Are Foliar Nutrition Status and Indicators of Oxidative Stress Associated with Tree Defoliation of Four Mediterranean Forest Species?
Abstract
1. Introduction
2. Results and Discussion
2.1. Foliar Nutrition Status of Four Selected Mediterranean Species
2.2. Content of Chl, H2O2, and MDA in Four Selected Mediterranean Species
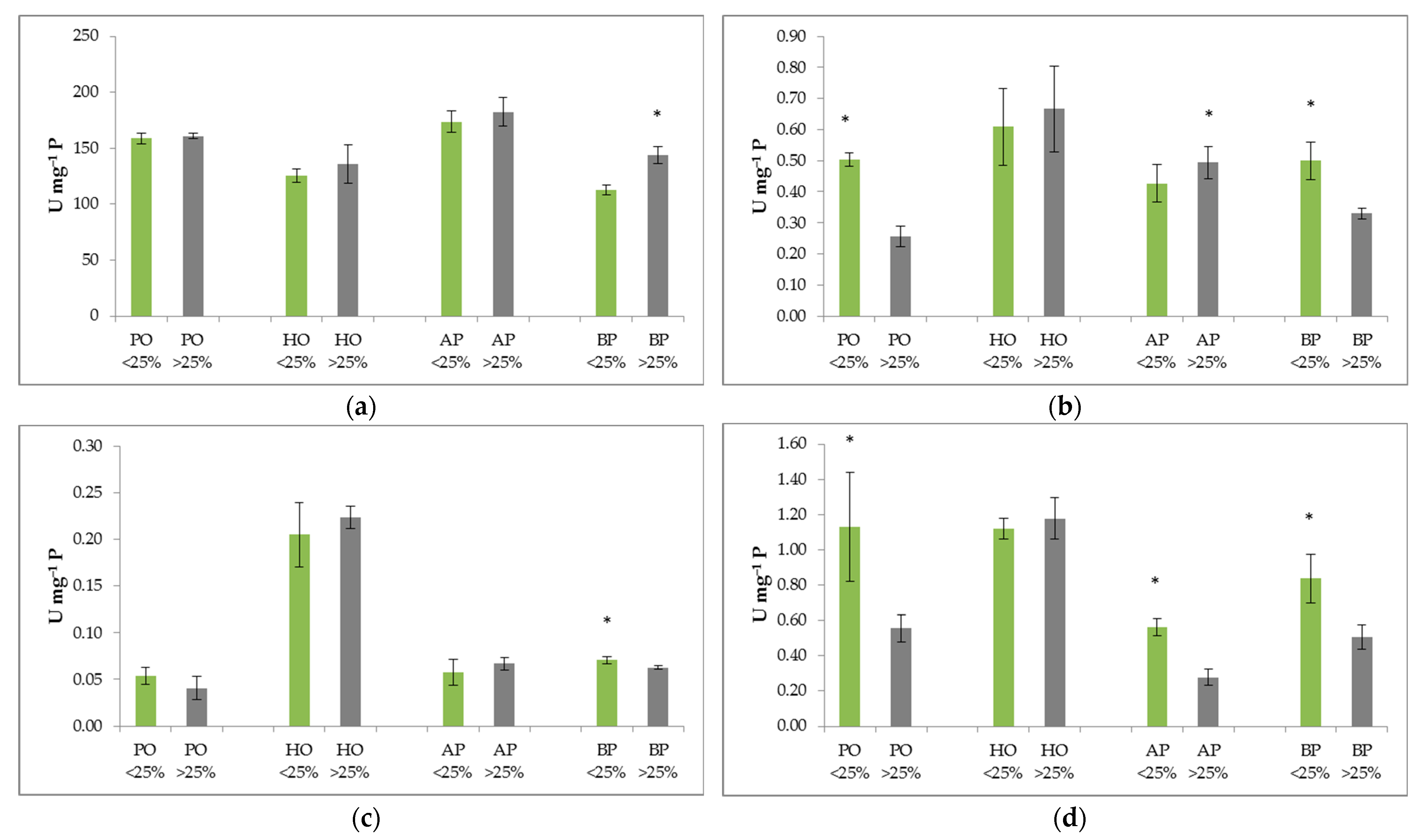
2.3. Activity of Antioxidative Enzymes in Four Selected Mediterranean Species
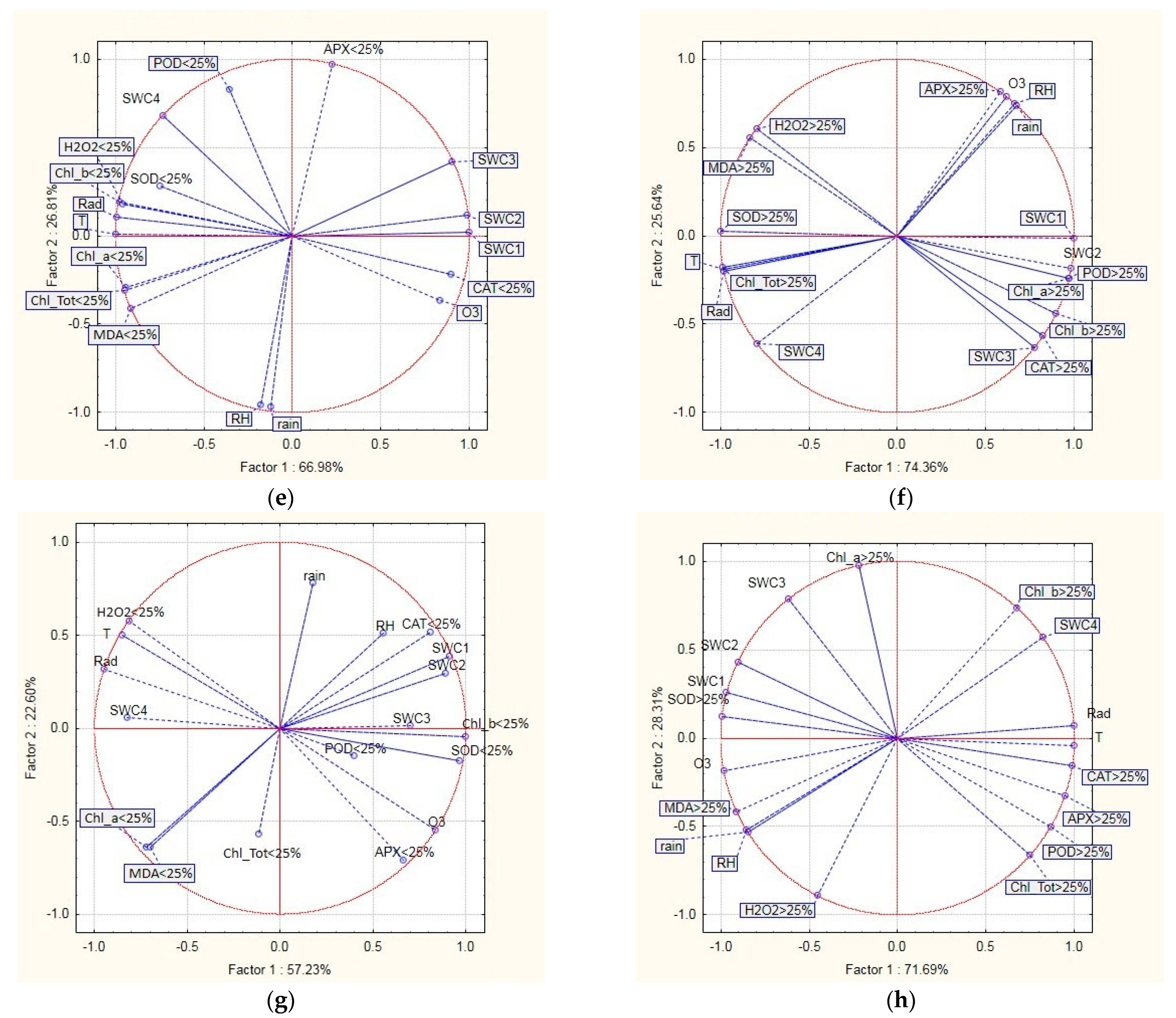
2.4. Relationship between Defoliation, Oxidative Stress Indicators, and Environmental Variables
3. Materials and Methods
3.1. Selected Plots
3.2. Crown Condition Assessment and Collection of Leaves and Needles
3.3. Foliar Nutrition Analysis
3.4. Estimation of Chlorophylls
3.5. Evaluation of Lipid Peroxidation (LPO) and Hydrogen Peroxide (H2O2)
3.6. Antioxidant Enzyme Extraction and Assay
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuttelod, A.; García, N.; Malak, D.A.; Temple, H.J.; Katariya, V. The Mediterranean: A Biodiversity Hotspot under Threat. In Wildlife in a Changing World—An Analysis of the 2008 IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2009; Volume 89, p. 9. [Google Scholar]
- Guidi, L.; Remorini, D.; Cotrozzi, L.; Giordani, T.; Lorenzini, G.; Massai, R.; Nali, C.; Natali, L.; Pellegrini, E.; Trivellini, A.; et al. The Harsh Life of an Urban Tree: The Effect of a Single Pulse of Ozone in Salt-Stressed Quercus ilex Saplings. Tree Physiol. 2017, 37, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Sicard, P.; Dalstein-Richier, L. Health and Vitality Assessment of Two Common Pine Species in the Context of Climate Change in Southern Europe. Environ. Res. 2015, 137, 235–245. [Google Scholar] [CrossRef] [PubMed]
- de Vries, W.; Dobbertin, M.H.; Solberg, S.; van Dobben, H.F.; Schaub, M. Impacts of Acid Deposition, Ozone Exposure and Weather Conditions on Forest Ecosystems in Europe: An Overview. Plant Soil 2014, 380, 1–45. [Google Scholar] [CrossRef]
- Šiljković, Ž.; Mamut, M. Forest Fires in Dalmatia. Bull. Geogr. Socio-Econ. Ser. 2016, 32, 117–130. [Google Scholar] [CrossRef]
- Anav, A.; De Marco, A.; Friedlingstein, P.; Savi, F.; Sicard, P.; Sitch, S.; Vitale, M.; Paoletti, E. Growing Season Extension Affects Ozone Uptake by European Forests. Sci. Total Environ. 2019, 669, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Jakovljević, T.; Lovreškov, L.; Jelić, G.; Anav, A.; Popa, I.; Fornasier, M.F.; Proietti, C.; Limić, I.; Butorac, L.; Vitale, M.; et al. Impact of Ground-Level Ozone on Mediterranean Forest Ecosystems Health. Sci. Total Environ. 2021, 783, 147063. [Google Scholar] [CrossRef]
- Jakovljević, T.; Marchetto, A.; Lovreškov, L.; Potočić, N.; Seletković, I.; Indir, K.; Jelić, G.; Butorac, L.; Zgrablić, Ž.; De Marco, A.; et al. Assessment of Atmospheric Deposition and Vitality Indicators in Mediterranean Forest Ecosystems. Sustainability 2019, 11, 6805. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A Global Overview of Drought and Heat-Induced Tree Mortality Reveals Emerging Climate Change Risks for Forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Proietti, C.; Anav, A.; De Marco, A.; Sicard, P.; Vitale, M. A Multi-Sites Analysis on the Ozone Effects on Gross Primary Production of European Forests. Sci. Total Environ. 2016, 556, 1–11. [Google Scholar] [CrossRef]
- Zhang, L.; Hoshika, Y.; Carrari, E.; Cotrozzi, L.; Pellegrini, E.; Paoletti, E. Effects of Nitrogen and Phosphorus Imbalance on Photosynthetic Traits of Poplar Oxford Clone under Ozone Pollution. J. Plant Res. 2018, 131, 915–924. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Remorini, D.; Pellegrini, E.; Landi, M.; Massai, R.; Nali, C.; Guidi, L.; Lorenzini, G. Variations in Physiological and Biochemical Traits of Oak Seedlings Grown under Drought and Ozone Stress. Physiol. Plant. 2016, 157, 69–84. [Google Scholar] [CrossRef] [PubMed]
- De Marco, A.; Proietti, C.; Anav, A.; Ciancarella, L.; D’Elia, I.; Fares, S.; Fornasier, M.F.; Fusaro, L.; Gualtieri, M.; Manes, F.; et al. Impacts of Air Pollution on Human and Ecosystem Health, and Implications for the National Emission Ceilings Directive: Insights from Italy. Environ. Int. 2019, 125, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Sicard, P.; Augustaitis, A.; Belyazid, S.; Calfapietra, C.; de Marco, A.; Fenn, M.; Bytnerowicz, A.; Grulke, N.; He, S.; Matyssek, R.; et al. Global Topics and Novel Approaches in the Study of Air Pollution, Climate Change and Forest Ecosystems. Environ. Pollut. 2016, 213, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Elvira, S.; González-Fernández, I.; Calvete, H.; García-Gómez, H.; Bermejo, V. Drought Stress Does Not Protect Quercus ilex L. from Ozone Effects: Results from a Comparative Study of Two Subspecies Differing in Ozone Sensitivity. Plant Biol. 2014, 16, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Proietti, C.; Fornasier, M.F.; Sicard, P.; Anav, A.; Paoletti, E.; De Marco, A. Trends in Tropospheric Ozone Concentrations and Forest Impact Metrics in Europe over the Time Period 2000–2014. J. For. Res. 2021, 32, 543–551. [Google Scholar] [CrossRef]
- Ferretti, M.; Fischer, R. Forest Monitoring: Methods for Terrestrial Investigations in Europe with an Overview of North America and Asia; Ferretti, M., Fischer, R., Eds.; Newnes: Oxford, UK, 2013. [Google Scholar]
- Eichhorn, J.; Roskams, P.; Potočic, N.; Timmermann, V.; Ferretti, M.; Mues, V.; Szepesi, A.; Durrant, D.; Seletković, I.; Schröck, H.; et al. Part IV: Visual Assessment of Crown Condition and Damaging Agents. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2020; p. 49 + Annex. [Google Scholar]
- Landmann, G. Forest Decline and Air Pollution Effects in the French Mountains: A Synthesis. In Forest Decline and Atmospheric Deposition Effects in the French Mountains; Springer: Berlin/Heidelberg, Germany, 1995; pp. 407–452. [Google Scholar]
- Johnson, J.; Jacob, M. Monitoring the Effects of Air Pollution on Forest Condition in Europe: Is Crown Defoliation an Adequate Indicator? iForest-Biogeosci. For. 2010, 3, 86–88. [Google Scholar] [CrossRef]
- Le Roncé, I.; Toïgo, M.; Dardevet, E.; Venner, S.; Limousin, J.-M.; Chuine, I. Resource Manipulation through Experimental Defoliation Has Legacy Effects on Allocation to Reproductive and Vegetative Organs in Quercus ilex. Ann. Bot. 2020, 126, 1165–1179. [Google Scholar] [CrossRef]
- Braun, S.; Schindler, C.; Rihm, B. Foliar Nutrient Concentrations of European Beech in Switzerland: Relations with Nitrogen Deposition, Ozone, Climate and Soil Chemistry. Front. For. Glob. Chang. 2020, 3, 33. [Google Scholar] [CrossRef]
- Wang, N.; Fu, F.; Wang, H.; Wang, P.; He, S.; Shao, H.; Ni, Z.; Zhang, X. Effects of Irrigation and Nitrogen on Chlorophyll Content, Dry Matter and Nitrogen Accumulation in Sugar Beet (Beta vulgaris L.). Sci. Rep. 2021, 11, 16651. [Google Scholar] [CrossRef]
- Tewari, R.K.; Yadav, N.; Gupta, R.; Kumar, P. Oxidative Stress Under Macronutrient Deficiency in Plants. J. Soil Sci. Plant Nutr. 2021, 21, 832–859. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, M.A. Plant Physiology, Development and Metabolism; Springer: New Delhi, India, 2018; ISBN 9811320233. [Google Scholar]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium Deficiency in Plants: An Urgent Problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- Jonard, M.; Fürst, A.; Verstraeten, A.; Thimonier, A.; Timmermann, V.; Potočić, N.; Waldner, P.; Benham, S.; Hansen, K.; Merilä, P.; et al. Tree Mineral Nutrition Is Deteriorating in Europe. Glob. Chang. Biol. 2015, 21, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Schwanz, P.; Polle, A. Antioxidative Systems, Pigment and Protein Contents in Leaves of Adult Mediterranean Oak Species (Quercus pubescens and Q. ilex) with Lifetime Exposure to Elevated CO2. New Phytol. 1998, 140, 411–423. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Gill, R.; Yadav, S.; Hasanuzzaman, M.; Fujita, M.; Mishra, P.; Sabat, S.C.; Tuteja, N. Superoxide Dismutase—Mentor of Abiotic Stress Tolerance in Crop Plants. Environ. Sci. Pollut. Res. 2015, 22, 10375–10394. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Contran, N.; Günthardt-Goerg, M.S.; Kuster, T.M.; Cerana, R.; Crosti, P.; Paoletti, E. Physiological and Biochemical Responses of Quercus pubescens to Air Warming and Drought on Acidic and Calcareous Soils. Plant Biol. 2013, 15, 157–168. [Google Scholar] [CrossRef]
- Lovreškov, L.; Limić, I.; Butorac, L.; Jakovljević, T. Nitrogen Deposition in Different Mediterranean Forest Types along the Eastern Adriatic Coast. South-East Eur. For. 2021, 12, 115–122. [Google Scholar] [CrossRef]
- Bobbink, R.; Hettelingh, J.P. Effects of Nitrogen Deposition on Woodland, Forest and Other Wooded Land (EUNIS Class G). In Review and Revision of Empirical Critical Loads and Dose–Response Relationships; RIVM Report 680359002; National Institute for Public Health and the Environment: Bilthoven, The Netherlands, 2011; pp. 135–171. [Google Scholar]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development; Sinauer Associates Incorporated: New York, NY, USA, 2018; ISBN 9781605357454. [Google Scholar]
- Ferretti, M.; Calderisi, M.; Marchetto, A.; Waldner, P.; Thimonier, A.; Jonard, M.; Cools, N.; Rautio, P.; Clarke, N.; Hansen, K.; et al. Variables Related to Nitrogen Deposition Improve Defoliation Models for European Forests. Ann. For. Sci. 2015, 72, 897–906. [Google Scholar] [CrossRef]
- Fürst, A.; Kowalska, A.; Brunialti, G.; Clarke, N.; Cools, N.; De Vos, B.; Derome, J.; Derome, K.; Ferretti, M.; Jakovljević, T.; et al. Part XVI: Quality Assurance and Control in Laboratories. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2020; p. 48. [Google Scholar]
- Cotrozzi, L.; Pellegrini, E.; Guidi, L.; Landi, M.; Lorenzini, G.; Massai, R.; Remorini, D.; Tonelli, M.; Trivellini, A.; Vernieri, P.; et al. Losing the Warning Signal: Drought Compromises the Cross-Talk of Signaling Molecules in Quercus ilex Exposed to Ozone. Front. Plant Sci. 2017, 8, 1020. [Google Scholar] [CrossRef] [PubMed]
- Cailleret, M.; Ferretti, M.; Gessler, A.; Rigling, A.; Schaub, M. Ozone Effects on European Forest Growth—Towards an Integrative Approach. J. Ecol. 2018, 106, 1377–1389. [Google Scholar] [CrossRef]
- Zhang, L.; Hoshika, Y.; Carrari, E.; Badea, O.; Paoletti, E. Ozone Risk Assessment Is Affected by Nutrient Availability: Evidence from a Simulation Experiment under Free Air Controlled Exposure (FACE). Environ. Pollut. 2018, 238, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Amores, G.; Bermejo, R.; Elustondo, D.; Lasheras, E.; Santamaría, J.M. Nutritional Status of Northern Spain Beech Forests Wate 4915. Water. Air. Soil Pollut. 2006, 177, 227–238. [Google Scholar] [CrossRef]
- Azim Nejad, Z.; Badehian, Z.; Rezaei Nejad, A.; Bazot, S. Do Soil Properties and Ecophysiological Responses of Oak (Quercus brantii Lindl.) Correlate with the Rate of Dieback? Trees 2021, 35, 1639–1650. [Google Scholar] [CrossRef]
- Potočić, N.; Ćosić, T.; Pilaš, I. The Influence of Climate and Soil Properties on Calcium Nutrition and Vitality of Silver Fir (Abies alba Mill.). Environ. Pollut. 2005, 137, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, A.S. Photosynthesis: A Comprehensive Treatise; Raghavendra, A.S., Ed.; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Agathokleous, E.; Feng, Z.; Peñuelas, J. Chlorophyll Hormesis: Are Chlorophylls Major Components of Stress Biology in Higher Plants? Sci. Total Environ. 2020, 726, 138637. [Google Scholar] [CrossRef]
- Landi, M.; Cotrozzi, L.; Pellegrini, E.; Remorini, D.; Tonelli, M.; Trivellini, A.; Nali, C.; Guidi, L.; Massai, R.; Vernieri, P.; et al. When “Thirsty” Means “Less Able to Activate the Signalling Wave Trigged by a Pulse of Ozone”: A Case of Study in Two Mediterranean Deciduous Oak Species with Different Drought Sensitivity. Sci. Total Environ. 2019, 657, 379–390. [Google Scholar] [CrossRef]
- Bassi, D.; Menossi, M.; Mattiello, L. Nitrogen Supply Influences Photosynthesis Establishment along the Sugarcane Leaf. Sci. Rep. 2018, 8, 2327. [Google Scholar] [CrossRef]
- Takashima, T.; Hikosaka, K.; Hirose, T. Photosynthesis or Persistence: Nitrogen Allocation in Leaves of Evergreen and Deciduous Quercus Species. Plant Cell Environ. 2004, 27, 1047–1054. [Google Scholar] [CrossRef]
- Poyatos, R.; Aguadé, D.; Galiano, L.; Mencuccini, M.; Martínez-Vilalta, J. Drought-Induced Defoliation and Long Periods of near-Zero Gas Exchange Play a Key Role in Accentuating Metabolic Decline of Scots Pine. New Phytol. 2013, 200, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive Oxygen Gene Network of Plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, L.; Palma, A.; Salvatori, E.; Basile, A.; Maresca, V.; Asadi Karam, E.; Manes, F. Functional Indicators of Response Mechanisms to Nitrogen Deposition, Ozone, and Their Interaction in Two Mediterranean Tree Species. PLoS ONE 2017, 12, e0185836. [Google Scholar] [CrossRef] [PubMed]
- Anav, A.; Proietti, C.; Menut, L.; Carnicelli, S.; De Marco, A.; Paoletti, E. Sensitivity of Stomatal Conductance to Soil Moisture: Implications for Tropospheric Ozone. Atmos. Chem. Phys. 2018, 18, 5747–5763. [Google Scholar] [CrossRef]
- Prpić, B.; Pernar, R.; Jurjević, P.; Milković, I.; Vrebčević, M.; Petreš, S. Kartiranje Općekorisnih Funkcija Šuma u Sredozemlju. In Šume Hrvatskog Sredozemlja; Matić, S., Ed.; Akademija Šumarskih Znanosti: Zagreb, Croatia, 2011; pp. 288–294. ISBN 978-953-985715-6. [Google Scholar]
- Gottardini, E.; Cristofolini, F.; Cristofori, A.; Pollastrini, M.; Camin, F.; Ferretti, M. A Multi-Proxy Approach Reveals Common and Species-Specific Features Associated with Tree Defoliation in Broadleaved Species. For. Ecol. Manag. 2020, 467, 118151. [Google Scholar] [CrossRef]
- Rautio, P.; Fürst, A.; Stefan, K.; Raitio, H.; Bartels, U. Part XII: Sampling and Analysis of Needles and Leaves. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2020; p. 19. [Google Scholar]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Ambriović-Ristov, A.; Brozović, A.; Bruvo Mađarić, B.; Ćetković, H.; Herak Bosnar, M.; Hranilović, D.; Katušić Hećimović, S.; Meštrović Radan, N.; Mihaljević, S.; Slade, N.; et al. Metode u Molekularnoj Biologiji; Institut Ruđer Bošković: Zagreb, Croatia, 2007; ISBN 9789536690725. [Google Scholar]
- Radojčić Redovniković, I.; De Marco, A.; Proietti, C.; Hanousek, K.; Sedak, M.; Bilandžić, N.; Jakovljević, T. Poplar Response to Cadmium and Lead Soil Contamination. Ecotoxicol. Environ. Saf. 2017, 144, 482–489. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. ISBN 0076-6879. [Google Scholar]
- Chance, B.; Maehly, A.C. Assay of Catalases and Peroxidases. In Methods in Enzymology; Academic Press: New York, NY, USA, 1955; Volume 2, pp. 764–775. ISBN 0076-6879. [Google Scholar]
- Hersbach, H.; Bell, B.; Berrisford, P.; Hirahara, S.; Horányi, A.; Muñoz-Sabater, J.; Nicolas, J.; Peubey, C.; Radu, R.; Schepers, D.; et al. The ERA5 Global Reanalysis. Q. J. R. Meteorol. Soc. 2020, 146, 1999–2049. [Google Scholar] [CrossRef]

| Species | Category | N | P | K | Ca | Mg |
|---|---|---|---|---|---|---|
| mg g−1 | mg g−1 | mg g−1 | mg g−1 | mg g−1 | ||
| Pubescent oak | Undefoliated trees (≤25%) | 16.95 | 1.22 | 6.62 | 10.72 | 1.23 |
| Defoliated trees (>25%) | 16.95 | 1.75 * | 7.50 * | 14.66 * | 1.97 * | |
| Holm oak | Undefoliated trees (≤25%) | 13.30 | 0.99 | 6.26 * | 6.21 | 1.22 |
| Defoliated trees (>25%) | 12.15 | 0.99 | 3.17 | 6.72 | 1.42 | |
| Aleppo pine | Undefoliated trees (≤25%) | 11.14 | 1.34 | 3.99 | 8.41 * | 1.65 |
| Defoliated trees (>25%) | 10.88 | 1.33 | 3.96 | 4.03 | 1.70 | |
| Black pine | Undefoliated trees (≤25%) | 10.11 | 1.20 | 4.98 | 5.24 * | 1.30 |
| Defoliated trees (>25%) | 9.69 | 1.51 * | 6.36 * | 2.52 | 1.22 |
| Species | Category | Chl-a | Chl-b | Chl-tot | H2O2 | MDA |
|---|---|---|---|---|---|---|
| µg g−1 FW | µg g−1 FW | µg g−1 FW | nmol g−1 FW | nmol g−1 FW | ||
| Pubescent oak | Undefoliated trees (≤25%) | 1503.93 ± 53.84 * | 366.39 ± 36.32 | 1870.32 ± 90.16 * | 44.04 ± 12.88 | 84.10 ± 7.33 * |
| Defoliated trees (>25%) | 1302.68 ± 57.91 | 347.22 ± 35.22 | 1649.90 ± 93.13 | 39.4 ± 5.67 | 48.95 ± 4.13 | |
| Holm oak | Undefoliated trees (≤25%) | 1673.62 ± 69.47 * | 466.84 ± 42.40 * | 2140.46 ± 111.87 * | 0.03 ± 0.01 | 93.65 ± 7.57 |
| Defoliated trees (>25%) | 1387.09 ± 55.64 | 357.96 ± 24.75 | 1745.05 ± 80.39 | 0.02 ± 0.00 | 140.63 ± 6.16 * | |
| Aleppo pine | Undefoliated trees (≤25%) | 406.74 ± 119.81 | 123.81 ± 25.77 | 530.55 ± 145.85 | 0.58 ± 0.09 | 16.49 ± 3.65 |
| Defoliated trees (>25%) | 562.72 ± 104.72 | 250.47 ± 34.61 * | 813.19 ± 139.33 | 0.61 ± 0.2 | 31.60 ± 4.31 * | |
| Black pine | Undefoliated trees (≤25%) | 666.30 ± 23.50 | 221.35 ± 13.81 | 887.65 ± 37.31 | 0.89 ± 0.21 * | 27.76 ± 5.06 |
| Defoliated trees (>25%) | 795.59 ± 8.42 * | 241.20 ± 26.52 | 1034.79 ± 34.94 * | 0.32 ± 0.06 | 24.57 ± 4.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lovreškov, L.; Radojčić Redovniković, I.; Limić, I.; Potočić, N.; Seletković, I.; Marušić, M.; Jurinjak Tušek, A.; Jakovljević, T.; Butorac, L. Are Foliar Nutrition Status and Indicators of Oxidative Stress Associated with Tree Defoliation of Four Mediterranean Forest Species? Plants 2022, 11, 3484. https://doi.org/10.3390/plants11243484
Lovreškov L, Radojčić Redovniković I, Limić I, Potočić N, Seletković I, Marušić M, Jurinjak Tušek A, Jakovljević T, Butorac L. Are Foliar Nutrition Status and Indicators of Oxidative Stress Associated with Tree Defoliation of Four Mediterranean Forest Species? Plants. 2022; 11(24):3484. https://doi.org/10.3390/plants11243484
Chicago/Turabian StyleLovreškov, Lucija, Ivana Radojčić Redovniković, Ivan Limić, Nenad Potočić, Ivan Seletković, Mia Marušić, Ana Jurinjak Tušek, Tamara Jakovljević, and Lukrecija Butorac. 2022. "Are Foliar Nutrition Status and Indicators of Oxidative Stress Associated with Tree Defoliation of Four Mediterranean Forest Species?" Plants 11, no. 24: 3484. https://doi.org/10.3390/plants11243484
APA StyleLovreškov, L., Radojčić Redovniković, I., Limić, I., Potočić, N., Seletković, I., Marušić, M., Jurinjak Tušek, A., Jakovljević, T., & Butorac, L. (2022). Are Foliar Nutrition Status and Indicators of Oxidative Stress Associated with Tree Defoliation of Four Mediterranean Forest Species? Plants, 11(24), 3484. https://doi.org/10.3390/plants11243484







