Responses of Caribbean Mangroves to Quaternary Climatic, Eustatic, and Anthropogenic Drivers of Ecological Change: A Review †
Abstract
1. Introduction
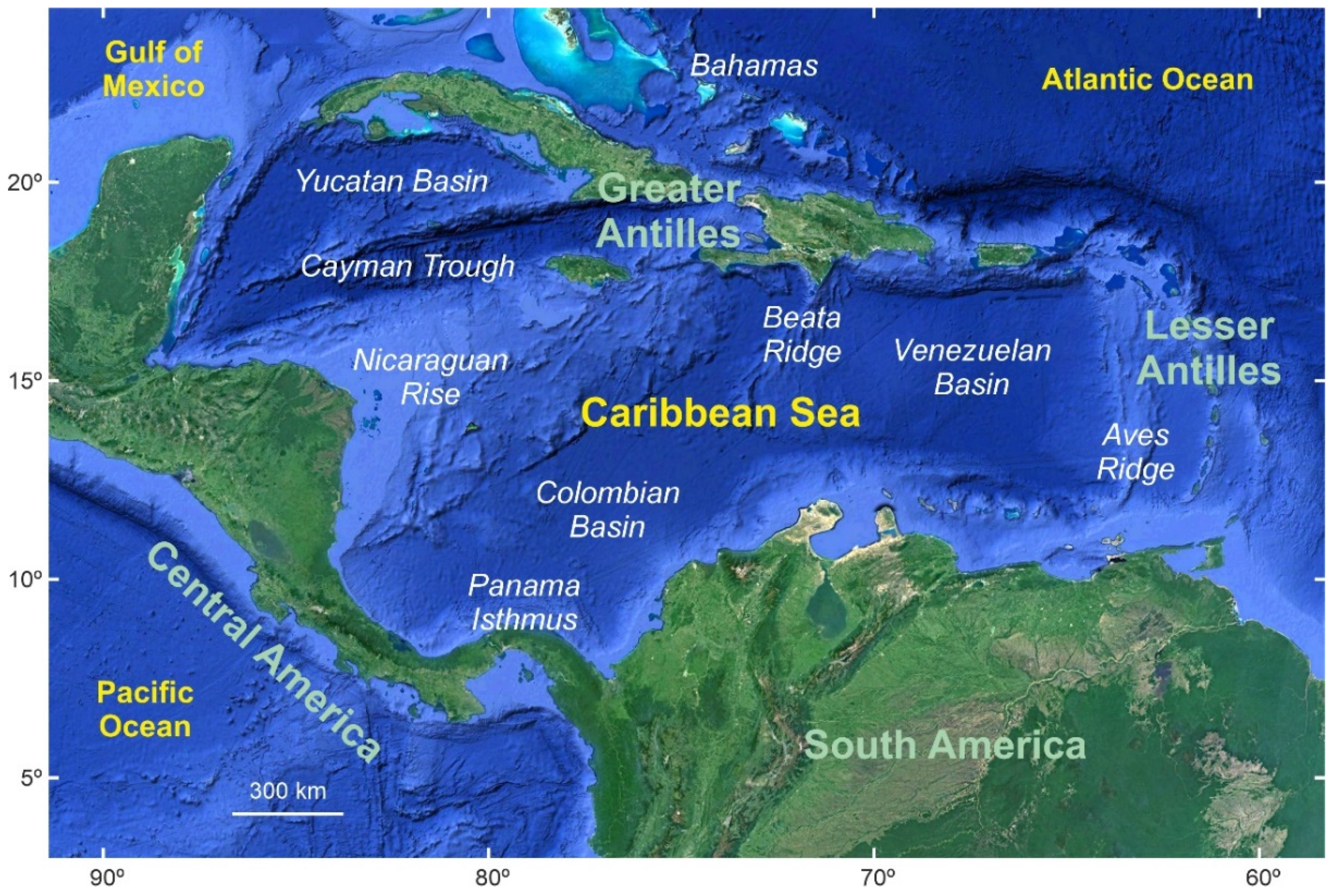
2. Study Area
2.1. Climate and Sea Level
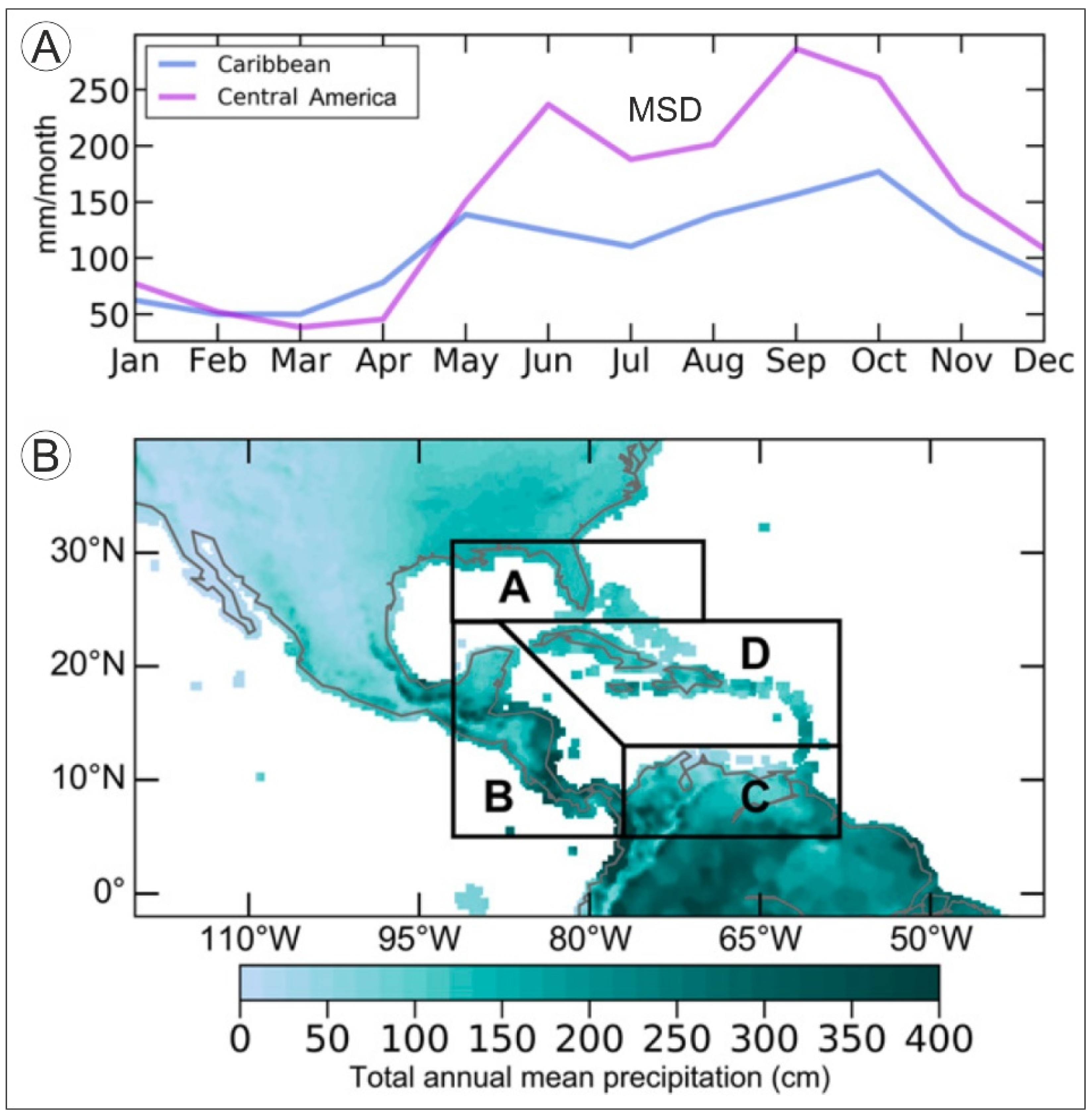
2.1.1. Temperature and Precipitation
2.1.2. Meteorological Hazards
2.1.3. Sea Level
2.1.4. Future Projections
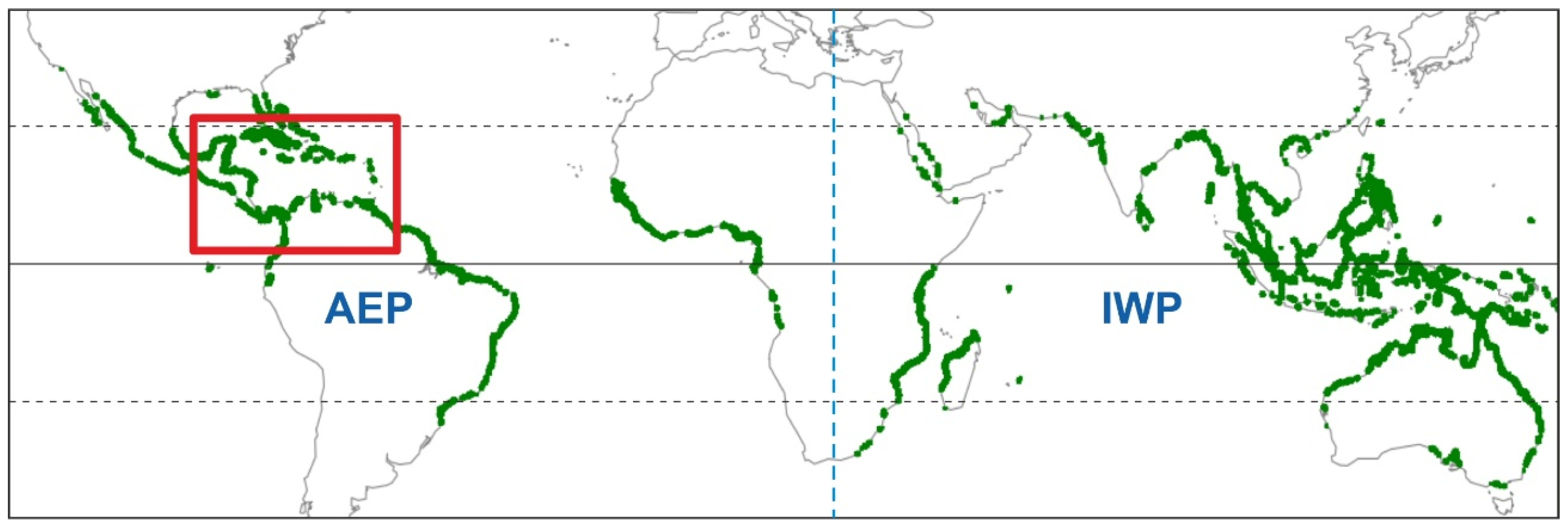
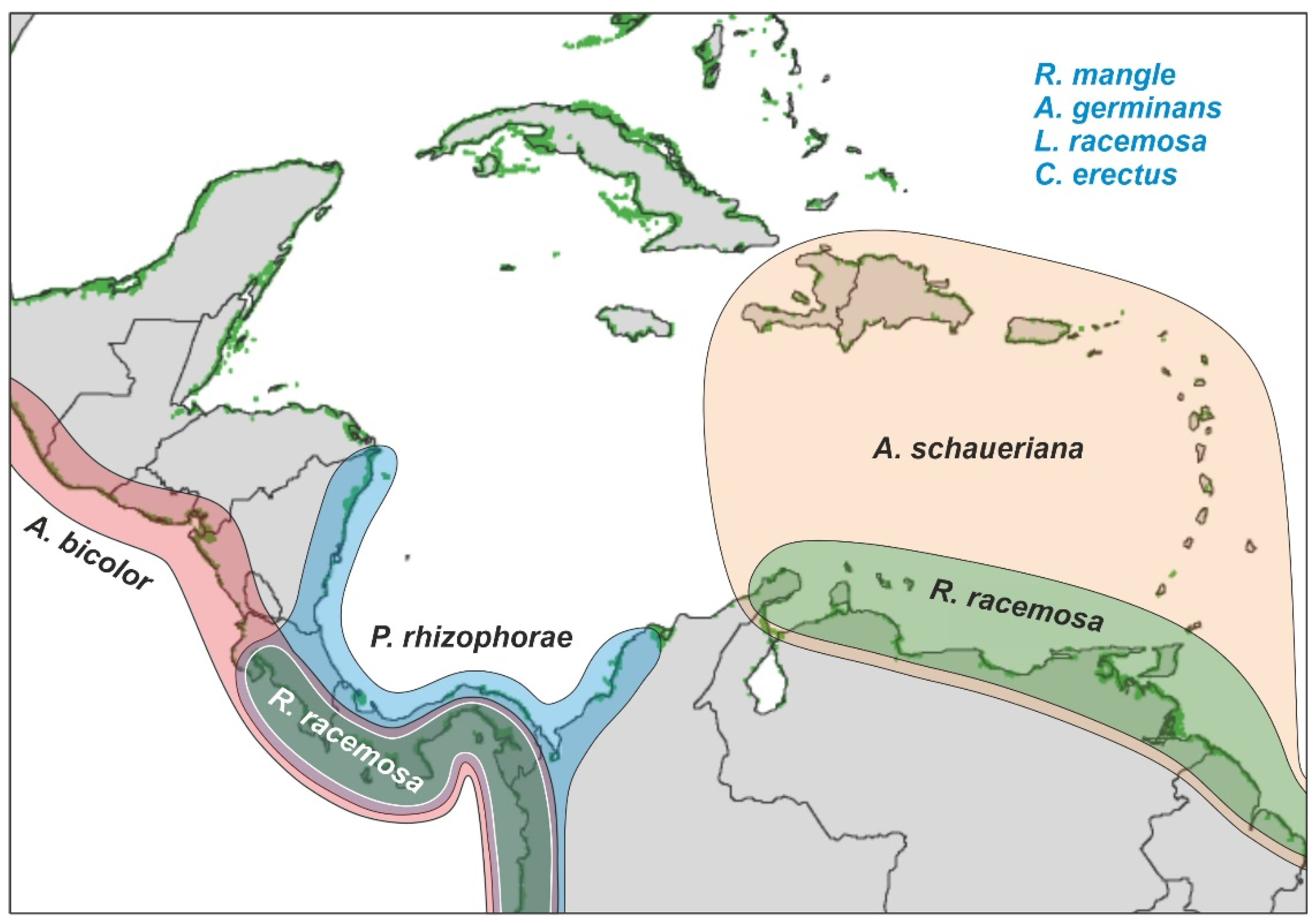
2.2. Mangrove Communities
2.2.1. Geographical Extent
| Code | Country/Island | 1980 [46] | 2010 [4] |
|---|---|---|---|
| An | Anguilla (UK) | 0.90 | 1.00 |
| AB | Antigua and Barbuda | 15.70 | 8.63 |
| Ar | Aruba | 4.20 | 0.26 |
| Bd | Barbados | 0.30 | 0.14 |
| Bz | Belize | 785.00 | 445.07 |
| Cy | Cayman Islands (UK) | 10.10 | 41.48 |
| Co | Colombia | 4400.00 | 2622.12 |
| CR | Costa Rica | 634.00 | 364.75 |
| Cu | Cuba | 5374.00 | 3328.16 |
| DR | Dominican Republic | 344.00 | 187.41 |
| ES | El Salvador | 467.00 | 375.89 |
| Gr | Grenada | 2.95 | 1.90 |
| Gp | Guadeloupe (France) | 30.00 | 37.13 |
| Gu | Guatemala | 186.00 | 235.23 |
| Gy | Guyana | 910.00 | 286.40 |
| Ht | Haiti | 178.00 | 144.32 |
| Ho | Honduras | 1525.00 | 597.32 |
| Ja | Jamaica | 120.00 | 94.11 |
| Mr | Martinique (France) | 19.00 | 20.52 |
| Ni | Nicaragua | 1034.00 | 739.88 |
| Pa | Panama | 2500.00 | 1533.37 |
| PR | Puerto Rico | 76.50 | 86.85 |
| SK | Saint Kitts and Nevis | 0.85 | 0.28 |
| SL | Saint Lucia | 2.00 | 16.40 |
| VG | Saint Vincent and the Grenadines | 0.55 | 0.31 |
| TT | Trinidad and Tobago | 75.00 | 76.96 |
| Ve | Venezuela | 2600.00 | 2753.25 |
| VI | Virgin Islands (US and UK) | 10.10 | 20.53 |
| Caribbean Total | 21,295.05 | 13,999.14 | |
| World total | 187,940.00 | 140,258.86 | |
| Caribbean/World (%) | 11.33 | 9.98 |
2.2.2. Floristics
2.2.3. Biogeography
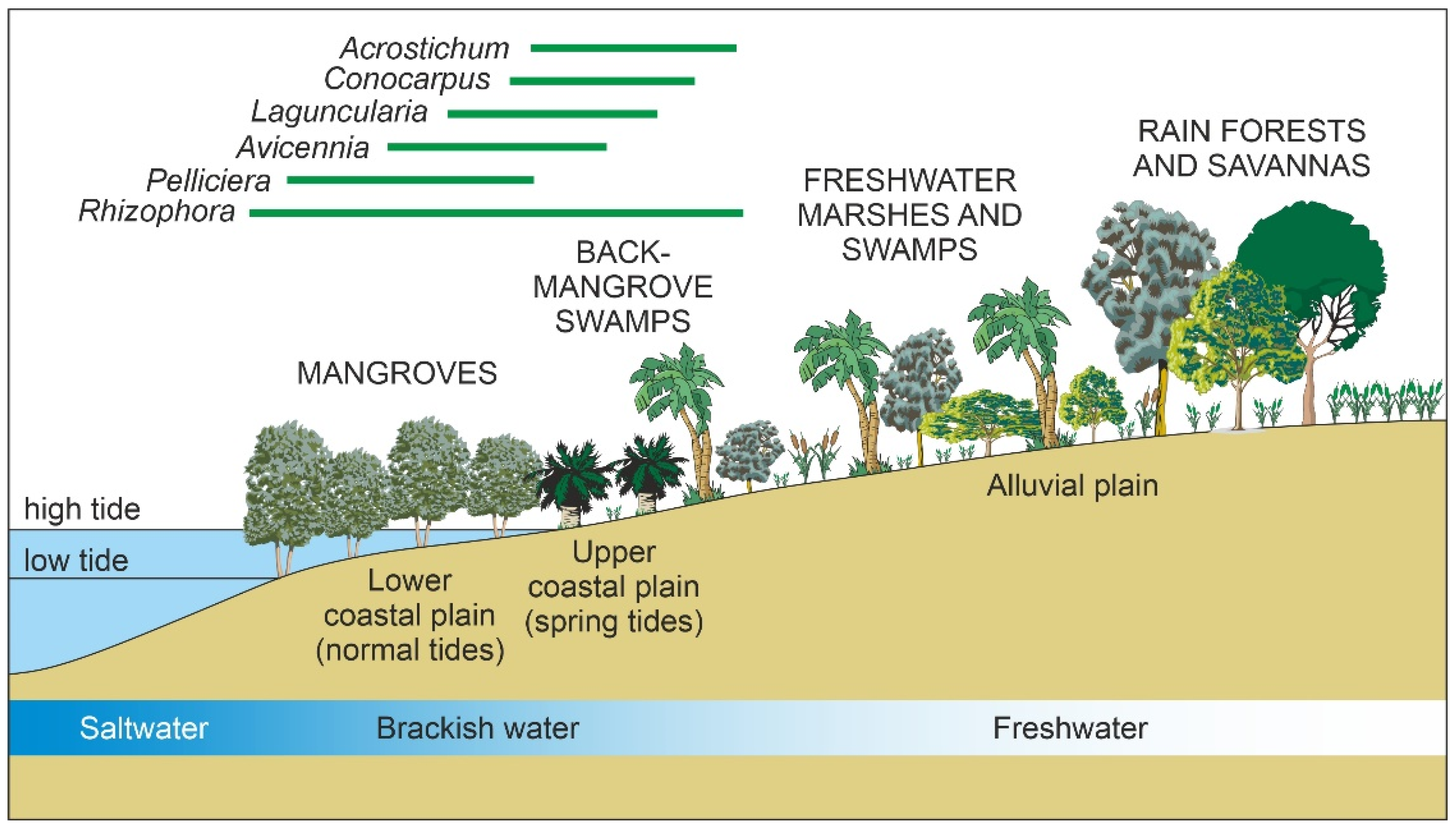
2.2.4. Ecology
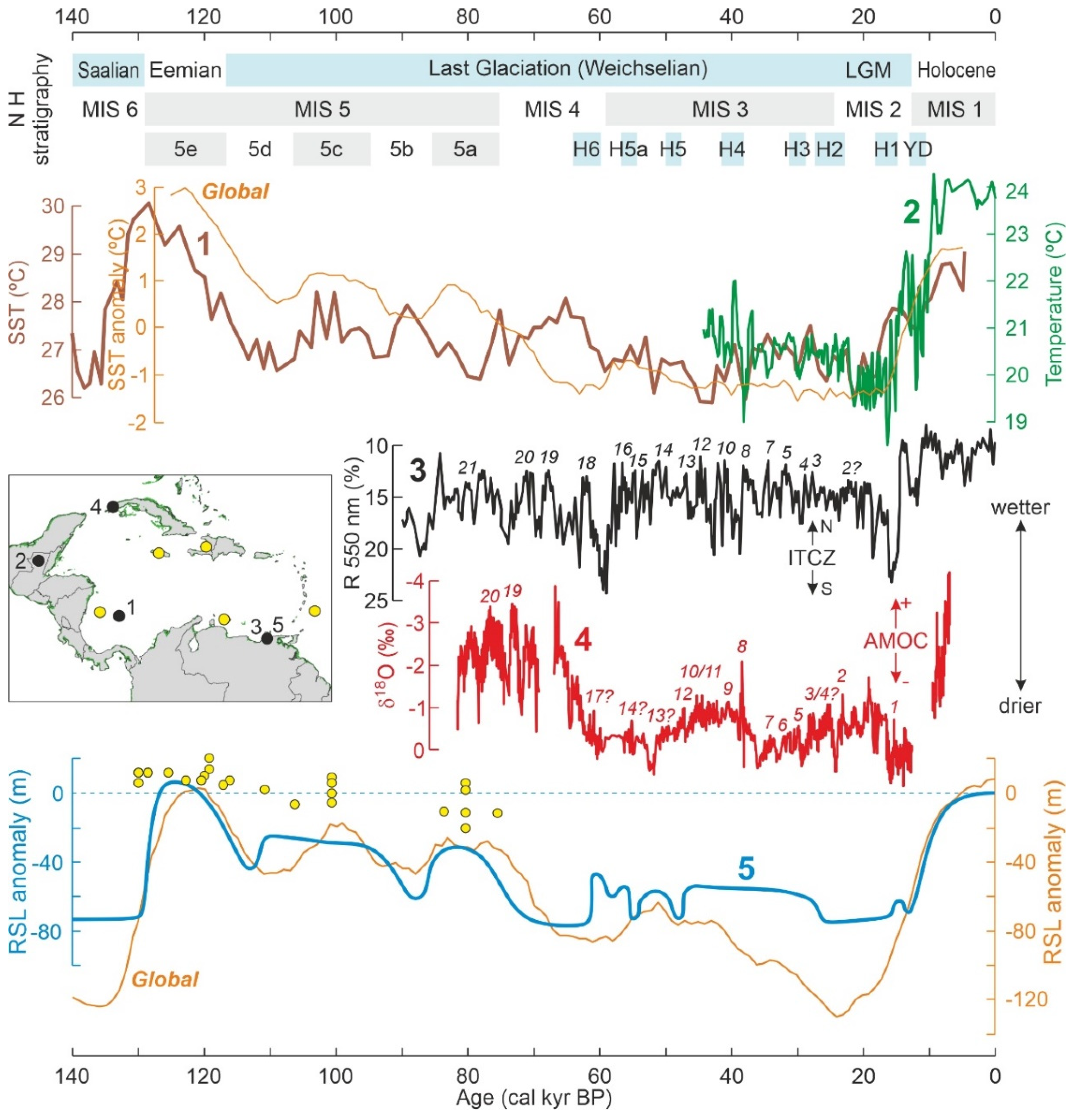
3. The Quaternary
3.1. Pleistocene Climatic and Sea-Level Changes
3.2. Late Glacial-Holocene Environmental Variability
3.3. Human Settlement
3.3.1. Late Pleistocene
3.3.2. Holocene
4. Mangrove Paleoecology
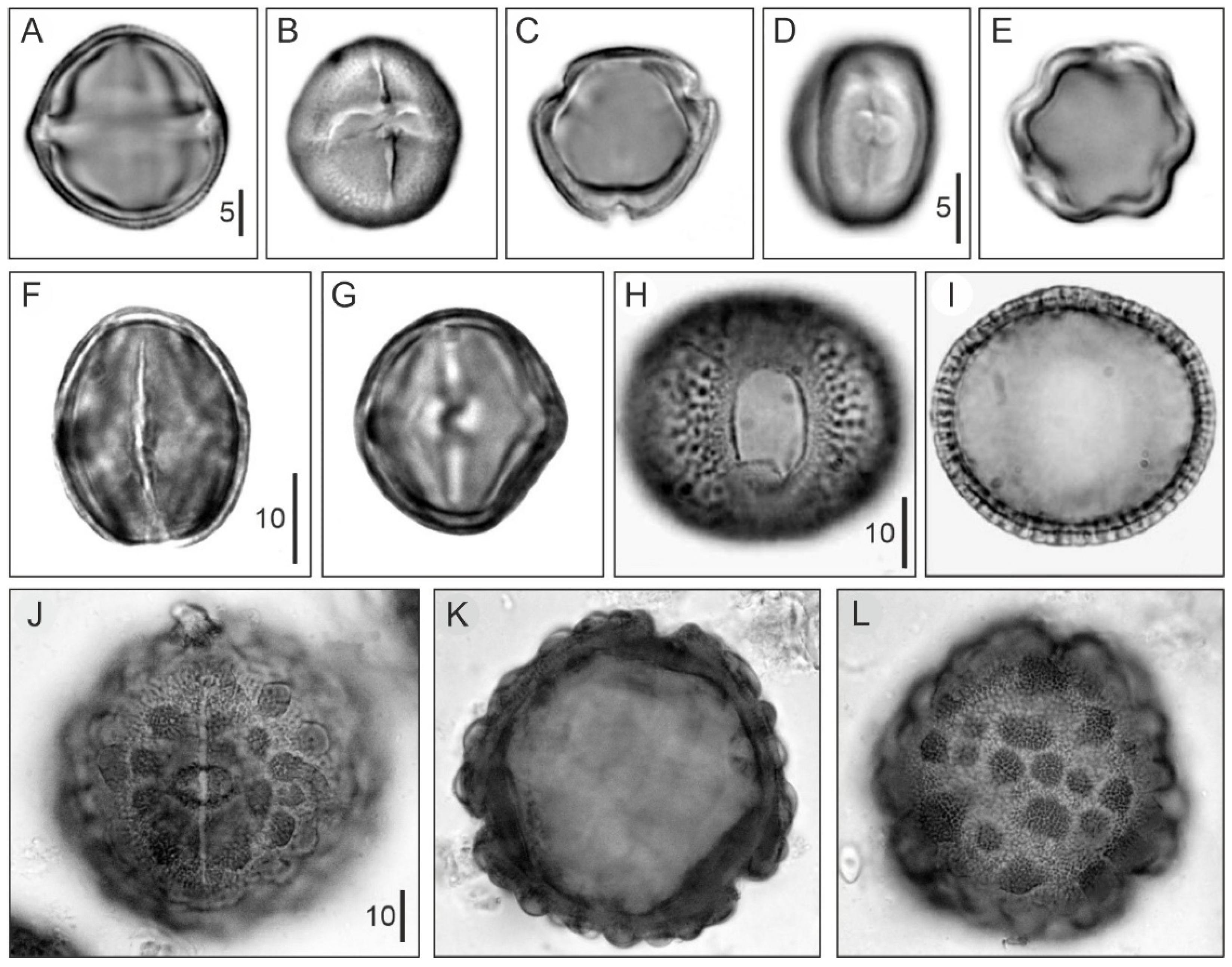
4.1. Modern Pollen Sedimentation
- The better-represented mangrove trees in modern pollen assemblages are Rhizophora and Avicennia, whereas Languncularia and Conocarpus are usually scarce or absent.
- The pollen of Rhizophora is the most abundant by far, reaching values of 80% in the source area, and is commonly overrepresented in relation to the abundance of the parent species. This pollen declines landward but is still present in low percentages beyond the inland boundary of the mangrove fringe. In shallow marine environments, Rhizophora pollen may be found >80 km offshore in high percentages (50%) [130].
- The pollen of Avicennia is usually underrepresented with respect to its parent species, and its abundance rarely exceeds 40% of the pollen assemblage, even in pure stands of these three, remaining below 25% in mixed Rhizophora-Avicennia stands [131]. This pollen settles comparatively sooner, and its abundance rapidly declines in both inland and offshore directions [130].
- The spores of the mangrove fern Acrostichum are more abundant behind the mangrove fringe, close to the habitat of the species, and are present in low abundances in other mangrove soils. It may also be found in moderate abundance on the sediments of river mouths [135]. It has been suggested that Acrostichum could be indicative of human disturbance [137].
- These patterns are linked to interspecific differences in pollen production, transport, sedimentation and reworking processes that are discussed in the original references. Noteworthy, Rhizophora is wind-pollinated and produces large amounts of pollen [51], whereas Avicennia, Laguncularia, and Conocarpus are pollinated by insects and produce far less pollen [58,59,63].
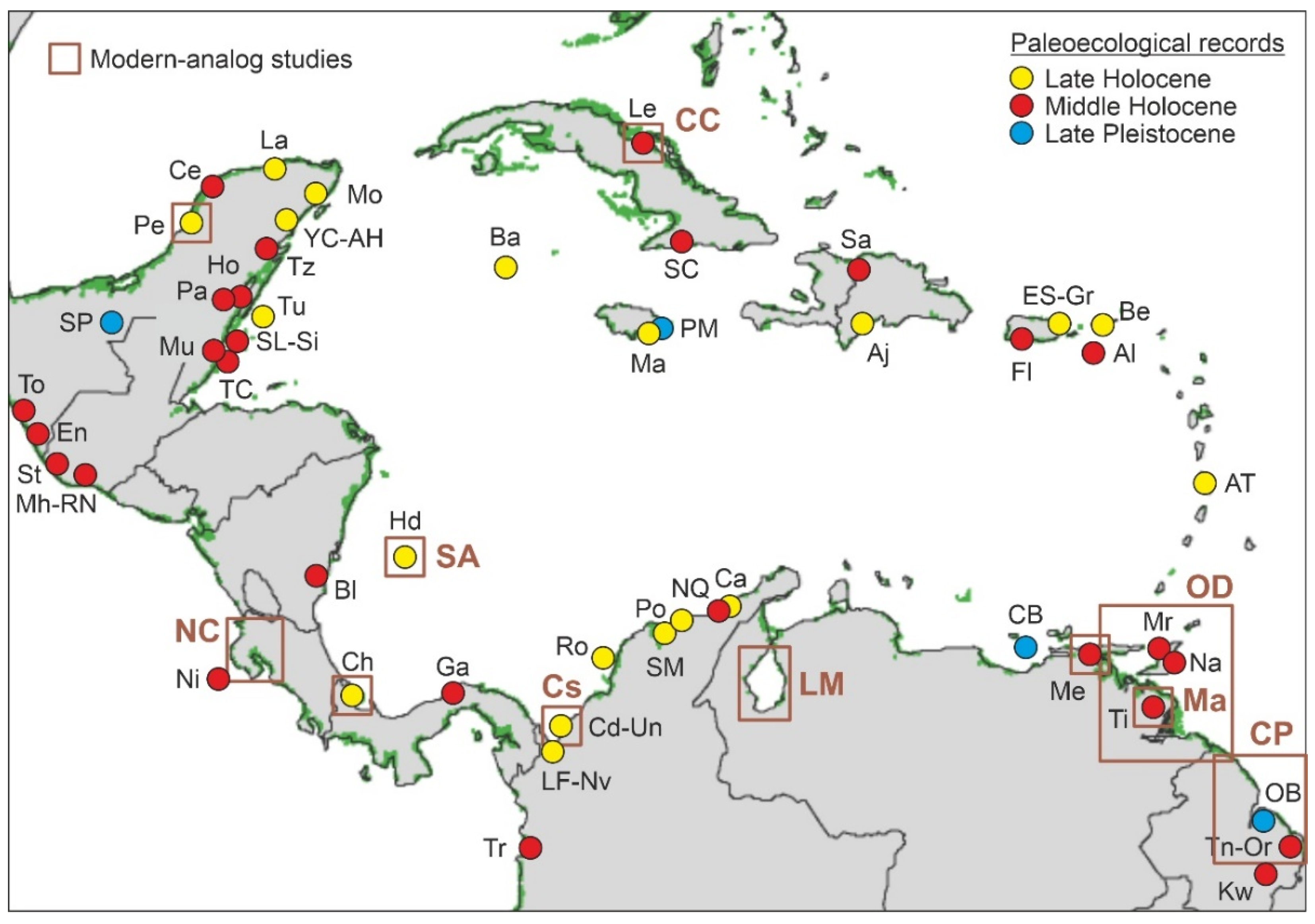
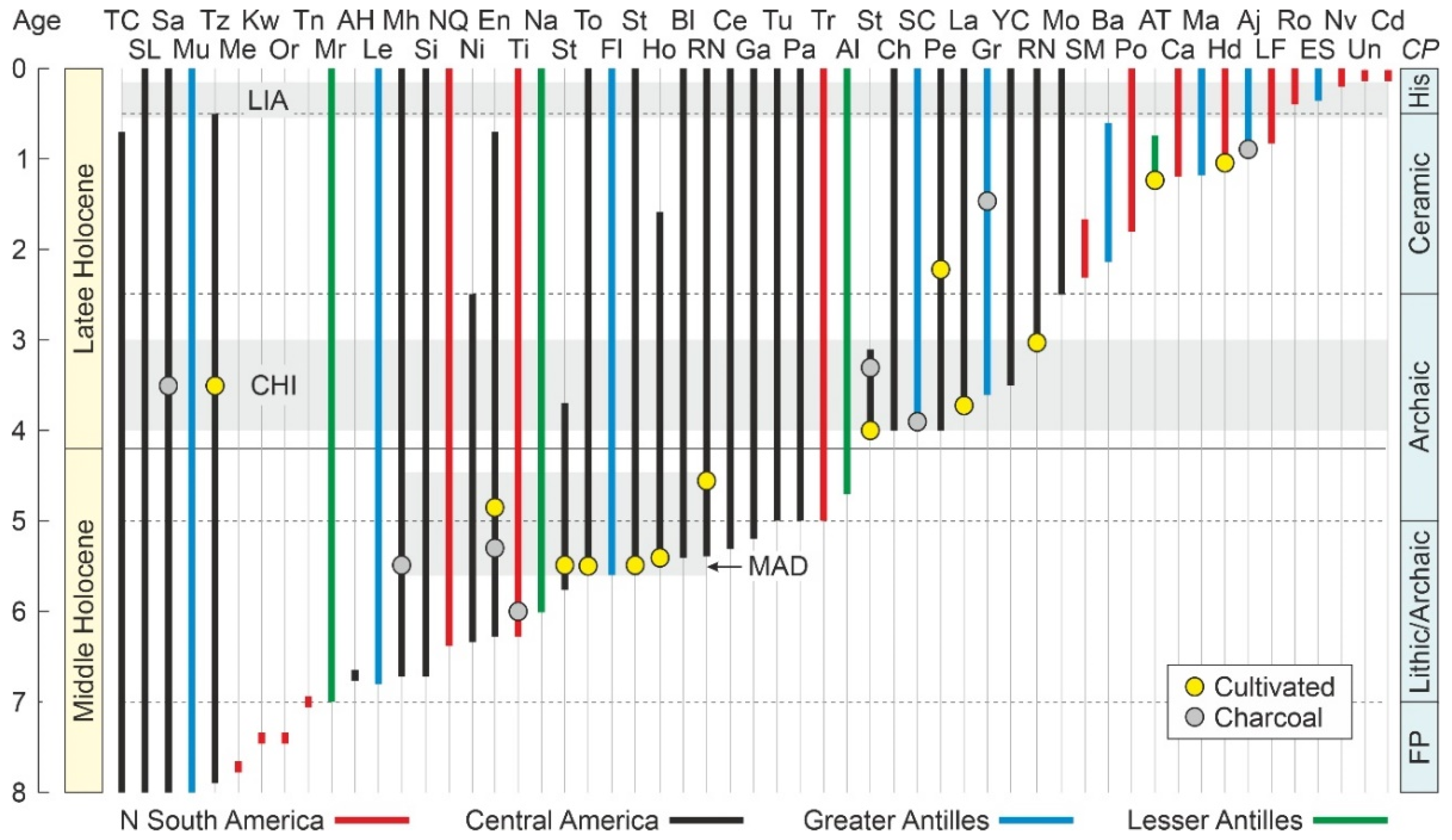
4.2. Paleoecological Sites
| Site (core) | Map | C/I | Coast | OA | Proxies | CP | Ch | References |
|---|---|---|---|---|---|---|---|---|
| Urabá (Candelaria) | Cd | Co | NSA | 100 | P | - | - | [148] |
| Urabá (Uno) | Un | Co | NSA | 100 | P | - | - | [148] |
| Cispatá (Navío) | Nv | Co | NSA | 200 | P/G | - | - | [149] |
| Espíritu Santo (BC7) | ES | PR | GA | 270 | G/F/T | - | - | [150] |
| ERIA (Rosario) | Ro | Co | NSA | 340 | P | - | - | [151] |
| Cispatá (Flotante) | LF | Co | NSA | 890 | P/G | - | - | [149] |
| Alejandro (ALEJ08) | Aj | DR | GA | 1000 | P/Ch/G/Py | - | 1000 | [152] |
| S. Andrés (Honda) | Hd | Co | NSA | 1100 | P | 1100 | - | [153] |
| Manatee (MB-7) | Ma | Ja | GA | 1200 | O/G | - | - | [154] |
| Guajira (Calancala) | Ca | Co | NSA | 1200 | P | - | - | [155] |
| Anse Trabaud | AT | Mr | LA | 1290 | A/St/Pt/Am | 1290 | - | [156] |
| Pozo (Neguanje) | Po | Co | NSA | 1800 | P/G/Pm | - | - | [157] |
| Barkers (BA 1-9) | Ba | Cy | GA | 2100 | S | - | - | [158] |
| Santa Marta | SM | Co | NSA | 2400 | P/Py | - | - | [159] |
| Morelos (MPM-1) | Mo | Mx | CA | 2520 | P | - | - | [160] |
| R. Naranjo (OC001) | RN | Gu | CA | 3050 | P/Ch/Ph | 3050 | 3050 | [124] |
| Yax Chen | YC | Mx | CA | 3500 | S | - | - | [161] |
| L. Grande | Gr | PR | GA | 3600 | P/Ch/S/D/F/G | - | 1550 | [162] |
| R. Lagartos | La | Mx | CA | 3870 | P | 3860 | - | [146] |
| Petenes (Xpuk chico) | Pe | Mx | CA | 4000 | P/G/Py/S | 2150 | - | [163,164] |
| Unnamed (SC01) | SC | Cu | GA | 4000 | P/Ch/F/S | - | 4000 | [165] |
| Changuinola | Ch | Pa | CA | 4000 | P | - | - | [133] |
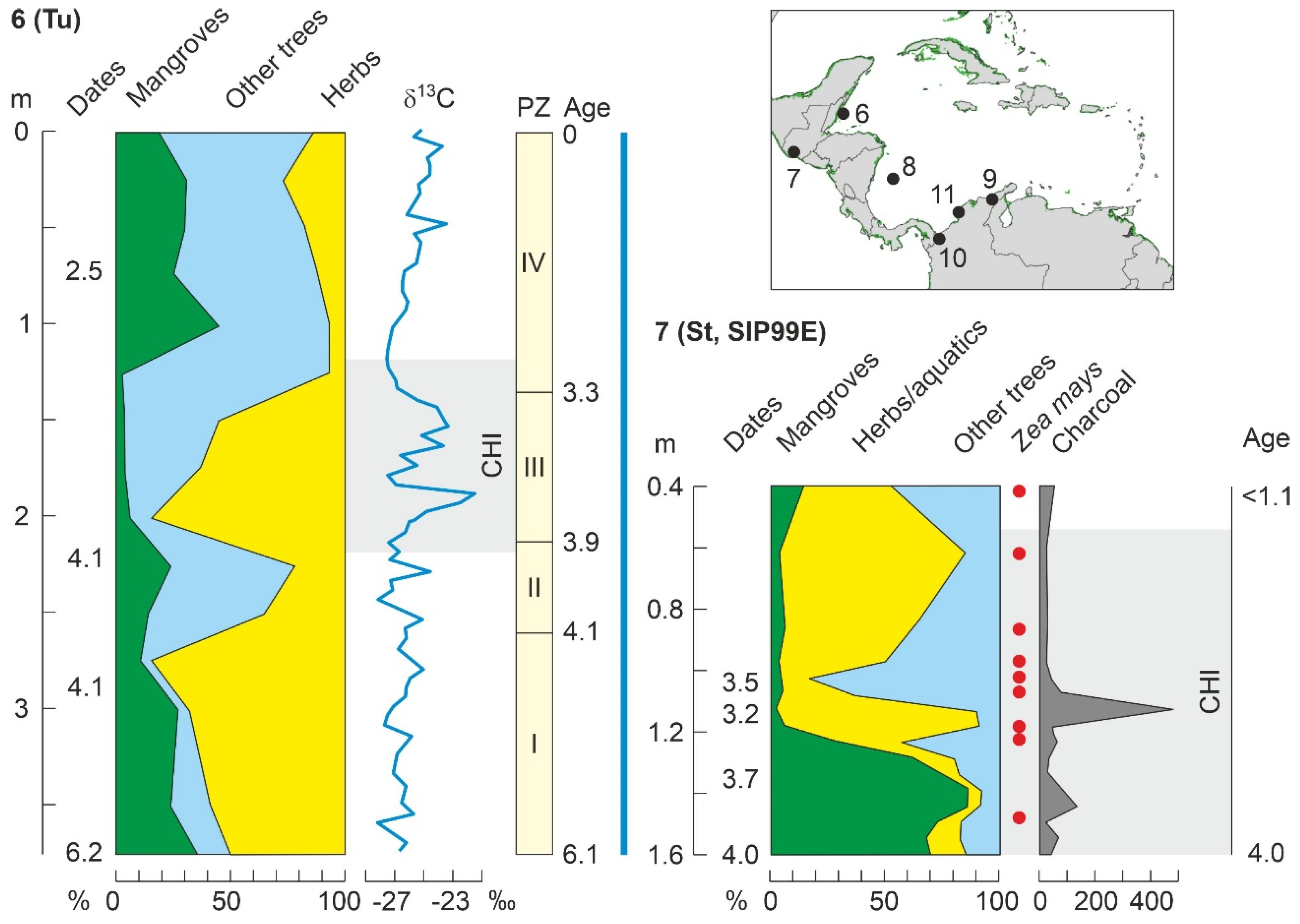
| Sipacate (SIP99E) | St | Gu | CA | 4020 | P/Ch/Ph | 4000 | 3200 | [124] |
| Altona (070321) | Al | SC | LA | 4700 | P/S/G | - | - | [166] |
| Tribugá | Tr | Co | NSA | 5000 | P/S | - | - | [167] |
| Palmar | Pa | Mx | CA | 5000 | P | - | - | [168] |
| Turneffe (TNF-1) | Tu | Bz | CA | 5000 | P/G | - | - | [169] |
| P. Galeta (GAL18) | Ga | Pa | CA | 5200 | P/G | - | - | [170] |
| Celestum (4A-4B) | Ce | Mx | CA | 5300 | F/G | - | - | [171] |
| R. Naranjo (TIL016) | RN | Gu | CA | 5390 | P/Ch/Ph | 4620 | 4620 | [124] |
| Bluefields (FBM1-5) | Bl | Ni | CA | 5400 | P | - | - | [172] |
| R. Hondo | Ho | Mx | CA | 5600 | P/G | 5600 | - | [173] |
| Sipacate (SIP014) | St | Gu | CA | 5600 | P/Ch/Ph | 5520 | 5520 | [124] |
| L. Flamenco (P01) | Fl | PR | GA | 5660 | G | - | - | [174,175] |
| Tonalá (La Joya) | To | Mx | CA | 5600 | P/Dt/G/Py | 5600 | - | [176] |
| Sipacate (SIP001) | St | Gu | CA | 5820 | P/Ch/Ph | 5520 | 5520 | [124] |
| Nariva | Na | TT | LA | 5990 | P | - | - | [177] |
| C. Tigre (A12) | Ti | Ve | NSA | 6200 | P/Ch/Py/G | - | 6000 | [143] |
| Encrucijada (C02) | En | Mx | CA | 6200 | P/Ch/F | 4900 | 5300 | [178] |
| Nicoya (DSDP-565) | Ni | CR | CA | 6255 | P | - | - | [139] |
| Guajira (N. Quebrado) | NQ | Co | NSA | 6280 | P | - | - | [155] |
| Sibun (SR-63) | Si | Bz | CA | 6780 | P/G | - | - | [179] |
| Manchón (MAN015) | Mh | Gu | CA | 6780 | P/Ch/Ph | - | 5520 | [124] |
| L. Leche (LL1) | Le | Cu | GA | 6800 | P/Pm/F/O/G | - | - | [180] |
| Aktun Ha | AH | Mx | CA | 6830 | P/G | - | - | [181] |
| Maracas | Mr | TT | LA | 7000 | P/G | - | - | [177,182] |
| Torani | Tn | Gy | NSA | 7030 | P/S | - | - | [131] |
| Oreala | Or | Gy | NSA | 7370 | P/S | - | - | [131] |
| Kwakwani | Kw | Gy | NSA | 7390 | P/S | - | - | [131] |
| P. Medina (PM-1) | Me | Ve | NSA | 7790 | P | - | - | [138] |
| L. Tzib | Tz | Mx | CA | 7900 | P/G | 3500 | - | [146] |
| Mullins-Sapodilla | Mu | Bz | CA | 7980 | S/G | - | - | [183] |
| Saladilla | Sa | DR | GA | 8030 | P/Ch/Dt | - | 3600 | [184] |
| S. Lookout (BT-79) | SL | Bz | CA | 8100 | P/S/G | - | - | [185] |
| Twin Cays | TC | Bz | CA | 8200 | S | - | - | [186] |
| Cariaco (MD03-2622) | CB | Ve | NSA | 68 k * | P | - | - | [187,188] |
| S. Pedro | SP | Mx | CA | 98 k * | Mp | - | - | [189] |
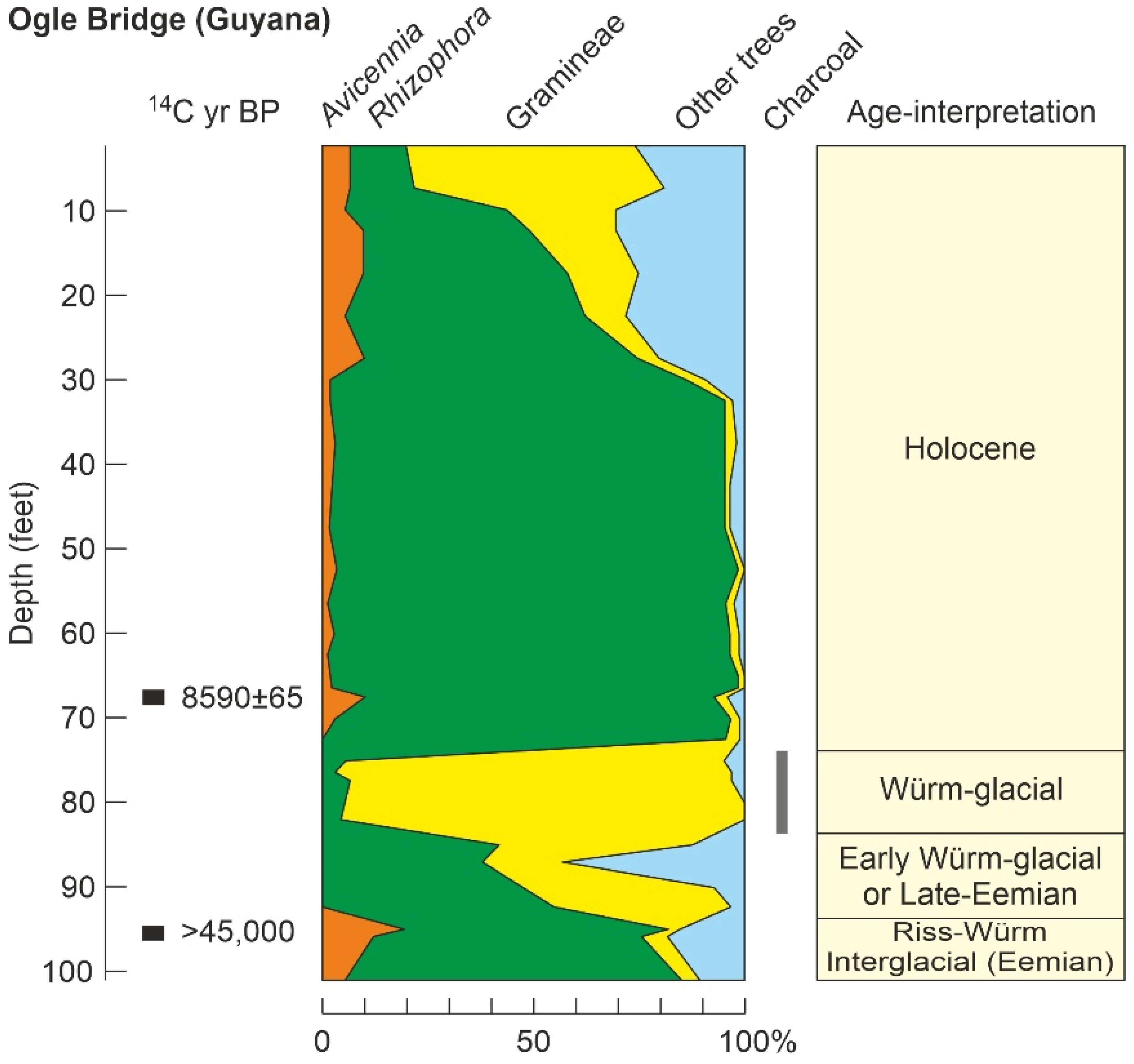
| Ogle Bridge (39) | OB | Gy | GA | >45 k * | P/Ch//S | - | Gl? | [131] |
| P. Morant | PM | Ja | GA | 132 k * | S/Am | - | - | [77] |
4.3. Pleistocene
4.3.1. Last Interglacial
4.3.2. Last Glaciation
4.4. Holocene
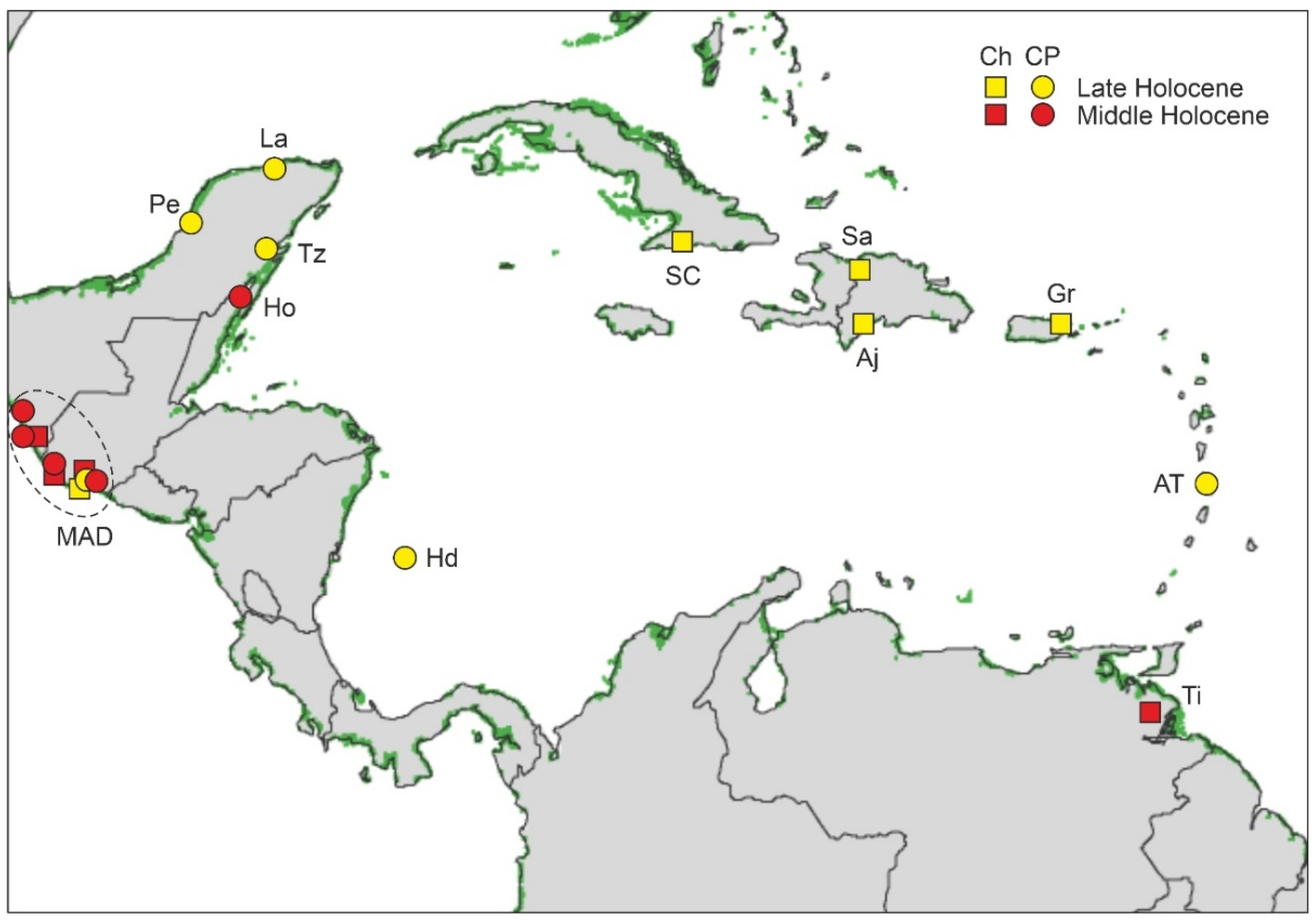
4.4.1. Middle Holocene
4.4.2. Late Holocene
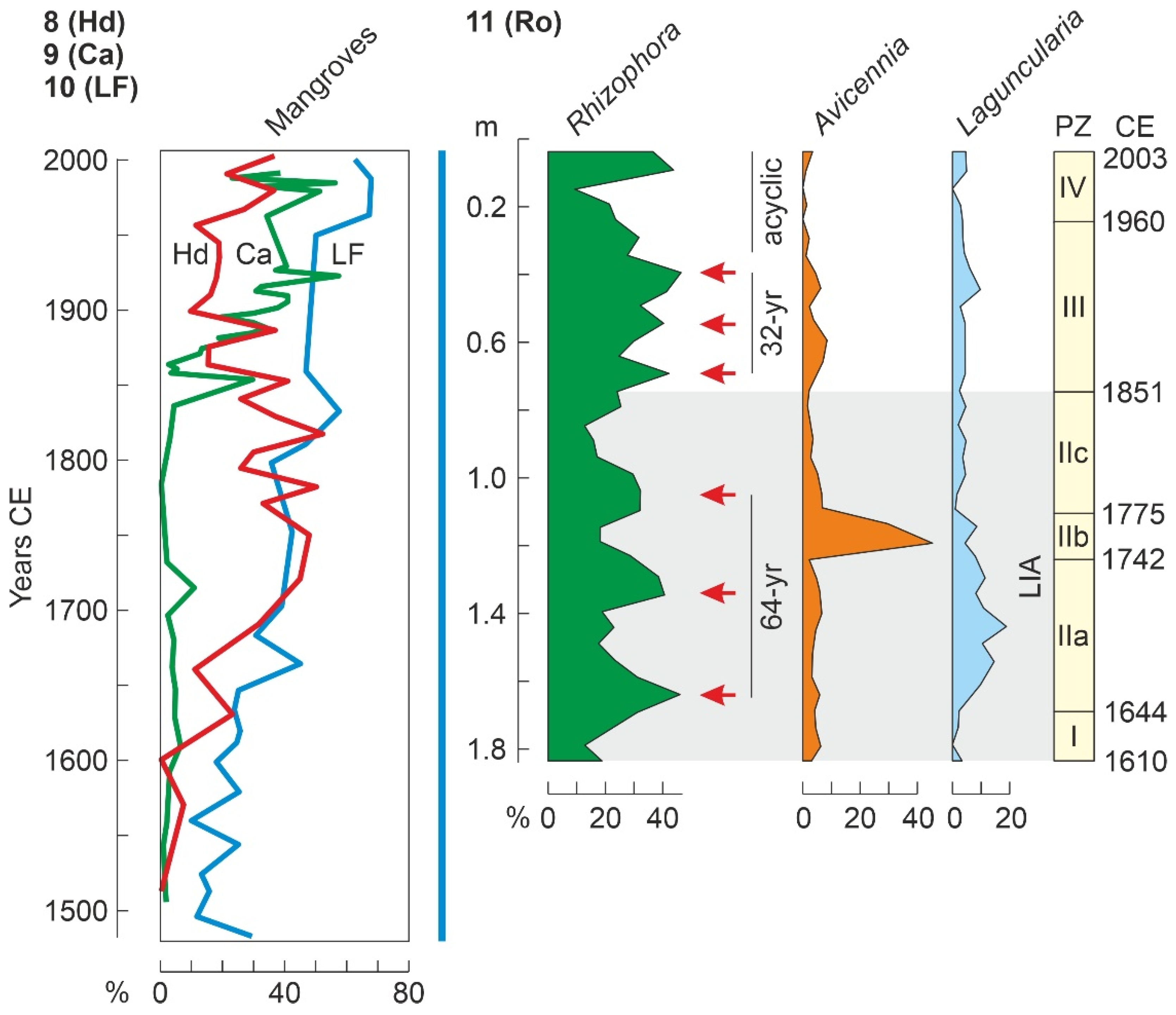
4.4.3. Last Millennium
5. Conclusions and Prospects for Future Studies
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spalding, M.; Kainuma, M.; Collins, L. Wolrd Atlas of Mangroves; Routledge: London, UK, 2010. [Google Scholar]
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Bunting, P.; Rosenqvist, A.; Lucas, R.; Rebelo, L.M.; Hilarides, L.; Thomas, N.; Hardy, A.; Itoh, T.; Shimada, M.; Finlayson, C. The Global Mangrove Watch—A New 2010 Global Baseline of Mangrove Extent. Remote Sens. 2018, 10, 1669. [Google Scholar] [CrossRef]
- Bunting, P.; Rosenqvist, A.; Hilarides, L.; Lucas, R.M.; Thomas, N. Global Mangrove Watch: Updated 2010 Mangrove Forest Extent (v2.5). Remote Sens. 2022, 14, 1034. [Google Scholar] [CrossRef]
- Walker, J.E.; Ankersen, T.; Barchiesi, S.; Meyer, C.K.; Altieri, A.H.; Osborne, T.Z.; Angelini, C. Governance and the mangrove commons: Advancing the cross-scale. nested framework for the global conservation and wise use of mangroves. J. Environ. Manag. 2022, 312, 114823. [Google Scholar] [CrossRef]
- Nizam, A.; Prasannakumari, S.; Kumar, A. Genetic and molecular mechanisms underlying mangrove adaptations to intertidal environments. ¡Science 2022, 25, 103547. [Google Scholar] [CrossRef]
- Worthington, T.A.; zu Ermgassen, P.S.E.; Fries, D.A.; Krauss, K.W.; Lovelock, C.E.; Thorley, J.; Tingey, R.; Woodrofe, C.D.; Bunting, P.; Cornier, N.; et al. A global biophysical typology of mangroves and its relevance for ecosystem structure and deforestation. Sci. Rep. 2020, 10, 14652. [Google Scholar] [CrossRef]
- Goldberg, L.; Lagomasino, D.; Thomas, N.; Fatoyinbo, T. Global declines in human-driven mangrove loss. Glob. Chang. Biol. 2020, 26, 5844–5855. [Google Scholar] [CrossRef]
- Duke, N.C.; Meyneccke, J.-O.; Dittmann, S.; Ellison, A.M.; Anger, K.; Berger, U.; Cannicci, S.; Diele, K.; Ewel, K.C.; Field, C.D.; et al. A world without mangroves? Science 2017, 317, 41b–42b. [Google Scholar] [CrossRef]
- Gilman, E.L.; Ellison, J.; Duke, N.C.; Field, C. Threats to mangroves from climate change and adaptation options: A review. Aquat. Bot. 2008, 89, 237–250. [Google Scholar] [CrossRef]
- Spalding, M.D.; Ruffo, S.; Lacambra, C.; Melianie, I.; Zeitlin, L.; Shepard, C.C.; Beck, M. The role of ecosystems in coastal protection: Adapting to climatic change and coastal hazards. Ocean Coast. Manag. 2014, 90, 50–57. [Google Scholar] [CrossRef]
- Biswas, P.L.; Biswas, S.R. Mangrove forests: Ecology. management. and threats. In Life on Land. Encyclopedia of the UN Sustainable Development Goals; Leal Filho, W., Azul, A., Branli, L., Özuyar, P., Wall, T., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 1–14. [Google Scholar]
- Wang, Y.-S.; Gu, J.-D. Ecological responses. adaptation and mechanisms of mangrove wetland ecosystem to global climate change and anthropogenic activities. Int. Biodeterior. Biodegradation 2021, 162, 105248. [Google Scholar] [CrossRef]
- Bosire, J.O.; Dahdouh-Guebas, F.; Walton, M.; Crona, B.I.; Lewis, R.R.; Field, C.; Kairo, J.G.; Koedam, N. Functionality of restored mangroves: A review. Aquat. Bot. 2008, 89, 251–259. [Google Scholar] [CrossRef]
- Saenger, P. Mangrove Ecology, Silviculture and Conservation; Kluwer: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Berger, U.; Rivera-Monroy, V.H.; Doyle, T.W.; Dahdouh-Guevas, F.; Duke, N.C.; Fontalvo-Herazo, M.L.; Hildebrandt, H.; Koedam, N.; Mehling, U.; Piou, C.; et al. Advances and limitations of individual-based models to analyze and predict dynamics of mangrove forests: A review. Aquat. Bot. 2008, 89, 260–274. [Google Scholar] [CrossRef]
- Duke, N.C. Mangrove floristics and biogeography revisited: Further deductions from biodiversity hot spots. ancestral discontinuities. and common evolutionary processes. In Mangrove Ecosystems: A Global Biogeographic Perspective; Rivera-Monroy, V.H., Lee, S.Y., Kristensen, E., Twilley, R.R., Eds.; Springer Nature: Cham, Switzerland, 2017; pp. 27–53. [Google Scholar]
- Tomlinson, P.B. The Botany of Mangroves; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Rull, V. Modern and Quaternary palynological studies in the Caribbean and Atlantic cosats of northern South America. Bol. Soc. Ven. Geól. 1998, 23, 5–24. [Google Scholar]
- Rull, V. The Caribbean mangroves: An Eocene innovation with no Cretaceous precursors. Earth Sci. Rev. 2022, 231, 104070. [Google Scholar] [CrossRef]
- Rull, V. Eocene/Oligocene global disruption and the revolution of Caribbean mangroves. EarthArXiv 2022. [Google Scholar] [CrossRef]
- Rull, V. Taxon cycles in Neotropical mangroves. bioRxiv 2022. [Google Scholar] [CrossRef]
- Graham, A. Diversification of Gulf/Caribbean mangrove communities through Cenozoic time. Biotropica 1995, 27, 20–27. [Google Scholar] [CrossRef]
- Taylor, M.A.; Alfaro, E.J. Climate of Central America and the Caribbean. In Encyclopedia of World Climates; Oliver, J.E., Ed.; Springer: Dordrecht, The Netherlands, 2005; pp. 183–189. [Google Scholar]
- Climate Studies Group Mona. The State of the Caribbean Climate; The University of the West Indies and The Caribbean Development Bank: Kingston, Jamaica, 2020. [Google Scholar]
- Herrera, D.A.; Ault, T.R.; Carrillo, C.M.; Fasullo, J.T.; Li, X.; Evans, C.P.; Alessi, M.J.; Mahowald, N. Dynamical characteristics of drought in the Caribbean from observations and simulations. J. Clim. 2020, 33, 10773–10797. [Google Scholar] [CrossRef]
- Yepes, J.; Poveda, G.; Mejía, J.F.; Moreno, L.; Rueda, C. CHOCO-JEX a research experiment focused on the Chocó low-level jet over the far eastern Pacific and western Colombia. Bull. Am. Meteorol. Soc. 2019, 100, 779–796. [Google Scholar] [CrossRef]
- Jones, P.D.; Harpham, C.; Harris, I.; Goodess, C.M.; Burton, A.; Centella-Artola, A.; Taylor, M.A.; Bezanilla-Morolot, A.; Campbell, D.; Stephenson, T.S.; et al. Long-term trends in precipitation and temperature across the Caribbean. Int. J. Climatol. 2015, 36, 3314–3333. [Google Scholar] [CrossRef]
- Intergovernmental Pannel on Climate Change (IPCC). Global Warming of 1.5 °C; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Magaña, V.; Amador, J.A.; Medina, S. The midsummer drought over Mexico and Central America. J. Clim. 1999, 12, 1577–1588. [Google Scholar] [CrossRef]
- Martinez, C.; Goddard, L.; Kushnir, Y.; Ting, M. Seasonal climatology and dynamical mechanisms of rainfall in the Caribbean. Clim. Dyn. 2019, 53, 825–846. [Google Scholar] [CrossRef]
- Burgess, C.; Taylor, M.; Spencer, N.; Jones, J.; Stephenson, T. Estimating damages from climate-related natural disasters for the Caribbean at 1.5 °C and 2 °C global warming above preindustrial levels. Reg. Environ. Chang. 2018, 18, 2297–2312. [Google Scholar] [CrossRef]
- Palmieri, S.; Teodonio, L.; Siani, A.-M.; Casale, G.R. Tropical storm impact in Central America. Meteorol. Appl. 2006, 13, 21–28. [Google Scholar] [CrossRef]
- Webster, P.J.; Holland, G.J.; Curry, J.A.; Chang, H.-R. Changes in tropical cyclone number. duration. and intensity in a warming environment. Science 2005, 309, 1844–1846. [Google Scholar] [CrossRef] [PubMed]
- Durán-Quesada, A.M.; Sori, R.; Ordoñez, P.; Gimeno, L. Climate perspectives in the Intra-Americas Seas. Atmosphere 2020, 11, 959. [Google Scholar] [CrossRef]
- Pielke, R.A.; Rubiera, J.; Landsea, C.; Fernández, M.L.; Klein, R. Hurricane Vulnerability in Latin America and The Caribbean: Normalized Damage and Loss Potentials. Nat. Hazards Rev. 2003, 4, 101–114. [Google Scholar] [CrossRef]
- Church, J.A.; White, N.J. A 20th century acceleration in global sea-level rise. Geophys. Res. Lett. 2006, 33, 94–97. [Google Scholar] [CrossRef]
- Palanisamy, H.; Becker, M.; Meyssignac, B.; Henry, O.; Cazenave, A. Regional sea level change and variability in the Caribbean Sea since 1950. J. Geodet. Sci. 2012, 2, 125–133. [Google Scholar] [CrossRef]
- Torres, R.R.; Tsimplis, M.N. Sea-level trends and interannual variability in the Caribbean Sea. J. Geophys. Res. 2013, 118, 2934–2947. [Google Scholar] [CrossRef]
- Taylor, M.A.; Clarke, L.A.; Centella, A.; Bezanilla, A.; Stephenson, T.S.; Jones, J.J.; Capmbell, J.D.; Vichot, A.; Charley, J. Future Caribbean climates in a world of rising temperatures: The 1.5 vs 2.0 dilemma. J. Clim. 2018, 31, 2907–2926. [Google Scholar] [CrossRef]
- Campbell, J.D.; Taylor, M.A.; Stephenson, T.S.; Watson, R.A.; Whyte, F.S. Future climate of the Caribbean from a regional climate model. Int. J. Climatol. 2011, 31, 1866–1878. [Google Scholar] [CrossRef]
- Stennett-Brown, R.K.; Jones, J.J.; Stephenson, T.S.; Taylor, M.A. Future Caribbean temperature and rainfall extremes from statistical downscaling. Int. J. Climatol. 2017, 37, 4828–4845. [Google Scholar] [CrossRef]
- Rahmstorf, S. A semi-empirical approach to projecting future sea level rise. Science 2007, 315, 368–370. [Google Scholar] [CrossRef]
- Perrette, M.; Launderer, F.; Riva, R.; Frieler, K.; Meinshausen, M.A. Scaling approach to project regional sea level rise and its uncertainties. Earth Syst. Dyn. 2013, 4, 11–29. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Borges, R.; Ferreira, A.C. Neotropical mangroves: Conservation and sustainable use in a scenario of global climate change. Aquat. Conserv. 2019, 29, 1347–1364. [Google Scholar] [CrossRef]
- FAO. The World’s Mangroves 1980–2005; Food and Agriculture Organization Forestry Paper 153; FAO: Rome, Italy, 2007. [Google Scholar]
- García-Fuentes, A.; Lendínez-Barriga, M.L.; Torres-Cordero, J.A.; Ruiz-Valenzuela, L.; Quesada, J.; León, Y.; Salazar-Medías, C. A study of the mangrove formations of the neotropicañ-Austroamerican Kngdom. Phytocoenologia 2020, 50, 137–162. [Google Scholar] [CrossRef]
- Graham, A. New records of Pelliciera (Theaceae/Pellicieraceae) in the Tertiary of the Caribbean. Biotropica 1977, 9, 48–52. [Google Scholar] [CrossRef]
- Lo, E.Y.Y.; Duke, N.C.; Sun, M. Phylogeographic pattern of Rhizophora (Rhizophoraceae) reveals the importance of both vicariance and long-distance oceanic dispersal to modern mangrove distriburion. BMC Evol. Biol. 2014, 14, 83. [Google Scholar] [CrossRef]
- Li, X.; Duke, N.C.; Yang, Y.; Huang, L.; Zhu, Y.; Zhang, Z.; Zhou, R.; Zhong, C.; Huang, Y.; Shi, S. Re-evaluation of phylogenetic relationships among species of the mangrove genus Avicennia from Indo-West Pacific based on multilocus analyses. PLoS ONE 2016, 11, e0164453. [Google Scholar] [CrossRef]
- DeYoe, H.; Lonard, R.I.; Judd, F.W.; Stalter, R.; Feller, I. Biological flora of the tropical and subtropical intertidal zone: Literature review for Rhizophora mangle L. J. Coast. Res. 2020, 36, 857–884. [Google Scholar] [CrossRef]
- Smith, T.J. Forest structure. In Tropical Mangrove Ecosystems; Robertson, A.I., Alongi, D.M., Eds.; American Geophysical Union: Washington, DC, USA, 1992; pp. 101–136. [Google Scholar]
- Lugo, A.E.; Snedaker, S.C. The ecology of mangroves. Ann. Rev. Ecol. Syst. 1974, 5, 39–64. [Google Scholar] [CrossRef]
- Rabinowitz, D. Early growth of mangrove seedlings in Panama and a hypothesis concernign the relayionship of dispersal and zonation. J. Biogeogr. 1978, 5, 113–133. [Google Scholar] [CrossRef]
- Woodroffe, C.D. Geomorphology and devlopment of mangrove swamps, Grand Cayman Island, West Indies. Bull. Mar. Sci. 1982, 32, 381–398. [Google Scholar]
- Sousa, W.P.; Kennedy, P.G.; Mitchell, B.I.; Ordóñez, B.M. Supply-side ecology in mangroves: Do propagule dispersal and seedling establishment explain forest structure? Ecol. Monogr. 2007, 77, 53–76. [Google Scholar] [CrossRef]
- Dangremond, E.M.; Feller, J.C.; Sousa, W.P. Environmental tolerances of rareand common mangroves along light and salinity gradients. Oecologia 2015, 179, 1187–1198. [Google Scholar] [CrossRef]
- Lonard, R.I.; Judd, F.W.; Summy, K.R.; DeYoe, H.; Stalter, R. The biological flora of coastal dunes and wetlands: Avicennia germinans (L.) L. J. Coastal Res. 2017, 33, 191–207. [Google Scholar] [CrossRef]
- Lonard, R.I.; Judd, F.W.; DeYoe, H.; Stalter, R. Biology and ecology of the halotype Laguncularia racemosa (L.) Gaertn. f.: A review. In Handbook of Halophytes; Grigore, M.-N., Ed.; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- Urrego, L.E.; Polanía, J.; Buitrago, M.F.; Cuartas, L.F.; Lema, A. Distribution of mangroves along environmental gradients on San Andres Island (Colombian Caribbean. Bull. Mar. Sci. 2009, 85, 27–43. [Google Scholar]
- Muller, E.; Lambs, L.; Fromard, F. Variations in water used by a mature mangrove of Avicennia germinans, French Guiana. Ann. Forest Sci. 2009, 66, 803. [Google Scholar] [CrossRef][Green Version]
- Medina, E.; Fernandez, W.; Barbosa, F. Element uptake accumulation and resorption in leaves of mangrove species with different mechanisms of salt regulation. Web Ecol. 2015, 15, 3–13. [Google Scholar] [CrossRef]
- Lonard, R.I.; Judd, F.W.; DeYoe, H.; Stalter, R. Biology of the mangal halotype Conocarpus erectus L.: A review. In Handbook of Halophytes; Grigore, M.-N., Ed.; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- McGirr, R.; Seton, M.; Williams, S. Kinematic and geodynamic evolution of the Isthmus of Panama region: Implications for Central American Seaway closure. Bull. Geol. Soc. Am. 2021, 133, 867–884. [Google Scholar] [CrossRef]
- Baker, P.A.; Fritz, S.C.; Dick, C.; Eckert, A.; Horton, B.K.; Manzoni, S.; Ribas, C.C.; Garzione, C.N.; Battisti, D.S. The emerging field of geogenomics: Constraining geological problems with genetic data. Earth Sci. Rev. 2014, 135, 38–47. [Google Scholar] [CrossRef]
- Cerón-Souza, I.; Rivera-Ocasio, E.; Medina, E.; Jiménez, J.A.; McMillan, W.O.; Bermingham, E. Hybridization and introgression in New World mangroves, Rhizophora (Rhizophoraceae). Am. J. Bot. 2010, 97, 945–957. [Google Scholar] [CrossRef]
- Cerón-Souza, I.; Bermingham, E.; McMillan, W.O.; Jones, F.A. Comparative genetic structure of two mangrove species in Caribbean and Pacific estuaries of Panama. BMC Ecol. Evol. 2012, 12, 205. [Google Scholar] [CrossRef]
- Nettel, A.; Dodd, R.S. Drifting propagules and receeding swamps: Genetic footprints of mangrove recolonization and dispersal along tropical coasts. Evolution 2007, 61, 958–971. [Google Scholar] [CrossRef]
- Nettel, A.; Dodd, R.S.; Afzal-Rafii, Z.; Tovilla-Hernández, C. Genetic diversity enhanced by ancient introgression and secondary contact in East Pacific black mangroves. Mol. Ecol. 2008, 17, 2680–2690. [Google Scholar] [CrossRef]
- Castillo-Cárdenas, M.F.; Díaz-Gonzáles, F.; Cerón-Souza, I.; Toro-Perea, N. Jumping a geographic barrier: Diversification of the mangrove species Pelliciera rhizophorae (Tetrameristaceae) across the Central American Isthmus. Tree Genet. Genom. 2015, 11, 822. [Google Scholar] [CrossRef]
- Van der Stocken, T.; Carroll, D.; Menemenlis, D.; Simard, M.; Koedam, N. Global scale dispersal and connectivity in mangroves. Proc. Natl. Acad. Sci. USA 2019, 116, 915–922. [Google Scholar] [CrossRef]
- Ravelo, A.C.; Andreasen, D.H.; Lyle, M.; Lyle, A.O.; Wara, M.W. Regional climate shifts caused by gradual global cooling in the Pliocene epoch. Nature 2004, 429, 263–267. [Google Scholar] [CrossRef]
- deMenocal, P.B.; Oppo, D.W.; Fairbanks, R.G. Pleistocene d13C variability of North Atlantic intermediate water. Paleoceanography 1992, 7, 229–250. [Google Scholar] [CrossRef]
- Haug, G.H.; Pedersen, T.F.; Sigman, D.M.; Calvert, S.E.; Nielsen, B.; Peterson, L.C. Glacial/Interglacial variations in production and nitrogen fixation in the Cariaco Basin during the last 580 kyr. Paleoceanography 1998, 13, 427–432. [Google Scholar] [CrossRef]
- Yarincik, K.M.; Murray, R.W. Climatically sensitive aeolian and hemipelagic deposition in the Cariaco Basin, Venezuela, over the past 578,000 years: Results from Al/Ti and K/Al. Paleoceanography 2000, 15, 210–228. [Google Scholar] [CrossRef]
- Martinez, J.I.; Mora, G.; Barrows, T.T. Paleoceangraphic conditions in the western Caribbean Sea for the last 560 kyr as inferred from planktonic foraminifera. Mar. Micopaleontol. 2007, 64, 177–188. [Google Scholar] [CrossRef]
- Michell, S.F.; Pickerill, R.K.; Stemann, T.A. The Port Moran Formation (Upper Pleistocene, Jamaica): High resolution sedimentology and paleoenvironmental analysis of a mixed carbonate clastic lagoonal succession. Sediment. Geol. 2001, 144, 291–306. [Google Scholar] [CrossRef]
- Rovere, A.; Raymo, M.E.; Vacchi, M.; Lorscheid, T.; Stocchi, P.; Gómez-Pujol, L.; Harris, D.L.; Casella, E.; O’Leary, M.J.; Hearty, P.J. The analysis of Last Interglacial (MIS5e) relative sea-level indicators: Reconstructing sea-level in a warmer world. Earth Sci. Rev. 2016, 159, 404–427. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Vautravers, M.J.; Spero, H.J. Western Caribbean sea surface temperatures during the late Quaternary. Geochem. Geophys. Geosys. 2006, 7, 2. [Google Scholar] [CrossRef]
- Grauel, A.-L.; Hodell, D.A.; Bernasconi, S.M. Quantitative estimates of tropical temperature change in lowland Central America during the last 42 ka. Earth Planet. Sci. Lett. 2016, 438, 37–46. [Google Scholar] [CrossRef]
- Peterson, L.C.; Haug, G.H.; Hughen, K.A.; Röhl, U. Rapid changes in the hydrologic cycle of the tropical Atlantic during the Last Glacial. Science 2000, 290, 1947–1951. [Google Scholar] [CrossRef]
- Boers, N.; Ghil, M.; Rousseau, D.-D. Ocean circulation, ice shelf, and sea ice interactions explain Dansgaard-Oeschger cycles. Proc. Natl. Acad. Sci. USA 2018, 115, E11005–E11014. [Google Scholar] [CrossRef]
- Warken, S.F.; Scholz, D.; Spötl, C.; Jochum, K.P.; Pajón, J.N.; Bahr, A.; Mangini, A. Caribbean hydroclimate and vegetation history across the last glacial period. Quat. Sci. Rev. 2019, 218, 75–90. [Google Scholar] [CrossRef]
- Van Daele, M.; van Welden, A.; Moernaut, J.; Beck, C.; Audemard, F.; Sanchez, J.; Jouanne, F.; Carrillo, E.; Malavé, G.; Lemus, A.; et al. Reconstruction of Late-Quaternary sea- and lake-level changes in a tectonically active marginal basin using seismic stratigraphy: The Gulf of Cariaco, NE Venezuela. Mar. Geol. 2011, 279, 37–51. [Google Scholar] [CrossRef]
- Hearthy, P.J.; Hollin, J.T.; Neumann, A.C.; O’Leary, M.J.; McCulloch, M. Global sea-level fluctuations during the Last Interglaciation (MIS 5e). Quat. Sci. Rev. 2007, 26, 2090–2112. [Google Scholar] [CrossRef]
- Coyne, M.K.; Jones, B.; Ford, D. Highstands during Marine Isotope Stage 5: Evidence from the ironshore Formarion of Grand Cayman, British West Indies. Quat. Sci. Rev. 2007, 26, 536–559. [Google Scholar] [CrossRef]
- Dumas, B.; Hoang, C.T.; Raffy, J. Record of MIS 5 sea-level highstands based on U/Th dated coral terraces of Haiti. Quat. Int. 2006, 145–146, 106–118. [Google Scholar] [CrossRef]
- Creveling, J.R.; Mitrovica, J.X.; Clarck, P.U.; Waelbroeck, C. Predicted bounds on peak global mean sea level during marine isotope stages 5a and 5c. Quat. Sci. Rev. 2017, 163, 193–208. [Google Scholar] [CrossRef]
- Rubio-Sandoval, K.; Rovere, A.; Cerrone, C.; Stocchi, P.; Torscheid, T.; Felis, T.; Petersen, A.-K.; Ryan, D.D. A review of last interglacial sea-level proxies in the western Atlantic and southwestern Caribbean, from Brazil to Honduras. Earth Syst. Sci. Data 2021, 13, 4819–4845. [Google Scholar] [CrossRef]
- Friedrich, T.; Timmermann, A. Using Late Pleistocene sea surface temperature reconstructions to constrain future greenhouse warming. Earth Planet. Sci. Lett. 2020, 530, 115911. [Google Scholar] [CrossRef]
- Spratt, R.M.; Liesecki, L.E. A Late Pleistocene sea level stack. Clim. Past. 2016, 12, 1079–1092. [Google Scholar] [CrossRef]
- Martinson, D.G.; Pisias, N.G.; Hays, J.D.; Imbrie, J.; Moore, T.C.; Schackleton, J. Age dating and the orbital theory of the ice ages: Development of a high-resolution 0 to 300,000-year chronology. Quat. Res. 1987, 27, 1–29. [Google Scholar] [CrossRef]
- Thompson, W.G.; Goldstein, S.L. A radiometric calibration of the SPECMAP timescale. Quat. Sci. Rev. 2006, 25, 3207–3215. [Google Scholar] [CrossRef]
- Hughes, P.D.; Gibbard, P.L.; Ehler, J. Timing of glaciation during the last glacial cycle: Evaluating the concept of a global “Last Glacial Maximum” (LGM). Earth Sci. Rev. 2013, 125, 171–198. [Google Scholar] [CrossRef]
- Lea, D.W.; Pak, D.K.; Peterson, L.C.; Hughen, K.A. Synchroneity of tropical and high-latitude Atlantic temperatures over the Last Glacial Termination. Science 2003, 301, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Wurtzel, J.B.; Black, D.E.; Thunell, R.C.; Peterson, L.C.; Tappa, E.J.; Rahman, S. Mechanisms of southern Caribbean SST variability over the last two millennia. Geophys. Res. Lett. 2013, 40, 5954–5958. [Google Scholar] [CrossRef]
- Haug, G.H.; Hughen, K.A.; Sigman, D.M.; Peterson, L.C.; Röhl, U. Southward migration of the Intertropical Convergence Zone through the Holocene. Science 2001, 293, 1304–1308. [Google Scholar] [CrossRef]
- Hodell, D.A.; Curtis, J.H.; Jones, G.A.; Higuera-Gundy, A.; Brenner, M.; Binford, M.W.; Dorsey, K.T. Reconstruction of Caribbean climate change over the last 10,500 years. Nature 1991, 352, 790–793. [Google Scholar] [CrossRef]
- Khan, N.S.; Ashe, E.; Horton, B.P.; Dutton, A.; Kopp, R.E.; Bocard, G.; Engelhart, S.E.; Hill, D.F.; Peltier, W.R.; Vane, C.H.; et al. Drivers of Holocene sea-level change in the Caribbean. Quat. Sci. Rev. 2017, 155, 13–36. [Google Scholar] [CrossRef]
- Fensterer, C.; Scholz, D.; Hoffmann, D.; Spotl, C.; Pajón, J.M.; Mangini, A. Cuban stalagmite suggests relationship between Caribbean precipitation and the Atlantic Multidecadal Oscillation during the past 1.3 ka. Holocene 2012, 22, 1405–1412. [Google Scholar] [CrossRef]
- Tamalavage, A.E.; van Hengstum, P.J.; Louchouarn, P.; Fall, P.L.; Donnelly, J.P.; Nancy, A.; Albury, N.A.; Coats, S.; Feakins, S.J. Plant wax evidence for precipitation and vegetation change from a coastal sinkhole lake in the Bahamas spanning the last 3000 years. Org. Geochem. 2020, 150, 104120. [Google Scholar] [CrossRef]
- Leyden, B.W. Late Quaternary aridity and Holocene moisture fluctuations in the Lake Valencia basin, Venezuela. Ecology 1985, 66, 1279–1295. [Google Scholar] [CrossRef]
- Hodell, D.A.; Brenner, M.; Curtis, J.H. Terminal Classic drought in the northern Maya lowlands inferred from multiple sediment cores in Lake Chichancanab (Mexico). Quat. Sci. Rev. 2005, 24, 1413–1427. [Google Scholar] [CrossRef]
- Haug, G.H.; Günther, D.; Peterson, L.C.; Sigman, D.M.; Hughen, K.A.; Aeschlimann, B. Climate and the collapse of Maya civilization. Science 2003, 299, 1731–1735. [Google Scholar] [CrossRef]
- Bryan, A.L.; Casamiquela, R.M.; Cruxent, J.M.; Gruhn, R.; Ochsenius, C. A El Jobo mastodon kill at Taima-taima, Venezuela. Science 1978, 200, 1275–1277. [Google Scholar] [CrossRef]
- Fiedel, S.J. Initial human colonization of the Americas: An overview of issues and evidence. Radiocarbon 2002, 44, 407–436. [Google Scholar] [CrossRef]
- Fiedel, S.J. Initial human colonization of the Americas, redux. Radiocarbon 2022, 64, 845–897. [Google Scholar] [CrossRef]
- Ranere, A.J.; Cooke, R.G. Late glacial and early Holocene migrations, and Middle Holocene settlement on the lower isthmian land-bridge. Quat. Int. 2021, 578, 20–34. [Google Scholar] [CrossRef]
- Ranere, A.J.; López, C.E. Cultural diversity in the Late Pleistocene/Early Holocene populations in northwest South America and Lower Central America. Int. J. S. Am. Archaeol. 2007, 1, 25–31. [Google Scholar]
- Cooke, R.G.; Ranere, A.J.; Pearson, G.A.; Dickau, R. Radiocarbon chronology of early human settlement of the Isthmus of Panama (13,000–7000 BP). Cultural affinities, environments and subsistence change. Quat. Int. 2013, 301, 3–22. [Google Scholar] [CrossRef]
- Pearson, G.A. Continental Paleoindian Expansions and Interactions as Viewed from the Earliest Lithic Industries of Lower Central America. Ph.D. Thesis, Univ. Kansas, Lawrence, KS, USA, 2002. [Google Scholar]
- Prufer, K.M.; Alsgaard, A.V.; Robinson, M.; Meredith, C.R.; Culleton, B.J.; Dennehy, T.; Magee, S.; Huckell, B.B.; Stemp, W.J.; Awe, J.J.; et al. Linking late Paleoindian stone tool technologies and populations in North, Central and South America. PLoS ONE 2019, 14, e021981. [Google Scholar] [CrossRef]
- Tankersly, K.B.; Dunning, N.P.; Owen, L.A.; Sparks, J. Geochronology and paleoenvironmental framework for the oldest archaeological site (7800-7900 cal BP) in the West Indies, Banwari Trace, Trinidad. Lat. Am. Antiq. 2018, 29, 681–695. [Google Scholar] [CrossRef]
- Nami, H.G. Fishtailed projectile points in the Americas: Remarks and hypotheses on the peopling of northern South America and beyond. Quat. Int. 2021, 578, 47–72. [Google Scholar] [CrossRef]
- Morrow, J.E.; Morrow, T.A. Geographic variation in fluted projectile points: A hemispheric perspective. Am. Antiq. 1999, 64, 215–231. [Google Scholar] [CrossRef]
- Pearson, G.A. Bridging the gap: An updated overivew of Clovis across Middle America and its techno-cultural relation with fluted point assemblages from South America. Paleoamerica 2017, 3, 203–230. [Google Scholar] [CrossRef]
- Fitzpatrick, S.M.; Keegan, W.F. Human impacts and adaptations in the Caribbean Islands: An historical ecology approach. Earth Environ. Trans. R. Soc. Edinb. 2007, 98, 29–45. [Google Scholar] [CrossRef]
- Keegan, W.F. West Indian archaeology. 3, ceramic age. J. Archaeol. Res. 2000, 8, 135–167. [Google Scholar] [CrossRef]
- Napolitano, M.F.; DiNapoli, R.; Stone, J.H.; Levin, M.J.; Jew, N.P.; Lane, B.G.; O’Connor, J.T.; Fitzpatrick, S.M. Reevaluating human colonization of the Caribbean using chronometric hygiene and Bayesian modeling. Sci. Adv. 2019, 5, eaar7806. [Google Scholar] [CrossRef]
- Prates, L.; Ivan Perez, S. Late Pleistocene South American megagafunal extinctions associated with rise of Fishtail points and human population. Nat. Commun. 2021, 12, 2175. [Google Scholar] [CrossRef]
- Keegan, W.F.; Hofman, C.L. The Caribbean before Columbus; Oxford Univ. Press: New York, NY, USA, 2017. [Google Scholar]
- Hofman, C.L.; Antczak, A.T. Early Settlers for the Insular Caribbean. Dearchaizing the Archaic; Sidestone Press: Leiden, The Netherlands, 2019. [Google Scholar]
- Leepart, T.P.; Cochrane, E.E.; Gaffney, D.; Hofman, C.L.; Laffoon, J.E.; Bunbury, M.M.E.; Broodbank, C. Global patterns in island colonization during the Holocene. J. Wolrd Prehist. 2022, 35, 163–232. [Google Scholar]
- Neff, H.; Pearsall, D.M.; Jones, J.G.; Arroyo, B.; Collins, S.K.; Freidel, D.E. Early Maya adaptive patterns: Mid-Late Holocene paleoenvironmental evidence from Pacific Guatemala. Lat. Am. Antiq. 2006, 17, 287–315. [Google Scholar] [CrossRef]
- Rouse, I. The Tainos: Rise and Decline of the people who Greeted Columbus; Yale Univ. Press: New Haven, CT, USA, 1992. [Google Scholar]
- Stokes, A.V. Ceramic-age dietary patterns in Puerto Rico: Stable isotopes and island biogeography. In Ancient Borinquen: Archaeology and Ethnohistory of Native Puerto Rico; Siegel, P.E., Ed.; Univ. Alabama Press: Tuscaloosa, AL, USA, 2005; pp. 185–201. [Google Scholar]
- Newson, L.A.; Wing, E.S. On Land and Sea: Native American Uses of Biological Resources in the West Indies; Univ. Alabama Press: Tuscaloosa, AL, USA, 2004. [Google Scholar]
- Romano, M. Reviewing the term uniformitarianism in modern Earth sciences. Earth Sci. Rev. 2015, 184, 65–106. [Google Scholar] [CrossRef]
- Jackson, S.T.; Williams, J.W. Modern analogs in Quaternary paleoecology: Here today, gone yesterday, gone tomorrow? Ann. Rev. Earth Planet. Sci. 2004, 32, 495–537. [Google Scholar] [CrossRef]
- Muller, J. Palynology of recent Orinoco delta and shelf sediments. Micropaleontology 1959, 5, 1–32. [Google Scholar] [CrossRef]
- Van der Hammen, T. A palynological study on the Quaternary of British Guiana. Leidse Geol. Meded. 1963, 29, 125–168. [Google Scholar]
- Davidson, D.E. Modern Pollen Spectra from Mangrove Ecosystems of the Sabana-Camagüey Archipelago and Ciego de Ávila, Cuba. Master’s Thesis, Univ. Toronto, Toronto, ON, Canada, 2007. [Google Scholar]
- Phillips, S.; Rouse, G.E.; Bustin, R.M. Vegetation zones and diagnostic pollen profiles of a coastal peat swamp, Bocas de Toro, Panamá. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1997, 128, 301–338. [Google Scholar] [CrossRef]
- Urrego, L.E.; Bernal, G.; Polanía, J. Comparison of pollen distribution patterns in surface sediments of a Colombian Caribbean mangrove with geomorphology and vegetation. Rev. Palaeobot. Palynol. 2009, 156, 358–375. [Google Scholar] [CrossRef]
- Tschudy, R.H. Relationship of palynomorphs to sedimentation. In Aspects of Palynology; Tschudy, R.H., Scott, R.A., Eds.; Wiley Interscience: New York, NY, USA, 1969; pp. 79–96. [Google Scholar]
- Hofman, C.-C. Pollen distribution in sub-Recent sedimentary environments of the Orinoco Delta (Venezuela)—An actuo-palaeobotanical study. Rev. Palaeobot. Palynol. 2002, 119, 191–217. [Google Scholar] [CrossRef]
- Rull, V.; Vegas-Vilarrúbia, T. Surface palynology of a small coastal basin from Venezuela and potential paleoecological applications. Micropaleontology 1999, 45, 365–393. [Google Scholar] [CrossRef]
- Rull, V.; Vegas-Vilarrúbia, T.; Espinoza, N. Palynological record of an Early-Mid Holocene mangrove in eastern Venezuela. Implications for sea-level rise and disturbance history. J. Coast. Res. 1999, 15, 496–504. [Google Scholar]
- Horn, S. Preliminary pollen analysis of Quaternary sediments from Deep Sea Drilling Project Site 565, western Costa Rica. Init. Rep. DSDP 1985, 84, 533–547. [Google Scholar]
- Rodgers, J.C.; Horn, S.P. Modern pollen spectra from Costa Rica. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1996, 124, 53–71. [Google Scholar] [CrossRef]
- Escarraga-Paredes, D.S.; Torrescano-Valle, N.; Islebe, G.A. Análisis de la relación vegetación-lluvia de polen actual de las comunidades vegetales en el nororoeste de la Península de Yucatán, México. Polibotánica 2014, 38, 27–52. [Google Scholar]
- Urrego, L.E.; González, C.; Urán, G.; Polanía, J. Modern pollen rain in mangroves from San Andrés Island, Colombian Caribbean. Rev. Palaeobot. Palynol. 2010, 162, 168–182. [Google Scholar] [CrossRef]
- Montoya, E.; Pedra-Méndez, J.; García-Falcó, E.; Gómez-Paccard, M.; Giralt, S.; Vegas-Vilarrúbia, T.; Stauffer, F.W.; Rull, V. Long-term vegetation dynamics of a tropical megadelta: Mid-Holocene palaeoecology of the Orinoco Delta (NE Venezuela). Quat. Sci. Rev. 2019, 221, 105874. [Google Scholar] [CrossRef]
- Islebe, G.A.; Villanueva-Gutiérrez, R.; Sánchez, O. Relación lluvia de polen-vegetación en selvas de Quintana Roo. Bol. Soc. Bot. México 2001, 69, 31–38. [Google Scholar]
- Torrescano, N. Actuo y Paleo Palinología de la Península de Yucatán: Estudios de Caso de Lluvia de Polen y Reconstrucciones Ambientales. Master’s Thesis, ECOSUR, Lerma, México, 2004. [Google Scholar]
- Carrillo-Bastos, A.; Islebe, G.A.; Torrescano-Valle, N. 3800 years of quantitative precipitation reconstruction from the northwest Yucatan Peninsula. PLoS ONE 2013, 8, e84333. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Beach, T.; Wahl, D. An analysis of modern pollen rain from the Maya lowlands of northern Belize. Rev. Palaeobot. Palynol. 2011, 164, 109–120. [Google Scholar] [CrossRef]
- Urrego, L.E.; Correa-Metrio, A.; González-Arango, C. Colombian Caribbean mangrove dynamics: Anthropogenic and environmental drivers. Bol. Soc. Geól. Mex. 2018, 70, 133–145. [Google Scholar] [CrossRef]
- Castaño, A.; Urrego, L.; Bernal, G. Dinámica del manglar en el complejo lagunar de Cispatá (Caribe colombiano) en los últimos 900 años. Rev. Biol. Trop. 2010, 58, 1347–1366. [Google Scholar] [CrossRef][Green Version]
- Khan, N.S.; Vane, C.H.; Engelhart, S.E.; Kendrick, C.; Horton, B.P. The application of δ13C, TOC and C/N geochemistry of mangrove sediments to reconstruct Holocene paleoenvironments and relative sea levels, Puerto Rico. Mar. Geol. 2019, 415, 105963. [Google Scholar] [CrossRef]
- Urrego, L.E.; Prado, M.A.; Bernal, G.; Galeano, A. Mangrove responses to droughts since the little ice age in the Colombian Caribbean. Est. Coast. Shelf Res. 2019, 230, 196432. [Google Scholar] [CrossRef]
- LeBlanc, A.R.; Kennedy, L.M.; Liu, K.-B.; Lane, C.S. Linking hurricane landfalls, precipitation variability, fires, and vegetation response over the past millennium from analysis of coastal lagoon sediments, southwestern Dominican Republic. J. Paleolimnol. 2017, 58, 135–150. [Google Scholar] [CrossRef]
- González, C.; Urrego, L.E.; Martínez, J.I.; Polanía, J.; Yokohama, Y. Mangrove dynamics in the southwestern Caribbean since the ‘Little Ice Age’: A history of human and natural disturbances. Holocene 2010, 20, 849–861. [Google Scholar] [CrossRef]
- Palmer, S.E.; Burn, M.J.; Holmes, J. A multiproxy analysis of extreme wave deposits in a tropical coastal lagoon in Jamaica, West Indies. Nat. Hazards 2020, 104, 2531–2560. [Google Scholar] [CrossRef]
- Urrego, L.E.; Correa-Metrio, A.; González, C.; Castaño, A.R.; Yokohama, Y. Contrasting responses of two Caribbean mangroves to sea-level rise in the Guajira Peninsula (Colombian Caribbean). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 370, 92–102. [Google Scholar] [CrossRef]
- Hofman, C.L.; Pagán-Jiménez, J.R.; Field, M.H.; Hooghiemstra, H.; Vermeer, J.A.M.; Jorissen, P.; Knippenberg, S.; Bérard, B.; Hoogland, M.L.P. Mangrove archives: Unravelling human-environment interactions from deeply buried deposits at the site Anse Trabaud, Martinique, Lesser Antilles (1290-780 cal BP). Environ. Archaeol. 2021. [Google Scholar] [CrossRef]
- García, Y.; Rangel, J.; Jaramillo, A. Environmental changes during the last 1800 years in the Neguanje mangrove, Tayrona National Natural Park, Colombian Caribbean. Rev. Acad. Colomb. Cienc. Ex. Fis. Nat. 2022, 46, 90–107. [Google Scholar]
- Woodroffe, C.D. Mangrove swamp stratigraphy and Holocene transgression, Grand Cayman Island, West Indies. Mar. Geol. 1981, 41, 271–294. [Google Scholar] [CrossRef]
- Cohen, A.; Wiedemann, H.U. Distribution and depositional history of some pre-lagoonal Holocene sediments in the Ciénaga Grande de Santa Marta, Colombia. Mitt. Inst. Colombo Alemán Invest. Cient. 1973, 7, 139–154. [Google Scholar] [CrossRef]
- Islebe, G.; Sánchez, O. History of Late Holocene vegetation at Quintana Roo, Caribbean coasts of Mexico. Plant Ecol. 2002, 160, 187–192. [Google Scholar] [CrossRef]
- Collins, S.V.; Reinhardt, E.G.; Werner, C.L.; Le Maillot, C.; Devos, F.; Rissolo, D. Late Holocene mangrove development and onset of sedimentation in the Yax Chen cave system (Ox Bel Ha) Yucatan, Mexico: Implications for using cave sediments as a sea-level indicator. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 438, 124–134. [Google Scholar] [CrossRef]
- Lane, C.S.; Clark, J.J.; Knudsen, A.; McFarlin, J. Late-Holocene paleoenvironmental history of bioluminiscent Laguna Grande, Puerto Rico. Palaeogeor. Palaeoclimatol. Palaeoecol. 2013, 369, 99–113. [Google Scholar] [CrossRef]
- Gutiérrez-Ayala, L.V.; Torrescano-Valle, N.; Islebe, G.A. Reconstrucción paleoambiental del Holoceno tardío de la reserva Los Petenes. Rev. Mex. Cien. Geól. 2012, 29, 749–763. [Google Scholar]
- Roy, P.D.; Torrescano-Valle, N.; Islebe, G.A.; Gutiérrez-Ayala, L.V. Late Holocene hydroclimate of the western Yucatan Peninsula (Mexico). J. Quat. Sci. 2017, 32, 1112–1120. [Google Scholar] [CrossRef]
- Peros, M.; Gregory, B.; Matos, F.; Reinhardt, E.; Desgloges, J. Late-Holocene record of lagoon evolution, climate change, and hurricane activity from southeastern Cuba. Holocene 2015, 25, 1483–1497. [Google Scholar] [CrossRef]
- Jessen, C.A.; Pedersen, J.B.T.; Bartholdy, J.; Seidenkrantz, M.-S.; Kuijpers, A. A late Holocene palaeoenvironmental record from Altona bay, St. Croix, US Virgin Islands. Geogr. Tidsskr. 2008, 108, 59–70. [Google Scholar] [CrossRef]
- Jaramillo, C.; Bayona, G. Mangrove distribution during the Holocene in Trbugá Gulf, Colombia. Biotropica 2000, 32, 14–22. [Google Scholar]
- Torrescano, N.; Islebe, G.A. Tropical forest and mangrove history from southeastern Mexico: A 5000 yr pollen record and implications for sea level rise. Veg. Hist. Archaeobot. 2006, 15, 191–195. [Google Scholar] [CrossRef]
- Wooler, M.J.; Behling, H.; Leon, J.; Jantz, N.; Zweigert, M.E. Late Holocene hydrologic and vegetation changes at Turneffe Atoll, Belize, compared with records from mainland Central America and Mexico. Palaios 2009, 24, 650–656. [Google Scholar] [CrossRef]
- Castañeda-Posadas, C.; Correa-Metrio, A.; Excobar, J.; Moreno, J.E.; Curtis, J.H.; Blaauw, M.; Jaramillo, C. Mid to la Holocene sea-level rise and precipitation variability recorded in the fringe mangroves of the caribbean coast of Panama. Palaeogeor. Palaeoclimatol. Palaeoecol. 2022, 592, 110918. [Google Scholar] [CrossRef]
- Hardage, K.; Street, J.; Herrera-Silveira, J.A.; Oberle, F.K.J.; Paytan, A. Late Holocene environmental change in Celestum Lagoon, Yucatan, Mexico. J. Paleolimnol. 2022, 67, 131–162. [Google Scholar] [CrossRef]
- McCloskey, T.A.; Liu, K.-B. A sedimentary-based history of hurricane strikes on the southern Caribbean coast of Nicaragua. Quat. Res. 2012, 78, 454–464. [Google Scholar] [CrossRef]
- Aragón-Moreno, A.A.; Islebe, G.A.; Roy, P.D.; Torrescano-Valle, N.; Mueller, A.D. Climate forcings on vegetation of the southern Yucatán Peninsula (Mexico) during the middle to late Holocene. Palaeogeor. Palaeoclimatol. Palaeoecol. 2018, 495, 214–226. [Google Scholar] [CrossRef]
- Cohen, M.C.L.; Lara, R.J.; Cuevas, E.; Mulero, E.; Da Silveira, L. Effects of sea-level rise and climatic changes on mangroves from southwestern littoral Puerto Rico during the middle and late Holocene. Catena 2016, 143, 187–200. [Google Scholar] [CrossRef]
- Xia, P.; Meng, X.; Zhang, Y.; Zhang, J.; Li, Z.; Wang, W. The potential of mangrove-derived organic matter in sediments for tracing mangrove development during the Holocene. Estuar. Coast. 2021, 44, 1020–1035. [Google Scholar] [CrossRef]
- Bocanegra-Ramírez, D.M.; Li, H.-C.; Domínguez, G.; Israde-Alcántara, I.; Bischoff, J.L. Holocene climate change and sea level oscillations in the pacific coast of Mexico. Quat. Int. 2019, 528, 100–108. [Google Scholar] [CrossRef]
- Ramcharan, E.K. Mid-to-Late Holocene sea level influence on coastal wetland development in Trinidad. Quat. Int. 2004, 120, 145–151. [Google Scholar] [CrossRef]
- Joo-Chang, J.C.; Islebe, G.A.; Torrescano-Valle, N. Mangrove history during middle- and late-Holocene in Pacific south-eastern Mexico. Holocene 2015, 25, 651–662. [Google Scholar] [CrossRef]
- Monacci, N.M.; Meier-Grünhagen, U.; Finney, B.P.; Behling, H.; Wooler, M.J. Paleoecology of mangroves along the Sibun River, Belize. Quat. Res. 2011, 76, 220–228. [Google Scholar] [CrossRef]
- Peros, M.C.; Reinhardt, E.G.; Schwarcz, H.P.; Davis, A.M. High-resolution paleosalinity reconstruction from Laguna de la Leche, north coastal Cuba, using Sr, O., and C isotopes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 245, 535–550. [Google Scholar] [CrossRef]
- Gabriel, J.J.; Reinhardt, E.G.; Peros, M.C.; Davidson, D.E.; van Hengstum, P.J.; Beddows, P.A. Palaeoenvironmental evolution of cenote Aktun Ha (Carwash) on the Yucatan Peninsula, Mexico and its response to Holocene sea-level rise. J. Paleolimnol. 2009, 42, 199–213. [Google Scholar] [CrossRef]
- Ramcharan, E.K.; McAndrews, J.H. Holocene development of coastal wetland at Maracas Bay, Trinidad, West Indies. J. Coastal Res. 2006, 22, 581–586. [Google Scholar] [CrossRef]
- Adomat, F.; Gischler, E. Sedimentary patterns and evolution of coastal environments during the Holocene in central Belize, Central America. J. Coast. Res. 2015, 31, 802–826. [Google Scholar] [CrossRef]
- Caffrey, M.A.; Horn, S.P.; Orvis, K.H.; Heryan, K.A. Holocene environmental change at Laguna Saladilla, coastal north Hispaniola. Palaeogeor. Palaeoclimatol. Palaeoecol. 2015, 436, 9–22. [Google Scholar] [CrossRef]
- Monacci, N.M.; Meier-Grünhagen, U.; Finney, B.P.; Behling, H.; Wooler, M.J. Mangrove ecosystem changes during the Holocene at Spanish Lookout Cay, Belize. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 280, 37–46. [Google Scholar] [CrossRef]
- MacIntyre, I.G.; Toscano, M.A.; Lighty, R.G.; Bond, G.B. Holocene history of the mangrove islands of Twin Cays, Belize, Central America. Atoll Res. Bull. 2004, 510, 1–16. [Google Scholar] [CrossRef]
- González, C.; Dupont, L.M. Tropical salt marsh succession as sea-level indicator during heinrich events. Quat. Sci. Rev. 2009, 28, 939–946. [Google Scholar] [CrossRef]
- González, C.; Dupont, L.M.; Behling, H.; Wefer, G. Neotropical vegetation response to rapid climate changes duing the last glacial period: Palynological evidence from the Cariaco Basin. Quat Res. 2008, 69, 217–230. [Google Scholar] [CrossRef]
- Aburto-Oropeza, O.; Burelo-Campos, C.M.; Ezcurra, E.; Ezcurra, P.; Herniquez, C.L.; Vanderplank, S.E.; Zapata, F. Relict inland mangrove ecosystem reveals Last Interglacial sea levels. Proc. Natl. Acad. Sci. USA 2021, 118, e2024518118. [Google Scholar] [CrossRef]
- Koch, M.S.; Snedaker, S.C. Factors influencing Rhizophora mangle L. seedling development in Everglades carbonate soils. Aquat. Bot. 1997, 59, 87–98. [Google Scholar] [CrossRef]
- Hanagata, N.; Takemura, T.; Karube, I.; Dubinsky, Z. Salt/water relationships in mangroves. Isr. J. Plant Sci. 1999, 47, 63–76. [Google Scholar] [CrossRef]
- Rinaldi, M. Palynological evidence of the Younger Dryas event in a core of Cariaco Basin, northeastern Venezuela (abstract). In Proceedings of the 30th International Geological Congress, Beijing, China, 4–14 August 1996. [Google Scholar]
- Vegas-Vilarrúbia, T.; Hernández, E.; Rull, V.; Rull-Vegas, E. The Orinoco megadelta as a conservation target in the face of the ongoing and future sea level rise. Sci. Total Environ. 2015, 515–516, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Pope, K.O.; Pohl, M.E.D.; Jones, J.G.; Lentz, D.L.; von Nagy, C.; Vega, F.J.; Quitmyer, I.B. Origin and environmental setting of ancient agriculture in the lowlands of Mesoamerica. Science 2001, 292, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Rull, V.; Montoya, E.; Vegas-Vilarrúbia, T.; Ballesteros, T. New insights on paleofires and savannisation in northern South America. Quat. Sci. Rev. 2015, 122, 158–165. [Google Scholar] [CrossRef]
- Bush, M.B.; Silman, M.R.; Listopad, C.M.C.S. A regional study of Holocene climate change and human occupation in Peruvian Amazonia. J. Biogeogr. 2007, 34, 1342–1356. [Google Scholar] [CrossRef]
- Faegri, K.; Iversen, J. Textbook of Modern Pollen Analysis; E. Munksgaard: Copennhagen, Denmark, 1950. [Google Scholar]
- Kennett, D.J.; Piperno, D.R.; Jones, J.G.; Neff, H.; Voorhies, B.; Walsh, M.K.; Culleton, B.J. Pre-pottery farmers on the Pacific coast of southern Mexico. J. Archaeol. Sci. 2010, 37, 3401–3411. [Google Scholar] [CrossRef]
- Torres, V.; Hooghiemstra, H.; Lourens, L.; Tzedakis, P.C. Astronomical tuning of long pollen records reveals the dynamic history of montane biomes and lake levels in the tropical high Andes during the Quaternary. Quat. Sci. Rev. 2013, 63, 59–72. [Google Scholar] [CrossRef]







| Groups | Associations | Main Trees | Other Trees | Exclusive Species |
|---|---|---|---|---|
| A | Lag-AviP | Ag | Lr | Sarcocornia pacifica |
| Lag-AviR | Ag-Lr | Ce | - | |
| Lon-LacC | Lr | Ag-Ce | Lonchocarpus sericeus, Mimosa pigra, Phyllathus elsiae | |
| Sta-LagC | Lr | Ag | - | |
| LagracR | Lr | - | - | |
| B1 | Acr-ConB | Ce-Aa | Lr | Cladium mariscus, Acrostichum danaefolium, Eleocharis cellulosa, Fimbristylis spadicea, Ipomoea triloba, Schoenoplectus americanus, Helietta plaeana, Quadrella odoratissima, Acacia macracantha |
| Lon-ConC | Ce | Rm-Lr-Ag | Lonchocarpus pycnophyllus | |
| Spo-ConM | Ce | - | Jacquinia keyensis, Pilosocereus royenii, Pithecelobium mucronatum, Tillandsia recurvata | |
| Dis-ConR | Ce | - | Paspalum distachyon, Chloris elara | |
| Bat-ConM | Ce | - | Evolvulus convolvuloides, Fimbristylis cimosa, Selenicereus grandiflorus, Broughtonia lindenii, Cienfuegosia yucatanensis, Suaeda linearis, Ambrosia hispida | |
| Rac-ConM | Ce | - | - | |
| ConereR | Ce | - | Lantana involucrata, Casasia clusiifolia, Ernodea littoralis | |
| Con-CocR | Ce | - | Thespecia populnea | |
| Gra-ConC | Ce | Lr-Ag | Pterocarpus acapulcensis, Phoradendron quadrangulare | |
| Lag-ConP | Ce | Lr | - | |
| B2 | Lyc-ConR | Ag-Ce | - | Chloris barbata, Cissus verticillata, Harrisia eriophora, Desmanthus virgatus, Lycium carolinianum, Opuntia dillenii |
| Sar-AviM | Ag | Lr | - | |
| Bat-AviB | Ag | Ce | Sporobolus pyramidatus, Heliotrophium curassavicum, Iva cheiranthifolia | |
| AvigerR | Ag | - | Bastardia viscosa | |
| Bat-AviP | Ag | - | Monanthochloe littoralis | |
| Avi-RhiR | Ag-Rm | Ce | - | |
| C1 | Pri-RhiC | Rm | Aa | Prioria copaifera, Bactris guineensis, Curcumis melo, Inga alba, Bignonia hyacinthina, Crescentia cujete, Dalbergia beownei, Melothria pendula |
| Avi-RhiB | Rm | - | Avicennia schaueriana | |
| Ann-FicC | Rm | - | Casearia aculeata, Erythrina fusca, Klarobelia anomala | |
| RhimanC | Rm | - | - | |
| C2 | Pel-RhiC | Rm-Lr-Pr | Ag | - |
| Rha-LagC | Lr | Rm-Ag | Cyperus alternifolius, Echinochloa polystachia, Eleocharis interstincta, Heterostachys ritteriana, Eleocharis mutata, Ludwigia octovalvis, Persicaria acuminata | |
| Mac-RhiC | Rm | Lr-Ag | Nephrolepis multiflora, Dalbergia berteroi, Sabal causiarum | |
| Dai-RhiB | Rm | Lr | Bucida palustris, Hohenbergia penduliflora, Tillandsia usneoides, Sabal palmetto, Roystonea regia, Cissus trifoliata, Tillandsia fasciculata, Tabebuia angustata | |
| Lag-RhiP | Rm-Lr | - | Paullinia fuscescens, Blepharodon mucronatum, Albizia saman, Eclipta prostrata, Ficus bullenei, Gliricidia sepium, Maclura tinctoria, Mesechites trifidus, Solanum jamaicense, Terminalia catappa, Sacrostemma glaucum, Wedelia fruticosa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rull, V. Responses of Caribbean Mangroves to Quaternary Climatic, Eustatic, and Anthropogenic Drivers of Ecological Change: A Review. Plants 2022, 11, 3502. https://doi.org/10.3390/plants11243502
Rull V. Responses of Caribbean Mangroves to Quaternary Climatic, Eustatic, and Anthropogenic Drivers of Ecological Change: A Review. Plants. 2022; 11(24):3502. https://doi.org/10.3390/plants11243502
Chicago/Turabian StyleRull, Valentí. 2022. "Responses of Caribbean Mangroves to Quaternary Climatic, Eustatic, and Anthropogenic Drivers of Ecological Change: A Review" Plants 11, no. 24: 3502. https://doi.org/10.3390/plants11243502
APA StyleRull, V. (2022). Responses of Caribbean Mangroves to Quaternary Climatic, Eustatic, and Anthropogenic Drivers of Ecological Change: A Review. Plants, 11(24), 3502. https://doi.org/10.3390/plants11243502





