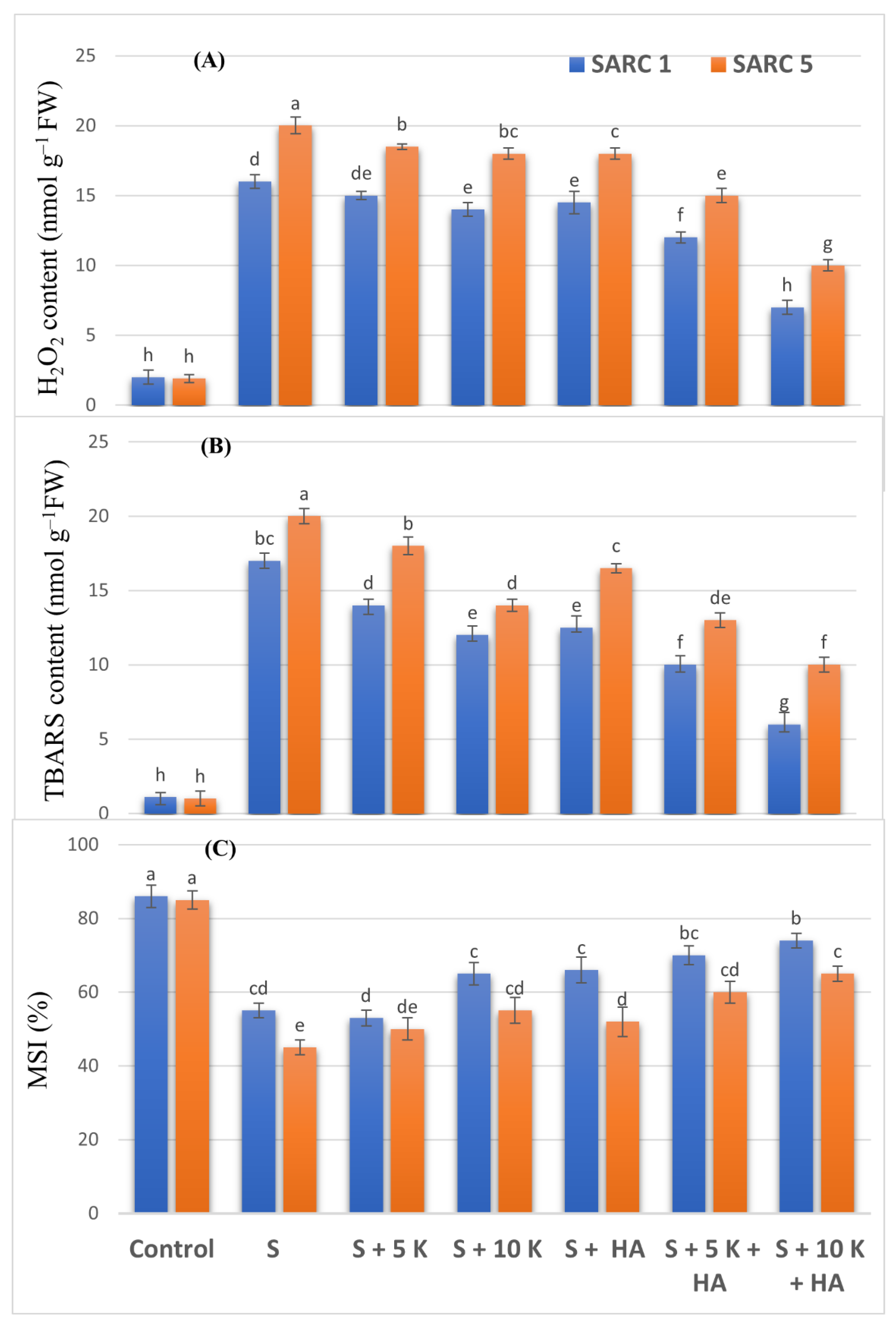
2.4. Oxidative Stress Attributes
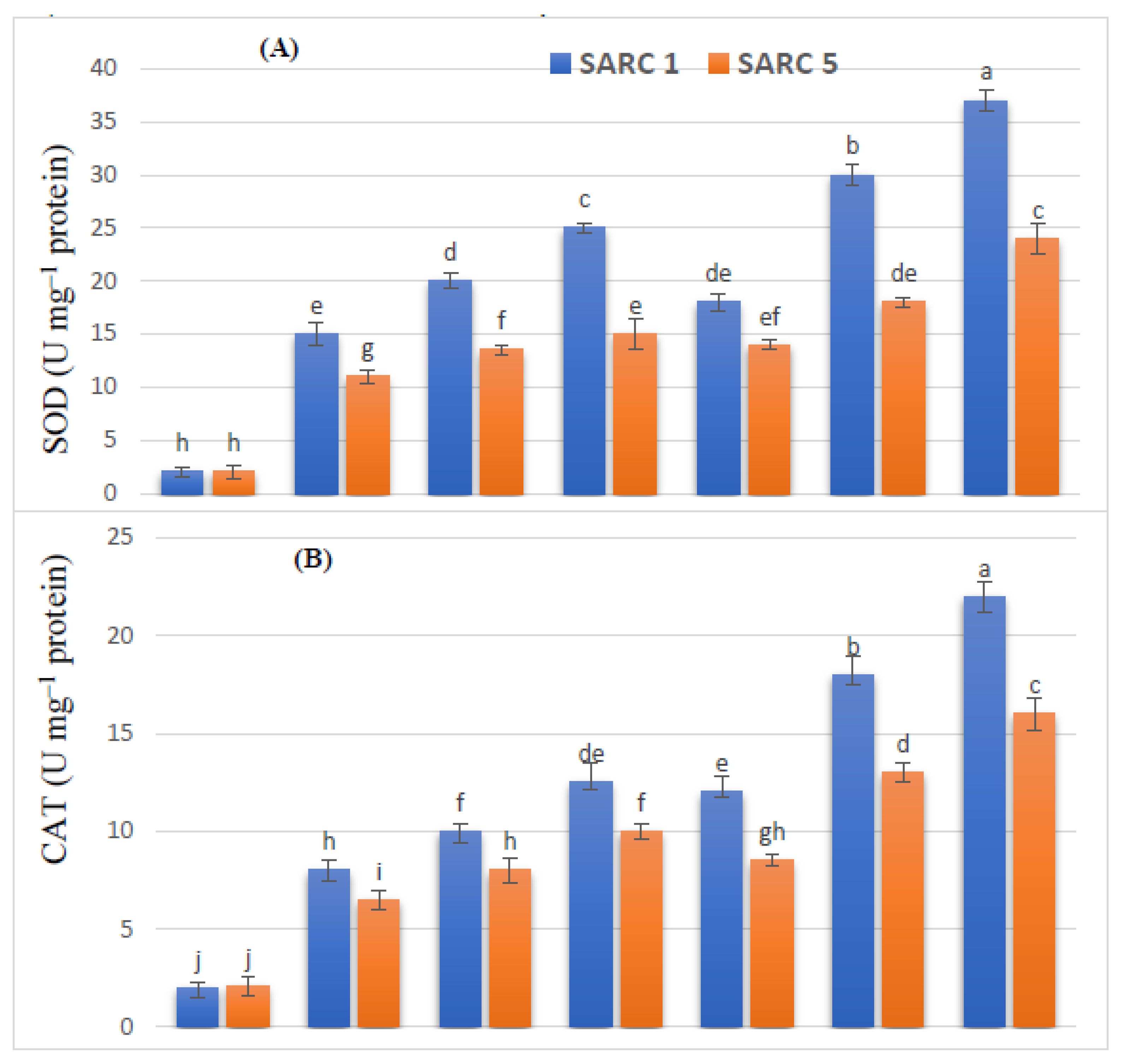
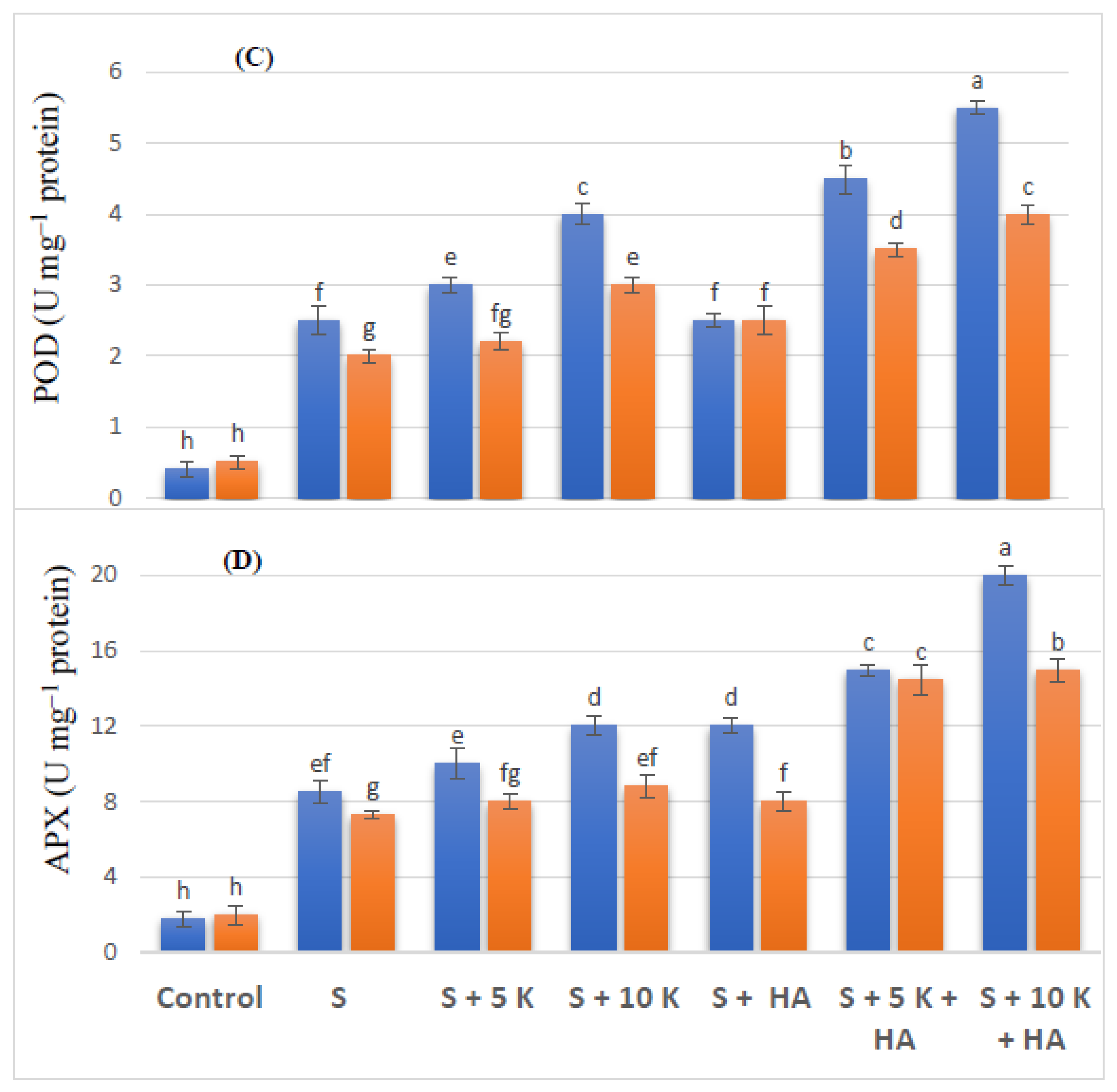
2.5. Antioxidant Enzymes
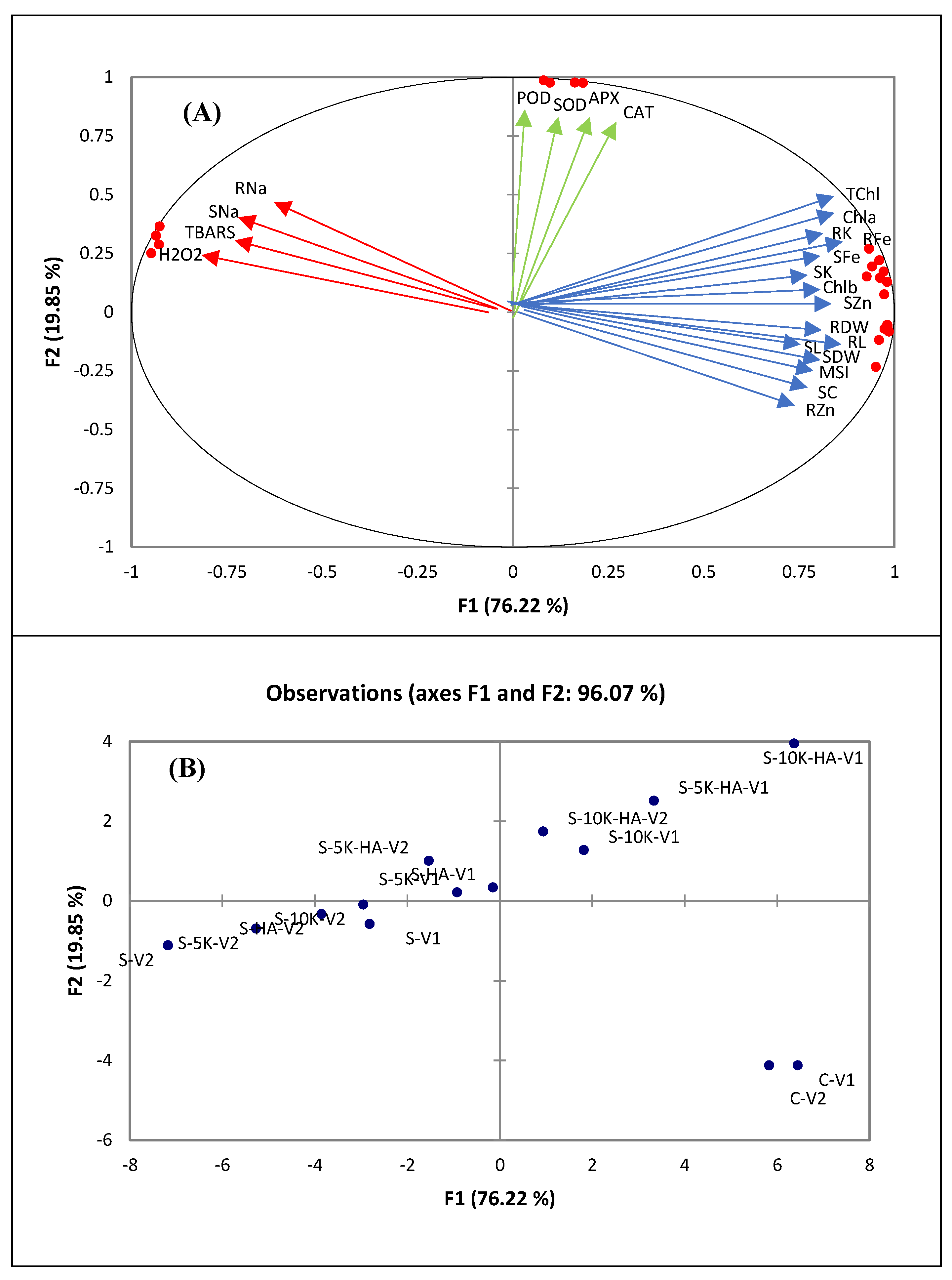
2.6. Multivariate Analyses
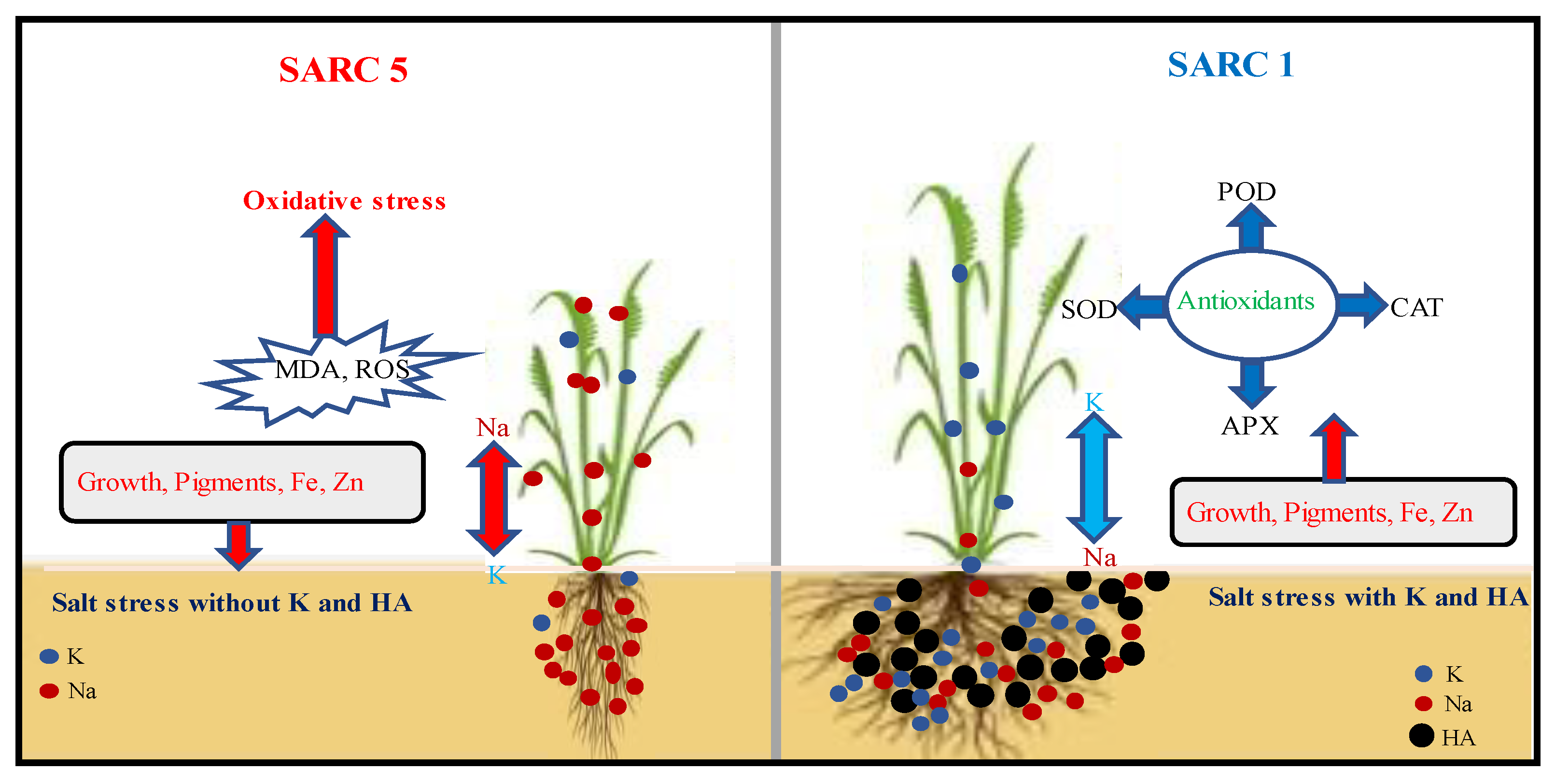
3. Discussion
4. Materials and Methods
4.1. Growth Conditions and Treatment Application
4.2. Harvesting and Elemental Analysis
4.3. Stomatal Conductance and Chlorophyll Contents
4.4. Oxidative Stress Attributes
4.5. Antioxidant Enzymes Activities
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saqib, M.; Akhtar, J.; Abbas, G.; Nasim, M. Salinity and drought interaction in wheat (Triticum aestivum L.) is affected by the genotype and plant growth stage. Acta Physiol. Plant. 2013, 35, 2761–2768. [Google Scholar] [CrossRef]
- Abbas, G.; Saqib, M.; Akhtar, J.; Murtaza, G.; Shahid, M. Effect of salinity on rhizosphere acidification and antioxidant activity of two acacia species. Can. J. For. Res. 2015, 45, 1–6. [Google Scholar] [CrossRef]
- Abbas, G.; Amjad, M.; Saqib, M.; Murtaza, B.; Asif Naeem, M.; Shabbir, A.; Murtaza, G. Soil sodicity is more detrimental than salinity for quinoa (Chenopodium quinoa Willd.): A multivariate comparison of physiological, biochemical and nutritional quality attributes. J. Agron. Crop. Sci. 2021, 207, 59–73. [Google Scholar] [CrossRef]
- Qadir, M.; Quille’rou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- FAO. FAO Land and Plant Nutrition Management Service 2008. Available online: http://www.fao.org/ag/agl/agll/spush (accessed on 25 April 2008).
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Zrig, A.; AbdElgawad, H.; Touneckti, T.; Mohamed, H.B.; Hamouda, F.; Khemira, H. Potassium and calcium improve salt tolerance of Thymus vulgaris by activating the antioxidant systems. Sci. Hort. 2021, 277, 109812. [Google Scholar] [CrossRef]
- Gul, M.; Wakeel, A.; Steffens, D.; Lindberg, S. Potassium-induced decrease in cytosolic Na+ alleviates deleterious effects of salt stress on wheat (Triticum aestivum L.). Plant Biol. 2019, 21, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Shah, M.A.; Khan, A.; Ahmad, A.M.; Hussain, F.M. Potassium enhanced grain zinc accumulation in wheat grown on a calcareous saline-sodic soil. Pakistan J. Bot. 2020, 52, 69–74. [Google Scholar] [CrossRef]
- Iftikhar, A.; Abbas, G.; Saqib, M.; Shabbir, A.; Amjad, M.; Shahid, M.; Ahmad, I.; Iqbal, S.; Qaisrani, S.A. Salinity modulates lead (Pb) tolerance and phytoremediation potential of quinoa: A multivariate comparison of physiological and biochemical attributes. Environ. Geochem. Health 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Saqib, Z.A.; Akhtar, J.; Bakhat, H.F.; Pasala, R.K.; Dietz, K.J. Protective role of silicon (Si) against combined stress of salinity and boron (B) toxicity by improving antioxidant enzymes activity in rice. Silicon 2019, 11, 2193–2197. [Google Scholar] [CrossRef]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Saifullah; Farooq, M.; Souri, Z.; Karimi, M.; Rengel, Z. Acquiring control: The evolution of ROS-induced oxidative stress and redox signaling pathways in plant stress responses. Plant. Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Intracellular redox compartmentation and ROS-related communication in regulation and signaling. Plant Physiol. 2017, 171, 1581–1592. [Google Scholar] [CrossRef]
- Shabbir, A.; Abbas, G.; Asad, S.A.; Razzaq, H.; Anwar-ul-Haq, M.; Amjad, M. Effects of arsenite on physiological, biochemical and grain yield attributes of quinoa (Chenopodium quinoa Willd.): Implications for phytoremediation and health risk assessment. Int. J. Phytoremediation 2021, 23, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Ashraf, M.Y.; Rafique, N.; Ashraf, M.; Azhar, N.; Marchand, M. Effect of supplemental potassium (K+) on growth, physiological and biochemical attributes of wheat grown under saline conditions. J. Plant Nutr. 2013, 36, 443–458. [Google Scholar] [CrossRef]
- Taha, R.; Seleiman, M.F.; Alotaibi, M.; Alhammad, B.A.; Rady, M.M.; Mahdi, A. Exogenous potassium treatments elevate salt tolerance and performances of Glycine max L. by boosting antioxidant defense system under actual saline field conditions. Agronomy 2020, 10, 1741. [Google Scholar] [CrossRef]
- Jan, A.U.; Hadi, F.; Nawaz, M.A.; Rahman, K. Potassium and zinc increase tolerance to salt stress in wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2017, 116, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.Y.; Sarwar, G. Salt tolerance potential in some members of Brassicaceae physiological studies on water relations and mineral contents. In Prospects for Saline Agriculture; Springer: Dordrecht, The Netherlands, 2002; pp. 237–245. [Google Scholar]
- Kausar, A.; Gull, M. Effect of potassium sulphate on the growth and uptake of nutrients in wheat (Triticum aestivum L.) under salt stressed conditions. J. Agric. Sci. 2014, 6, 101. [Google Scholar] [CrossRef][Green Version]
- Abdal, N.; Abbas, G.; Asad, S.A.; Ghfar, A.A.; Shah, G.M.; Rizwan, M.; Ali, S.; Shahbaz, M. Salinity mitigates cadmium-induced phytotoxicity in quinoa (Chenopodium quinoa Willd.) by limiting the Cd uptake and improved responses to oxidative stress: Implications for phytoremediation. Environ. Geochem. Health 2021. [Google Scholar] [CrossRef]
- Weng, L.; Van Riemsdijk, W.H.; Koopal, L.K.; Hiemstra, T. Adsorption of humic substances on goethite: Comparison between humic acids and fulvic acids. Environ. Sci. Technol. 2006, 40, 7494–7500. [Google Scholar] [CrossRef]
- Aydin, A.; Kant, C.; Turan, M. Humic acid application alleviates salinity stress of bean (Phaseolus vulgaris L.) plants decreasing membrane leakage. Afr. J. Agric. Res. 2012, 7, 1073–1086. [Google Scholar] [CrossRef]
- Mazhar, A.A.M.; Shedeed, S.I.; Abdel-Aziz, N.G.; Mahgoub, M.H. Growth, flowering and chemical constituents of Chrysanthemum indicum L. plant in response to different levels of humic acid and salinity. J. Appl. Sci. Res. 2012, 8, 3697–3706. [Google Scholar]
- Ali, A.Y.A.; Ibrahim, M.E.H.; Zhou, G.; Nimir, N.E.A.; Jiao, X.; Zhu, G.; Elsiddig, A.M.I.; Zhi, W.; Chen, X.; Lu, H. Ameliorative effects of jasmonic acid and humic acid on antioxidant enzymes and salt tolerance of forage sorghum under salinity conditions. Agron. J. 2019, 111, 3099–3108. [Google Scholar] [CrossRef]
- Hatami, E.; Shokouhian, A.A.; Ghanbari, A.R.; Naseri, L.A. Alleviating salt stress in almond rootstocks using of humic acid. Sci. Hortic. 2018, 237, 296–302. [Google Scholar] [CrossRef]
- Khaled, H.; Fawy, H.A. Effect of different levels of humic acids on the nutrient content, plant growth, and soil properties under conditions of salinity. Soil Water Res. 2011, 6, 21–29. [Google Scholar] [CrossRef]
- Beni, M.; Hatamzadeh, A.; Nikbakht, A.; Ghasemnezhad, M.; Zarchini, M. Improving physiological quality of cut tuberose (Polianthes tuberosa cv. Single) flowers by continues treatment with humic acid and nano-silver particles. J. Ornamen. Hortic. Plant 2013, 3, 133–141. [Google Scholar]
- Dinçsoy, M.; Sönmez, F. The effect of potassium and humic acid applications on yield and nutrient contents of wheat (Triticum aestivum L. var. Delfii) with same soil properties. J. Plant Nutr. 2019, 33, 2757–2772. [Google Scholar] [CrossRef]
- Genc, Y.; Mcdonald, G.K.; Tester, M. Reassessment of tissue Na+ concentration as a criterion for salinity tolerance in bread wheat. Plant Cell Enviro. 2007, 30, 1486–1498. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Cha, J.Y.; Kang, S.H.; Ji, M.G.; Shin, G.I.; Jeong, S.Y.; Ahn, G.; Kim, M.G.; Jeon, J.R.; Kim, W.Y. Transcriptome changes reveal the molecular mechanisms of humic acid-induced salt stress tolerance in Arabidopsis. Molecules. 2021, 26, 782. [Google Scholar] [CrossRef] [PubMed]
- Saqib, M.; Abbas, G.; Akhtar, J. Root-mediated acidification and resistance to low calcium improve wheat (Triticum aestivum) performance in saline-sodic conditions. Plant Physiol. Biochem. 2020, 156, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Agarwal, R.M. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L) as influenced by potassium supplementation. Plant Physiol. Biochem. 2017, 115, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Jatav, K.S.; Agarwal, R.M.; Tomar, N.S.; Tyagi, S.R. Nitrogen metabolism, growth and yield responses of wheat (Triticum aestivum L.) to restricted water supply and varying potassium treatments. J. Indian Bot. Soc. 2014, 93, 177–189. [Google Scholar]
- Mohammed, A.K. Effect of urea and humic acid fertilization on some chemical/physical properties and yield of cow pea Vigna unguiculata (L.) walp. Uni Thi-Qar J. Sci. 2012, 3, 82–88. [Google Scholar]
- Jain, M.; Rivera, S.; Monclus, E.A.; Synenki, L.; Zirk, A.; Eisenbart, J.; Feghali-Bostwick, C.; Mutlu, G.M.; Budinger, G.S.; Chandel, N.S. Mitochondrial reactive oxygen species regulate transforming growth factor-β signaling. J. Biol. Chem. 2013, 288, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Cotrozzi, L.; Lorenzini, G.; Nali, C.; Pisuttu, C.; Pampana, S.; Pellegrini, E. Transient waterlogging events impair shoot and root physiology and reduce grain yield of durum wheat cultivars. Plants 2021, 10, 2357. [Google Scholar] [CrossRef]
- Mustafa, T.; Sattar, A.; Sher, A.; Ul-Allah, S.; Ijaz, M.; Irfan, M.; Butt, M.; Cheema, M. Exogenous application of silicon improves the performance of wheat under terminal heat stress by triggering physio-biochemical mechanisms. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Weisany, W.; Sohrabi, Y.; Heidari, G.; Siosemardeh, A.; Ghassemi-Golezani, K. Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (Glycine max L.). Plant Omics 2012, 5, 60–67. [Google Scholar]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Lee, J.; Jeon, J.H.; Shin, J.; Jang, H.M.; Kim, S.; Song, M.S.; Kim, Y.M. Quantitative and qualitative changes in antibiotic resistance genes after passing through treatment processes in municipal wastewater treatment plants. Sci. Total Environ. 2017, 605, 906–914. [Google Scholar] [CrossRef]
- Pottosin, I.; Velarde-Buendía, A.M.; Zepeda-Jazo, I.; Dobrovinskaya, O.; Shabala, S. Synergism between polyamines and ROS in the induction of Ca2+ and K+ fluxes in roots. Plant Signal. Behav. 2012, 7, 1084–1087. [Google Scholar] [CrossRef]
- Mohamed, W.H. Effects of humic acid and calcium forms on dry weight and nutrient uptake of maize plant under saline condition. Aus. J. Basic App. Sci. 2012, 6, 597–604. [Google Scholar]
- Shabala, S.; Shabala, L.; Cuin, T.A.; Pang, J.; Percey, W.; Chen, Z.; Conn, S.; Eing, C.; Wegner, L.H. Xylem ionic relations and salinity tolerance in barley. Plant J. 2010, 61, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Pottosin, I. Regulation of potassium transport in plants under hostile conditions: Implications for abiotic and biotic stress tolerance. Physiol. Plant 2014, 151, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Amjad, M.; Akhtar, J.; Murtaza, B.; Abbas, G.; Jawad, H. Differential accumulation of potassium results in varied salt-tolerance response in tomato (Solanum lycopersicum L.) cultivars. Hort. Environ. Biotechnol. 2016, 57, 248–258. [Google Scholar] [CrossRef]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant. Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Salama, Z.A.; El Hariri, D.M. Some biochemical markers for evaluation of flax cultivars under salt stress conditions. J. Nat. Fibers 2008, 5, 316–330. [Google Scholar] [CrossRef]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef]
- Sabagh, A.E.L.; Islam, M.S.; Skalicky, M.; Raza, M.A.; Singh, K.; Hossain, M.A.; Hossain, A.; Mahboob, W.; Iqbal, M.A.; Ratnasekera, D.P.; et al. Adaptation and management strategies of wheat (Triticum aestivum L.) against salinity stress to increase yield and quality. Front. Agron. 2021, 3, 43. [Google Scholar] [CrossRef]
- Mirzapour, M.H.; Khoshgoftar, A.H.; Mirnia, S.K.; Bahrami, H.A.; Naeini, M.R. Interactive effects of potassium and magnesium on growth and yield of sunflower in a saline soil. Iran. J. Soil Water Sci. 2004, 17, 132–139. [Google Scholar]
- Abbas, G.; Saqib, M.; Akhtar, J.; Haq, M.A.U. Interactive effects of salinity and iron deficiency on different rice genotypes. J. Plant Nut. Soil Sci. 2015, 178, 306–311. [Google Scholar] [CrossRef]
- Kaya, C.; Kirnak, H.; Higgs, D. Enhancement of growth and normal growth parameters by foliar application of potassium and phosphorus on tomato cultivars grown at high (NaCl) salinity. J. Plant Nutr. 2001, 24, 357–367. [Google Scholar] [CrossRef]
- Zientara, M. Effect of sodium humate on membrane potential in internodal cells of Nitellopsis obtuse. Acta Soc. Bot. Pol. 1983, 52, 271–277. [Google Scholar] [CrossRef][Green Version]
- David, P.P.; Nelson, P.V.; Sanders, D.C. A humic acid improves growth of tomato seedling in solution culture. J. Plant Nutr. 1994, 17, 173–184. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Islam, E.; Liu, D.; Li, T.; Yang, X.; Jin, X.; Mahmood, Q.; Tian, S.; Li, J. Effect of Pb toxicity on leaf growth, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J. Hazard. Mater. 2008, 154, 914–926. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Sairam, R.K.; Rao, K.V.; Srivastava, G.C. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Gupta, A.S.; Webb, R.P.; Holaday, A.S.; Allen, R.D. Overexpression of superoxide dismutase protects plants from oxidative stress (induction of ascorbate peroxidase in superoxide dismutase-overexpressing plants). Plant Physiol. 1993, 103, 1067–1073. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Meth Enzymol. 1984, 105, 121–126. [Google Scholar]
- Amako, K.; Chen, G.X.; Asada, K. Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol. 1994, 35, 497–504. [Google Scholar]
- Hemeda, H.M.; Klein, B. Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J. Food Sci. 1990, 55, 184–185. [Google Scholar] [CrossRef]
- Steel, R.; Torrie, J.; Dickey, D. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw-Hill: New York, NY, USA, 1997. [Google Scholar]


| Treatments | Shoot Length (cm) | Root Length (cm) | Shoot Dry Weight (g plant −1) | Root Dry Weight (g plant −1) | ||||
|---|---|---|---|---|---|---|---|---|
| SARC 1 | SARC 5 | SARC 1 | SARC 5 | SARC 1 | SARC 5 | SARC 1 | SARC 5 | |
| Control | 49.0 a | 47.3 a | 47.9 a | 45.0 a | 0.46 a | 0.45 ab | 0.15 a | 0.15 a |
| S | 32.5 efg | 27.0 h | 31.2 cde | 25.0 h | 0.30 ef | 0.23 h | 0.10 def | 0.08 f |
| S + 5 K | 34.2 def | 28.0 h | 34.3 c | 27.0 gh | 0.33 de | 0.26 gh | 0.12 bcd | 0.09 ef |
| S + 10 K | 39.0 c | 31.0 g | 39.0 b | 30.0 efg | 0.38 c | 0.27 fg | 0.13 abc | 0.10 def |
| S + HA | 35.0 de | 28.0 h | 34.2 c | 28.0 fgh | 0.35 cd | 0.26 gh | 0.11 cd | 0.09 ef |
| S + 5 K + HA | 43.0 b | 32.0 fg | 40.1 b | 31.0 def | 0.33 b | 0.30 ef | 0.13 abc | 0.10 def |
| S + 10 K + HA | 48.2 a | 36.0 d | 46.0 a | 34.0 cd | 0.44 ab | 0.33 de | 0.15 a | 0.11 cd |
| Treatments | Chlorophyll a (µg g−1) | Chlorophyll b (µg g−1) | Total Chlorophyll (µg g−1) | Stomatal Conductance (mmol m−2 s−1) | ||||
|---|---|---|---|---|---|---|---|---|
| SARC 1 | SARC 5 | SARC 1 | SARC 5 | SARC 1 | SARC 5 | SARC 1 | SARC 5 | |
| Control | 290 b | 285 b | 180 b | 175 b | 470 b | 460 b | 440 a | 435 a |
| S | 230 ef | 200 g | 140 d | 120 f | 370 e | 320 g | 350 de | 295 f |
| S + 5 K | 245 e | 220 f | 145 d | 125 ef | 390 d | 345 f | 370 d | 320 e |
| S + 10 K | 280 bc | 235 ef | 160 c | 132 ef | 440 c | 367 ef | 405 b | 340 de |
| S + HA | 265 d | 255 d | 146 d | 136 e | 411 d | 391 e | 365 d | 325 e |
| S + 5 K + HA | 295 b | 255 d | 172 bc | 145 d | 467 b | 410 e | 395 bc | 350 de |
| S + 10 K + HA | 320 a | 272 c | 192 a | 160 c | 512 a | 430 c | 438 a | 360 d |
| Traits | SL | RL | SDW | RDW | SNa | RNa | SK | RK | H2O2 | TBARS | MSI | SOD | CAT | POD | APX | SFe | RFe | SZn | RZn | Chla | Chlb | TChl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RL | 0.99 | 1.00 | ||||||||||||||||||||
| SDW | 0.99 | 0.99 | 1.00 | |||||||||||||||||||
| RDW | 0.98 | 0.99 | 0.98 | 1.00 | ||||||||||||||||||
| SNa | −0.94 | −0.95 | −0.93 | −0.94 | 1.00 | |||||||||||||||||
| RNa | −0.94 | −0.94 | −0.94 | −0.93 | 0.99 | 1.00 | ||||||||||||||||
| SK | 0.95 | 0.94 | 0.96 | 0.95 | −0.86 | −0.84 | 1.00 | |||||||||||||||
| RK | 0.93 | 0.92 | 0.90 | 0.91 | −0.86 | −0.84 | 0.94 | 1.00 | ||||||||||||||
| H2O2 | −0.93 | −0.92 | −0.91 | −0.90 | 0.97 | 0.97 | −0.85 | −0.89 | 1.00 | |||||||||||||
| TBARS | −0.94 | −0.94 | −0.92 | −0.92 | 0.97 | 0.97 | −0.87 | −0.91 | 0.98 | 1.00 | ||||||||||||
| MSI | 0.95 | 0.94 | 0.94 | 0.91 | −0.96 | −0.97 | 0.88 | 0.88 | −0.96 | −0.98 | 1.00 | |||||||||||
| SOD | 0.11 | 0.09 | 0.12 | 0.11 | 0.17 | 0.21 | 0.33 | 0.28 | 0.14 | 0.11 | −0.09 | 1.00 | ||||||||||
| CAT | 0.12 | 0.09 | 0.12 | 0.09 | 0.15 | 0.18 | 0.35 | 0.33 | 0.10 | 0.05 | −0.04 | 0.97 | 1.00 | |||||||||
| POD | 0.02 | 0.00 | 0.02 | 0.02 | 0.24 | 0.29 | 0.25 | 0.23 | 0.21 | 0.17 | −0.17 | 0.98 | 0.97 | 1.00 | ||||||||
| APX | 0.02 | 0.00 | 0.03 | 0.00 | 0.22 | 0.26 | 0.27 | 0.25 | 0.16 | 0.13 | −0.12 | 0.95 | 0.98 | 0.96 | 1.00 | |||||||
| SFe | 0.92 | 0.94 | 0.94 | 0.93 | −0.88 | −0.86 | 0.95 | 0.94 | −0.85 | −0.91 | 0.92 | 0.22 | 0.25 | 0.15 | 0.17 | 1.00 | ||||||
| RFe | 0.90 | 0.91 | 0.94 | 0.92 | −0.82 | −0.81 | 0.97 | 0.86 | −0.80 | −0.79 | 0.83 | 0.32 | 0.30 | 0.21 | 0.23 | 0.91 | 1.00 | |||||
| SZn | 0.90 | 0.90 | 0.90 | 0.91 | −0.81 | −0.79 | 0.95 | 0.92 | −0.79 | −0.86 | 0.85 | 0.34 | 0.37 | 0.27 | 0.26 | 0.96 | 0.88 | 1.00 | ||||
| RZn | 0.93 | 0.93 | 0.91 | 0.92 | −0.93 | −0.92 | 0.90 | 0.94 | −0.93 | −0.97 | 0.94 | 0.02 | 0.07 | −0.03 | −0.02 | 0.95 | 0.84 | 0.90 | 1.00 | |||
| Chla | 0.88 | 0.89 | 0.89 | 0.87 | −0.78 | −0.76 | 0.93 | 0.94 | −0.78 | −0.82 | 0.84 | 0.40 | 0.43 | 0.33 | 0.35 | 0.96 | 0.88 | 0.93 | 0.88 | 1.00 | ||
| Chlb | 0.97 | 0.96 | 0.96 | 0.94 | −0.88 | −0.87 | 0.97 | 0.98 | −0.89 | −0.90 | 0.90 | 0.29 | 0.31 | 0.21 | 0.22 | 0.94 | 0.92 | 0.92 | 0.92 | 0.96 | 1.00 | |
| TChl | 0.92 | 0.92 | 0.92 | 0.90 | −0.82 | −0.81 | 0.95 | 0.97 | −0.83 | −0.86 | 0.87 | 0.36 | 0.39 | 0.29 | 0.31 | 0.96 | 0.90 | 0.93 | 0.90 | 0.99 | 0.98 | 1.00 |
| SC | 0.98 | 0.99 | 0.98 | 0.99 | −0.94 | −0.93 | 0.95 | 0.92 | −0.90 | −0.92 | 0.92 | 0.12 | 0.11 | 0.03 | 0.03 | 0.95 | 0.92 | 0.91 | 0.93 | 0.89 | 0.95 | 0.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, G.; Rehman, S.; Siddiqui, M.H.; Ali, H.M.; Farooq, M.A.; Chen, Y. Potassium and Humic Acid Synergistically Increase Salt Tolerance and Nutrient Uptake in Contrasting Wheat Genotypes through Ionic Homeostasis and Activation of Antioxidant Enzymes. Plants 2022, 11, 263. https://doi.org/10.3390/plants11030263
Abbas G, Rehman S, Siddiqui MH, Ali HM, Farooq MA, Chen Y. Potassium and Humic Acid Synergistically Increase Salt Tolerance and Nutrient Uptake in Contrasting Wheat Genotypes through Ionic Homeostasis and Activation of Antioxidant Enzymes. Plants. 2022; 11(3):263. https://doi.org/10.3390/plants11030263
Chicago/Turabian StyleAbbas, Ghulam, Sadia Rehman, Manzer H. Siddiqui, Hayssam M. Ali, Muhammad Ansar Farooq, and Yinglong Chen. 2022. "Potassium and Humic Acid Synergistically Increase Salt Tolerance and Nutrient Uptake in Contrasting Wheat Genotypes through Ionic Homeostasis and Activation of Antioxidant Enzymes" Plants 11, no. 3: 263. https://doi.org/10.3390/plants11030263
APA StyleAbbas, G., Rehman, S., Siddiqui, M. H., Ali, H. M., Farooq, M. A., & Chen, Y. (2022). Potassium and Humic Acid Synergistically Increase Salt Tolerance and Nutrient Uptake in Contrasting Wheat Genotypes through Ionic Homeostasis and Activation of Antioxidant Enzymes. Plants, 11(3), 263. https://doi.org/10.3390/plants11030263








