Optimization of Microwave Assisted Extraction Conditions to Improve Phenolic Content and In Vitro Antioxidant and Anti-Microbial Activity in Quercus cerris Bark Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Bacterial Strains
2.2. Plant Sample
2.3. Optimization and Extraction/Design of Experiments (DoE)
2.4. Quantification of Total Phenolics
2.5. Quantification of Tannins
2.6. Antioxidant In Vitro Assays
2.6.1. Determination of DPPH Radical Scavenging Activity
2.6.2. Determination of ABTS Free Radical-Scavenging Activity
2.7. Antibacterial Activity
2.8. Statistical Analysis
3. Results and Discussions
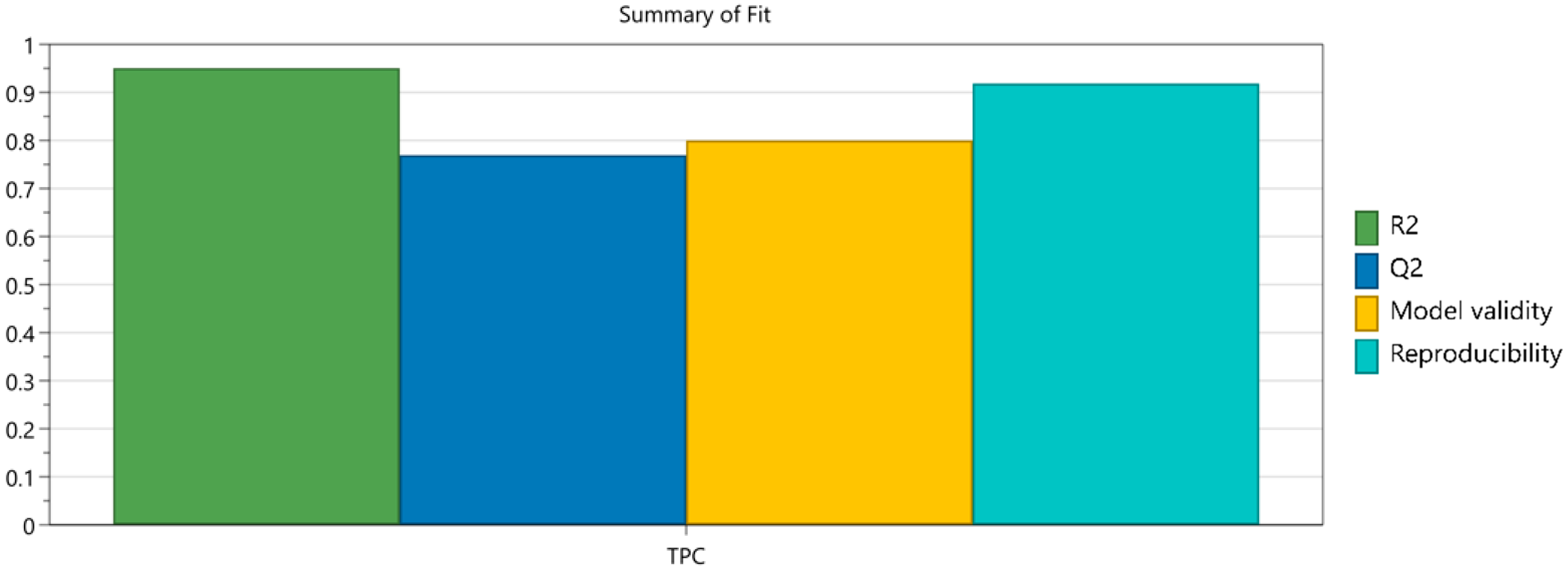
3.1. Design of Experiments
3.2. Effects of Process Variables on the Extracted TPC
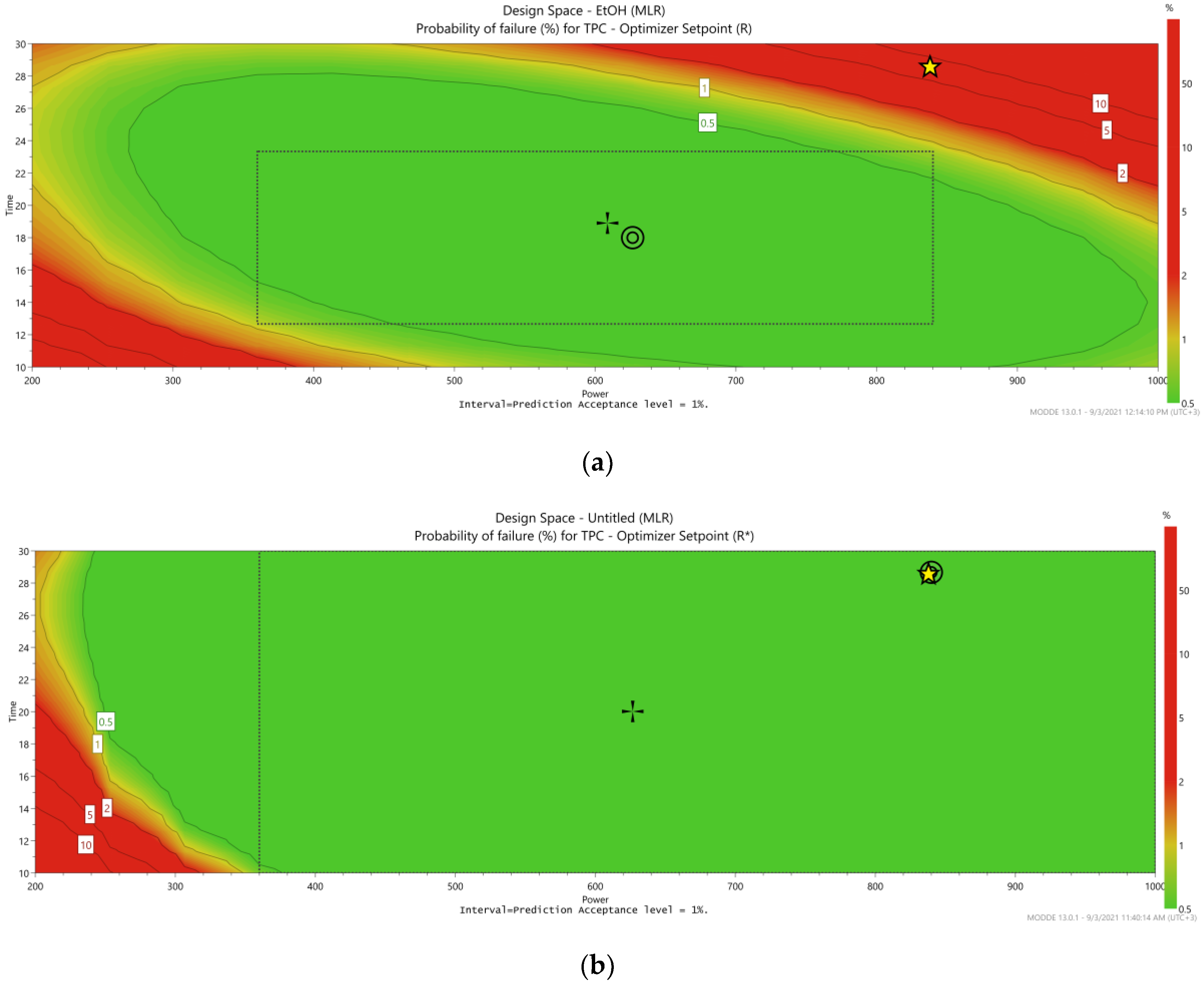
3.3. Design Space and Process Optimization
3.4. Total Polyphenolic and Tannin Content
3.5. Antioxidant Activity
3.6. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tantray, Y.R.; Wani, M.S.; Hussain, A. Genus Quercus: An Overview. Inter. J. Adv. Res. Sci. Eng. 2017, 6, 1880–1886. [Google Scholar]
- Giertych, M.J.; Chmielarz, P. Size Variability in Embryonic Axes, Cotyledons, Acorns and Seedlings in Fifteen Species of the Genus Quercus. Trees 2020, 34, 593–601. [Google Scholar] [CrossRef]
- Popović, B.M.; Štajner, D.; Ždero, R.; Orlović, S.; Galić, Z. Antioxidant Characterization of Oak Extracts Combining Spectrophotometric Assays and Chemometrics. Sci. World J. 2013, 2013, 134656. [Google Scholar] [CrossRef] [PubMed]
- Mölder, A.; Meyer, P.; Nagel, R.-V. Integrative Management to Sustain Biodiversity and Ecological Continuity in Central European Temperate Oak (Quercus robur Q. petraea) Forests: An Overview. For. Ecol. Manag. 2019, 437, 324–339. [Google Scholar] [CrossRef]
- Burlacu, E.; Nisca, A.; Tanase, C. A Comprehensive Review of Phytochemistry and Biological Activities of Quercus Species. Forests 2020, 11, 904. [Google Scholar] [CrossRef]
- Elansary, H.; Szopa, A.; Kubica, P.; Ekiert, H.; A Mattar, M.; Al-Yafrasi, M.A.; El-Ansary, D.O.; Zin El-Abedin, T.K.; Yessoufou, K. Polyphenol Profile and Pharmaceutical Potential of Quercus Spp. Bark Extracts. Plants 2019, 8, 486. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Kim, H.H.; Hwang, I.H.; Kim, D.H.; Lee, M.W. Anti-Inflammatory Effects of Phenolic Compounds Isolated from Quercus mongolica Fisch. Ex Ledeb. on UVB-Irradiated Human Skin Cells. Molecules 2019, 24, 3094. [Google Scholar] [CrossRef] [Green Version]
- Panchal, S.K.; Brown, L. Cardioprotective and Hepatoprotective Effects of Ellagitannins from European Oak Bark (Quercus petraea L.) Extract in Rats. Eur. J. Nutr. 2013, 52, 397–408. [Google Scholar] [CrossRef]
- Fodor, E.; Timofte, A.; Geambașu, T. Mycorrhizal Status of Several Quercus Species in Romania (Quercus cerris, Q. frainetto, Q. robur) and the Optimization Perspective of Growth Conditions for in Vitro Propagated Plants Transplanted in the Field. Ann. For. Res. 2011, 54, 57–71. [Google Scholar]
- Chisăliță, I.; Dincă, L.; Spârchez, G.; Crăciunescu, A.; Vişoiu, D. The nfluence of some stagnoluvosols characteristics on the productivity of Quercus cerris and Quercus frainetto stands from os Faget, ds Timiş. Res. J. Agric. Sci. 2015, 47, 23–28. [Google Scholar]
- Adam, I. The Spreading of the Turkey Oak (Quercus cerris L.) in Romania. Forestry Sites and Turkey Oak Stands. J. Hortic. For. Biotechnol. 2009, 13, 309–314. [Google Scholar]
- Şen, A.; Miranda, I.; Pereira, H. Temperature-Induced Structural and Chemical Changes in Cork from Quercus cerris. Ind. Crops Prod. 2012, 37, 508–513. [Google Scholar] [CrossRef]
- Lakreb, N.; As, N.; Gorgun, V.; Sen, U.; Gomes, M.G.; Pereira, H. Production and Characterization of Particleboards from Cork-Rich Quercus cerris Bark. Eur. J. Wood Wood Prod. 2018, 76, 989–997. [Google Scholar] [CrossRef]
- Şen, A.; Quilhó, T.; Pereira, H. The Cellular Structure of Cork from Quercus cerris Var. Cerris Bark in a Materials’ Perspective. Ind. Crops Prod. 2011, 34, 929–936. [Google Scholar] [CrossRef]
- Sen, A.; Miranda, I.; Esteves, B.; Pereira, H. Chemical Characterization, Bioactive and Fuel Properties of Waste Cork and Phloem Fractions from Quercus cerris L. Bark. Ind. Crops Prod. 2020, 157, 112909. [Google Scholar] [CrossRef]
- Cetera, P.; Russo, D.; Milella, L.; Todaro, L. Thermo-Treatment Affects Quercus cerris L. Wood Properties and the Antioxidant Activity and Chemical Composition of Its By-Product Extracts. Ind. Crops Prod. 2019, 130, 380–388. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Sabuncuoğlu, S.; Harput, Ü.Ş. Evaluation of Antioxidative, Protective Effect against H2O2 Induced Cytotoxicity, and Cytotoxic Activities of Three Different Quercus Species. Food Chem. Toxicol. 2012, 50, 141–146. [Google Scholar] [CrossRef]
- Tanase, C.; Coșarcă, S.; Muntean, D.-L. A Critical Review of Phenolic Compounds Extracted from the Bark of Woody Vascular Plants and Their Potential Biological Activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef] [Green Version]
- Meullemiestre, A.; Petitcolas, E.; Maache-Rezzoug, Z.; Chemat, F.; Rezzoug, S.A. Impact of Ultrasound on Solid-Liquid Extraction of Phenolic Compounds from Maritime Pine Sawdust Waste. Kinetics, Optimization and Large Scale Experiments. Ultrason. Sonochem. 2016, 28, 230–239. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically Assisted Extraction (UAE) and Microwave Assisted Extraction (MAE) of Functional Compounds from Plant Materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of Microwave-Assisted Extraction of Polyphenols from Myrtus communis L. Leaves. Food Chem. 2015, 166, 585–595. [Google Scholar] [CrossRef]
- Eriksson, L.; Johansson, E.; Kettaneh-Wold, N.; Wikström, C.; Wold, S. Design of Experiments. In Principles and Applications; Learn Ways AB: Stockholm, Sweden, 2000. [Google Scholar]
- European Medicines Agency, ICH Guideline Q8 (R2) on Pharmaceutical Development. 2009. Available online: https://www.ema.europa.eu/en/ich-q8-r2-pharmaceutical-development (accessed on 17 December 2021).
- Moldovan, C.; Frumuzachi, O.; Babotă, M.; Menghini, L.; Cesa, S.; Gavan, A.; Sisea, C.R.; Tanase, C.; Dias, M.I.; Pereira, C.; et al. Development of an Optimized Drying Process for the Recovery of Bioactive Compounds from the Autumn Fruits of Berberis vulgaris L. and Crataegus monogyna Jacq. Antioxidants 2021, 10, 1579. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A Reproducible, Rapid and Inexpensive Folin-Ciocalteu Micro-Method in Determining Phenolics of Plant Methanol Extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 2nd ed.; Council of Europe: Strasbourg, France, 2019; ISBN 92-871-8505-0. [Google Scholar]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity Using the DPPH.Free Radical Method. LWT-Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Nisca, A.; Ștefănescu, R.; Stegăruș, D.I.; Mare, A.D.; Farczadi, L.; Tanase, C. Comparative Study Regarding the Chemical Composition and Biological Activity of Pine (Pinus nigra and P. sylvestris) Bark Extracts. Antioxidants 2021, 10, 327. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Tănase, C.; Coşarcă, S.; Toma, F.; Mare, A.; Man, A.; Miklos, A.; Imre, S.; Boz, I. Antibacterial Activities of Beech Bark (Fagus sylvatica, L.) Polyphenolic Extract. Environ. Engin. Manag. J. 2018, 17, 877–884. [Google Scholar] [CrossRef]
- Vera Candioti, L.; De Zan, M.M.; Cámara, M.S.; Goicoechea, H.C. Experimental Design and Multiple Response Optimization. Using the Desirability Function in Analytical Methods Development. Talanta 2014, 124, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J.; Whitcomb, P.J. RSM Simplified: Optimizing Processes Using Response Surface Methods for Design of Experiments; Productivity Press: New York, NY, USA, 2016; ISBN 1315382326. [Google Scholar]
- Delgado-Moreno, L.; Peña, A.; Mingorance, M.D. Design of Experiments in Environmental Chemistry Studies: Example of the Extraction of Triazines from Soil after Olive Cake Amendment. J. Hazard. Mater. 2009, 162, 1121–1128. [Google Scholar] [CrossRef]
- Moldovan, C.; Babotă, M.; Mocan, A.; Menghini, L.; Cesa, S.; Gavan, A.; Sisea, C.; Vodnar, D.C.; Dias, M.I.; Pereira, C.; et al. Optimization of the Drying Process of Autumn Fruits Rich in Antioxidants: A Study Focusing on Rosehip (Rosa canina L.) and Sea Buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) and Their Bioactive Properties. Food Funct. 2021, 12, 3939–3953. [Google Scholar] [CrossRef]
- Moreira, M.M.; Barroso, M.F.; Boeykens, A.; Withouck, H.; Morais, S.; Delerue-Matos, C. Valorization of Apple Tree Wood Residues by Polyphenols Extraction: Comparison between Conventional and Microwave-Assisted Extraction. Ind. Crops Prod. 2017, 104, 210–220. [Google Scholar] [CrossRef] [Green Version]
- Bouras, M.; Chadni, M.; Barba, F.J.; Grimi, N.; Bals, O.; Vorobiev, E. Optimization of Microwave-Assisted Extraction of Polyphenols from Quercus Bark. Ind. Crops Prod. 2015, 77, 590–601. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Awad, M.A.; Al-Qurashi, A.D. Antioxidant Activity, Antioxidant Compounds, Antioxidant and Hydrolytic Enzymes Activities of ‘Barhee’ Dates at Harvest and during Storage as Affected by Pre-Harvest Spray of Some Growth Regulators. Sci. Hortic. 2014, 167, 91–99. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH Assays to Measure Antioxidant Capacity in Popular Antioxidant-Rich US Foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Semwal, P.; Painuli, S.; Badoni, H.; Bacheti, R.K. Screening of Phytoconstituents and Antibacterial Activity of Leaves and Bark of Quercus leucotrichophora A. Camus from Uttarakhand Himalaya. Clin. Phytosci. 2018, 4, 30. [Google Scholar] [CrossRef]
- Valencia-Avilés, E.; Martínez-Flores, H.-E.; García-Pérez, M.; Meléndez-Herrera, E.; García-Pérez, M.-E. Investigation of the Antibacterial Activity and Subacute Toxicity of a Quercus crassifolia Polyphenolic Bark Extract for Its Potential Use in Functional Foods. J. Food Sci. 2019, 84, 1692–1702. [Google Scholar] [CrossRef]


| Exp No | Exp Name | Run Order | Power (W) | Time (min) |
|---|---|---|---|---|
| 1 | N1 | 11 | 200 | 10 |
| 2 | N2 | 13 | 1000 | 10 |
| 3 | N3 | 2 | 1000 | 10 |
| 4 | N4 | 4 | 500 | 10 |
| 5 | N5 | 10 | 200 | 30 |
| 6 | N6 | 12 | 1000 | 30 |
| 7 | N7 | 3 | 1000 | 30 |
| 8 | N8 | 1 | 500 | 30 |
| 9 | N9 | 14 | 200 | 20 |
| 10 | N10 | 9 | 1000 | 20 |
| 11 | N11 | 6 | 500 | 20 |
| 12 | N12 | 7 | 500 | 20 |
| 13 | N13 | 8 | 500 | 20 |
| 14 | N14 | 5 | 500 | 20 |
| Sample | TPC (mg GAE/g d.w.) | IC50 DPPH (µg/mL) | IC50 ABTS (µg/mL) | TTC (%) |
|---|---|---|---|---|
| QCA | 382.26 ± 0.97 a | 2.446 ± 0.24 a | 6.211 ± 0.51 | 49.14 ± 1.36 a |
| QCE | 403.73 ± 7.35 b | 6.92 ± 0.39 b | 6.19 ± 0.35 | 45.68 ± 1.75 a |
| Bacterial Strain | MIC (mg/mL) | |
|---|---|---|
| QCA | QCE | |
| Gram-positive bacteria | ||
| Staphylococcus aureus ATCC 25923 | 0.625 | 0.625 |
| Methicillin-resistant Staphylococcus aureus ATCC 43300 | 1.25 | 0.625 |
| Gram-negative bacteria | ||
| Escherichia coli ATCC 25922 | >5 | 2.5 |
| Klebsiella pneumoniae ATCC 13883 | 1.25 | 1.25 |
| Pseudomonas aeruginosa ATCC 27853 | >5 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisca, A.; Ștefănescu, R.; Moldovan, C.; Mocan, A.; Mare, A.D.; Ciurea, C.N.; Man, A.; Muntean, D.-L.; Tanase, C. Optimization of Microwave Assisted Extraction Conditions to Improve Phenolic Content and In Vitro Antioxidant and Anti-Microbial Activity in Quercus cerris Bark Extracts. Plants 2022, 11, 240. https://doi.org/10.3390/plants11030240
Nisca A, Ștefănescu R, Moldovan C, Mocan A, Mare AD, Ciurea CN, Man A, Muntean D-L, Tanase C. Optimization of Microwave Assisted Extraction Conditions to Improve Phenolic Content and In Vitro Antioxidant and Anti-Microbial Activity in Quercus cerris Bark Extracts. Plants. 2022; 11(3):240. https://doi.org/10.3390/plants11030240
Chicago/Turabian StyleNisca, Adrian, Ruxandra Ștefănescu, Cadmiel Moldovan, Andrei Mocan, Anca Delia Mare, Cristina Nicoleta Ciurea, Adrian Man, Daniela-Lucia Muntean, and Corneliu Tanase. 2022. "Optimization of Microwave Assisted Extraction Conditions to Improve Phenolic Content and In Vitro Antioxidant and Anti-Microbial Activity in Quercus cerris Bark Extracts" Plants 11, no. 3: 240. https://doi.org/10.3390/plants11030240
APA StyleNisca, A., Ștefănescu, R., Moldovan, C., Mocan, A., Mare, A. D., Ciurea, C. N., Man, A., Muntean, D.-L., & Tanase, C. (2022). Optimization of Microwave Assisted Extraction Conditions to Improve Phenolic Content and In Vitro Antioxidant and Anti-Microbial Activity in Quercus cerris Bark Extracts. Plants, 11(3), 240. https://doi.org/10.3390/plants11030240











