Induction of Male Sterility by Targeted Mutation of a Restorer-of-Fertility Gene with CRISPR/Cas9-Mediated Genome Editing in Brassica napus L.
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Materials
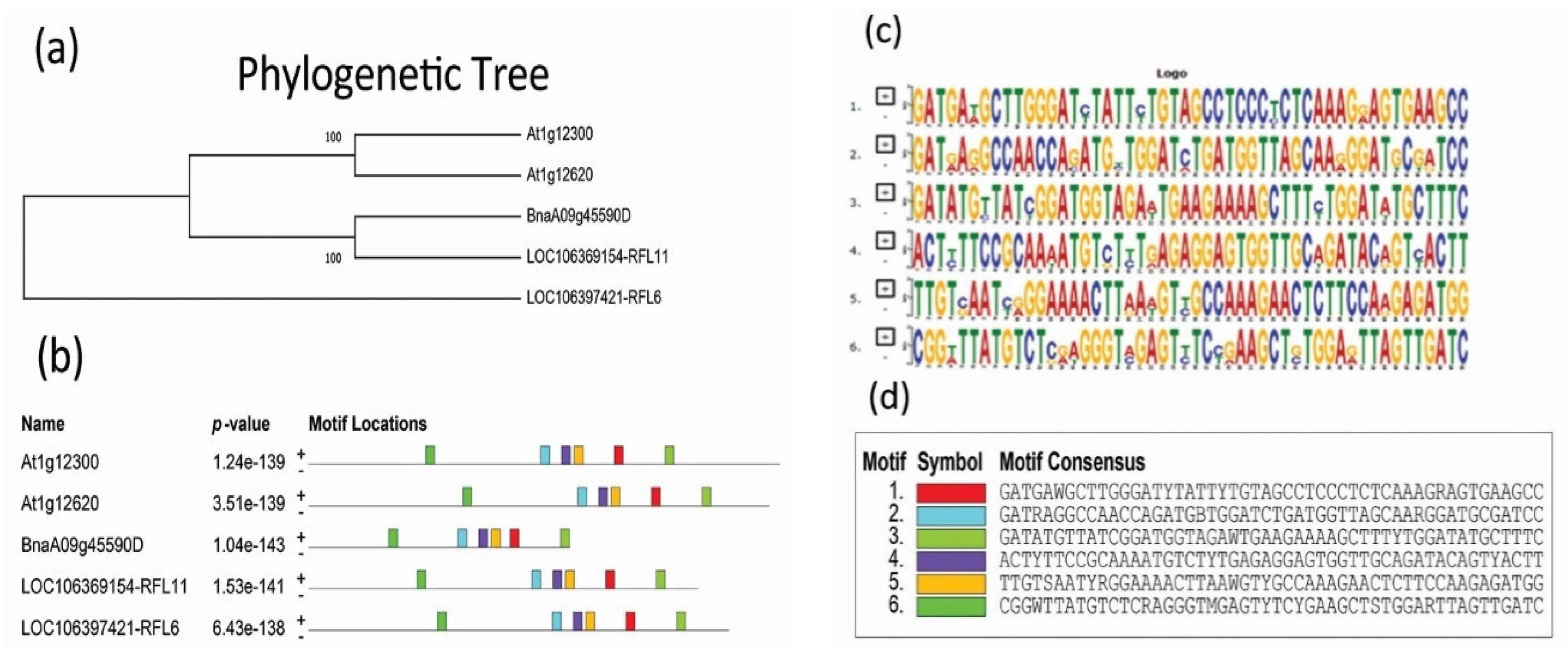
2.2. Phylogenetic and Bioinformatics Analysis
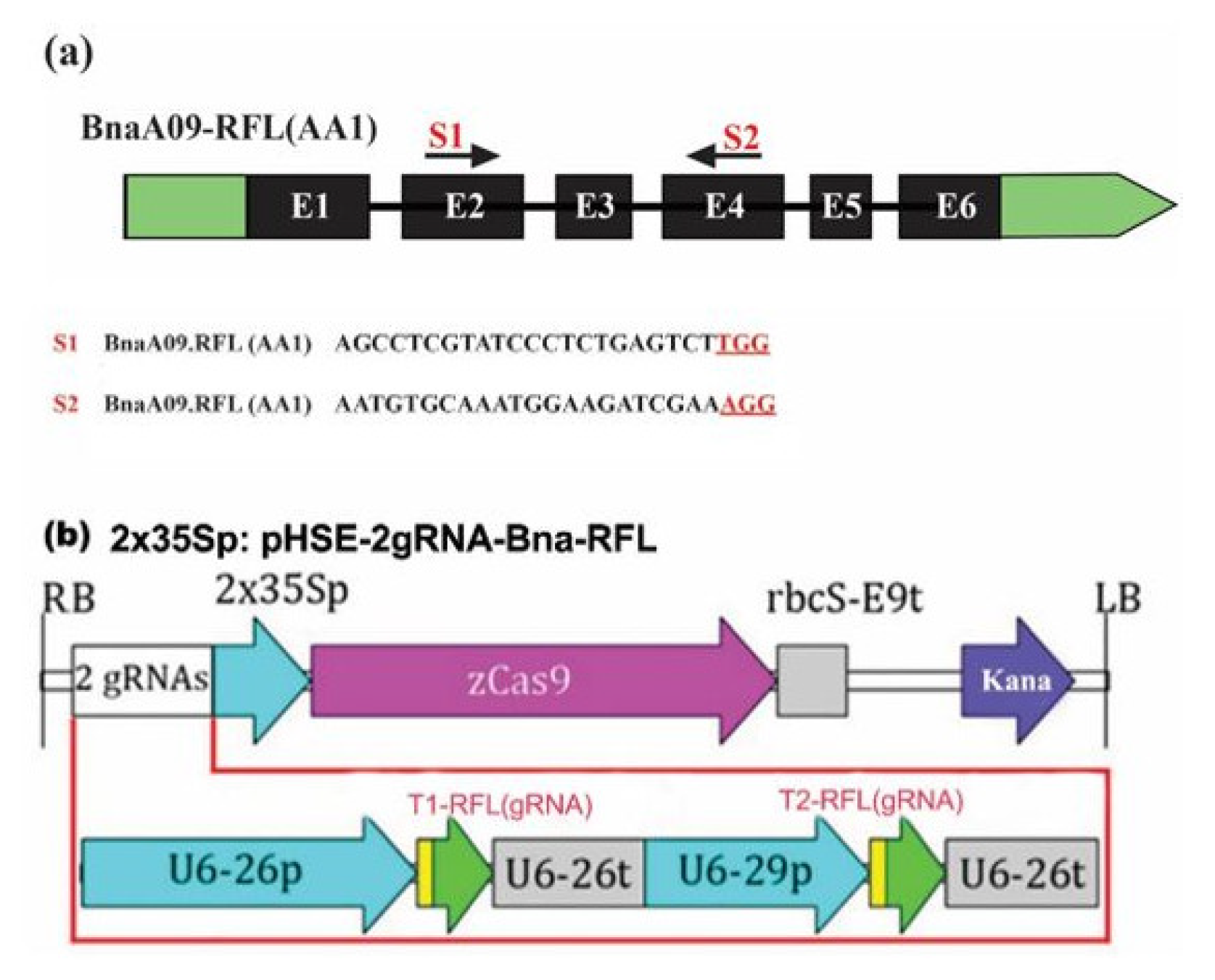
2.3. Computational sgRNA Design and Selection
2.4. Construction of Binary Vectors and Genetic Transformation of Plants
2.5. Identification of Transgenic Mutant Plants and Potential Off-Targets
2.6. RNA Extraction, RT-PCR
2.7. Histological Analyses
2.7.1. Scanning Electron Microscopy Analysis
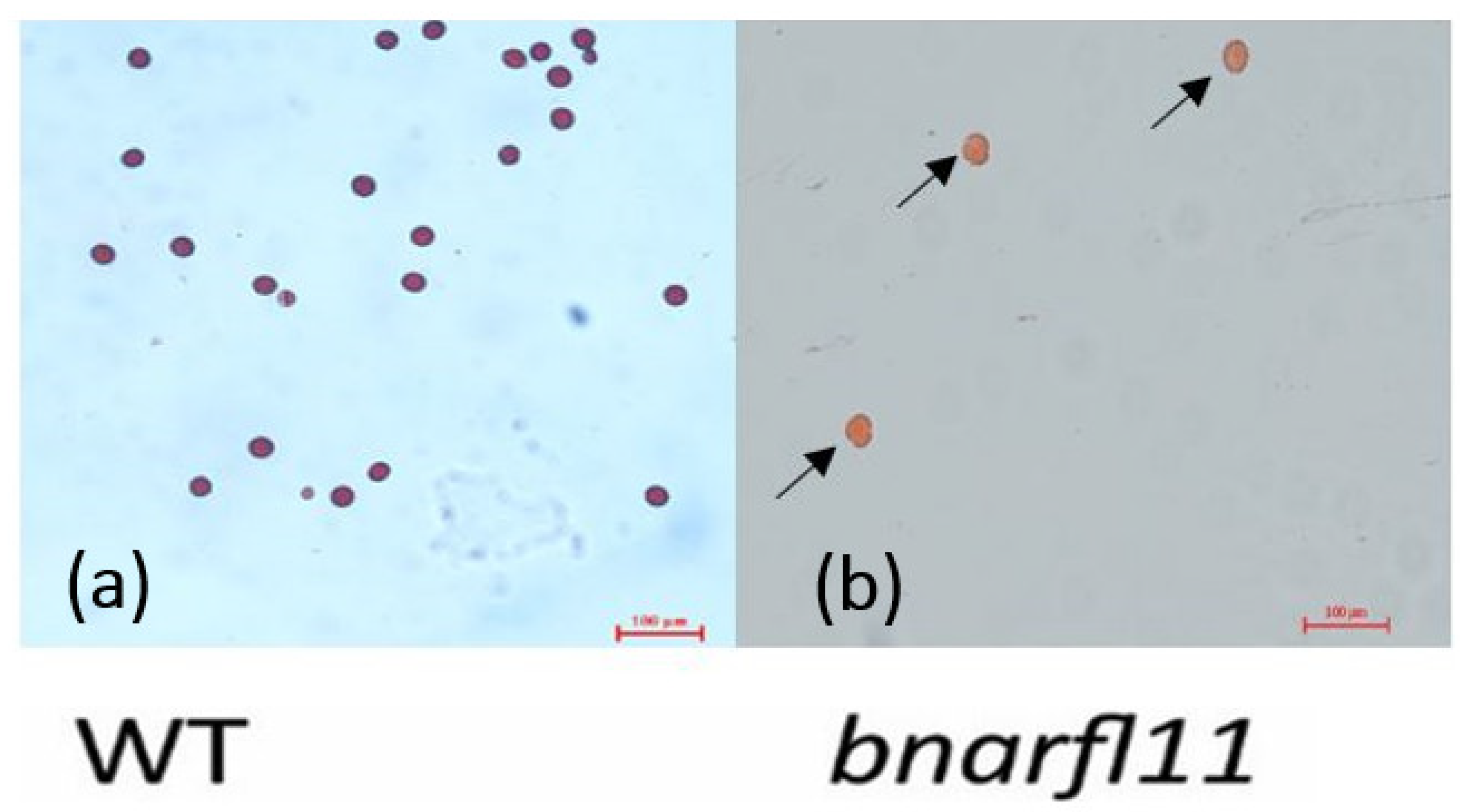
2.7.2. Pollen Fertility Examination
2.7.3. Cytological Analysis
2.8. Field Experiments and Phenotyping
3. Results
3.1. Bioinformatic Analysis of BnaRFL11 Gene in Westar
3.2. CRISPR/Cas9 Vector Construction to Knock out the BnaRFL11 Gene in Rapeseed
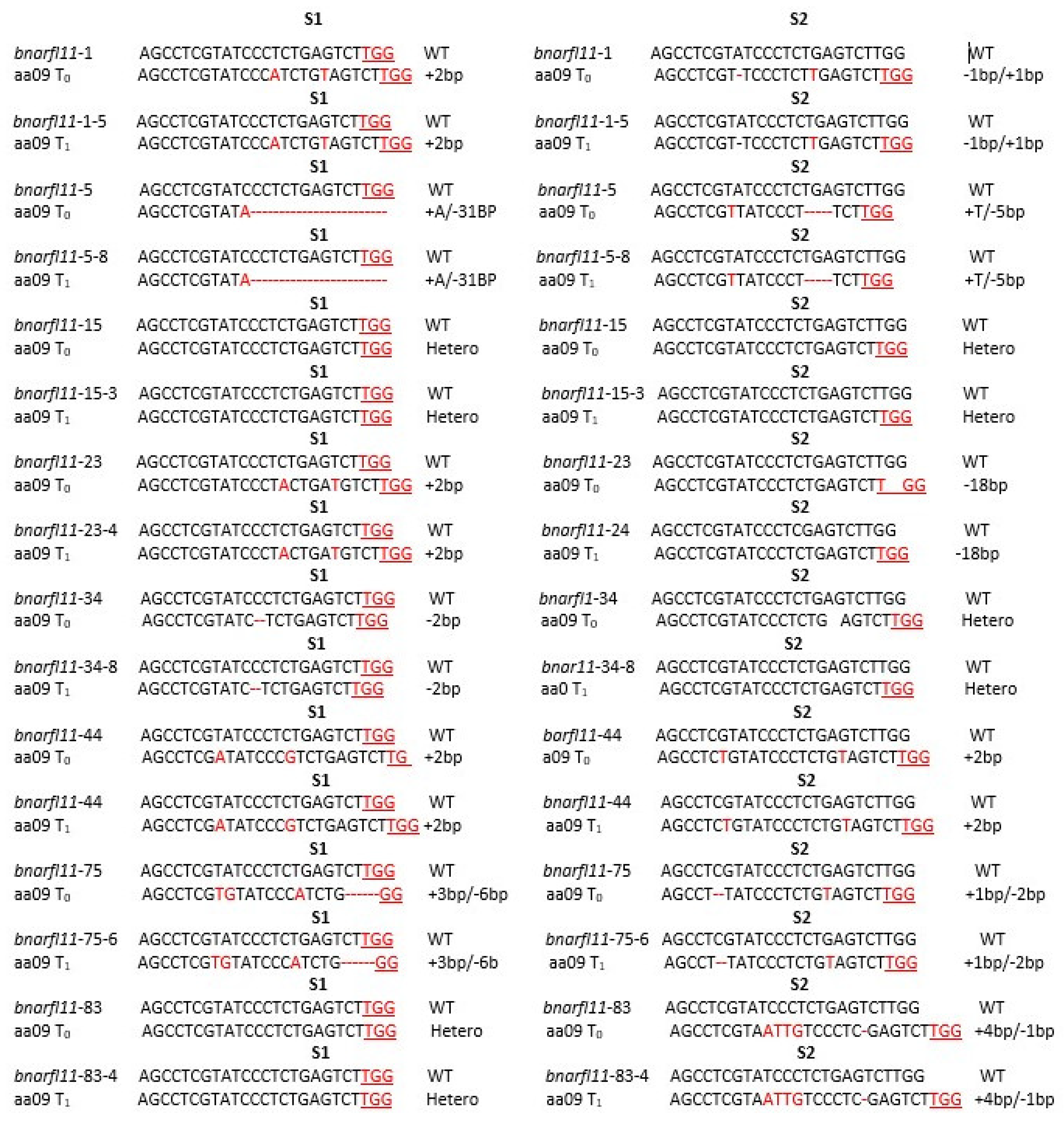
3.3. Identification of Mutation Patterns in the Transgenic Plants
3.4. Isolation of Mutants with Transgenic Elements in T0 and T1 Generations
3.5. CRISPR’s Off-Target Effects on Mutant Lines (T0) in B. napus
3.6. Editing Efficiency of sgRNAs in BnaRFL11
3.7. Morphological Analysis of bnarfl11 Mutant Plants and Pollen Viability Test
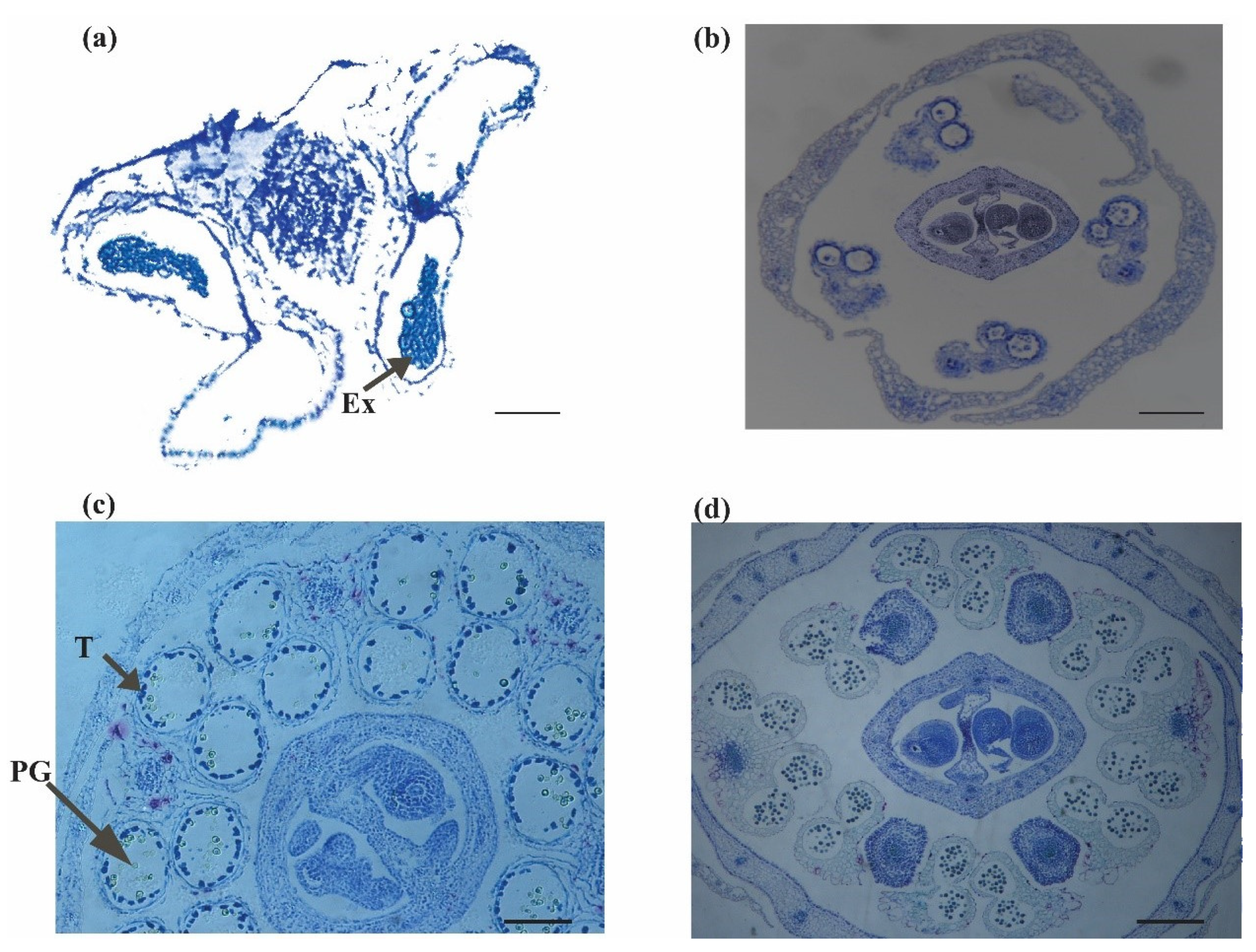
3.8. Cytological Analysis of Anther Abortion in bnarfl11
Development of bnarfl11 Anthers
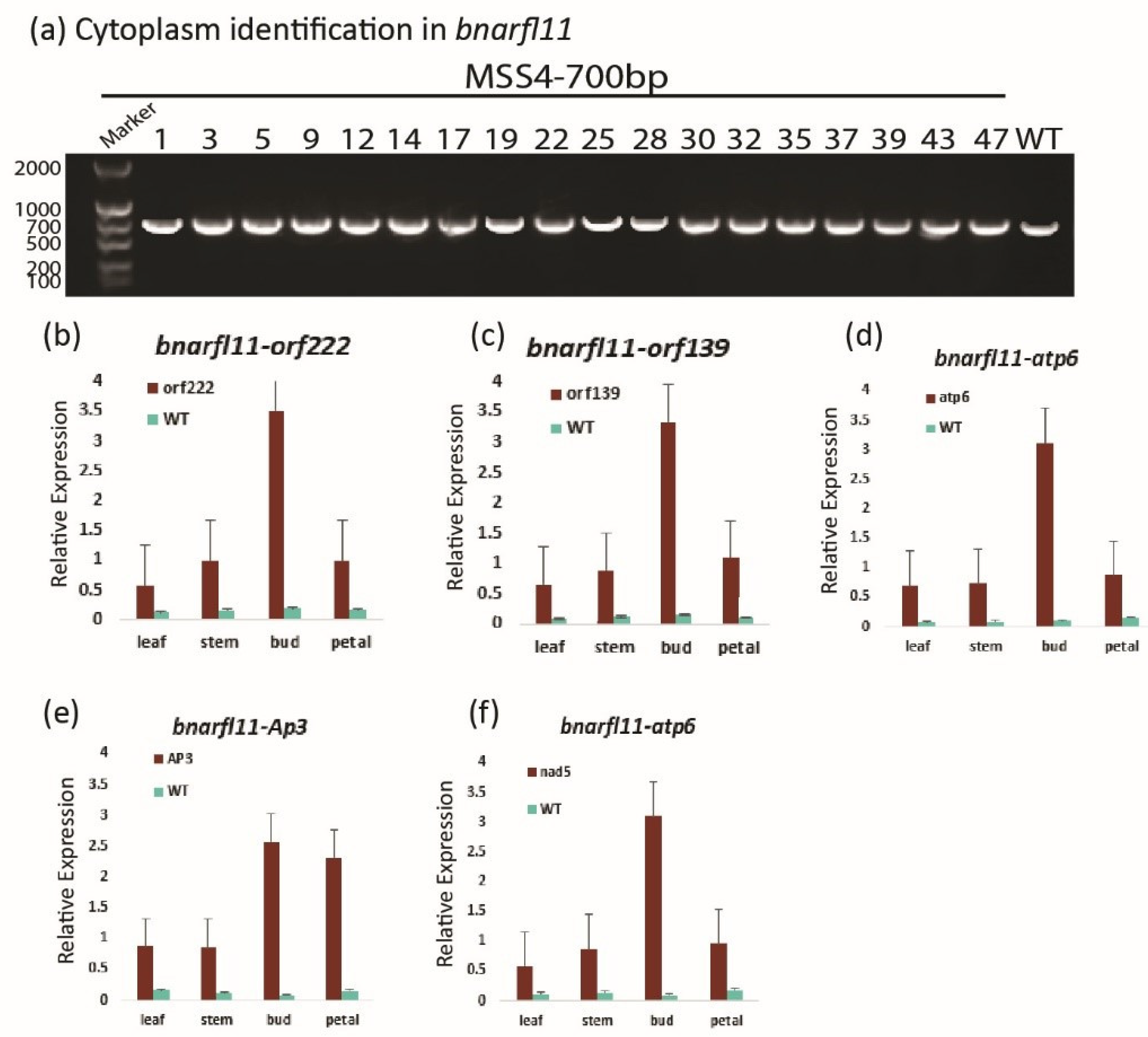
3.9. The Behavior of nap-CMS Causing Gene in bnarfl11
4. Discussion
4.1. In B. napus, the CRISPR/Cas9 System Is Highly Effective in Producing Targeted Mutations
4.2. Precise Identification of Allelic Variation with T.A. Clone following Sequencing
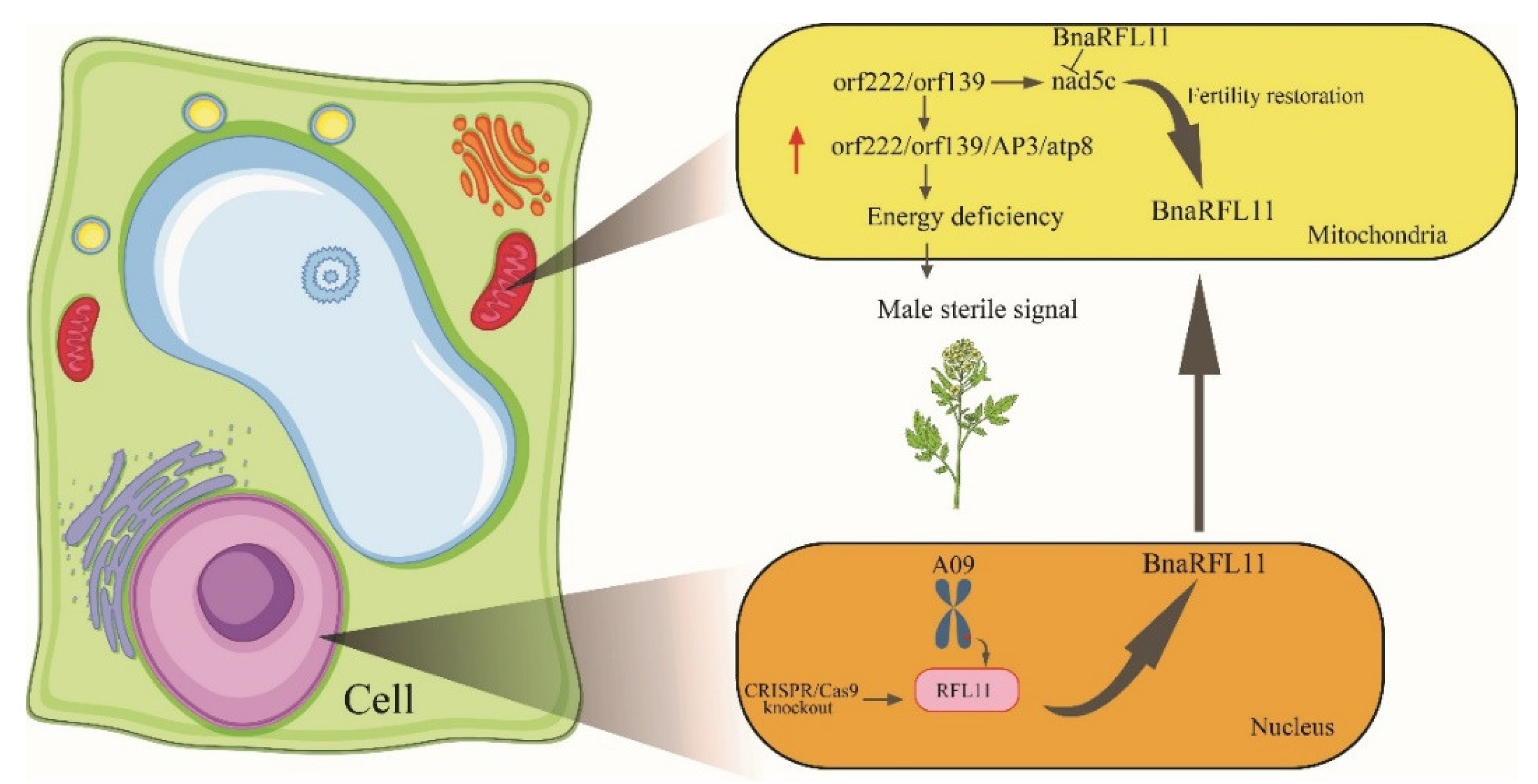
4.3. BnaRFL11 Gene Plays an Essential Role in Fertility Restoration in B. napus
5. Conclusions
- (1)
- In a single copy of bnarfl11, heritable mutant plants develop different mutant lines with a constant mutation transmission in the T0 and T1 generations. Free T-DNA mutant lines were generated across two successive generations.
- (2)
- The phenotypic analysis of bnarfl11 revealed defects in the floral structure, leaf size, branch number, and seed production. The phenotypic analysis showed that the mutant plant lines had male sterility with multiple branches and a reduced leaf size compared to the W.T. plant lines.
- (3)
- To determine whether the off-target effect occurred in this study, we used the “CRISPR-P” software to identify a supposed off-target site in the rapeseed genome that is homologous to the two designed sgRNAs. The locations of potential off-target sites in the genome were noted. According to the sequencing (TA-Clone) of the PCR products, seven possible off-target sites from several T0 mutated plants showed no mutations.
- (4)
- The editing efficiency of the two sgRNAs of the bnarfl11 gene ranged from 0% to 65.10% in T0. We analyzed the editing efficiency of each sgRNA (S1–S2) that targets the bnarfl11 gene.
6. Future Prospective
- (1)
- To further identify the signaling pathway and molecular mechanism of BnaRFL11 in controlling other related genes involved in male sterility.
- (2)
- The development of markers for the polymorphic identification of variations in different mutation sites for CMS breeding in B. napus.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiang, N.T.; Barbetti, M.J.; Jacqueline, B. Current Status and Challenges in Identifying Disease Resistance Genes in Brassica napus. Front. Plant Sci. 2017, 8, 1788. [Google Scholar]
- Van de Wouw, A.P.; Idnurm, A.; Davidson, J.A.; Sprague, S.J.; Khangura, R.K.; Ware, A.H.; Lindbeck, K.D.; Marcroft, S.J. Fungal diseases of canola in Australia: Identification of trends, threats and potential therapies. Australas. Plant Pathol. 2016, 45, 415–423. [Google Scholar] [CrossRef]
- Bharti, S.; Pedro, M.J.; Chrungu, K.B.; Aparajita, M. Chloroplast DNA Variations in Wild Brassicas and Their Implication in Breeding and Population Genetics Studies. Scientifica 2015, 2015, 952395. [Google Scholar]
- Yamagishi, H.; Bhat, S.R. Cytoplasmic male sterility in Brassicaceae crops. Breed. Sci. 2014, 64, 38. [Google Scholar] [CrossRef]
- Yang, L.Y.; Liu, P.W.; Yang, G.S. Development of Polima temperature-sensitive cytoplasmic male sterile lines of Brassica napus through isolated microspore culture. Plant Breed. 2010, 125, 368–371. [Google Scholar] [CrossRef]
- Hompson, K.F. Cytoplasmic male-sterility in oil-seed rape. Heredity 1972, 29, 253–257. [Google Scholar] [CrossRef]
- Ogura, H. Studies on the new male sterility in Japanese radish, with special references on the utilization of this sterility towards the practical raising of hybrid seeds. Mem. Fac. Agric. Kagoshima Univ. 1968, 6, 40–75. [Google Scholar]
- Hu, Q.; Hua, W.; Yin, Y.; Zhang, X.; Liu, L.; Shi, J.; Zhao, Y.; Qin, L.; Chen, C.; Wang, H. Rapeseed research and production in China. Crop J. 2017, 5, 127–135. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Wang, H.; Yu, L.; Li, D. Sequence analysis and expression of orf224 gene associated with two types of cytoplasmic male sterility in Brassica napus L. Z. Nat. C J. Biosci. 2010, 65, 395–402. [Google Scholar] [CrossRef]
- Banga, S.S.; Labana, K.S.; Banga, S.K. Male sterility in Indian mustard (Brassica juncea (L.) Coss.)—A biochemical characterization. Theor. Appl. Genet. 1984, 67, 515–519. [Google Scholar] [CrossRef]
- Liu, J.; Xiang, R.; Wang, W.; Mei, D.; Li, Y.; Mason, A.S.; Fu, L.; Hu, Q. Cytological and molecular analysis of Nsa CMS in Brassica napus L. Euphytica 2015, 206, 279–286. [Google Scholar] [CrossRef]
- Heng, S.; Wei, C.; Jing, B.; Wan, Z.; Wen, J.; Yi, B.; Ma, C.; Tu, J.; Fu, T.; Shen, J. Comparative analysis of mitochondrial genomes between the hau cytoplasmic male sterility (CMS) line and its iso-nuclear maintainer line in Brassica juncea to reveal the origin of the CMS-associated gene orf288. BMC Genom. 2014, 15, 322. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Andersen, S.; Dixelius, C.; Hansen, L. Production of fertile intergeneric somatic hybrids between Brassica napus and Sinapis arvensis for the enrichment of the rapeseed gene pool. Plant Cell Rep. 2002, 21, 147–152. [Google Scholar]
- Li, P.; Kang, L.; Wang, A.; Cui, C.; Li, Z. Development of a Fertility Restorer for inap CMS (Isatis indigotica) Brassica napus through Genetic Introgression of One Alien Addition. Front. Plant Sci. 2019, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Castandet, B.; Araya, A. The nucleocytoplasmic conflict, a driving force for the emergence of plant organellar RNA editing. IUBMB Life 2012, 64, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Barkan, A.; Small, I. Pentatricopeptide Repeat Proteins in Plants. Annu. Rev. Plant Biol. 2014, 65, 415–442. [Google Scholar] [CrossRef] [PubMed]
- Bentolila, S.; Alfonso, A.A.; Hanson, M.R. A pentatricopeptide repeat-containing gene restores fertility to cytoplasmic male-sterile plants. Proc. Natl. Acad. Sci. USA 2002, 99, 10887–10892. [Google Scholar] [CrossRef]
- Akagi, H.; Nakamura, A.; Yokozeki-Misono, Y.; Inagaki, A.; Takahashi, H.; Mori, K.; Fujimura, T. Positional cloning of the rice Rf-1 gene, a restorer of BT-type cytoplasmic male sterility that encodes a mitochondria-targeting PPR protein. Theor. Appl. Genet. 2004, 108, 1449–1457. [Google Scholar] [CrossRef]
- Fujii, S.; Kazama, T.; Ito, Y.; Kojima, S.; Toriyama, K. A candidate factor that interacts with RF2, a restorer of fertility of Lead rice-type cytoplasmic male sterility in rice. Rice 2014, 7, 21. [Google Scholar] [CrossRef]
- Brown, G.G.; Formanová, N.; Jin, H.; Wargachuk, R.; Dendy, C.; Patil, P.; Laforest, M.; Zhang, J.; Cheung, W.Y.; Landry, B.S. The radish Rfo restorer gene of Ogura cytoplasmic male sterility encodes a protein with multiple pentatricopeptide repeats. Plant J. 2003, 35, 262–272. [Google Scholar] [CrossRef]
- Desloire, S.; Gherbi, H.; Laloui, W.; Marhadour, S.; Clouet, V.; Cattolico, L.; Falentin, C.; Giancola, S.; Renard, M.; Budar, F. Identification of the fertility restoration locus, Rfo, in radish, as a member of the pentatricopeptide-repeat protein family. EMBO Rep. 2003, 4, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, P.; Mullet, J.; Minx, P.; Rooney, W.; Schertz, K. Fertility restorer locus Rf1 of sorghum (Sorghum bicolor L.) encodes a pentatricopeptide repeat protein not present in the colinear region of rice chromosome 12. Theor. Appl. Genet. 2005, 111, 994–1012. [Google Scholar] [CrossRef] [PubMed]
- Heng, S.; Chen, F.; Wei, C.; Hu, K.; Yang, Z.; Wen, J.; Yi, B.; Ma, C.; Tu, J.; Si, P.; et al. identification of different cytoplasms based on newly developed mitotype-specific markers for marker-assisted selection breeding in Brassica napus L. Plant Cell Rep. 2017, 36, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Zhang, D. Molecular Control of Male Fertility for Crop Hybrid Breeding. Trends Plant Sci. 2018, 23, 53–65. [Google Scholar] [CrossRef]
- Pahwa, R.; Banga, S.; Gogna, K.; Banga, S. Tournefortii male sterility system in Brassica napus. Identification, expression and genetic characterization of male fertility restorers. Plant Breed. 2004, 123, 444–448. [Google Scholar] [CrossRef]
- Kang, L.; Li, P.; Wang, A.; Ge, X.; Li, Z. A Novel Cytoplasmic Male Sterility in Brassica napus (inap CMS) with Carpelloid Stamens via Protoplast Fusion with Chinese Woad. Front. Plant Sci. 2017, 8, 529. [Google Scholar] [CrossRef]
- Iwabuchi, M.; Koizuka, N.; Fujimoto, H.; Sakai, T.; Imamura, J. Identification and expression of the kosena radish (Raphanus sativus cv. Kosena) homologue of the ogura radish CMS-associated gene, orf138. Plant Mol. Biol. 1999, 39, 183–188. [Google Scholar] [CrossRef]
- Sang, S.-F.; Mei, D.-S.; Liu, J.; Zaman, Q.U.; Zhang, H.-Y.; Hao, M.-Y.; Fu, L.; Wang, H.; Cheng, H.-T.; Hu, Q. Organelle genome composition and candidate gene Identification for Nsa cytoplasmic male sterility in Brassica napus. BMC Genom. 2019, 20, 813. [Google Scholar] [CrossRef]
- Ning, L.; Wang, H.; Li, D.; Li, Y.; Chen, K.; Chao, H.; Li, H.; He, J.; Li, M. Genome-wide identification of the restorer-of-fertility-like (RFL) gene family in Brassica napus and expression analysis in Shaan2A cytoplasmic male sterility. BMC Genom. 2020, 21, 765. [Google Scholar] [CrossRef]
- Melonek, J.; Stone, J.D.; Small, I. Evolutionary plasticity of restorer-of-fertility-like proteins in rice. Sci. Rep. 2016, 6, 35152. [Google Scholar] [CrossRef]
- Sykes, T.; Yates, S.; Nagy, I.; Asp, T.; Small, I.; Studer, B. In silico identification of candidate genes for fertility restoration in cytoplasmic male sterile perennial ryegrass (Lolium perenne L.). Genome Biol. Evol. 2017, 9, 351–362. [Google Scholar] [PubMed]
- Kazama, T.; Nakamura, T.; Watanabe, M.; Sugita, M.; Toriyama, K. Suppression mechanism of mitochondrial ORF79 accumulation by Rf1 protein in BT-type cytoplasmic male sterile rice. Plant J. 2008, 55, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Uyttewaal, M.; Arnal, N.; Quadrado, M.; Martin-Canadell, A.; Vrielynck, N.; Hiard, S.; Gherbi, H.; Bendahmane, A.; Budar, F.; Mireau, H. Characterization of Raphanus sativus pentatricopeptide repeat proteins encoded by the fertility restorer locus for Ogura cytoplasmic male sterility. Plant Cell 2008, 20, 3331–3345. [Google Scholar] [CrossRef] [PubMed]
- Barr, C.M.; Fishman, L. The nuclear component of a cytonuclear hybrid incompatibility in Mimulus maps to a cluster of pentatricopeptide repeat genes. Genetics 2010, 184, 455–465. [Google Scholar] [CrossRef]
- Fujii, S.; Yamada, M.; Fujita, M.; Itabashi, E.; Hamada, K.; Yano, K.; Kurata, N.; Toriyama, K. Cytoplasmic–nuclear genomic barriers in rice pollen development revealed by comparison of global gene expression profiles among five independent cytoplasmic male sterile lines. Plant Cell Physiol. 2010, 51, 610–620. [Google Scholar] [CrossRef]
- Luo, D.; Xu, H.; Liu, Z.; Guo, J.; Li, H.; Chen, L.; Fang, C.; Zhang, Q.; Bai, M.; Yao, N. A detrimental mitochondrial-nuclear interaction causes cytoplasmic male sterility in rice. Nat. Genet. 2013, 45, 573–577. [Google Scholar] [CrossRef]
- Meyer, J.; Pei, D.; Wise, R.P. Rf8-mediated T-urf13 transcript accumulation coincides with a pentatricopeptide repeat cluster on maize chromosome 2L. Plant Genome 2011, 4. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Z.; Wang, X.; Li, K.; An, H.; Liu, J.; Yang, G.; Fu, T.; Yi, B.; Hong, D. A Mitochondria-Targeted PPR Protein Restores pol Cytoplasmic Male Sterility by Reducing orf224 Transcript Levels in Oilseed Rape. Mol. Plant 2016, 9, 1082–1084. [Google Scholar] [CrossRef]
- Liu, X.-Q.; Liu, Z.-Q.; Yu, C.-Y.; Dong, J.-G.; Hu, S.-W.; Xu, A.-X. TGMS in rapeseed (Brassica napus) resulted in aberrant transcriptional regulation, asynchronous microsporocyte meiosis, defective tapetum, and fused sexine. Front. Plant Sci. 2017, 8, 1268. [Google Scholar] [CrossRef]
- Zhai, Y.; Yu, K.; Cai, S.; Hu, L.; Amoo, O.; Xu, L.; Yang, Y.; Ma, B.; Jiao, Y.; Zhang, C. Targeted mutagenesis of BnTT8 homologs controls yellow seed coat development for effective oil production in Brassica napus L. Plant Biotechnol. J. 2020, 18, 1153–1168. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ding, Y.; Zhou, Y.; Jin, W.; Xie, K.; Chen, L.L. CRISPR-P 2.0: An improved CRISPR-Cas9 tool for genome editing in plants. Mol. Plant 2017, 10, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.D.; Scott, D.A.; Weinstein, J.A.; Ran, F.; Konermann, S.; Agarwala, V.; Li, Y.; Fine, E.J.; Wu, X.; Shalem, O. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 2013, 31, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.-L.; Dong, L.; Wang, Z.-P.; Zhang, H.-Y.; Han, C.-Y.; Liu, B.; Wang, X.-C.; Chen, Q.-J. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 2014, 14, 327. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, H.; Gilmer, S.; Whitwill, S.; Keller, W.; Fowke, L. Control of petal and pollen development by the plant cyclin-dependent kinase inhibitor ICK1 in transgenic Brassica plants. Planta 2002, 215, 248–257. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, Y.; Yu, S.; Lu, L.; Ding, M.; Cheng, J.; Song, G.; Gao, X.; Yao, L.; Fan, D. An efficient genotyping method for genome-modified animals and human cells generated with CRISPR/Cas9 system. Sci. Rep. 2014, 4, 6420. [Google Scholar] [CrossRef]
- Peng, Y.; Shi, D.; Zhang, T.; Li, X.; Fu, T.; Xu, Y.; Wan, Z. Development and utilization of an efficient cytoplasmic male sterile system for Cai-xin (Brassica rapa L.). Sci. Hortic. 2015, 190, 36–42. [Google Scholar] [CrossRef]
- Fujii, S.; Suzuki, T.; Giegé, P.; Higashiyama, T.; Koizuka, N.; Shikanai, T. The Restorer-of-fertility-like 2 pentatricopeptide repeat protein and R.N. ase P are required for the processing of mitochondrial orf291 RNA in Arabidopsis. Plant J. 2016, 86, 504–513. [Google Scholar] [CrossRef]
- Bohra, A.; Jha, U.C.; Adhimoolam, P.; Bisht, D.; Singh, N.P. Cytoplasmic male sterility (CMS) in hybrid breeding in field crops. Plant Cell Rep. 2016, 35, 967–993. [Google Scholar] [CrossRef]
- Lei, Y.; Lu, L.; Liu, H.-Y.; Li, S.; Xing, F.; Chen, L.-L. CRISPR-P: A web tool for synthetic single-guide RNA design of CRISPR-system in plants. Mol. Plant 2014, 7, 1494–1496. [Google Scholar] [CrossRef]
- Hussain, B.; Lucas, S.J.; Budak, H. CRISPR/Cas9 in plants: At play in the genome and at work for crop improvement. Brief. Funct. Genom. 2018, 17, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Sander, J.D.; Joung, J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014, 32, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, Q.; Zhu, Q.; Liu, W.; Chen, Y.; Qiu, R.; Wang, B.; Yang, Z.; Li, H.; Lin, Y. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant 2015, 8, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, K.; Li, H.; Han, S.; Meng, Q.; Khan, S.U.; Fan, C.; Xie, K.; Zhou, Y. Precise editing of CLAVATA genes in Brassica napus L. regulates multilocular silique development. Plant Biotechnol. J. 2018, 16, 1322–1335. [Google Scholar] [CrossRef] [PubMed]
- Jonietz, C.; Forner, J.; Hildebrandt, T.; Binder, S. RNA PROCESSING FACTOR3 is crucial for the accumulation of mature ccmC transcripts in mitochondria of Arabidopsis accession Columbia. Plant Physiol. 2011, 157, 1430–1439. [Google Scholar] [CrossRef]
- Fujii, S.; Bond, C.S.; Small, I.D. Selection patterns on restorer-like genes reveal a conflict between nuclear and mitochondrial genomes throughout angiosperm evolution. Proc. Natl. Acad. Sci. USA 2011, 108, 1723–1728. [Google Scholar] [CrossRef]
- Schleicher, S.; Binder, S. In Arabidopsis thaliana mitochondria 5’ end polymorphisms of nad4L-atp4 and nad3-rps12 transcripts are linked to RNA PROCESSING FACTORs 1 and 8. Plant Mol. Biol. 2021, 106, 335–348. [Google Scholar] [CrossRef]
- Gutmann, B.; Gobert, A.; Giegé, P. PRORP proteins support RNase P activity in both organelles and the nucleus in Arabidopsis. Genes Dev. 2012, 26, 1022–1027. [Google Scholar] [CrossRef]
- Geddy, R.; Mahé, L.; Brown, G.G. Cell-specific regulation of a Brassica napus CMS-associated gene by a nuclear restorer with related effects on a floral homeotic gene promoter. Plant J. 2005, 41, 333–345. [Google Scholar] [CrossRef]
- Sabar, M.; Gagliardi, D.; Balk, J.; Leaver, C.J. ORFB is a subunit of F1 FO-ATP synthase: Insight into the basis of cytoplasmic male sterility in sunflower. EMBO Rep. 2003, 4, 381–386. [Google Scholar] [CrossRef]
- Devenish, R.J.; Prescott, M.; Roucou, X.; Nagley, P. Insights into ATP synthase assembly and function through the molecular genetic manipulation of subunits of the yeast mitochondrial enzyme complex. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2000, 1458, 428–442. [Google Scholar] [CrossRef]





| Target Gene | Number of Targets | Generation | Transgene Rate (%) | Positive Rate (%) |
|---|---|---|---|---|
| BnaRFL11 | 2 | T0 | 81% (88/108) | 89% (97/108) |
| 2 | T1 | 75% (110/145) | 90% (131/145) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, Z.; Nouman Riaz, M.; Farooq, M.S.; Li, Y.; Wang, H.; Ahmad, M.; Tu, J.; Ma, C.; Dai, C.; Wen, J.; et al. Induction of Male Sterility by Targeted Mutation of a Restorer-of-Fertility Gene with CRISPR/Cas9-Mediated Genome Editing in Brassica napus L. Plants 2022, 11, 3501. https://doi.org/10.3390/plants11243501
Farooq Z, Nouman Riaz M, Farooq MS, Li Y, Wang H, Ahmad M, Tu J, Ma C, Dai C, Wen J, et al. Induction of Male Sterility by Targeted Mutation of a Restorer-of-Fertility Gene with CRISPR/Cas9-Mediated Genome Editing in Brassica napus L. Plants. 2022; 11(24):3501. https://doi.org/10.3390/plants11243501
Chicago/Turabian StyleFarooq, Zunaira, Muhammad Nouman Riaz, Muhammad Shoaib Farooq, Yifan Li, Huadong Wang, Mayra Ahmad, Jinxing Tu, Chaozhi Ma, Cheng Dai, Jing Wen, and et al. 2022. "Induction of Male Sterility by Targeted Mutation of a Restorer-of-Fertility Gene with CRISPR/Cas9-Mediated Genome Editing in Brassica napus L." Plants 11, no. 24: 3501. https://doi.org/10.3390/plants11243501
APA StyleFarooq, Z., Nouman Riaz, M., Farooq, M. S., Li, Y., Wang, H., Ahmad, M., Tu, J., Ma, C., Dai, C., Wen, J., Shen, J., Fu, T., Yang, S., Wang, B., & Yi, B. (2022). Induction of Male Sterility by Targeted Mutation of a Restorer-of-Fertility Gene with CRISPR/Cas9-Mediated Genome Editing in Brassica napus L. Plants, 11(24), 3501. https://doi.org/10.3390/plants11243501









