A Critical Appraisal of the Most Recent Investigations on the Hepatoprotective Action of Brazilian Plants
Abstract
1. Introduction
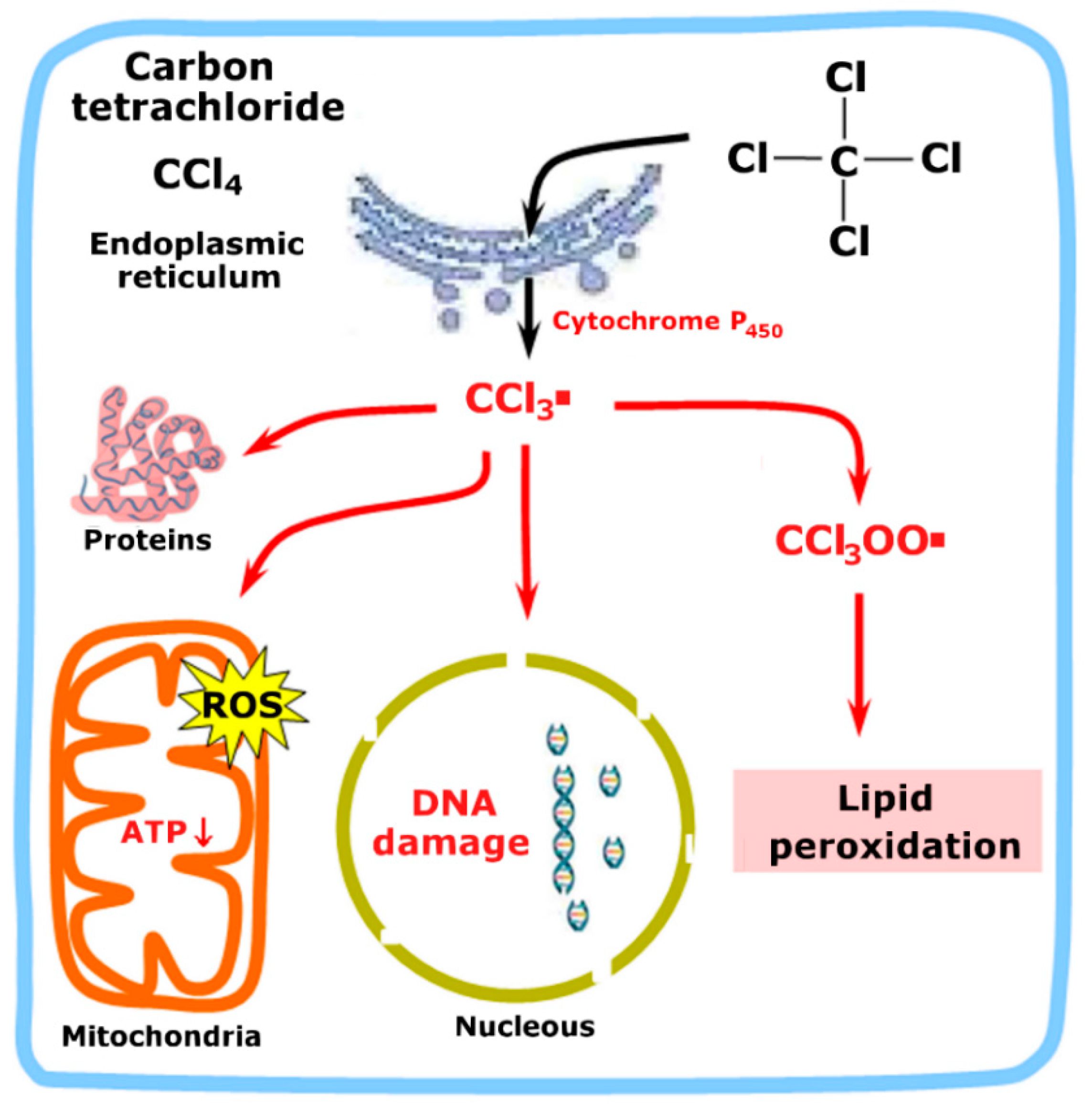
2. Hepatoxicity and the Main Toxic Agents Utilized When Studying Hepatoprotection
3. Methods for Evaluating Hepatotoxicity
4. Extract Preparation and Characterization Procedures
5. Brazilian Native Plants with Potential Hepatoprotective Action Preparation and Characterization
5.1. General Aspects
5.2. Characteristics of the Plants with Hepatoprotective Activity and Effectiveness
| Scientific Name (Family)/Popular Designation/ Brazilian Biome | Plant Part/Extraction Solvent or Mode | Doses and Main Compounds | Assay; Injury Inducer | Main Results Obtained | Ref. |
|---|---|---|---|---|---|
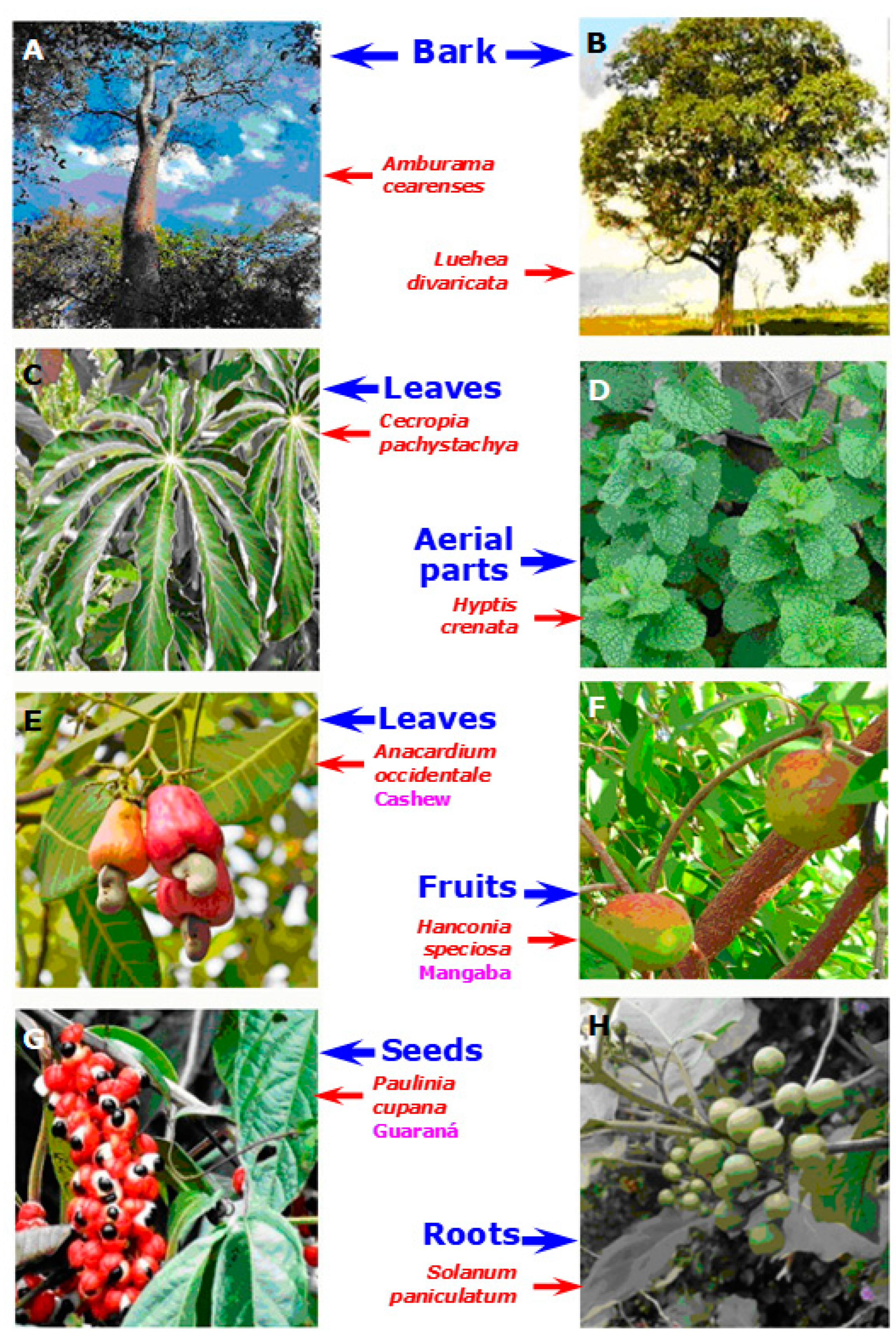
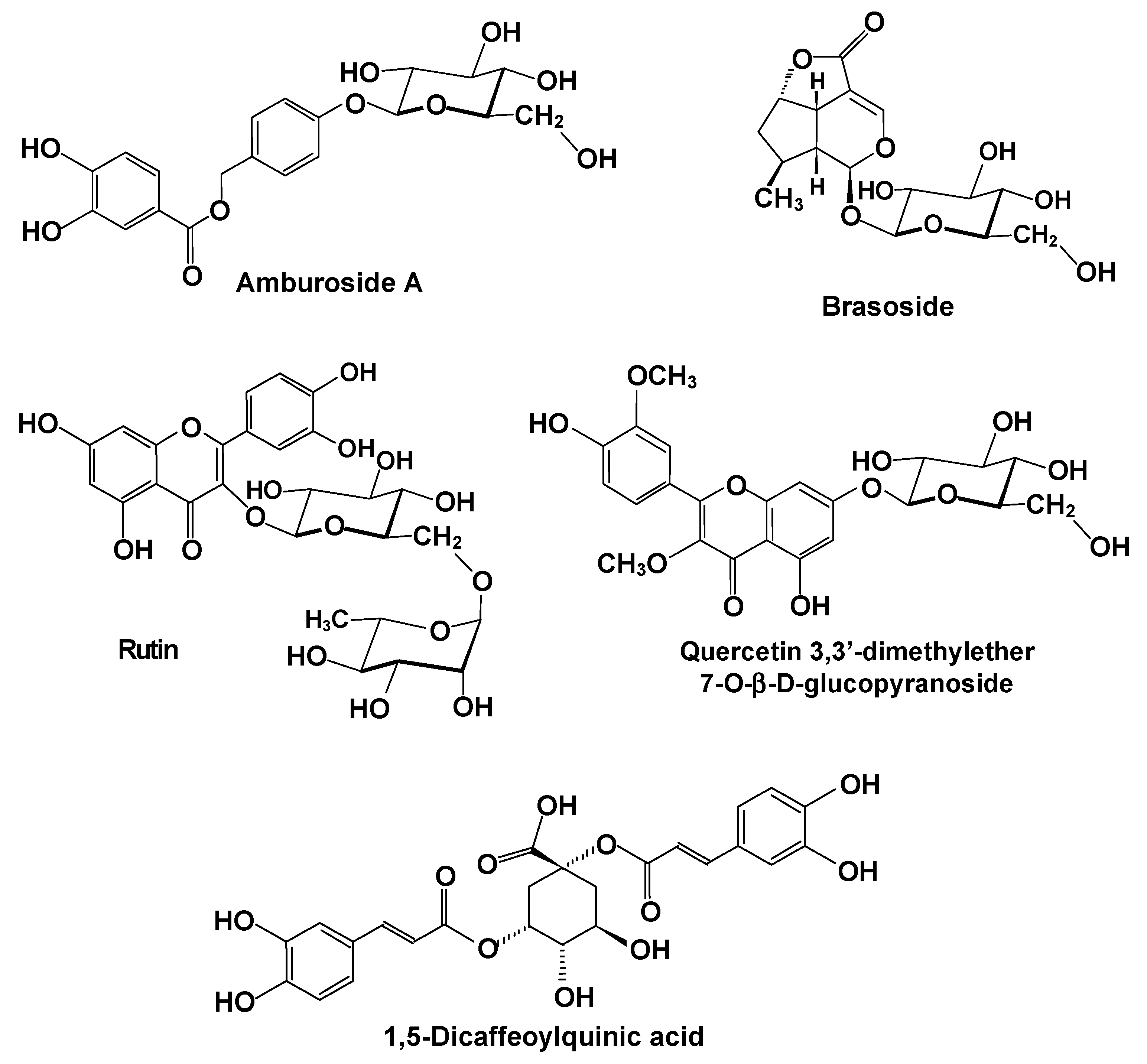
| Amburana cearenses (Figure 3A; Fabaceae)/ umburana de cheiro/ Cerrado and Caatinga | Bark/ethanol | 25 and 50 mg/kg amburoside A (Figure 4) | In vivo, rats; CCl4 | The treatment avoided the increase in plasma AST and ALT and attenuated necrosis and infiltration of inflammatory cells in the liver. Oxidative stress was attenuated with a diminution of lipid peroxidation, restoration of the catalase activity, and reversion of the diminution in the reduced glutathione levels. | [38] |
| Anacardium occidentale (Figure 3E; Anacardia-ceae)/cashew/ Amazon rainforest, Cerrado and Caatinga | Leaves/methanol | 500 and 1000 mg extract/kg, 35.5% in total phenolics (e.g., glycosylated quercetin, amentoflavone derivative) | In vivo, rats; CCl4 | The treatment preserved the liver histo-architecture and significantly reduced the serum AST, ALT, and ALP activities. | [40,41] |
| Annona crassiflora (Anonnaceae)/araticum/ Cerrado | Bark and seeds/ethanol | Extract with 50 mg equivalents of gallic acid/kg (quercetin and rutin (Figure 4) and several organic acids) | In vivo, rats; CCl4 | The treatment prevented lipid peroxidation, the increase in GSH, and the decrease in CAT activity. However, it did not affect significantly the changes induced by CCl4 on cytochrome P450, b5, and SOD. | [39,42] |
| Annona crassiflora (Anonnaceae) araticum/ Cerrado | Fruit peel/ethanol | 10–100 mg ethanolic extract/kg and 10–100 mg polyphenol-rich extract/kg | In vivo, mice. Triton WR-1339- induced hyperlipidemic mice | Lipid-lowering actions and hepatoprotective activities were found. The poly-phenols-rich fraction showed markedly stronger effects than the crude extract, with emphasis on the reduction of lipid peroxidation and protein carbonylation, in addition to the increase in thiol content and restoration of G6PD, GSH-Px, GSH-R, and GSH in the liver. | [43] |
| Annona crassiflora (Annonaceae)/araticum/ Cerrado | Fruit peel/ethanol → n-butanol | 25, 50, and 100 mg polyphenol-rich extract per kg (procyanidin B2, epicatechin, catechin, chlorogenic acid, and caffeoyl-glucoside) | In vivo, rats; diabetes-induced oxidative and nitrosative stress | Treatment decreased serum ALT, AST, and ALP as well as hepatic lipid peroxidation, protein carbonylation and nitration, and inducible nitric oxide synthase level. The antioxidant capacity was increased as well as the glutathione reductase activity and the reduced glutathione level. A general preservation of the liver histoarchitecture was observed. | [44] |
| Baccharis trimera (Asteraceae)/gorse/ Atlantic forest | Aerial parts/hydroethanolic mixture | 600 mg extract rich in quercetin (Figure 4) and flavone derivatives per kg | In vivo, rats; paracetamol | The treatment attenuated the increases in plasma ALT and AST caused by paracetamol. In addition, the treatment in-creased the CAT activity and the total concentration of glutathione but diminished the SOD activity. Histohepathologic analysis revealed a reduction in the injury caused by paracetamol. | [45] |
| Baccharis trimera (Asteraceae)/gorse/ Atlantic forest | Aerial parts/ water → ethanol precipitation | 1 mg/kg of a polysaccharide fraction containing an inulin-type polysaccharide (Figure 4) | In vivo, mice; CCl4 | At the low dose of 1 mg/kg, the preparation significantly reduced the blood levels of ALT, AST, and ALP. It also diminished lipid peroxidation and increased both the catalase activity and the levels of reduced glutathione in the liver. Administration of the polysaccharide preparation at the very high dose of 100 mg/kg increased GSH levels in the liver of heathy mice. | [46] |
| Bidens pilosa (Asteraceae)/picão-preto/ Pampa | Aerial parts/ hydro-ethanolic mixture → ethylacetate | 15 mg fraction rich in quercetin derivatives (Figure 4) per kg | In vivo, mice; CCl4 | The treatment protected the hepatic tissue by blocking lipid peroxidation, protein carbonylation, and DNA fragmentation. In addition, the plasma antioxidant capacity was preserved and there was a reduction in the elevation of the serum transaminases and lactate de-hydrogenase. | [47] |
| Campomanesia adamantium (Myrtaceae)/gabiroba/ Cerrado and Atlantic forest | Pulp and seeds/ hydro-ethanolic mixture | 800 and 1000 µg extract per mL (flavonoids mainly of the flavanone and chalcone class; 138.09 mg/g) | In vitro, mice HEPG2 cells; CCl4 | There was protection against cytotoxicity caused by CCl4 exposure (cell viability) and maintenance of the morphological characteristics (general and nuclear) of the cells. The treatment also reduced the appearance of AST in the supernatant of the cellular incubation medium. | [48,49] |
| Caryocar brasiliense (Caryocaraceae)/ pequi/ Cerrado | Seeds/hand pressing and cold pressing oil extraction | 3 and 6 mL of hand-made and cold-pressed almond oil (fatty acids, phenolic compounds, tocopherols, carotenoids, and phytosterols) per kg. | In vivo, rats; CCl4 | Treated rats showed diminished plasma ALT and AST levels and reduced hepatic injury scores. The plasma serum high-density lipoprotein levels were increased. In addition, the treatment improved the antioxidant capacity as revealed by the increased hepatic glutathione peroxidase and glutathione reductase activities, as well as by the reduced circulating concentrations of leptin and inflammatory mediators. | [50] |
| Cecropia glaziovii (Urticaceae)/embaúba-vermelha | Leaves/hydro- ethanolic mixture | 20 mg extract/kg extract (flavone derivatives and chlorogenic acid) | In vivo, rats; CCl4 | The extract inhibited hepatic lipid peroxidation, diminished the serum levels of ALT and AST, and increased the activities of SOD and CAT in the liver. | [51] |
| Cecropia pachystachya (Figure 3C; Urticaceae)/embaúba | Leaves/ ethylacetate | 20 mg extract/kg (chlorogenic acid, iso-orientin, and orientin) | In vivo, mice; non-alcoholic liver disease induced by hyper-caloric diet | Treatment with the extract prevented the increase in liver weight and lipid per-oxidation caused by liver disease. Serum levels of ALT and AST were not affected by the treatment. Although the treatment reduced steatosis, it remained higher than in healthy animals. | [52] |
| Eugenia uniflora (Myrtaceae)/pitangueira/red Brazilian cherry)/ Cerrado, Atlantic forest and Pampa | Leaves/ ethylacetate | 200 mg/kg extract (quercetin and myricetin derivatives) | In vivo, rats; CCl4 | The treatment prevented the elevation in the serum levels of ALT, AST, total bilirubin, total cholesterol, and triglycerides. In the liver, it prevented lipid peroxidation and restored the activity of SOD and glutathione (GSH) levels. In addition, the treatment effectively attenuated the CCl4-induced histopathological changes. | [20] |
| Hancornia speciosa (Figure 3F; Apocynaceae)/ mangaba/ Cerrado | Fruit/freeze-dried extract | 200 mg/kg extract (chlorogenic acid (150 mg/g and rutin (120 mg/g) | In vivo, rats; paracetamol | The treatment maintained the nuclear envelope integrity of the hepatocytes against damage induced by acetaminophen. It was also effective in reducing liver function markers AST, ALT, and GGT in serum. The extract also diminished lipid peroxidation and significantly improved the oxidative status of the liver by increasing the levels of antioxidant enzymes. | [53] |
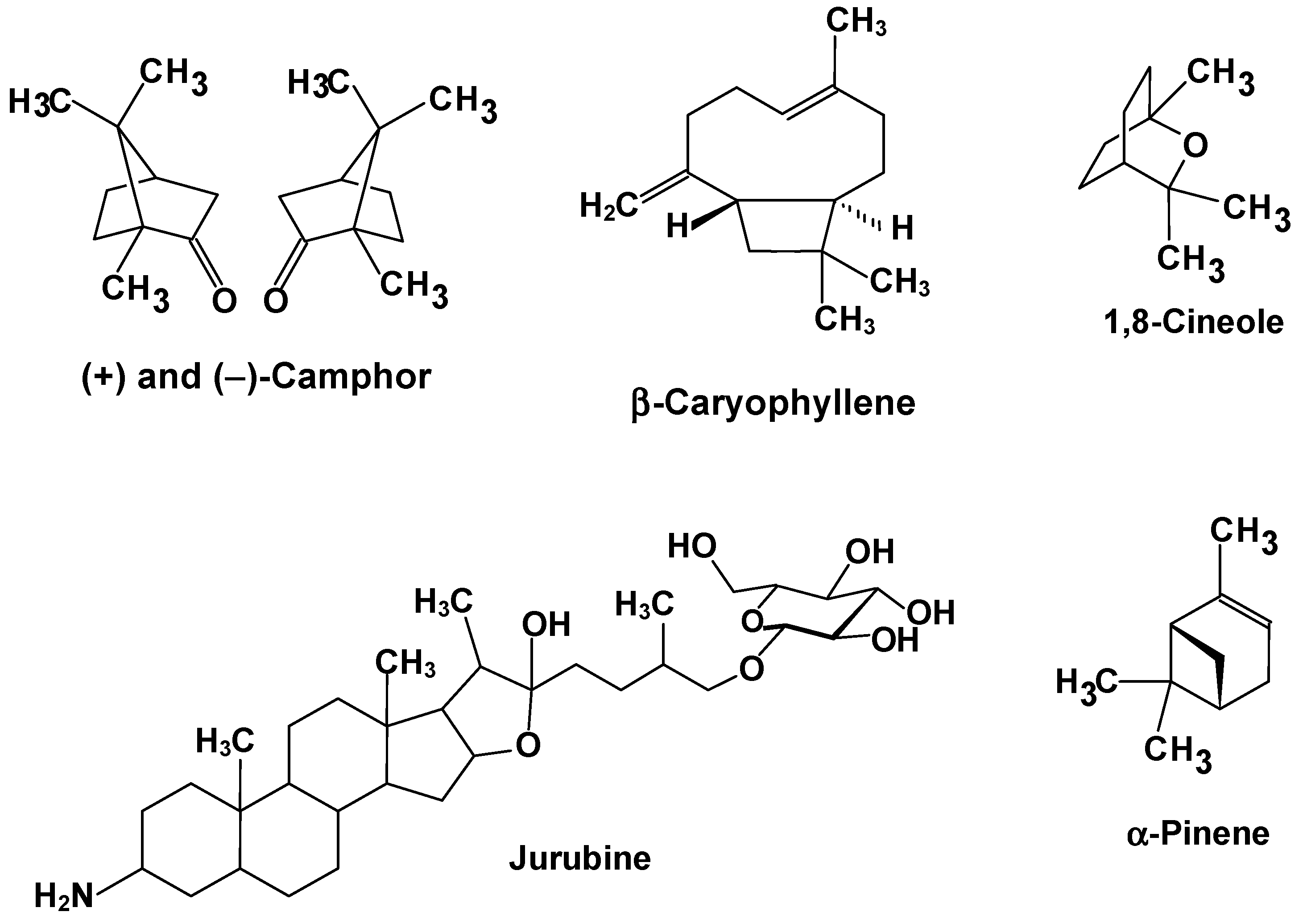
| Hyptis crenata (Figure 3D; Lamiaceae)/Cerrado | Aerial parts/ steam distillation | 300 mg essential oil/kg (32.8% camphor, 18.0% 1,8-cineole, 13.4% α-pinene, 12.9% β-caryophyllene; Figure 5) | In vivo, rats; poly-microbial sepsis | The essential oil normalized serum ALP, ALT, and bilirubin levels and prevented morphological changes. Additionally, the essential oil inhibited elevation in hepatic lipid peroxidation and reduction of the glutathione peroxidase activity. | [54] |
| Indigofera suffruticosa (Fabaceae)/ All biomes | Leaves/methanol | 50 mg/kg (alkaloids, flavonoids, steroids, proteins, carbohydrates, triterpenes, and indigo coumarin) | In vivo, mice; paracetamol | Histopathological and histomorphometric analyses revealed the reorganization of structural units of cells, nuclei, and sinusoidal capillaries of hepatocytes by the treatment, thus reducing the damage to liver tissue and increasing organ regeneration rate. The plasma levels of the marker enzymes ALT and AST, as well as the plasma levels of bilirubin, were also restored by the treatment. | [55] |
| Luehea divaricata (Figure 3B; Malvaceae)/whips horse/Atlantic forest | Bark/hydro-ethanolic mixture | 200 mg/kg (6.6 mg/kg of a β-type (epi)catechin dimer + several catechin trimers and pentamers) | In vivo, rats; paracetamol | Liver injury in rats was substantially attenuated in animals treated with the hydro-alcoholic extract (200 mg kg−1 day−1). This was deduced from aspartate aminotransferase and alanine amino-transferase measurements in plasma as well as from the hepatic activities of catalase and superoxide dismutase. The anti-inflammatory activity of the L. divaricata extract, as evidenced by the inhibitory activity of nitric oxide production, may be partly responsible for the observed hepatoprotective effects. | [56] |
| Maytenus robusta (Celastraceae)/cafezinho-do-mato/ Atlantic forest | Aerial parts/ hydro-ethanolic mixture | 100 mg/kg (13.7% in phenolic compounds) | In vivo, mice; CCl4 | Treatment with the extract reduced the hepatic histological changes and normalized the serum ALT levels. Lipo-peroxidation was diminished and the reduced glutathione levels were augmented. The activities of the following antioxidant enzymes were increased: SOD, CAT, and glutathione-S-transferase. The myeloperoxidase activity was de-creased as well as the TNFα and inter-leukin-6 levels. | [57] |
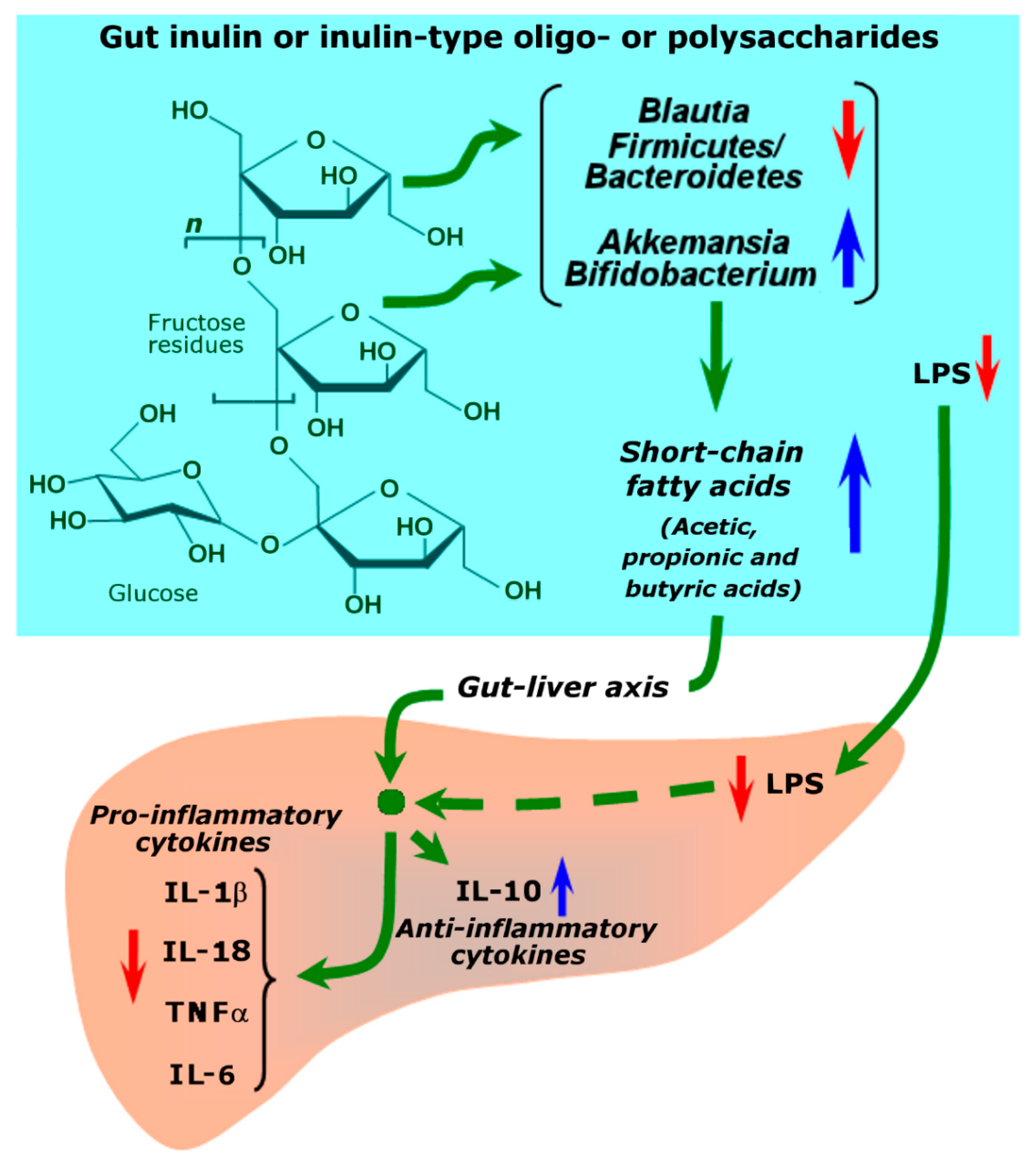
| Mikania glomerata (Asteraceae)/guaco/ Atlantic forest | Aerial parts/ water | Inulin type fructan: 100 mg/kg Fructooligosaccharides: 10 mg/kg (see Figure 4) | In vivo, mice; CCl4 | The pretreatment with both the inulin-type fructan and the fructooligosaccharides attenuated the elevations in the serum levels of ALT, AST, and alkaline phosphatase. Further, the same pretreatments also partially prevented lipid peroxidation, the diminution in the GSH levels, and the CAT activity. | [58] |
| Paullinia cupana (Figure 3G; Sapindaceae)/guaraná/ Amazon rainforest | Seeds/water | 100, 300, and 600 mg/kg extracts (vitamin C, methylxantines, catechin and epi-catechin derivatives, and pro-anthocyanidins) | In vivo, rats; CCl4 | The guarana extracts diminished the increased serum activities of ALT and AST and prevented DNA strand breakage. | [59,60] |
| Rourea induta (Connaraceae)/chapeudinha/ Cerrado | Leaves/hydro- ethanolic mixture | 500 mg extract/kg (derivatives of quercetin and hyperin) | In vivo, rats; CCl4 | The treatment reduced the elevated serum markers ALT, AST, and bilirubin, and improved the parameters CAT, SOD, GPx, GSH, and TBARS in the hepatic tissue. Histopathologic modifications were prevented. | [61] |
| Solanum fastigiatum (Solanaceae)/falsa jurubeba/ Atlantic forest | Leaves/water | 100 and 200 mg extract/kg | In vivo, mice; paracetamol | The treatment prevented the changes in the TBARS levels and non-protein thiol levels. | [62] |
| Solanum paniculatum (Figure 3H; Solanaceae)/ All biomes | Leaves/water → ethylacetate | Aqueous extract: 600–1200 mg/kg; Ethyl-acetate fraction: 300 mg/kg | In vivo, mice; paracetamol | Both the aqueous extract and its ethyl acetate fraction antagonized the rises in serum ALT and AST, though at different doses. In the liver, the same preparations antagonized the drop in reduced GSH and the increase in lipid peroxidation. The liver protective effects of the ethyl-acetate fraction were similar to those of N-acetyl-cysteine. | [63] |
| Solanum paniculatum (Figure 3H; Solanaceae)/ All biomes | Roots/hydro-methanolic mixture → ethylacetate | 100 mg crude extract/kg; 200 mg 3-amino-spirostane alkaloids (jurubine, etc.) fraction per kg (Figure 5) | In vivo, mice; CCl4 | In the groups treated with both the crude extract and the alkaloid fraction hepatic degeneration was diminished, although cytoplasmic vacuolization was still evident. Serum ALT activity was diminished, but the AST activity was not affected. | [64] |
| Verbena litoralis and Verbena Montevidensis (Verbenaceae)/fel-da-terra/ Atlantic forest | Aerial parts/water and methanol | Aqueous and methanolic extracts: 0.1–100 µg/mL Brasoside: 10–100 µM (Figure 4) | In vitro, mice HEPG2 cells; ethanol-induced injury | Toxicity was evaluated by measuring the cellular dehydrogenase activity and neutral red dye incorporation. Both aqueous and methanolic extracts of both species as well as brasoside were hepato-protective. The effects were attributed to brasoside, present in both extracts. | [65] |
| Vernonia condensata (Asteraceae)/alumã, figatil or necroton | Leaves/ethanol → ethylacetate | 50–200 mg ethyl-acetate fraction per kg (rich in 1,5-di-O-caffeoylquinic acid; Figure 4) | In vivo, rats; paracetamol | All doses of the ethylacetate fraction reduced and eventually normalized the serum AST, ALT, and ALP levels. Moreover, all doses were able to inhibit malonaldehyde formation and increase the GSH levels. Treated rats did not present degenerative changes maintaining an almost normal histological aspect. | [66] |
5.3. The Folk Medicinal Perception of Brazilian Hepatoprotective Plants
6. Compounds Possibly Involved in Hepatoprotection and Molecular Mechanisms
7. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ullah, H.; Khan, A.; Rehman, N.U.; Halim, S.A.; Khan, H.; Khan, I.; Csuk, R.; Al Rawahi, A.; Al-Hatmi, S.; Al Harrasi, A. Lophenol and lathosterol from resin of Commiphora kua possess hepatoprotective effects in vivo. J. Ethnopharmacol. 2020, 252, 112558. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Ullah, H.; Nabavi, S.M. Mechanistic insights of hepatoprotective effects of curcumin: Therapeutic updates and prospects. Food Chem. Toxicol. 2019, 124, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, N.; Hong, M.; Tan, H.Y.; Pan, G.; Feng, Y. Hepatoprotective effects of a functional formula of three Chinese medicinal herbs: Experimental evidence and network pharmacology-based identification of mechanism of action and potential bioactive components. Molecules 2018, 23, 352. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Mendoza, N.; Madrigal-Santillán, E.; Morales-Gonzáles, Á.; Esquivel-Soto, J.; Esquivel-Cuirino, C.; Gonzáles-Rúbio, M.G.L.; Gayosso-de-Lucio, J.A.; Moráles-Gonzáles, J.A. Hepatoprotective effect of silymarin. World J. Hepatol. 2014, 6, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Fan, Y.; Yan, Q.; Fan, X.; Wu, B.; Han, Y.; Zhang, Y.; Chen, Y.; Zhang, H.; Niu, J. The therapeutic effect of silymarin in the treatment of nonalcoholic fatty disease. A meta-analysis (PRISMA) of randomized control trials. Medicine 2017, 9, 49. [Google Scholar]
- Adewusi, E.A.; Afolayan, A.J. A review of natural products with hepatoprotective activity. J. Med. Plants Res. 2010, 4, 1318–1334. [Google Scholar]
- Madrigal-Santillán, E.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Sumaya-Martinez, M.T.; Gutiérrez-Salinas, J.; Bautista, M.; Morales-Gonzáles, Á.; González-Rubio, M.G.G.; Aguilar-Faisal, J.L.; Morales-González, J.A. Review of natural products with hepatoprotective effects. World J. Gastroenterol. 2014, 20, 14787–14804. [Google Scholar] [CrossRef]
- Pereira, C.; Barros, L.; Ferreira, I.C.F. Extraction, identification, fractionation and isolation of phenolic compounds in plants with hepatoprotective effects. J. Sci. Food Agric. 2016, 96, 1068–1084. [Google Scholar] [CrossRef]
- Antunes, C.; Arbo, M.D.; Konrath, E.D. Hepatoprotective native plants documented in Brazilian traditional medicine literature: Current knowledge and prospects. Chem. Biodivers. 2022, 19, e202100933. [Google Scholar] [CrossRef]
- Zimmerman, H.J. Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver, 2nd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1999. [Google Scholar]
- Health Jade Team. Hepatotoxicity Homepage on the Internet. Health Jade Medical Group. Available online: https://healthjade.net/hepatotoxicity/#Hepatotoxicitycauses (accessed on 13 May 2022).
- McGill, M.R.; Jaescke, H. Animal models of drug-induced liver injury. BBA Mol. Basis. Dis. 2019, 185, 1031–1039. [Google Scholar] [CrossRef]
- Dassarma, B.; Nandi, D.K.; Gangopadhyay, S.; Samanta, S. Hepatoprotective effect of food preservatives (butylated hydroxyanisole, butylated hydroxytoluene) on carbon tetrachloride-induced hepatotoxicity in rat. Toxicol. Rep. 2018, 5, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.A.; Sá-Nakanishi, A.B.; Bracht, A.; da Costa, S.M.G.; Koehnlein, A.; de Souza, C.G.M.; Peralta, R.M. Hepatoprotective effects of mushrooms. Molecules 2013, 18, 7609–7630. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, C.I.; Pérez María, J.; Manautou, J.E.; Mottino, A.D. Acetaminophen from liver to brain: New insights into drug pharmacological action and toxicity. Pharmacol. Res. 2016, 109, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.A. Management of acetaminophen and ibuprofen toxicoses in dogs and cats. J. Vet. Emerg. Crit. Care 2000, 10, 285–291. [Google Scholar] [CrossRef]
- Im, Y.R.; Hunter, H.; Hahn, D.G.; Duret, A.; Cheah, Q.; Dong, J.; Fairey, M.; Hjalmarsson, C.; Li, A.; Lim, H.K.; et al. A systematic review of animal models of NAFLD finds high-fat, high-fructose diets most closely resemble human NAFLD. Hepatology 2021, 74, 1884–1901. [Google Scholar] [CrossRef] [PubMed]
- Jeyadevi, R.; Sivasudha, T.; Rameshkumar, A.; Harnly, J.M.; Lin, L.Z. Phenolic profiling by UPLC–MS/MS and hepatoprotective activity of Cardiospermum halicacabum against CCl4 induced liver injury in Wistar rats. J. Funct. Foods 2013, 5, 289–298. [Google Scholar] [CrossRef]
- Esmaeili, M.A.; Alilou, M. Naringenin attenuates CCl4-induced hepatic inflammation by the activation of an Nrf2-mediated pathway in rats. Clin. Exp. Pharmacol. Physiol. 2014, 41, 416–422. [Google Scholar] [CrossRef]
- Sobeh, M.; Hamza, M.S.; Ashou, M.L.; Elkhatieb, M.; El Raey, M.A.; Abdel-Naim, A.B.; Wink, M. A polyphenol-rich fraction from Eugenia uniflora exhibits antioxidant and hepatoprotective activities in vivo. Pharmaceuticals 2020, 13, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.M.; Nassan, M.A.; Ismail, T.A. Immunohistochemical and molecular study on the protective effect of curcumin against hepatic toxicity induced by paracetamol in Wistar rats. BMC Complement. Altern. Med. 2014, 14, 457. [Google Scholar] [CrossRef] [PubMed]
- Abascal, K.; Ganora, L.; Yarnell, E. The effect of freeze-drying and its implications for botanical medicine: A review. Phytother. Res. 2005, 19, 655–660. [Google Scholar] [CrossRef]
- Mumper, R.J.; Dai, J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar]
- Shi, J.; Nawaz, H.; Pohorly, J.; Mittal, G.; Kakuda, Y.; Jiang, Y. Extraction of polyphenolics from plant material for functional foods-engineering and technology. Food Rev. Int. 2005, 21, 139–166. [Google Scholar] [CrossRef]
- Chatterjee, A.; Rakshit, A.; Singh, N.N. Interfacing external peripherals with a mobile robot. In Vision Based Autonomous Robot Navigation, Studies in Computational Intelligence; Springer: Berlin/Heidelberg, Germany, 2013; Volume 455, pp. 21–46. [Google Scholar]
- Huang, H.W.; Hsu, C.P.; Yang, B.B.; Wang, C.Y. Advances in the extraction of natural ingredients by high pressure extraction technology. Trends Food Sci. Technol. 2013, 33, 54–62. [Google Scholar] [CrossRef]
- Moreira, S.A.; Alexandre, E.M.C.; Pintado, M.; Saraiva, J.A. Effect of emergent non-thermal extraction technologies on bioactive individual compounds profile from different plant materials. Food Res. Int. 2018, 115, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.A.; Soares, A.A.; Correa, R.C.G.; Barros, L.; Haminiuk, C.W.I.; Peralta, R.M.; Ferreira, I.C.F.R.; Bracht, A. Merlot grape pomace hydroalcoholic extract improves the oxidative and inflammatory states of rats with adjuvant-induced arthritis. J. Funct. Foods 2017, 33, 408–418. [Google Scholar] [CrossRef]
- Castilho, P.A.; Bracht, L.; Barros, L.; Albuquerque, B.R.; Dias, M.I.; Ferreira, I.C.F.R.; Comar, J.F.; da Silva, T.B.V.; Peralta, R.M.; Sá-Nakanishi, A.B.; et al. Effects of a Myrciaria jaboticaba peel extract on starch and triglyceride absorption and the role of cyanidin-3-O-glucoside. Food Funct. 2021, 12, 2644–2659. [Google Scholar] [CrossRef]
- Buzgaia, N.; Lee, S.Y.; Rukayadi, Y.; Abas, F.; Shaari, K. Antioxidant Activity, α-Glucosidase Inhibition and UHPLC–ESI–MS/MS Profile of Shmar (Arbutus pavarii Pamp). Plants 2021, 10, 1659. [Google Scholar] [CrossRef]
- El-Shahir, A.A.; El-Wakil, D.A.; Latef, A.A.H.A.; Youssef, N.H. Bioactive Compounds and Antifungal Activity of Leaves and Fruits Methanolic Extracts of Ziziphus spina-christi L. Plants 2022, 11, 746. [Google Scholar] [CrossRef]
- Gazwi, H.S.S.; Shoeib, N.A.; Mahmoud, M.E.; Soltan, O.I.A.; Hamed, M.M.; Ragab, A.E. Phytochemical Profile of the Ethanol Extract of Malvaviscus arboreus Red Flower and Investigation of the Antioxidant, Antimicrobial, and Cytotoxic Activities. Antibiotics 2022, 11, 1652. [Google Scholar] [CrossRef]
- Li, F.; Boateng, I.D.; Yang, X.; Li, Y. Extraction, Purification, and Elucidation of Six Ginkgol Homologs from Ginkgo biloba Sarcotesta and Evaluation of Their Anti-cancer Activities. Molecules 2022, 27, 7777. [Google Scholar] [CrossRef]
- Salih, A.M.; Al-Qurainy, F.; Tarroum, M.; Khan, S.; Nadeem, M.; Shaikhaldein, H.O.; Alansi, S. Phytochemical Compound Profile and the Estimation of the Ferruginol Compound in Different Parts (Roots, Leaves, and Seeds) of Juniperus procera. Separations 2022, 9, 352. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Zengin, G.; Sinan, K.I.; Yildiztugay, E.; Mahomoodally, M.F.; Ak, G.; Picot-Allain, C.N.; Gevrenova, R. Identification of bioactive compounds from Rhaponticoides iconiensis extracts and their bioactivities: An endemic plant to Turkey flora. J. Pharm. Biom. Anal. 2020, 190, 113537. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, K.; Riese, U.; Hamburger, M. HPLC-based bioactivity profiling of plant extracts: A kinetic assay for the identification of monoamine oxidase-A inhibitors using human recombinant monoamine oxidase-A. Phytochemistry 2004, 65, 2885–2891. [Google Scholar] [CrossRef] [PubMed]
- Francelin, M.F.; Vieira, T.F.; Andrade Garcia, J.A.; Correa, R.C.G.; Monteiro, A.R.G.; Bracht, A.; Peralta, R.M. Phytochemical, nutritional and pharmacological properties of unconventional native fruits and vegetables from Brazil. In Phytochemicals in Vegetables: A Valuable Source of Bioactive Compounds, 1st ed.; Petropoulos, S.A., Ferreira, I.C.F.R., Barros, L., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2018; pp. 444–472. [Google Scholar]
- Leal, L.K.; Fonseca, F.N.; Pereira, F.A.; Canuto, K.M.; Felipe, C.F.; Fontenele, J.F.; Pitombeira, M.V.; Silveira, E.R.; Viana, G.S. Protective effects of amburoside A, a phenol glucoside from Amburana cearensis, against CCl4-induced hepatotoxicity in rats. Planta Med. 2008, 74, 497–502. [Google Scholar] [CrossRef]
- Roesler, R. Effect of extracts from araticum (Annona crassiflora) on CCl4-induced liver damage in rats. Food Sci. Technol. 2011, 31, 93–100. [Google Scholar] [CrossRef]
- Ikyembe, D.; Pwavodi, C.; Agbon, A.N. Hepatoprotective effect of methanolic leaf extract of Anacardium occidentale (cashew) on carbon-tetrachloride-induced liver toxicity in wistar rats. Sub-Saharan Afr. J. Med. 2014, 1, 124–131. [Google Scholar]
- Konan, N.A.; Bacchi, E.M. Antiulcerogenic effect and acute toxicity of a hydroethanolic extract from the cashew (Anacardium occidentale L.) leaves. J. Ethnopharmacol. 2007, 112, 237–242. [Google Scholar] [CrossRef]
- Roesler, R.; Catharino, R.R.; Malta, L.G.; Eberlin, M.N.; Pastore, G.M. Antioxidant activity of Annona crassiflora: Characterization of major components by electrospray ionization mass spectrometry. Food Chem. 2007, 104, 1048–1054. [Google Scholar] [CrossRef]
- Ramos, L.P.A.; Justino, A.B.; Tavernelli, N.; Saraiva, A.L.; Franco, R.R.; de Souza, A.V.; Silva, H.C.G.; de Moura, F.B.R.; Botelho, F.V.; Espindola, F.S. Antioxidant compounds from Annona crassiflora fruit peel reduce lipid levels and oxidative damage and maintain the glutathione defense in hepatic tissue of Triton WR-1339-induced hyperlipidemic mice. Biomed. Pharmacother. 2021, 142, 112049. [Google Scholar] [CrossRef]
- Justino, A.B.; Pereira, M.N.; Peixoto, L.G.; Vilela, D.D.; Caixeta, D.C.; de Souza, A.V.; Teixeira, R.R.; Silva, H.C.G.; de Moura, F.B.R.; Moraes, I.B.; et al. Hepatoprotective properties of a polyphenol-enriched fraction from Annona crassiflora Mart. Fruit peel against diabetes-induced oxidative and nitrosative stress. J. Agric. Food Chem. 2017, 65, 4428–4438. [Google Scholar] [CrossRef]
- Chaves, P.F.P.; Adami, E.R.; Acco, A.; Iacomini, M.; Cordeiro, L.M.C. Chemical characterization of polysaccharides from Baccharis trimera (Less.) DC Infusion and its hepatoprotective effects. Food Res. Int. 2020, 136, 109510. [Google Scholar] [CrossRef] [PubMed]
- Pádua, B.C.; Rossoni, B., Jr.; Magalhães, C.L.B.; Chaves, M.M.; Silva, M.E.; Pedrosa, M.L.; de Souza, G.H.B.; Brandão, G.C.; Rodrigues, I.V.; Lima, W.G.; et al. Protective effect of Baccharis trimera extract on acute hepatic injury in a model of inflammation induced by acetaminophen. Mediat. Inflamm. 2014, 2014, 196598. [Google Scholar] [CrossRef] [PubMed]
- Kviecinski, M.R.; Felipe, K.B.; Correia, J.F.G.; Ferreira, E.A.; Rossi, M.H.; Gatti, F.M.; Wilhelm-Filho, D.; Pedrosa, R.C. Brazilian Bidens pilosa Linné yields fraction containing quercetin-derived flavonoid with free radical scavenger activity and hepatoprotective effects. Libyan J. Med. 2011, 6, 5651. [Google Scholar] [CrossRef]
- Fernandes, T.O.; de Ávila, R.I.; de Moura, S.C.; Ribeiro, G.A.; Naves, M.M.V.; Valadares, M.C. Campomanesia adamantium (Myrtaceae) fruits protect HEPG2 cells against carbon tetrachloride-induced toxicity. Toxicol. Rep. 2015, 2, 184–193. [Google Scholar] [CrossRef]
- Lescano, C.H.; de Lima, F.F.; Mendes-Silvério, C.B.; Justo, A.F.O.; Baldivia, D.S.; Vieira, C.P.; Sanjinez-Argandoña, E.J.; Cardoso, C.A.L.; Mónica, F.Z.; de Oliveira, I.P. Effect of polyphenols from Campomanesia adamantium on platelet aggregation and inhibition of cyclooxygenases: Molecular docking and in vitro analysis. Front. Pharmacol. 2018, 9, 617. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.R.; Santana, F.C.; Torres-Leal, F.L.; de Melo, I.L.P.; Yoshime, L.T.; Matos-Neto, E.M.; Seelaender, M.C.L.; Araújo, C.M.M.; Cogliati, B.; Mancini-Filho, J. Pequi (Caryocar brasiliense Camb.) almond oil attenuates carbon tetrachloride-induced acute hepatic injury in rats: Antioxidant and anti-inflammatory effects. Food Chem. Toxicol. 2016, 97, 205–216. [Google Scholar] [CrossRef]
- Petronilho, F.; Dal-Pizzol, F.; Costa, G.M.; Kappel, V.D.; de Oliveira, S.Q.; Fortunato, J.; Cittadini-Zanette, V.; Moreira, J.C.F.; Simões, C.M.O.; Dal-Pizzol, F.; et al. Hepatoprotective effects and HSV-1 activity of the hydroethanolic extract of Cecropia glaziovii (embaúba-vermelha) against acyclovir-resistant strain. Pharm. Biol. 2012, 50, 911–918. [Google Scholar] [CrossRef]
- Campos, M.L.; Castro, M.B.; Campos, A.D.; Fernandes, M.F.; Conegundes, J.L.M.; Rodrigues, M.N.; Mügge, F.L.B.; da Silva, A.M.; Sabarense, C.M.; Castañon, M.C.M.N.; et al. Antiobesity, hepatoprotective and anti-hyperglycemic effects of a pharmaceutical formulation containing Cecropia pachystachya Trécul in mice fed with a hypercaloric diet. J. Ethnopharmacol. 2021, 280, 114418. [Google Scholar] [CrossRef]
- Santos, R.S.; Chaves-Filho, A.B.; Silva, L.A.S.; Garcia, C.A.B.; Silva, A.R.S.T.; Dolabella, S.S.; Costa, S.S.L.; Miyamoto, S.; Matos, H.R. Bioactive compounds and hepatoprotective effect of Hancornia speciosa Gomes fruit juice on acetaminophen-induced hepatotoxicity in vivo. Nat. Prod. Res. 2021, 3, 2565–2569. [Google Scholar] [CrossRef]
- Lima, G.C.; Vasconcelos, Y.A.G.; de Santana Souza, M.T.; Oliveira, A.S.; Bomfim, R.R.; Albuquerque-Júnior, R.L.C.; Camargo, E.A.; Portella, V.G.; Coelho-de-Souza, A.N.; Diniz, L.R.L. Hepatoprotective effect of essential oils from Hyptis crenata in sepsis-induced liver dysfunction. J. Med. Food. 2018, 21, 709–715. [Google Scholar] [CrossRef]
- Lima, I.R.; Silva, I.B.; Lima, R.M.L.; Silva, T.M.S.; Maia, M.B.S. Hepatoprotective efficacy of methanolic extract of Indigofera suffruticosa (Mill) on paracetamol-induced liver damage in mice. Arq. Gastroenterol. 2019, 56, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manieri, A.A.; Correa, V.G.; Corrêa, R.C.G.; Dias, M.I.; Calhelha, R.C.; Ivanov, M.; Sokovic, M.; Barros, L.; Ferreira, I.C.F.R.; Bracht, A.; et al. Polyphenolic profile and pharmacological activities of whips horse (Luehea divaricata) bark extracts studied using in vitro and in vivo systems. Biocatal. Agric. Biotechnol. 2022, 45, 102530. [Google Scholar] [CrossRef]
- Thiesen, L.C.; da Silva, L.M.; Santin, J.R.; Bresolin, T.M.B.; de Andrade, S.F.; Amorim, C.M.; Merlin, L.; de Freitas, R.A.; Niero, R.; Netz, D.J.A. Hepatoprotective effect of Maytenus robusta Reiss extract on CCl4-induced hepatotoxicity in mice and HepG2 cells. Regul. Toxicol. Pharmacol. 2017, 86, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Chaves, P.F.P.; Adami, E.R.; Corso, C.R.; Milani, L.; de Oliveira, N.M.T.; da Silva, L.C.M.; Acco, A.; Iacomini, M.; Cordeiro, L.M.C. Carbohydrates from Mikania glomerata Spreng tea: Chemical characterization and hepatoprotective effects. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100227. [Google Scholar] [CrossRef]
- Yonekura, L.; Martins, C.A.; Sampaio, G.R.; Monteiro, M.P.; César, L.A.M.; Mioto, B.M.; Mori, C.S.; Mendes, T.M.N.; Ribeiro, M.L.; Arçari, D.P.; et al. Bioavailability of catechins from guarana (Paullinia cupana) and its effect on antioxidant enzymes and other oxidative stress markers in healthy human subjects. Food Funct. 2016, 7, 2970–2978. [Google Scholar] [CrossRef]
- Kober, H.; Tatsch, E.; Torbitz, V.D.; Cargnin, L.P.; Sangoi, M.B.; Bochi, G.V.; da Silva, A.R.H.; Barbisan, F.; Ribeiro, E.E.; da Cruz, I.B.M.; et al. Genoprotective and hepatoprotective effects of guarana (Paullinia cupana Mart. var. sorbilis) on CCl4-induced liver damage in rats. Drug Chem. Toxicol. 2016, 39, 48–52. [Google Scholar] [CrossRef]
- Kalegari, M.; Gemin, C.A.B.; Araújo-Silva, G.; de Brito, N.J.N.; López, J.A.; Tozetto, S.O.; Almeida, M.G.; Miguel, M.D.; Stien, D.; Miguel, O.G. Chemical composition, antioxidant activity and hepatoprotective potential of Rourea induta Planch. (Connaraceae) against CCl4-induced liver injury in female rats. Nutrition 2014, 30, 713–718. [Google Scholar] [CrossRef]
- Sabir, S.M.; Rocha, J.B.T. Antioxidant and hepatoprotective activity of aqueous extract of Solanum fastigiatum (false “Jurubeba”) against paracetamol-induced liver damage in mice. J. Ethnopharmacol. 2008, 120, 226–232. [Google Scholar] [CrossRef]
- de Souza, G.R.; de Oliveira, A.C.A.X.; Soares, V.; Chagas, L.F.; Barbi, N.S.; Paumgartten, F.J.R.; da Silva, A.J.R. Chemical profile, liver protective effects and analgesic properties of a Solanum paniculatum leaf extract. Biomed. Pharmacother. 2019, 110, 129–138. [Google Scholar] [CrossRef]
- Gazolla, M.C.; Marques, L.M.M.; Silva, M.G.; Araújo, T.M.F.; Mendes, R.L.; Almeida, J.R.G.S.; Vessecchi, R.; Lopes, N.P. Characterization of 3-aminospirostane alkaloids from roots of Solanum paniculatum L. with hepatoprotective activity. Rapid Commun. Mass Spectrom. 2020, 34, e8705. [Google Scholar] [CrossRef]
- Vestena, A.; Piton, Y.; de Loretto Bordignon, S.A.; Garcia, S.; Arbo, M.D.; Zuanazzi, J.A.; von Poser, G. Hepatoprotective activity of Verbena litoralis, Verbena montevidensis and their main iridoid, brasoside. J. Ethnopharmacol. 2019, 239, 111906. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.B.D.; Mendes, R.F.; Tomasco, V.; Pinto, N.C.C.; de Oliveira, L.G.; Rodrigues, M.N.; Aragão, D.M.O.; de Aguiar, J.A.K.; Alves, M.S.; Castañon, M.C.N.M.; et al. New aspects on the hepatoprotective potential associated with the antioxidant, hypocholesterolemic and anti-inflammatory activities of Vernonia condensata Baker. J. Ethnopharmacol. 2017, 198, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2007, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Kalyani Nair, K.; Kharb, S.; Thompkinson, D.K. Inulin dietary fiber with functional and health attributes—A review. Food Rev. Int. 2010, 26, 189–203. [Google Scholar] [CrossRef]
- Roshanravan, N.; Mahdavi, R.; Alizadeh, E.; Ghavami, A.; Saadat, Y.R.; Alamdari, N.M.; Alipour, S.; Dastouri, M.R.; Ostadrahimi, A. The effects of sodium butyrate and inulin supplementation on angiotensin signaling pathway via promotion of Akkermansia muciniphila abundance in type 2 diabetes; a randomized, double-blind, placebo-controlled trial. J. Cardiovasc. Thorac. Res. 2017, 9, 183–190. [Google Scholar] [CrossRef]
- Bao, T.; He, F.; Zhang, X.; Zhu, L.; Wang, Z.; Lu, H.; Wang, T.; Li, Y.; Yang, S.; Wang, H. Inulin exerts beneficial effects on non-alcoholic fatty liver disease via modulating gut microbiome and suppressing the lipopolysaccharide-toll-like receptor 4-Mψ-nuclear factor-κB-nod-like receptor protein 3 pathway via gut-liver axis in mice. Front. Pharmacol. 2020, 11, 558525. [Google Scholar] [CrossRef]
- Comelli, M.C.; Mengs, U.; Schneider, C.; Prosdocimi, M. Toward the definition of the mechanism of action of silymarin: Activities related to cellular protection from toxic damage induced by chemotherapy. Integr. Cancer Ther. 2007, 6, 120–129. [Google Scholar] [CrossRef]
- Lee, C.C.; Shen, S.R.; Lai, Y.J.; Wu, S.C. Rutin and quercetin, bioactive compounds from tartary buckwheat, prevent liver inflammatory injury. Food Funct. 2013, 4, 794–802. [Google Scholar] [CrossRef]
- Domitrović, R.; Jakovac, H.; Marchesi, V.V.; Vladimir-Knežević, S.; Cvijanović, O.; Tadić, Ž.; Romić, Ž.; Rahelić, D. Differential hepatoprotective mechanisms of rutin and quercetin in CCl4-intoxicated BALB/cN mice. Acta Pharmacol. Sin. 2012, 33, 1260–1270. [Google Scholar] [CrossRef]
- De David, C.; Rodrigues, G.; Bona, S.; Meurer, L.; González-Gallego, J.; Tuñón, M.J.; Marroni, N.P. Role of quercetin in preventing thioacetamide-induced liver injury in rats. Toxicol. Pathol. 2011, 39, 949–957. [Google Scholar] [CrossRef]
- De Vries, J.H.; Hollman, P.C.; Meyboom, S.; Buysman, M.N.; Zock, P.L.; van Staveren, W.A.; Katan, M.B. Plasma concentrations and urinary excretion of the antioxidant flavonols quercetin and kaempferol as biomarkers for dietary intake. Am. J Clin. Nutr. 1998, 68, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scarbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Zhou, Q.; Yang, R.; Zeng, J.; Li, X.; Zhang, R.; Tang, W.; Li, H.; Wang, S.; Shen, T.; et al. Naringin attenuates high fat diet induced non-alcoholic fatty liver disease and gut bacterial dysbiosis in mice. Front. Microbiol. 2020, 11, 585066. [Google Scholar] [CrossRef] [PubMed]
- Afifi, N.A.; Ibrahim, M.A.; Galal, M.K. Hepatoprotective influence of quercetin and ellagic acid on thioacetamide-induced hepatotoxicity in rats. Can. J. Physiol. Pharmacol. 2018, 96, 624–629. [Google Scholar] [CrossRef]
- Zhao, L.; Mehmood, A.; Soliman, M.M.; Iftikhar, A.; Iftikhar, M.; Aboelenin, S.M.; Wang, C. Protective effects of ellagic acid against alcoholic liver disease in mice. Front. Nutr. 2021, 14, 744520. [Google Scholar] [CrossRef]
- Silacci, P.; Tretola, M. Pomegranate’s ellagitannins: Metabolism and mechanisms of health promoting properties. Nutri. Food Sci. Int. J. 2019, 9, 555766. [Google Scholar] [CrossRef]
- Al-Sayed, E.; Korinek, M.; Esmat, A.; Chen, G.Y.; Cheng, Y.B.; Hsieh, P.W.; Chen, B.H.; Hwang, T.L. Anti-inflammatory, hepatoprotective and antioxidant activity of ellagitannin isolated from Melaleuca styphelioides. Phytochemistry 2020, 177, 112429. [Google Scholar] [CrossRef]
- Schreiber, K.; Ripperger, H. Jurubine, a novel type of steroidal saponin with (25S)-3β-amino-5α-furostane-22α. 26-diol o (26)-β-D-Glucopyranoside structure from Solanum paniculatum L. Tetrahedron Lett. 1966, 48, 5997–6002. [Google Scholar] [CrossRef]
- Böttcher, S.; Drusch, S. Saponins—Self-assembly and behavior at aqueous interfaces. Adv. Colloid Interface Sci. 2017, 243, 105–113. [Google Scholar] [CrossRef]
- Daoudi, N.E.; Bnouham, M. Hepatoprotective essential oils: A review. J. Pharmacopuncture 2020, 23, 124–141. [Google Scholar] [CrossRef]
- Arizuka, N.; Murakami, T.; Suzuki, K. The effect of β-caryophyllene on nonalcoholic steatohepatitis. J. Toxicol. Pathol. 2017, 30, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Ames-Sibin, A.P.; Barizão, C.L.; Castro-Ghizoni, C.V.; Silva, F.M.S.; Sá-Nakanishi, A.B.; Bracht, L.; Bersani-Amado, C.A.; Marçal-Natali, M.R.; Bracht, A.; Comar, J.F. β-Caryophyllene, the major constituent of copaiba oil, reduces systemic inflammation and oxidative stress in arthritic rats. J. Cell. Biochem. 2018, 119, 10262–10277. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Manieri, J.A.A.; Correa, V.G.; Backes, E.; de Sá-Nakanishi, A.B.; Bracht, L.; Comar, J.F.; Corrêa, R.C.G.; Peralta, R.M.; Bracht, A. A Critical Appraisal of the Most Recent Investigations on the Hepatoprotective Action of Brazilian Plants. Plants 2022, 11, 3481. https://doi.org/10.3390/plants11243481
Garcia-Manieri JAA, Correa VG, Backes E, de Sá-Nakanishi AB, Bracht L, Comar JF, Corrêa RCG, Peralta RM, Bracht A. A Critical Appraisal of the Most Recent Investigations on the Hepatoprotective Action of Brazilian Plants. Plants. 2022; 11(24):3481. https://doi.org/10.3390/plants11243481
Chicago/Turabian StyleGarcia-Manieri, Jéssica Amanda Andrade, Vanesa Gesser Correa, Emanueli Backes, Anacharis Babeto de Sá-Nakanishi, Lívia Bracht, Jurandir Fernando Comar, Rúbia Carvalho Gomes Corrêa, Rosane Marina Peralta, and Adelar Bracht. 2022. "A Critical Appraisal of the Most Recent Investigations on the Hepatoprotective Action of Brazilian Plants" Plants 11, no. 24: 3481. https://doi.org/10.3390/plants11243481
APA StyleGarcia-Manieri, J. A. A., Correa, V. G., Backes, E., de Sá-Nakanishi, A. B., Bracht, L., Comar, J. F., Corrêa, R. C. G., Peralta, R. M., & Bracht, A. (2022). A Critical Appraisal of the Most Recent Investigations on the Hepatoprotective Action of Brazilian Plants. Plants, 11(24), 3481. https://doi.org/10.3390/plants11243481








