Abstract
The concept of the taxon cycle involves successive range expansions and contractions over time, through which a species can indefinitely maintain its core distribution. Otherwise, it becomes extinct. Taxon cycles have been defined mostly for tropical island faunas; examples from continental areas are scarce, and similar case studies for plants remain unknown. Most taxon cycles have been identified on the basis of phylogeographic studies, and straightforward empirical evidence from fossils is lacking. Here, empirical fossil evidence is provided for the recurrent Eocene to the present expansion/contraction cycles in a mangrove taxon (Pelliciera) after a Neotropical-wide study of the available pollen records. This recurrent behavior is compatible with the concept of the taxon cycle from biogeographical, chronological and ecological perspectives. The biotic and abiotic drivers potentially involved in the initiation and maintenance of the Pelliciera expansion/contraction cycles are analyzed, and the ecological and evolutionary implications are discussed. Whether this could be a trend toward extinction is considered under the predictions of the taxon cycle theory. The recurrent expansion and contraction cycles identified for Pelliciera have strong potential for being the first empirically and unequivocally documented taxon cycles and likely the only taxon cycles documented to date for plants.
1. Introduction
The concept of the taxon cycle was introduced by Wilson [1] to describe the biogeographical and evolutionary dynamics of species that experience successive range expansions and contractions over time linked to adaptive ecological shifts. According to this author, a taxon can maintain its core distribution area, which he called the headquarters, in a given land mass indefinitely by expanding and contracting its geographical range recurrently. Otherwise, it becomes extinct. In the taxon cycle, expanding and contracting species populations have disparate geographical patterns and adaptive features that allow the subdivision of the process into four main stages [2] (Figure 1). In stage I, high-density expanding populations rapidly colonize new environments but bear low morphological differentiation across their geographical range. These taxa have high reproductive potential and broad habitat tolerance and have been called supertramps [3]. Expansion slows down in stage II, and population differentiation significantly increases, especially near the range margins. The taxa corresponding to this stage are known as great speciators [4]. Stage III is characterized by geographical stasis and local extinction leading to fragmented distributions and incipient speciation, which may trigger the onset of a new cycle. If this is not the case, a gradual decline in range size and intraspecific diversity takes place, leading to a progressive relictualization (stage IV) and eventually to extinction [5]. The main contribution of the taxon cycle concept to biogeography is the focus on the evolutionary consequences of ecological interactions among colonizing and autochthonous (resident) species, which influence their extinction dynamics and shape their geographical distribution patterns [6].
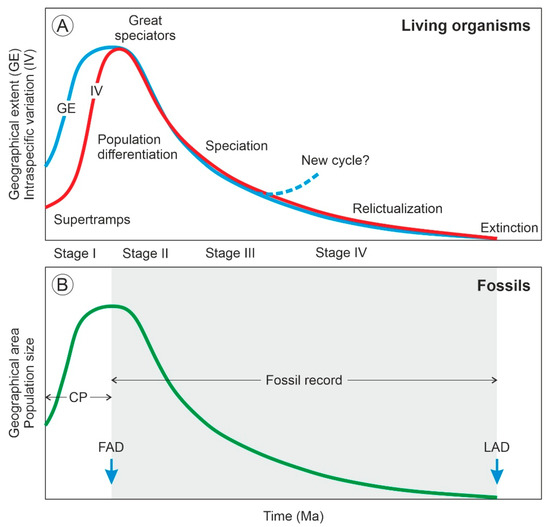
Figure 1.
The taxon cycle and its fossil expression. (A) Variations in geographical extent and intraspecific differentiation along the stages of the taxon cycle (redrawn from Ref. [5]). (B) Variations in the geographical area of fossils according to the asymmetric model [7]. The stages represented in the fossil record (gray area) and the condensed phase (CP), which is apparently unrecorded, are indicated. FAD, first appearance datum; LAD, last appearance datum.
Originally, taxon cycles were defined to explain the biogeographical patterns of island biotas [8], and although it has been suggested that they may also occur in continental environments [6], archipelagos remain the most common targets. Special emphasis has been placed on animal groups (crustaceans, insects, reptiles, birds) from tropical islands and archipelagos (Caribbean Antilles, Indonesia, Melanesia, New Guinea, Philippines, Madagascar) [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. Few case studies are available from continental areas. For example, a meta-analysis of a widely distributed bird group (Campefagidae) from tropical Asia, Africa and Australia, including insular and continental areas, has been considered to be consistent with the taxon cycle concept [5]. Another case is the diversification of Central American salamanders of the supergenus Bolitoglossa (Plethodontidae) [25]. In plants, similar case studies seem to be lacking. In agreement with previous reports [26], the author was unable to find examples providing empirical support for the predictions of the taxon cycle theory. A single molecular phylogenetic study on the plant tribe Gaultherieae (Ericaceae) was found, suggesting that fruit color might follow a process similar to a taxon cycle [27].
Initially, the occurrence of taxon cycles was attributed to changing biotic interactions, notably competition and predation, rather than to shifts in environmental drivers [1,2]. Later, it was realized that the estimated duration of taxon cycles in Caribbean birds, based on molecular phylogenetic analyses, was on the order of 105–107 years [6], which is much longer than the period of most climatic drivers of cyclic nature, especially the Pleistocene glacial–interglacial cycles, which have periods of 0.02–0.1 million years (my) [28]. Therefore, the idea of a biotic origin and control was reinforced. Further studies using similar methods estimated taxon cycle periodicities of ~5 my for Indo-Pacific birds [5,15] and reaffirmed the idea that Pleistocene climatic cycles could have not been important drivers. However, these authors suggested that other environmental shifts of greater periodicities, such as plate collision or orogenesis, could have been involved [5]. It has also been suggested that biotic drivers play a major role in the expansion phase, whereas abiotic drivers are more influential in the retraction phase [29]. In all these works, the duration of taxon cycles was deduced from phylogenetic divergence times, which are usually estimated using molecular clock assumptions or are modeled using a variety of indirect methods [30]. Therefore, phylogenetic divergence times are hypotheses, not empirical evidence [31].
The fossil record could provide straightforward evidence and more reliable chronologies, but unequivocal fossil evidence for taxon cycles is still lacking. Range expansion–contraction cycles have been observed in the fossil record [29,32,33,34] but have not been analyzed from a taxon cycle perspective [5], which could be due to the difficulty of detecting the chronological and geographical origin of a species [7]. Some paleontologists argue that the waxing and waning of fossil species follow symmetric trends [32] but others consider the taxon cycle model as a good example of a time-asymmetric biogeographical and evolutionary process, as the initial dispersion phase (stage I) is usually much faster—and, hence, much more difficult to document in the fossil record—than the ensuing contraction/diversification and further extinction phases (stages II to IV) [7]. As a result, fossils would be able to account mainly for phases II to IV, whereas phase I would remain hidden (likely condensed) and be represented only by the seemingly synchronous appearance of the species in a more or less wide area [35] (Figure 1). According to the punctuated equilibrium model of evolution, this asymmetry should be viewed as an intrinsic feature of the fossil record—and, therefore, of the evolutionary process itself—rather than an imperfection, as is usually considered [36,37]. In summary, according to the asymmetric model, the last appearance datum (LAD) of a fossil reliably records its extinction, but its first appearance datum (FAD) does not record its actual time and place of origin but of its initial spreading (Figure 1).
Pelliciera Planch. and Triana (Triana and Planchon)—also reported as Pelliceria in some early publications—is a genus of Neotropical mangrove trees of the family Tetrameristaceae—formerly in the Theaceae or the Pellicieraceae, as reported in a number of classical papers—which has traditionally been considered monotypic (P. rhizophorae) but has recently been split into two species: P. rhizophorae (Planch. and Triana) N.C. Duke and P. benthamii Planch. and Triana [38]. These species are currently restricted to a small patch along the Caribbean and Pacific coasts of Central America and northwestern South America—called here the present Pelliciera range or PPR (Figure 2)—which has been considered to be a relict of the larger, nearly pan-Neotropical distribution attained by Pelliciera during Tertiary times [39,40,41,42,43].
Presently, Pelliciera is rare and restricted to sites with low or moderate salinity in the understory of Rhizophora-dominated mangrove forests [44]. Recent autecological studies have shown that Pelliciera is highly sensitive to light intensity and salinity, and the combination of high levels of these environmental stressors leads to increased mortality, lower photosynthesis rates and reduced growth. When this species grows in shade conditions, however, it can tolerate high salinities, which suggests that light intensity is the main limiting factor. As a result, this taxon is unable to establish itself in sites with an open canopy and grows in the understory beneath the canopy of other tree species that, in the case of Central America, is provided by Rhizophora mangle, which is more tolerant to environmental stressors [44]. It has also been reported that the scarcity of nutrients such as nitrogen and phosphorus can limit the growth of Pelliciera, leading to the development of dwarf forms [45]. In general, Pelliciera is considered to be a stenotopic taxon bearing a relatively narrow and specialized niche.
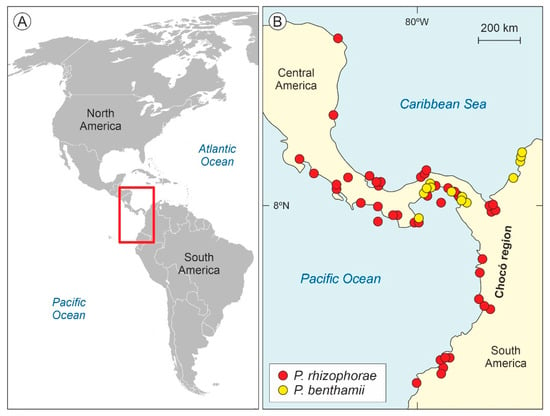
Figure 2.
Present distribution of Pelliciera species. (A) Map of the Americas with the distribution area of Pelliciera highlighted by a red box. (B) Close up of the occurrence patterns of the two Pelliciera species (modified from Ref. [38]). The Colombian Chocó region is highlighted because it is one of the most humid regions of the world, with precipitation values up to 13,000 mm y−1 [46].
Reconstructions of past biogeographical patterns and trends of Pelliciera are based on its fossil representative, the morphological pollen species Lanagiopollis crassa (van der Hammen and Wijmstra) Frederiksen, which also appears in the literature under the synonym Psilatricolporites crassus van der Hamen and Wijmstra [39]. This pollen originated locally in the Early Eocene but did not attain significant abundances until the Middle Eocene [47], when it became the dominant tree in Neotropical mangrove communities [48]. By that time, Rhizophora (represented by the fossil pollen Zonocostites ramonae Germeraad, Hopping and Muller), currently the most abundant mangrove-forming tree in Neotropical coasts, was still absent in the region and arrived later, in the Mid–Late Eocene, likely by long-distance dispersal from the Indo-Pacific region, crossing the Atlantic through the Tethys seaway [49]. Rhizophora acquired its present dominant status during the Eocene/Oligocene transition (hereafter EOT), which represented a global disruption characterized by a rapid cooling (which inaugurated the Cenozoic icehouse Earth state) and a sea level fall that heavily influenced the Earth’s biota in the form of enhanced extinction and intense biotic turnover [50,51].
In the Neotropics, the EOT signified a revolution for mangrove communities as the former Eocene Pelliciera-dominated mangroves disappeared and this tree turned into a minor subordinate element of Rhizophora-dominated communities. However, this did not lead to the extinction of Pelliciera. Rather, this taxon was much more widespread across the Neotropics after losing its dominance than it was before and is today [39,40,41,42,43], which represents a major biogeographical challenge. Several explanations have been proposed to account for the Miocene–present Pelliciera reduction, including climatic and/or salinity stress, sea-level shifts or competition with Rhizophora, among others [39,40,41,42,43,52,53]. However, there are some methodological weaknesses that should be addressed before analyzing the potential causes for the Pelliciera biogeographical trends. First, although the idea of a post-Miocene range reduction was based on the analysis of barely a dozen fossil records, this view has perpetuated until today with no further reconsideration based on an updated fossil database. Second, this reduction is only part of the story about the Cenozoic range shifts of Pelliciera, as pre-Miocene evidence has also not been analyzed under the same premises of a representative spatiotemporal fossil record. Third, most pollen records on which previous hypotheses were based consisted of qualitative (presence/absence) and pseudoquantitative (abundant, common, scarce) records, and it has been demonstrated that quantitative data are essential to properly record and understand the evolution of Neotropical mangroves [48].
This paper uses an updated fossil pollen database of almost 80 widely distributed qualitative and quantitative Neotropical pollen records to reconstruct the biogeographical trends of Pelliciera since its early Eocene origin to the present. This analysis revealed the occurrence of a long-term expansion–contraction loop that would be compatible with the concept of the taxon cycle [1]. Therefore, this would be not only the first evidence for a taxon cycle in plants but also strong support for a taxon cycle, in general, as it is based on empirical, straightforward and chronologically accurate evidence rather than on phylogenetic assumptions and modeling. The biogeographical cycle identified in this way is discussed in ecological and evolutionary terms using the known ecological traits of the taxa involved, primarily Pelliciera and Rhizophora, under the above-mentioned predictions of the taxon cycle model and further updates. External (environmental) drivers potentially involved in the Pelliciera taxon cycle are also discussed. Considering the present features of Pelliciera populations, in comparison with its previous biogeographical history, possible future developments are also evaluated.
2. Materials and Methods
The data used in this paper were obtained from the available literature; more information exists in the databases of oil companies but is not publicly available. Efforts to make this information public [47,54] are worth undertaking. After a comprehensive literature survey, almost 80 fossil mangrove pollen records were identified with useful data to reconstruct the biogeographical history of Pelliciera and Rhizophora in the Neotropics from the Eocene to the present. Almost half of these sites (45%) had quantitative data (percentages), and slightly more than a quarter (26%) had only presence/absence records; the rest had subjective measures such as abundant, common or rare. Ages were provided as geological epochs (Eocene, Oligocene, Miocene, Pliocene, Pleistocene and Holocene). In some cases, there was not enough chronological precision to resolve between them and longer ranges were used (e.g., Oligo–Miocene, Mio–Pliocene). The lack of site coordinates in many original references prevented precise estimations of range sizes using statistical methods [55,56,57].
3. Results
The results obtained are displayed in Figure 3. Raw data (Table S1) and detailed figures for each time interval (Figures S1 to S4) are provided in the Supplementary Material. During the Eocene (17 sites, including the two labeled as Eocene/Oligocene), Pelliciera was common or abundant in most localities, with maximum percentages up to 60%. The distribution area was restricted to NW South America (presently Colombia and Venezuela), with one site in eastern central America (Panama) and another on the Caribbean island of Jamaica. Rhizophora was present only in six localities, attaining some relevance (≤10%) in only one site from Panama. In the Oligocene and Oligo–Miocene (18 sites), a significant abundance decline was observed in Pelliciera, which fell to values below 5% except in two localities from Venezuela. Rhizophora showed a reverse trend and was common or abundant in a significant number of sites, attaining values between 50 and 90% in four of them, which were situated in Guyana, Mexico, Venezuela and Puerto Rico. Regarding the distribution, Pelliciera showed a dramatic expansion in both latitudinal and longitudinal senses, ranging from Mexico and Puerto Rico to Brazil.
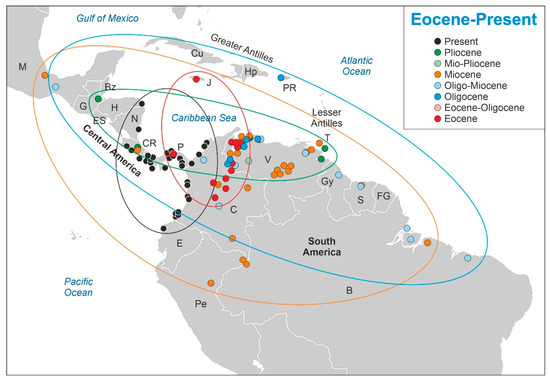
Figure 3.
Graphical display of the results from Table S1 (Supplementary Material). Present Pelliciera localities were taken from Figure 2. Countries: B, Brazil; Bz, Belize; C, Colombia; CR, Costa Rica; Cu, Cuba; E, Ecuador; ES, El Salvador; FG, French Guiana; G, Guatemala; Gy, Guyana; H, Honduras; Hp, Hispaniola (Haiti and Santo Domingo); J, Jamacia; M, Mexico; N, Nicaragua; P, Panama; Pe, Peru; PR, Puerto Rico; S, Surinam; T, Trinidad and Tobago; V, Venezuela.
During the Miocene (36 sites), the decline in the abundance of Pelliciera continued and disappeared from eight localities, reaching values of 3% in only five Venezuelan and one Panamanian site. The geographical distribution was similar to that in the Oligocene but with a slight displacement toward the SW. In contrast, Rhizopohora became abundant in most Miocene records with values up to 90%. In the Pliocene (eight localities, including some labeled as Mio–Pliocene), Pelliciera was only present and its range was restricted to the southern Caribbean margin (northern South America and Central America), whereas Rhizophora attained values of 70–100% in three sites and was present in others. No records exist for the Pleistocene and a few records were available for the Holocene, which were restricted to the PPR [58].
The shifts in the geographical range of Pelliciera represented in Figure 3 could be subdivided into four main phases (Figure 4). During the first phase (Eocene to Oligocene), the range expanded to most of the Neotropics, but the populations experienced a significant reduction, which resulted in a more diluted distribution. This phase is called thinning expansion here, as the term dilution has already been used in biogeography for other processes [59,60]. The second phase (Oligocene to Miocene) was a displacement phase, where the range slightly migrated to the NW, with no significant differences with respect to the Oligocene situation. During the third phase (Miocene to Pliocene), the range underwent a major contraction toward the southern Caribbean margin. Finally, in the fourth phase (Pliocene to present) the range did not experience further reductions but showed a longitudinal contraction around Central America and a southward migration toward the Equator, which is considered a spatial reorganization. In summary, the range of Pelliciera significantly expanded from its original NW South American cradle until a Miocene maximum, encompassing most of the Neotropics to initiate a retraction that ended in the present PPR, which is very similar in location and extent to the Eocene range. This biogeographical loop was accompanied by a dramatic decline in the Pelliciera populations, which was abrupt in the EOT and gradual between the Oligocene and the present (Figure 5). If we consider that the Eocene Pelliciera mangroves attained their maximum extent in the Lutetian (Middle Eocene) [42,48], the minimum duration of the overall Pelliciera biogeographical loop was approximately 45 my.
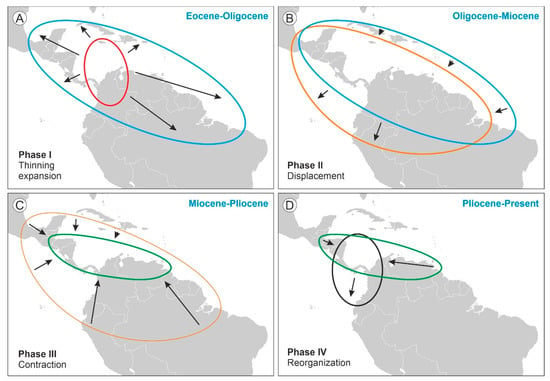
Figure 4.
Pelliciera range shifts between the Eocene and the present subdivided into four chronological phases. (A) Phase I, thinning expansion (Eocene-Oligocene). (B) Phase II, displacement (Oligocene-Miocene). (C) Phase III, contraction (Miocene-Pliocene). (D) Phase IV, reorganization (Pliocene-Present).
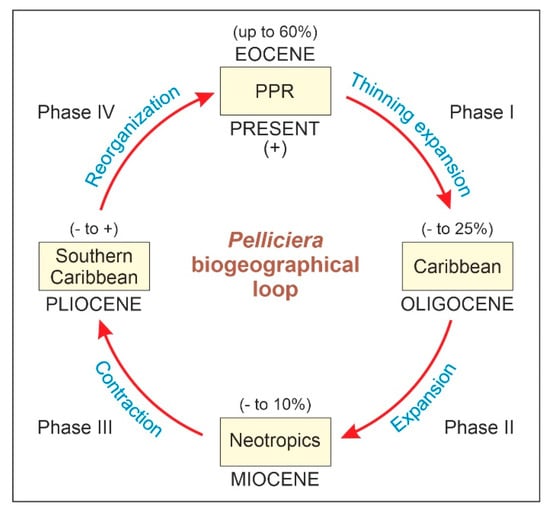
Figure 5.
Diagram of the Pelliciera biographical loop with indication of the ranges of abundance of its fossil pollen in each geological epoch. PPR, present Pelliciera range.
4. Discussion
The Pelliciera loop, as documented in the fossil record, is straightforward empirical evidence for an expansion–contraction cycle, whose duration (~4·107 y) and biogeographical expression are consistent with the concept of taxon cycle [1]. Whether this cycle truly corresponds to a taxon cycle is discussed in this section in more depth, with special consideration of the potential ecological and evolutionary implications.
First, it should be noted that the expansion phase (Eocene to Miocene) was much longer than expected under the asymmetric model [7], which postulated that phase I was too fast to be detectable in the fossil record (Figure 1). According to the asymmetric predictions, phase I should be condensed in the Eocene, when Pelliciera had already expanded across NW South America, Central America and the Greater Antilles (Figure 3). To verify this possibility, the Eocene records (Figure 2) were subdivided into the Early Eocene, Middle Eocene and Late Eocene, revealing the occurrence of an intra-Eocene contraction–expansion loop (Figure 6). According to this reconstruction, Pelliciera would have originated in the Early Eocene in western Venezuela and expanded relatively fast, attaining a maximum in the Middle Eocene, to further retreat (Late Eocene) to an area similar but not identical to its original range (Figure 6). Therefore, the true stage I would have occurred between the Early and Middle Eocene. The exact duration of this loop was difficult to establish, but a maximum estimate could be the total duration of the Eocene epoch, which is 22 my (~2·107 y). Using the same reasoning, stage I would have lasted a maximum of 10 my (107 y).
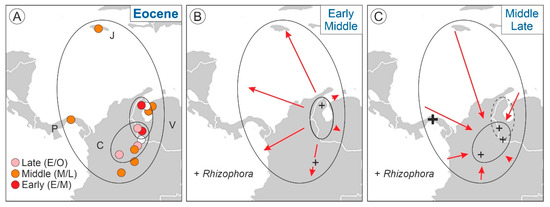
Figure 6.
The Eocene expansion–contraction Pelliciera loop. (A) Eocene localities (Figure 3) subdivided into Early, Middle and Late Eocene ages (Table S1 of the Supplementary Material). Countries: C, Colombia; J, Jamaica; P, Panama; V, Venezuela. (B) Early to Middle Eocene expansion. (C) Middle to Late Eocene contraction. The initial Early Eocene range (A) is indicated by a broken line.
Evidence for population differentiation and eventual speciation should be sought for in pollen morphology, which has been demonstrated to be useful for differentiating extant Pelliciera species and subspecific taxa [38,61,62]. The main diagnostic characteristics are size and exine (the outer pollen wall) sculpture. Fossil Pelliciera pollen (L. crassa or P. crassus) also exhibits significant morphological variability in these diagnostic characteristics from the Eocene to the Pliocene [47,54,63,64,65]. Unfortunately, no systematic records of this variability exist in the fossil record that enable distinguishment among the possible taxonomic categories. This should be addressed in future studies, but with the available fossil evidence, the occurrence of different species and/or subspecies cannot be dismissed.
Regarding ecological preferences, most paleoecological studies using pollen rely on a reasonable degree of niche constancy over time (niche conservatism), especially at the genus level, in long-lasting communities [66,67,68], which is the case of mangroves. Therefore, it is likely that fossil Pelliciera species were also stenotopic, as the extant species are. This would be especially true in the Eocene, as the maximum recorded expansion of the Pelliciera range did not progress beyond tropical warm and wet climates, and was very similar to the present range, which is characterized by average temperatures of ~27 °C and total annual precipitation values up to ~3000 mm [45,62]. It is especially noteworthy that extant Pelliciera grows around one of the most humid areas of the world, the Chocó region (Figure 2), with precipitation values reaching 13,000 mm y−1 [46]. During the Eocene, climates were significantly warmer than at present, and global average temperatures were ~8–14 °C above present temperatures [69], which would indicate macrothermal conditions for fossil Pelliciera species.
The first records of Rhizophora dated from the Middle Eocene and extended up to the Late Eocene in the form of scattered appearances, which were always around the initial and final Eocene ranges of Pelliciera. The only exception was a Panamanian site (67), where Rhizophora reached 10% of the pollen assemblage during the Late Eocene. These occurrences represent the first stages of colonization of the Neotropics by Rhizophora, which coincided with the results of recent molecular phylogeographical studies that situated the origin of this genus in the Indo-Pacific region and its worldwide spreading in the Mid–Late Eocene [49]. According to the same studies, Rhizophora could have reached the Neotropics by the Atlantic Ocean via the Tethys seaway. The use of this pathway cannot be supported or dismissed by paleogeographic reconstructions and fossil pollen records, as both the Atlantic and Pacific seaways to the Caribbean were open for dispersal during the whole Eocene [70,71], and the fossil records do not show a clear Atlantic or Pacific pattern (Figure 6). Once more, the only exception was site 67, which was situated in the Pacific island arc of the western Caribbean plate margin during the Eocene, which opens the door to a possible Pacific dispersal pathway, but more studies are needed for a sound assessment.
The Oligocene-to-Miocene Pelliciera expansion (thinning expansion) started from a small area in NW South America at the intersection between the Eocene and the present ranges (Figure 4 and Figure 6). Since the beginning, this expansion followed the expansion of Rhizophora, the dominant mangrove tree. This change in dominance would have been greatly influenced by the EOT environmental disruption in combination with biotic interactions. The EOT cooling would have affected a stenotypic macrothermic taxon such as Pelliciera and favored the development of the more eurytopic and climatically tolerant Rhizophora. In addition to its wider environmental tolerance, Rhizphora is an aggressive colonizer with a high dispersal power, as its propagules are able to remain floating and viable for a year or more in saltwater. In contrast, Pelliciera propagules have a maximum flotation period of barely a week and a maximum viability of roughly a couple of months, and their dispersal occurs primarily over short distances transported by coastal currents [72,73]. The whole picture may suggest that the competitive superiority of Rhizophora could have led to the extinction of Pelliciera, but in contrast, this taxon not only survived but also expanded its range at the same pace as Rhizophora (Figure 4). This could be explained by a combination of two types of biotic interactions known as facilitation and niche segregation.
Facilitation occurs when a species provides refuge to another in the face of environmental stress, predation or competition, thus allowing its survival [74,75,76,77]. Niche segregation refers to the spatial, temporal or functional divergence of the niches of two competing species that allows the survival of both [78,79,80]. As quoted above for extant mangroves, Pelliciera is highly sensitive to environmental stressors, and its growth is facilitated by R. mangle, which provides a favorable microhabitat in the mangrove understory [44]. The maintenance of this specialized microhabitat could be explained by the fact that although eurytopic generalists are apt to live in a wide range of environmental conditions—and, therefore, able to invade the specialist’s microhabitat—stenotopic specialists are more efficient within the restricted set of conditions in which they can develop [81].
Using these ecological relationships as modern analogs for EOT mangroves, the Pelliciera-to-Rhizophora dominance shift without competitive exclusion could be viewed as a tradeoff, in which the newly arrived eurytopic generalist (Rhizophora) outcompeted the resident stenotopic specialist (Pelliciera) in terms of dominance and, in exchange, facilitated a microhabitat to the loser, which “accepted” playing a secondary role in order to survive. In addition to survival, Pelliciera acquired the opportunity to expand its range across the whole Neotropic area beyond its macroenvironmental niche boundaries during the Oligocene and the Miocene. This would have hardly been possible without the facilitation of Rhizophora, as suggested by the permanent restriction of Pelliciera within its comfort zone (or the headquarters, in the words of Wilson [1]) during the whole Eocene when Rhizophora was absent. Given the dispersal mode of Pelliciera, which is typically over short distances through coastal currents, its expansion should have been by diffusion, that is, gradual migration across hospitable terrains, rather than by long-distance dispersal, which implies crossing inhospitable lands [82]. This suggests that Rhizophora must have been the pioneering colonizer, and once the mangrove community was developed and a microhabitat suitable for Pelliciera was created, this taxon would have been able to establish. Therefore, during its maximum Oligo–Miocene expansion, Pelliciera likely survived as a diffuse network of small populations restricted to favorable microhabitats, which is supported by mangrove fossil pollen records of this age, which were widespread but showed very low pollen percentages when present. This spatial arrangement and ecological dynamics fit with the concept of microrefugia, specifically the diffuse type, which promotes genetic differentiation among populations of the same species [83]. The ecological loser could have had unprecedented opportunities for diversification and become an evolutionary winner [84].
From an environmental point of view, the expansion of Pelliciera occurred in a phase of extended climatic stability spiked only by minor shifts [69], which suggests that biotic interactions were the main drivers in this part of the cycle. It is important to emphasize that the Oligo–Miocene spreading—whose duration is difficult to estimate due to the lack of enough temporal resolution in the fossil record—was the second expansion of Pelliciera, after the first occurred during the Early–Mid Eocene around the original range and its further Late Eocene contraction. Therefore, Pelliciera experienced at least two expansion/contraction cycles since the Eocene. The causes for the occurrence of the first cycle remain unknown, but the second was likely triggered by the Late Eocene arrival of Rhizophora, which not only provided new spreading and diversification opportunities but also physically mediated the process as a niche builder and an indirect dispersal agent (actually an ecological nurse). It is not possible to know whether Pelliciera would have become extinct without the arrival of Rhizophora as its range was receding, but, if so, Rhizophora could have provided the conditions for Pelliciera to overcome this bottleneck. Therefore, the ecological winner would have been not only the ecological nurse for the ecological loser but also its evolutionary rescuer. Considering the evolutionary dimension of these ecological interactions, Rhizophora, which likely began as a competitor, would have been much more than a lifesaver for Pelliciera, becoming with time a real sponsor/benefactor.
The Miocene–Pliocene contraction occurred after a significant cooling known as the Middle Miocene Cooling Transition (MMCT) [69]. No evident changes were observed in the fossil pollen record, and hence, this contraction could have been more influenced by climatic than by biotic drivers. Obviously, the Pliocene restriction to the southern Caribbean margin was due to the local extinction of all Miocene Pelliciera populations outside this area, as predicted by the taxon cycle theory. It is possible that Pelliciera was unable to endure a second cooling, even under the protection of Rhizophora, and survived only in the sector of its range where temperature and precipitation remained favorable. This would be supported by the fact that the observed contraction had a clear latitudinal component. The reorganization that occurred between the Pliocene and the present is more enigmatic for two main reasons. On the one hand, the present distribution is based on actual records of living populations, while former distributions were inferred from fossil pollen assemblages, which are subjected to a variety of taphonomic processes not affecting living plants. On the other hand, there is a gap between the Pliocene and the present [58]. This prevents us from knowing how Pleistocene glaciations, the coolest phases of the whole Cenozoic era, affected the range of Pelliciera. Knowing the macrothermic nature of this taxon, it is expected that glaciations significantly affected its populations, but no evidence of this possibility is available to date.
An additional factor characteristic of the last millennia, which was absent in former geological epochs, is the presence of humans, whose influence on Pelliciera is largely unknown, except for the last decades. P. rhizophorae has been listed as “Vulnerable”—that is, under a high risk of extinction in the wild due to its small (500–2000 km2) and fragmented distribution area—in the IUCN Red List of Threatened Species [85,86,87]. Urban expansion has been recognized as a major threat to Pelliciera populations, which are being heavily fragmented and threatened by habitat loss [88]. From a biogeographical perspective, the available evidence suggests that, rather than shrinking the distribution area of Pelliciera as a whole, human activities have caused its severe fragmentation, which affects population viability and increases the sensitivity to extreme events and global warming [88]. In the context of the taxon cycle, it could be asked whether the total extinction of Pelliciera is approaching and how human activities could contribute to accelerating this end. It is possible that, if the IUCN considers this view, Pelliciera would be transferred to the “Critically Endangered” category and considered a conservation priority. It is difficult to envisage the emergence of another evolutionary rescuer similar to Rhizophora that prevented the extinction of Pelliciera and promoted its expansion, thus initiating an eventual third cycle. Humans could be able to achieve this through conservation/restoration actions, but we should seriously ask ourselves if we have the right to artificially preserve a taxon that is naturally headed to extinction [89].
To summarize, the Pelliciera expansion–contraction cycles documented in the fossil pollen record since the Eocene have strong potential for being considered taxon cycles, as per Wilson [1], from chronological, biogeographical and ecological perspectives. Evolutionary predictions are more difficult to evaluate solely on the basis of the available pollen records and complementary evidence is needed for a sound assessment. Two main aspects remain to be analyzed in more detail in further studies, namely phase chronology and population diversification. Regarding the first, more precision is needed to accurately estimate the duration of the cycles and their respective phases. With reference to the second, more systematic pollen morphological studies, along with the use of molecular phylogenetic techniques, are required to document potential differentiation patterns among fossil populations. This paper tried to maximize the utility of the information available in the literature in this sense. However, the existing studies were not aimed at demonstrating the existence of taxon cycles, which hindered more robust conclusions. With this in mind, further studies can be devised with the specific target of testing the evolutionary predictions of the taxon cycle model. Hopefully, this paper has provided a number of testable hypotheses that are worth exploring in this line. If confirmed, the Pelliciera cycles would be the first taxon cycles supported by straightforward empirical evidence and the first known taxon cycle for plants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12020244/s1, Figure S1 to S4: Maps showing the location of the studied sites grouped by geological epochs; Table S1: Eocene to Pliocene Pelliciera (represented by Lanagiopollis crassa and Psilatricolporites crassus) and Rhizophora (represented by Zonocostites ramonae and other species of this genus) records from the Neotropics, with indication of the relative abundance according to the original data reported in the corresponding papers (+ present, - absent or not mentioned).
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data provided in the Supplementary Material.
Acknowledgments
The comments by two anonymous reviewers are greatly appreciated.
Conflicts of Interest
The author declares no conflict of interest.
References
- Wilson, E.O. The nature of the taxon cycle in the Melanesian ant fauna. Am. Natur. 1961, 95, 169–193. [Google Scholar] [CrossRef]
- Ricklefs, R.E.; Cox, G.C. Taxon cycles in the West Indian avifauna. Am. Natur. 1972, 106, 195–219. [Google Scholar] [CrossRef]
- Diamond, J.M. Colonization of exploded volcanic islands by birds: The supertramp strategy. Science 1974, 184, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J.M.; Gilpin, M.E.; Mayr, E. Species-distance relation for birds of Solomon archipelago, and paradox of great speciators. Proc. Natl. Acad. Sci. USA 1976, 73, 2160–2164. [Google Scholar] [CrossRef]
- Pepke, M.L.; Irestedt, M.; Fjeldså, J.; Rahbeck, C.; Jønsson, K.A. Reconciling supertramps, great speciators and relict species with the taxon cycle stages of a large island radiation (Aves: Campephagidae). J. Biogeog. 2019, 46, 1214–1225. [Google Scholar] [CrossRef]
- Ricklefs, R.E.; Bermingham, E. The concept of the taxon cycle in biogeography. Glob. Ecol. Biogeog. 2002, 11, 353–361. [Google Scholar] [CrossRef]
- Lopez-Martinez, N. Time asymetry in the palaeobiogeographic history of species. Bull. Soc. Geol. Française 2009, 180, 445–455. [Google Scholar]
- Wilson, E.O. Adaptive shifts and dispersal in a tropical ant fauna. Evolution 1959, 13, 122–144. [Google Scholar] [CrossRef]
- MacLean, W.P.; Holt, R.D. Distributional patterns in St. Croix Sphaerodactylus lizards: The taxon cycle in action. Biotropica 1979, 11, 189–195. [Google Scholar] [CrossRef]
- Losos, J.B. A critical comparison of the taxon-cycle and character-displacement models for size evolution of Anolis lizards in the Lesser Antilles. Copeia 1992, 2, 279–288. [Google Scholar] [CrossRef]
- Jones, M.J.; Sullivan, M.S.; Marsden, S.J.; Lindsley, M.D. Correlates of extinction risk of birds from two Indonesian islands. Biol. J. Linn. Soc. 2001, 73, 65–79. [Google Scholar] [CrossRef]
- Cook, B.D.; Pringle, C.M.; Hughes, J.M. Molecular evidence for sequential colonization and taxon cycling in freshwater decapod shrimps on a Caribbean Island. Mol. Ecol. 2008, 17, 1066–1075. [Google Scholar] [CrossRef]
- Simberloff, D.; Collins, M.D. The domain of the dynamic equilibrium theory and assembly rules, with comments on the taxon cycle. In The Theory of Island Biogeography Revisited; Losos, J.B., Ricklefs, R.E., Eds.; Princeton University Press: Princeton, NJ, USA, 2010; pp. 237–263. [Google Scholar]
- Economo, E.P.; Sarnat, E.M. Revisiting the ants of Melanesia and the taxon cycle: Historical and human-mediated invasions of a tropical archipelago. Am. Natur. 2012, 180, E1–E16. [Google Scholar] [CrossRef] [PubMed]
- Jønsson, K.A.; Irestedt, M.; Christidis, L.; Clegg, S.M.; Holt, B.G.; Fjeldså, J. Evidence of taxon cycles in an Indo-Pacific passerine bird radiation (Aves: Pachycephala). Proc. R. Soc. B 2014, 281, 20131727. [Google Scholar] [CrossRef]
- Economo, E.P.; Sarnat, E.M.; Janda, M.; Clouse, R.; Klimov, P.B.; Fischer, G.; Blanchard, B.D.; Ramirez, L.N.; Andersen, A.N.; Berman, M.; et al. Breaking out biogeographical modules: Range expansion and taxon cycles in the hyperdiverse ant genus Pheidole. J. Biogeog. 2015, 42, 2289–2301. [Google Scholar] [CrossRef]
- Fuchs, J.; Lemoine, D.; Parra, J.L.; Pons, J.-M.; Raherilalao, M.J.; Prys-Jones, R.; Thebaud, C.; Warren, B.H.; Goodman, S.M. Long-distance dispersal and inter-island colonization across the western Malagasy Region explain diversification in brush-warblers (Passeriformes: Nesillas). Biol. J. Linn. Soc. 2016, 119, 873–889. [Google Scholar] [CrossRef]
- Dalsgaard, B.; Kennedy, J.; Simmons, B.; Baquero, A.C.; González, A.M.M.; Timmermann, A.; Maruyama, P.K.; A McGuire, J.; Ollerton, J.; Sutherland, W.; et al. Trait evolution, resource specialization and vulnerability to plant extinctions among Antillean hummingbirds. Proc. R. Soc. B 2018, 285, 20172754. [Google Scholar] [CrossRef]
- Matos-Maraví, P.; Matzke, N.J.; Larabee, F.J.; Clouse, R.M.; Wheeler, W.C.; Sorger, D.M.; Suarez, A.V.; Janda, M. Taxon cycle predictions supported by model-based inference in Indo-Pacific trap-jaw ants (Hymenoptera: Formicidae: Odontomachus). Mol. Ecol. 2017, 27, 4090–4107. [Google Scholar] [CrossRef]
- Oliver, P.M.; Brown, R.M.; Kraus, F.; Rittmeyer, E.; Travers, S.L.; Siler, C.D. Lizards of the lost arcs: Mid-Cenozoic diversification, persistence and ecological marginalization in the West Pacific. Proc. R. Soc. B 2018, 285, 20171760. [Google Scholar] [CrossRef]
- O’Connell, D.P.; Kelly, D.J.; Lawless, N.; Karya, A.; Analuddin, K.; Marples, N.M. Diversification of a ‘great speciator’ in the Wallacea region: Differing responses of closely related resident and migratory kingfischer species (Aves: Alceinidae: Todiramphus). Ibis 2019, 161, 806–823. [Google Scholar] [CrossRef]
- Cozzarolo, C.-S.; Balke, M.; Buerki, S.; Arrigo, N.; Pitteloud, C.; Gueuning, M.; Salamin, N.; Sartori, M.; Alvarez, N. Biogeography and ecological diversification of a mayfly clade in New Guinea. Front. Ecol. Evol. 2019, 7, 233. [Google Scholar] [CrossRef]
- Liu, C.; Sarnat, E.M.; Friedman, N.R.; Hita Garcia, F.; Darwell, C.; Booher, D.; Kubota, Y.; Mikheyev, A.S.; Economo, E.P. Colonize, radiate, decline: Unravelling the dynamics of island community assembly with Fijian trap-jaw ants. Evolution 2020, 74, 1082–1097. [Google Scholar] [CrossRef] [PubMed]
- Cognato, A.I.; Smith, S.M.; Jordal, B.H. Patterns of host tree use within a lineage of saproxlic snout-less weevils (Coleoptera; Curculionidae; Scolytinae; Scolytini). Mol. Phylogenet. Evol. 2021, 159, 107107. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Paris, M.; Good, D.A.; Parra-Olea, G.; Wake, D.B. Biodiversity of Costa Rican salamanders: Implications of high levels of genetic differentiation and phylogeographic structure for species formation. Proc. Natl. Acad. Sci. USA 2000, 97, 1640–1647. [Google Scholar] [CrossRef]
- Sheh, S.N.; Morueta-Holm, N.; Angert, A.L. Determinants of geographic range size in plants. New Phytol. 2018, 226, 650–665. [Google Scholar] [CrossRef]
- Lu, L.; Fritsch, P.W.; Matzke, N.J.; Wang, H.; Kron, K.A.; Li, D.-Z.; Wiens, J.J. Why is fruit color so variable? Phylogenetic analyses reveal relationships between fruit-color evolution, biogeography and diversification. Glob. Ecol. Biogeog. 2019, 28, 891–903. [Google Scholar] [CrossRef]
- Hays, J.D.; Imbrie, J.; Shackleton, N.J. Variations in Earth’s orbit: Pacemaker of ice ages. Science 1976, 194, 1121–1132. [Google Scholar] [CrossRef]
- Žliobaitė, I.; Fortelius, M.; Stenseth, N.C. Reconciling taxon senescence with the Red Queen’s hypothesis. Nature 2017, 552, 92–95. [Google Scholar] [CrossRef]
- Ho, S.Y.W. The Molecular Evolutionary Clock. Theory and Practice; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- Parenti, L.R.; Ebach, M.C. Evidence and hypothesis in biogeography. J. Biogeog. 2013, 40, 813–820. [Google Scholar] [CrossRef]
- Foote, M. Symmetric waxing and waning of marine invertebrate genera. Paleobiology 2007, 33, 517–529. [Google Scholar] [CrossRef]
- Foote, M.; Crampton, J.S.; Beu, A.G.; Marshall, B.A.; Cooper, R.A.; Maxwell, P.A.; Matcham, I. Rise and fall of species occupancy in Cenozoic fossil mollusks. Science 2017, 318, 1131–1134. [Google Scholar] [CrossRef]
- Liow, L.H.; Stenseth, N.C. The rise and fall of species: Implications for macroevolutionary and macroecological studies. Proc. R. Soc. B 2007, 274, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
- Brunet, M.; Beauvilain, A.; Coppens, Y.; Heintz, E.; Moutaye, A.H.E.; Pildeam, D. The first Australopithecine 2500 km west of the Rift Valley (Chad). Nature 1995, 378, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Eldredge, N.; Gould, S.J. Punctuated equilibria: An alternative to phyletic gradualism. In Models in Paleobiology; Schopf, T.J.M., Ed.; Freeman Cooper: San Francisco, CA, USA, 1972; pp. 193–223. [Google Scholar]
- Gould, S.J.; Eldredge, N. Punctuated equilibria: The tempo and mode of evolution reconsidered. Paleobiology 1977, 3, 115–151. [Google Scholar] [CrossRef]
- Duke, N.C. A systematic revision of the vulnerable mangrove genus Pelliciera (Tetramerisraceae) in equatorial America. Blumea 2020, 65, 107–120. [Google Scholar] [CrossRef]
- Wijmstra, T.A. The identity of Psilatricolporites and Pelliciera. Acta Bot. Neerl. 1968, 17, 114–116. [Google Scholar] [CrossRef]
- Graham, A. New records of Pelliciera (Theaceae/Pellicieriaceae) in the Tertiary of the Caribbean. Biotropica 1977, 9, 48–52. [Google Scholar] [CrossRef]
- Graham, A. Diversification of Caribbean/Gulf mangrove communities through Cenozoic time. Biotropica 1995, 27, 20–27. [Google Scholar] [CrossRef]
- Rull, V. Middle Eocene mangroves and vegetation changes in the Maracaibo Basin, Venezuela. Palaios 1998, 13, 287–296. [Google Scholar] [CrossRef]
- Rull, V. A quantitative palynological record from the early Miocene of western Venezuela, with emphasis on mangroves. Palynology 2001, 25, 109–126. [Google Scholar] [CrossRef]
- Dangremond, E.M.; Feller, I.V.; Sousa, W.P. Environmental tolerances of rare and common mangroves along light and salinity gradients. Oecologia 2015, 179, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Dangremond, E.M.; Feller, I.C. Functional traits and nutrient limitation in the rare mangrove Pelliciera rhizophorae. Aquat. Bot. 2014, 116, 1–7. [Google Scholar] [CrossRef]
- Yepes, J.; Poveda, G.; Mejía, J.F.; Moreno, L.; Rueda, C. CHOCO-JEX a research experiment focused on the Chocó low-level jet over the far eastern Pacific and western Colombia. Bull. Am. Meteorol. Soc. 2019, 100, 779–796. [Google Scholar] [CrossRef]
- Germeraad, J.H.; Hopping, C.A.; Muller, J. Palynology of Tertiary sediments from tropical areas. Rev. Palaeobot. Palynol. 1968, 6, 189–348. [Google Scholar] [CrossRef]
- Rull, V. The Caribbean mangroves: An Eocene innovation with no Cretaceous precursors. Earth-Sci. Rev. 2022, 231, 104070. [Google Scholar] [CrossRef]
- Takayama, K.; Tateishi, Y.; Kaijita, T. Global phylogeography of a pantropical mangrove genus Rhizophora. Sci. Rep. 2021, 11, 7228. [Google Scholar] [CrossRef]
- Coxall, H.K.; Pearson, P.N. The Eocene-Oligocene transition. In Deep-Time Perspectives on Climate Change: Marrying the Signal from Computer Models and Biological Proxies; Williams, M., Haywood, A.M., Gregory, F.J., Scmidt, D.N., Eds.; The Geological Society: London, UK, 2007; pp. 351–387. [Google Scholar]
- Hutchinson, D.K.; Coxall, H.K.; Lunt, D.J.; Steinthorsdottir, M.; de Boer, A.M.; Baatsen, M.; von der Heydt, A.; Huber, M.; Kennedy-Asser, A.T.; Kunzmann, L. The Eocene-Oligocene transition: A review of marine and terrestrial proxy data, models and model-data comparisons. Clim. Past 2021, 17, 269–315. [Google Scholar] [CrossRef]
- Fuchs, H.P. Ecological and palynological notes on Pelliciera rhizophorae. Acta Bot. Neerl. 1970, 19, 884–894. [Google Scholar] [CrossRef]
- Jiménez, J.A. A hypothesis to explain the reduced distribution of the mangrove Pelliciera rhizophorae Tr and Pl. Biotropica 1984, 16, 304–308. [Google Scholar] [CrossRef]
- Lorente, M.A. Palynology and palynofacies of the Upper Tertiary in Venezuela. Dissert. Bot. 1986, 99, 1–222. [Google Scholar]
- Darroch, S.A.; Saupe, E.E. Reconstructing geographic range-size dynamics from fossil data. Paleobiology 2018, 44, 25–39. [Google Scholar] [CrossRef]
- Darroch, S.A.; Casey, M.M.; Antell, G.S.; Sweeney, A.; Saupe, E.E. High preservation potential of paleogeographic range size distributions in deep time. Am. Natur. 2020, 196, 454–471. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, F.; Di Febbraro, M.; Mondanaro, A.; Castiglione, S.; Serio, C.; Melchionna, M.; Rook, L.; Raia, P. MInOSSE: A new method to reconstruct geographic ranges of fossil species. Meth. Ecol. Evol. 2020, 11, 1121–1132. [Google Scholar] [CrossRef]
- Rull, V. Responses of Caribbean mangroves to Quaternary climatic, eustatic and anthropogenic drivers of ecological change: A review. Plants 2022, 11, 3502. [Google Scholar] [CrossRef] [PubMed]
- Keesing, F.; Holt, R.D.; Ostfeld, R.S. Effects of species diversity on disease risk. Ecol. Lett. 2006, 9, 485–498. [Google Scholar] [CrossRef]
- Keesing, F.; Belden, L.K.; Daszak, P.; Dobson, A.; Harvell, C.D.; Holt, R.D.; Hudson, P.; Jolles, A.E.; Jones, K.E.; Mitchell, C.E.; et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 2010, 468, 647–652. [Google Scholar] [CrossRef]
- Castillo-Cárdenas, M.F.; Díaz-Gonzales, F.; Cerón-Souza, I.; Sanjur, O.; Toro-Perea, N. Jumping a geographical barrier: Diversification of the mangrove species Pelliciera rhizophorae (Tetrameristaceae) across the Central American Isthmus. Tree Genet. Genom. 2014, 11, 1–11. [Google Scholar]
- Castillo-Cárdenas, M.F.; Ramirez-Silva, J.A.; Sanjur, O.; Toro-Perea, N. Evidence of incipient speciation in the Neotropical mangrove Pelliciera rhizophorae (Tetrameristaceae) as revealed by molecular, morphological, physiological and climatic characteristics. Bot. J. Linn. Soc. 2015, 179, 499–510. [Google Scholar] [CrossRef]
- Frederiksen, N. Review of Early Tertiary sporomorph paleoecology. Am. Assoc. Strat. Palynol. Contr. Ser. 1985, 19, 1–92. [Google Scholar]
- Muller, J.; Di Giacomo, E.; Ven Erve, A.W. A palynological zonation for the Cretaceous, Tertiary and Quaternary of Northern South America. Am. Assoc. Strat. Palynol. Contr. Ser. 1987, 19, 7–76. [Google Scholar]
- Jaramillo, C.; Dilcher, D.L. Middle Paleogene palynology of Central Colombia, South America: A study of pollen and spores from tropical latitudes. Palaeontogr. B 2001, 258, 87–213. [Google Scholar] [CrossRef]
- Wiens, J.J.; Graham, C.H. Niche conservatism: Integrating evolution, ecology, and conservation biology. Ann. Rev. Ecol. Evol. Syst. 2005, 36, 519–539. [Google Scholar] [CrossRef]
- Wiens, J.J.; Ackerly, D.D.; Allen, A.P.; Anacker, B.L.; Buckley, L.B.; Cornell, H.V.; Damschen, E.I.; Davies, T.J.; Grytnes, J.-A.; Harrison, S.P.; et al. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 2010, 13, 1310–1324. [Google Scholar] [CrossRef]
- Hadly, E.A.; Spaeth, P.A.; Li, C. Niche conservatism above the species level. Proc. Natl. Acad. Sci. USA 2009, 106, 19707–19714. [Google Scholar] [CrossRef] [PubMed]
- Westerhold, T.; Marwan, N.; Drury, A.J.; Liebrand, D.; Agnini, C.; Anagnostou, E.; Barnet, J.S.K.; Bohaty, S.M.; De Vleeschouwer, D.; Florindo, F.; et al. An astronomically dated record of Earth’s climate and its predictability over the last 66 million years. Science 2020, 369, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Mann, P. Gulf of Mexico, Central America, and the Caribbean. In Encyclopedia of Geology; Alderton, D., Elias, S.A., Eds.; Academic Press: London, UK, 2021; pp. 47–67. [Google Scholar]
- Romito, S.; Mann, P. Tectonic terrains underlying the present-day Caribbean plate: Their tectonic origin, sedimentary thickness, subsidence histories and regional controls on hydrocarbon resources. In The Basins, Orogens, and Evolution of the Southern Gulf of Mexico and Northen Caribbean; Davidson, I., Hull, J.N.F., Pindell, J., Eds.; Geological Society: London, UK, 2020; pp. 343–378. [Google Scholar]
- Rabinowitz, D. Dispersal properties of mangrove propagules. Biotropica 1978, 10, 47–57. [Google Scholar] [CrossRef]
- Van der Stocken, T.; Carroll, D.; Menemenlis, D.; Simard, M.; Koedam, N. Global-scale dispersal and connectivity in mangroves. Proc. Natl. Acad. Sci. USA 2019, 116, 915–922. [Google Scholar] [CrossRef]
- Boucher, D.H.; James, S.; Keeler, K.H. The ecology of mutualism. Ann. Rev. Ecol. Syst. 1982, 13, 315–347. [Google Scholar] [CrossRef]
- Callaway, R.M. Positive interactions among plants (interpreting botanical progress). Bot. Rev. 1995, 61, 306–349. [Google Scholar] [CrossRef]
- Stachowicz, J.J. Mutualism, facilitation, and the structure of ecological communities. BioScience 2001, 51, 235–246. [Google Scholar] [CrossRef]
- Bruno, J.F.; Stachowicz, J.J.; Bertness, M.D. Inclusion of facilitation into ecological theory. Tree 2003, 18, 119–125. [Google Scholar] [CrossRef]
- MacArthur, R.; Levins, R. The limiting of similarity, convergence, and divergence of coexisting species. Am. Natur. 1976, 10, 377–385. [Google Scholar] [CrossRef]
- Violle, C.; Nemergut, D.R.; Pu, Z.; Jiang, L. Phylogenetic limiting similarity and competitive exclusion. Ecol. Lett. 2011, 14, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Kosicki, J.Z. Niche segregation on the landscape scale of two co-existing related congeners in the sympatric zone—Modelling approach. Ecol. Model. 2022, 468, 109960. [Google Scholar] [CrossRef]
- Futuyma, D.J.; Moreno, G. The evolution of ecological specialization. Ann. Rev. Ecol. Syst. 1988, 19, 207–233. [Google Scholar] [CrossRef]
- Pielou, E.C. Biogeography; Wiley & Sons: New York, NY, USA, 1977. [Google Scholar]
- Rull, V. Microrefugia. J. Biogeog. 2009, 36, 481–484. [Google Scholar] [CrossRef]
- Fernández-Palacios, J.M.; Otto, R.; Borregaard, M.K.; Kreft, H.; Price, J.P.; Steinbauer, M.J.; Weigelt, P.; Whittaker, R.J. Evolutionary winners are ecological losers among oceanic island plants. J. Biogeog. 2021, 48, 2186–2198. [Google Scholar] [CrossRef]
- Polidoro, B.A.; Carpenter, K.E.; Collins, L.; Duke, N.C.; Ellison, A.M.; Ellison, J.C.; Farnsworth, E.J.; Fernando, E.S.; Kathiresan, K.; Koedam, N.E.; et al. The loss of species: Mangrove extinction risk and geographic areas of global concern. PLoS ONE 2010, 5, e10095. [Google Scholar] [CrossRef]
- Blanco, J.F.; Estrada, E.A.; Ortiz, L.F.; Urrego, L.E. Ecosystem-wide impacts of deforestation in mangroves: The Urabá Gulf (Colombian Caribbean) case study. ISRN Ecol. 2012, 2012, 958709. [Google Scholar] [CrossRef]
- Bhowmik, A.K.; Padmanaban, R.; Cabral, P.; Romeiras, M.M. Global mangrove deforestation and its interacting social-ecological drivers: A systematic review and synthesis. Sustainability 2022, 14, 4433. [Google Scholar] [CrossRef]
- Blanco-Libreros, J.F.; Ramírez-Ruiz, K. Threatened mangroves in the Anthropocene: Habitat fragmentation in urban coastalscapes of Pelliciera spp. (Tetrameristaceae) in northern South America. Front. Mar. Sci. 2021, 8, 670354. [Google Scholar] [CrossRef]
- Rull, V. Biodiversity crisis or sixth mass extinction? EMBO Rep. 2022, 23, e54193. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).