Nephroprotective Activity of Papaloquelite (Porophyllum ruderale) in Thioacetamide-Induced Injury Model
Abstract
1. Introduction
2. Results
2.1. Anti-Inflammatory Activity
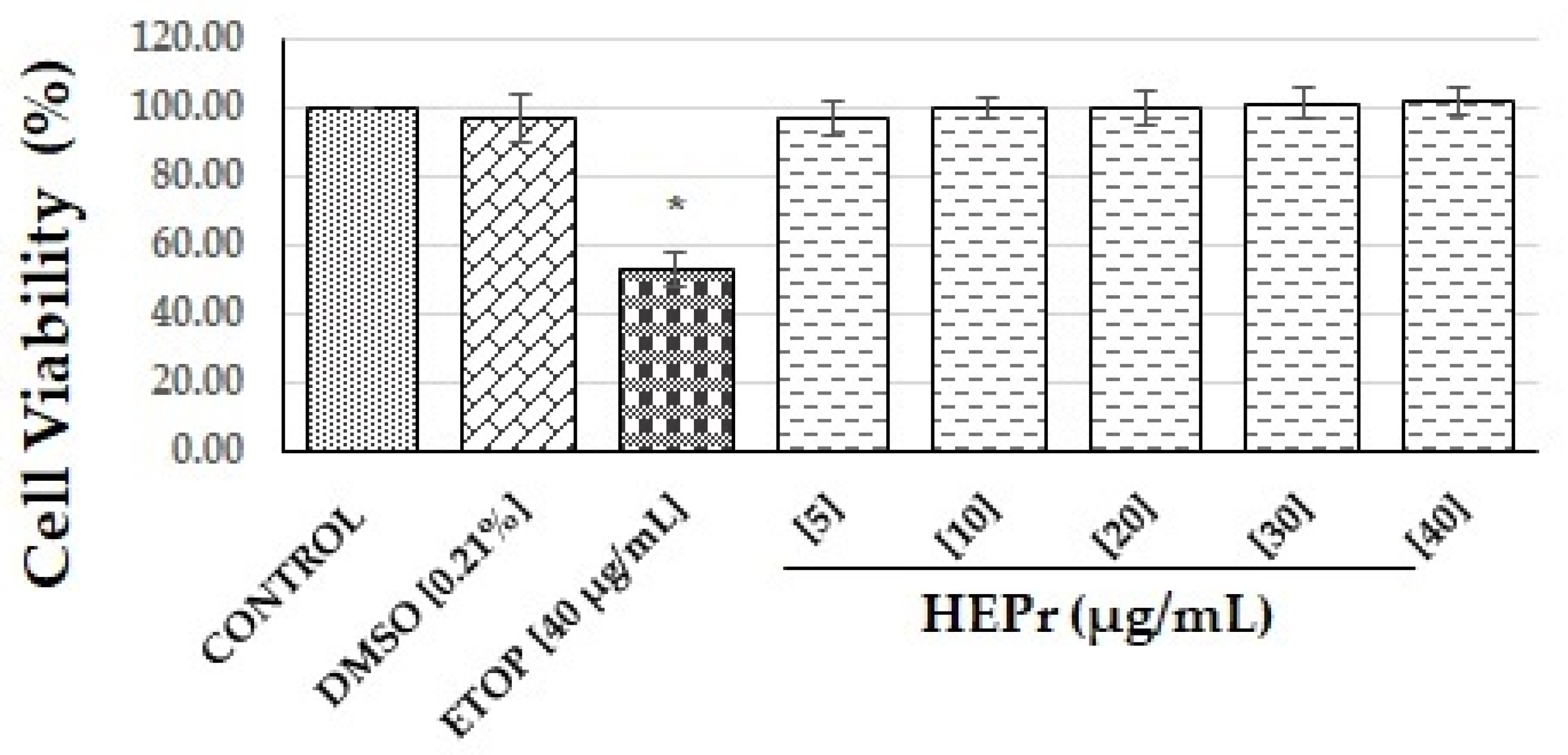
2.1.1. Cell Viability Tests
2.1.2. Inhibition of Nitric Oxide (NO) Production
2.2. Antioxidant Activity
2.3. Acute Oral Toxicity
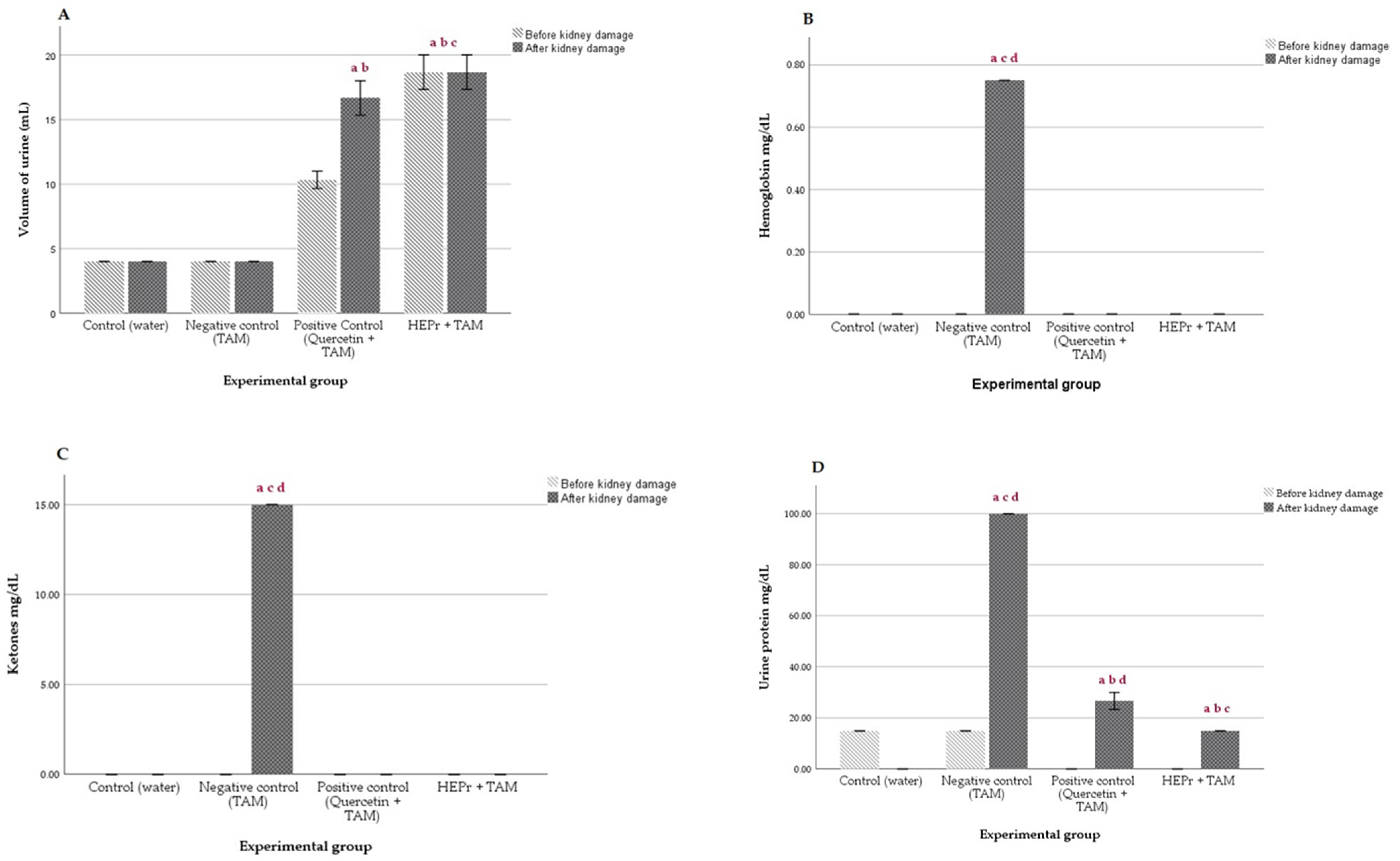
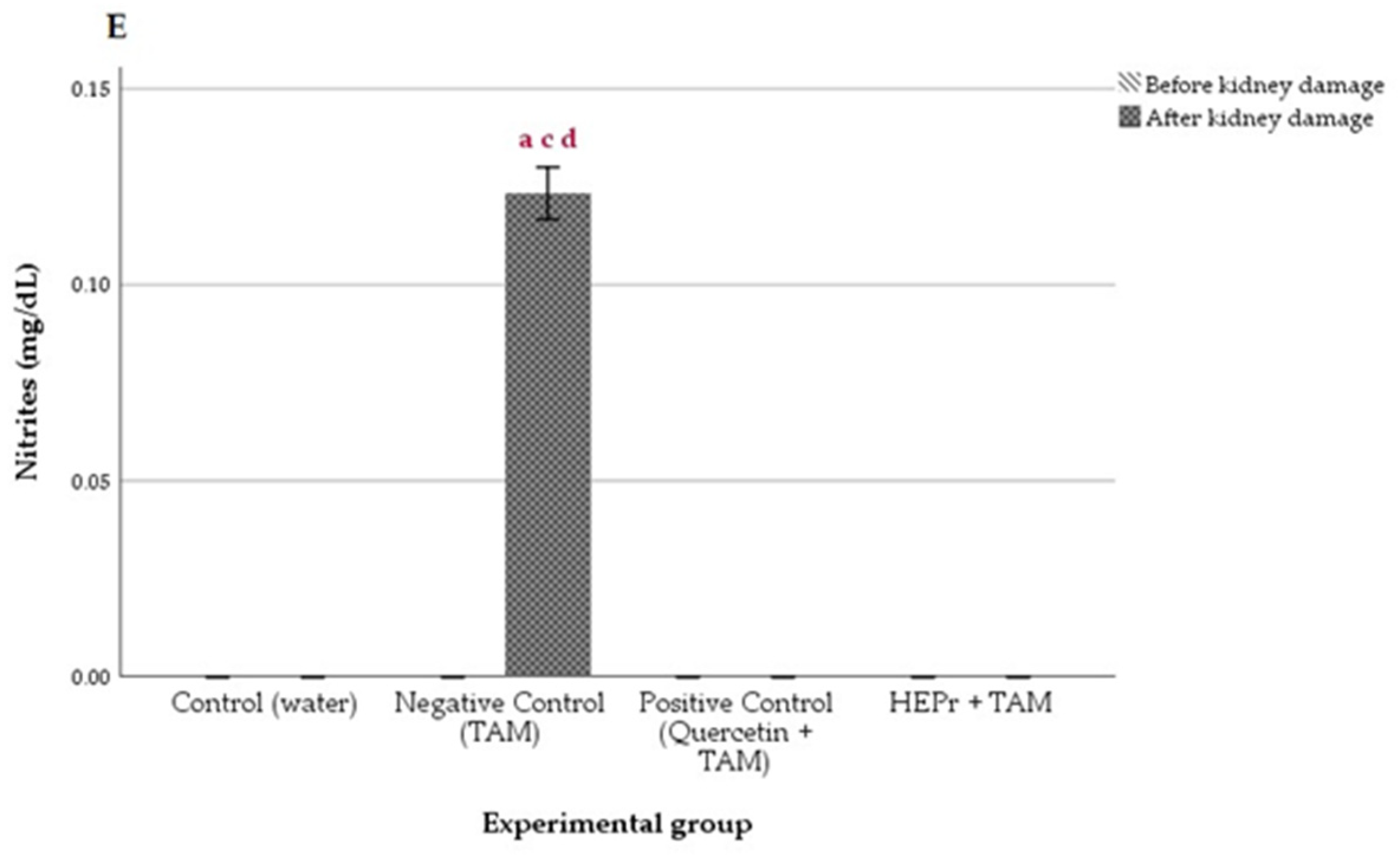
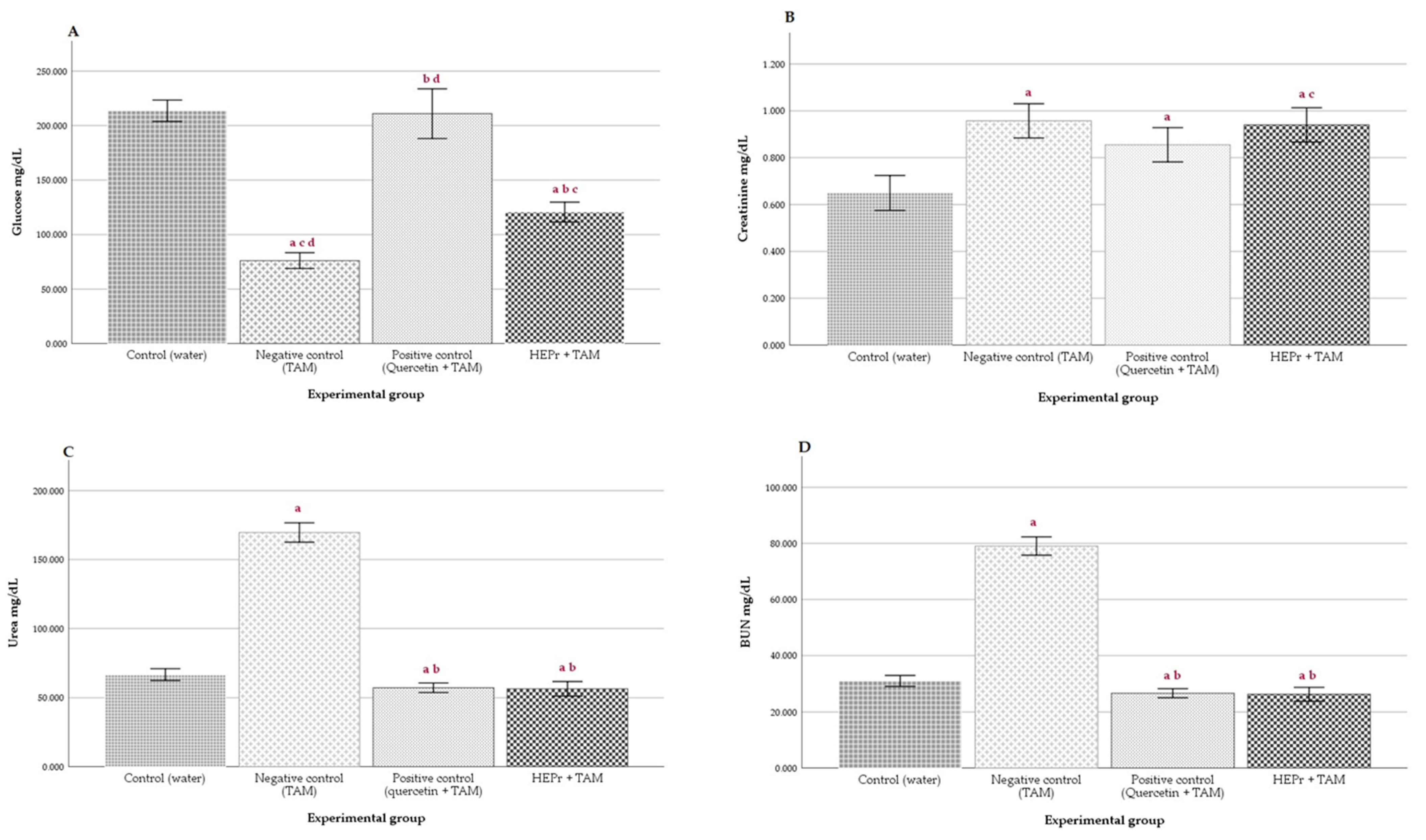
2.4. In Vivo Nephroprotective Activity
2.5. Major Compounds
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Hydroalcoholic Extract (HEPr)
4.3. In Vitro and In Vivo Test
4.3.1. Anti-Inflammatory Activity
Cell Culture
Cell Viability by MTS Assay
Treatment of Macrophages with Lipopolysaccharide (LPS)
Determination of NO Concentration
4.3.2. In Vitro Antioxidant Activity Assays
DPPH Radical Scavenging Assay
ABTS Radical Scavenging Assay
Ferric Reducing Antioxidant Power (FRAP) Assay
Total Phenolic Content
4.3.3. In vivo Acute Oral Toxicity
4.3.4. In Vivo Nephroprotective Activity
Biochemical Assays
4.4. Identification of Major Compounds of HEPr
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellomo, R.; Kellum, J.A.; Ronco, C. Acute kidney injury. Lancet 2012, 380, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.J. Acute kidney injury. Nat. Rev. Dis. Prim. 2021, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Burki, S.; Burki, Z.G.; Asghar, M.A.; Ali, I.; Zafar, S. Phytochemical, acute toxicity and renal protective appraisal of Ajuga parviflora hydromethanolic leaf extract against CCl4 induced renal injury in rats. BMC Complement. Med. Ther. 2021, 21, 198. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Iñiguez, J.S.; García-García, G.; Lombardi, R. Epidemiología y desenlaces de la lesión renal aguda en Latinoamérica. Gac. Med. Mex. 2018, 1, 6–14. [Google Scholar] [CrossRef]
- Breton, S.; Brown, D. Novel Proinflammatory Function of Renal Intercalated Cells. Ann. Nutr. Metab. 2018, 72, 11–16. [Google Scholar] [CrossRef]
- Boubekri NBelloum, Z.; Boukaabache, R.; Amrani, A.; Kahoul, N.; Hamama, W.; Zama, D.; Boumaza, O.; Bouriche, H.; Benayache, F.; Benayache, S. In vivo anti-inflammatory and in vitro antioxidant activities of Genista quadriflora Munby extracts. Sch. Res. Libr. 2014, 6, 1–7. [Google Scholar]
- Mancuso, P. The role of adipokines in chronic inflammation. ImmunoTargets Ther. 2016, 5, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Caravaca, F.; Sánchez-Casado, E. Inflamación en la enfermedad renal crónica avanzada: Características clínicas asociadas y valor pronóstico. DYT Premio Gen. Lab 2004, 25, 3–16. [Google Scholar]
- Papi, S.; Ahmadizar, F.; Hasanvand, A. The role of nitric oxide in inflammation and oxidative stress. Immunopathol. Persa 2019, 5, e08. [Google Scholar] [CrossRef]
- Modlinger, P.S.; Wilcox, C.S.; Aslam, S. Nitric Oxide, oxidative stress, and progression of chronic renal failure. Semin. Nephrol. 2004, 24, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Abdelaaty, A.S.; Heba, D.H.; Riaz, U.; Ali, S.A.; Husseiny, A.H.; Alicja, K.; Abdel-Razik, H.F. Renoprotective and Cardioprotective Potential of Moricandia sinaica (Boiss.) against Carbon Tetrachloride-Induced Toxicity in Rats. Evid. Based Complement. Altern. Med. 2022, 2022, 8545695. [Google Scholar]
- Cengiz, M. Renoprotective effects of Silybum marianum (L.) Gaertn (Silymarin) on thioacetamide-induced renal injury: Biochemical and histopathological approach. Pak. J. Pharm. Sci. 2018, 31, 2137–2141. [Google Scholar]
- Alomar, M.Y. Physiological and histopathological study on the influence of Ocimum basilicum leaves extract on thioacetamide-induced nephrotoxicity in male rats. Saudi J. Biol. Sci. 2020, 27, 1843–1849. [Google Scholar] [CrossRef]
- Wang, K.; Deng, Y.; Zhang, J.; Cheng, B.; Huang, Y.; Meng, Y.; Zhong, K.; Xiong, G.; Guo, J.; Liu, Y.; et al. Toxicity of thioacetamide and protective effects of quercetin in zebrafish (Danio rerio) larvae. Environ. Toxicol. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zargar, S.; Alonazi, M.; Rizwana, H.; Wani, T.A. Resveratrol reverses thioacetamide-induced renal assault with respect to oxidative stress, renal function, DNA damage, and cytokine release in Wistar rats. Oxid. Med. Cell Longev. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shirai, M.; Arakawa, S.; Miida, H.; Matsuyama, T.; Kinoshita, J.; Makino, T.; Kai, K.; Teranishi, M. Thioacetamide-induced hepatocellular necrosis is attenuated in diet-induced obese mice. J. Toxicol. Pathol. 2013, 26, 175–186. [Google Scholar] [CrossRef][Green Version]
- Universidad Nacional Autónoma de México (UNAM). Biblioteca Digital de la Medicina Tradicional Mexicana. Pápalo o Papaloquelite. Available online: http://www.medicinatradicionalmexicana.unam.mx/apmtm/termino.php?l=3&t=papalo-papaloquelite (accessed on 2 October 2022).
- Castro Lara, D.; Basurto Peña, F.; Mera Ovando, L.M.; Bye Boettler, R.A. Los Quelites, Tradición Milenaria en México, 1st ed.; León Márquez Ortíz.: Chapingo, Mexico, 2022; p. 32. [Google Scholar]
- Castro Lara, D.; Bye Boettler, R.A.; Mera Ovando, L.M. Diagnóstico del Pápaloquelite en México, 1st ed.; León Márquez Ortíz.: Chapingo, Mexico, 2011; pp. 1–55. [Google Scholar]
- Fukalova Fukalova, T.; García-Martínez, M.D.; Raigón, M.D. Nutritional composition, bioactive compounds, and volatiles profile characterization of two edible undervalued plants: Portulaca oleracea L. and Porophyllum ruderale (Jacq.) Cass. Plants 2022, 11, 377. [Google Scholar] [CrossRef]
- Milan, P.; Hayashi, A.H.; Appezzato-da-Glória, B. Comparative leaf morphology and anatomy of three Asteraceae species. Braz. Arch. Biol. Technol. 2006, 49, 135–144. [Google Scholar] [CrossRef]
- Postigo, A.; Funes, M.; Petenatti, E.; Bottai, H.; Pacciaroni, A.; Sortino, M. Antifungal photosensitive activity of Porophyllum obscurum (Spreng.) DC.: Correlation of the chemical composition of the hexane extract with the bioactivity. Photodiagnosis Photodyn. Ther. 2017, 20, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Villavicencio-Araujo, N.Y. Evaluación de la Actividad Antiespasmódica del Extracto Hidroalcohólico de Hojas de Porophyllum Ruderale (Jac.) Cassini “Rupay Wachi”, Sobre el Íleon Aislado de Cavia Porcellus “Cobayo”, Ayacucho 2016; Universidad Nacional de San Cristóbal de Huamanga: Huamanga, Perú, 2017. [Google Scholar]
- Takahashi, H.T.; Novello, C.R.; Ueda-Nakamura, T.; Filho, B.P.D.; Palazzo de Mello, J.C.; Nakamura, C.V. Thiophene Derivatives with Antileishmanial Activity Isolated from Aerial Parts of Porophyllum ruderale (Jacq.) Cass. Molecules 2011, 16, 3469–3478. [Google Scholar] [CrossRef] [PubMed]
- Enciso Gutiérrez, J.; Amiel Pérez, J.; Guija Poma, E. Antioxidant activity of hydroalcoholic extract of medicinal plants and stimulation of fibroblast proliferation. Rev. Soc. Quím. Perú 2010, 76, 73–79. [Google Scholar]
- Lima, G.M.; Bonfim, R.R.; Silva, M.R.; Thomazzi, S.M.; Santos, M.R.V.; Quintans-Júnior, L.J.; Bonjardim, L.R.; Araújo, A.A.S. Assessment of antinociceptive and anti- inflammatory activities of Porophyllum ruderale aqueous extract. Rev. Braz. J. Pharmacogn. 2011, 21, 486–490. [Google Scholar] [CrossRef]
- Robles-Zepeda, R.E.; Velázquez-Contreras, C.A.; Garibay-Escobar, A.; Galvéz-Ruíz, J.C.; Ruiz-Bustos, E. Antimicrobial activity of Northwestern Mexican plants against Helicobacter pylori. J. Med. Food 2011, 14, 1280–1283. [Google Scholar] [CrossRef] [PubMed]
- Conde-Hernández, L.A.; Guerrero-Beltrán, J.Á. Total phenolics and antioxidant activity of Piper auritum and Porophyllum ruderale. Food Chem. 2014, 142, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Conde-Hernández, L.A.; Espinosa-Victoria, J.R.; Guerrero-Beltrán, J.Á. Supercritical extraction of essential oils of Piper auritum and Porophyllum ruderale. J. Supercrit. Fluids 2017, 127, 97–102. [Google Scholar] [CrossRef]
- Pawłowska, K.A.; Baracz, T.; Skowronska, W.; Piwowarski, J.P.; Majdna, M.; Malarz, J.; Stojakowska, A.; Zidorn, C.; Granica, S. The contribution of phenolics to the anti-inflammatory potential of the extract from Bolivian coriander (Porophyllum ruderale subsp. ruderale). Food Chem. 2022, 371, 131116. [Google Scholar] [CrossRef]
- Sánchez-Ramos, M.; Álvarez, L.; Romero-Estrada, A.; Bernabé-Antonio, A.; Marquina-Bahena, S.; Cruz-Sosa, F. Establishment of a Cell Suspension Culture of Ageratina pichinchensis (Kunth) for the Improved Production of Anti-Inflammatory Compounds. Plants 2020, 9, 1398. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Rufián-Henares, J.A.; Morales, F.J. Assessing the Antioxidant Activity of Melanoidins from Coffee Brews by Different Antioxidant Methods. J. Agric. Food Chem. 2005, 53, 7832–7836. [Google Scholar] [CrossRef]
- Holser, R. Principal Component Analysis of Phenolic Acid Spectra. Int. Sch. Res. Netw. 2012, 2012, 493203. [Google Scholar] [CrossRef]
- Santibáñez, A.; Herrera-Ruiz, M.; González-Cortazar, M.; Nicasio-Torres, P.; Sharma, A.; Jiménez-Ferrer, E. Pharmacokinetics and Tissue Distribution of Coumarins from Tagetes lucida in an LPS-Induced Neuroinflammation Model. Plants 2022, 11, 2805. [Google Scholar] [CrossRef] [PubMed]
- Garro, A.; Cardona, W.; Rojano, B.; Robledo, S.M.; Alzate, F. Actividad antioxidante y citotóxica de extractos de Pilea dauciodora Weed (Urticaceae). Rev. Cuba. Plantas Med. 2015, 20, 88–97. [Google Scholar]
- Yang, F.; Qi, Y.; Liu, W.; Li, J.; Wang, D.; Fang, L.; Zhang, Y. Separation of Five Flavonoids from Aerial Parts of Salvia Miltiorrhiza Bunge Using HSCCC and Their Antioxidant Activities. Molecules 2019, 24, 3448. [Google Scholar] [CrossRef]
- Souza, M.C.; Siani, A.C.; Ramos, M.F.S.; Menezes-de-Lima, O., Jr.; Henriques, M.G.M.O. Evaluation of anti-inflammatory activity of essential oils from two Asteraceae species. Pharmazie 2003, 58, 582–586. [Google Scholar] [PubMed]
- Segura-Cobos, D.; Martínez-Juárez, M.E.; Casas-García, G.; Martínez-Cortés, G.; Guzmán-Hernández, E.A.; Vázquez-Cruz, B. Anti-inflammatory and antinociceptive properties of the extracts from the leaves of Porophyllum tagetoides and Annona reticulata. J. Med. Plants Stud. 2019, 7, 50–54. [Google Scholar]
- Vázquez-Atanacio, M.J.; Bautista-Ávila, M.; Velázquez-González, C.; Castañeda-Ovando, A.; González-Cortázar, M.; Sosa-Gutiérrez, C.; Ojeda-Ramírez, D. Porophyllum Genus Compounds and Pharmacological Activities: A Review. Sci. Pharm. 2021, 89, 7. [Google Scholar] [CrossRef]
- Lee, H.J.; Jeong, H.S.; Kim, D.J.; Noh, Y.H.; Yuk, D.Y.; Hong, J.T. Inhibitory effect of Citral on NO production by suppression of iNOS expression and NF-κB activation in RAW264.7 cells. Arch. Pharm. Res. 2008, 31, 342–349. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z., Jr.; Scheerens, C.J.; Raymond Miller, A. Modified 2,2-Azino-bis-3-ethylbenzothiazoline-6-sulfonic Acid (ABTS) Method to Measure Antioxidant Capacity of Selected Small Fruits and Comparison to Ferric Reducing Antioxidant Power (FRAP) and 2,2′-Diphenyl-1-picrylhydrazyl (DPPH) Methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Kim, D.-O.; Lee, C.Y. Comprehensive study on vitamin C equivalent antioxidant capacity (VCEAC) of various polyphenolics in scavenging a free radical and its structural relationship. Crit. Rev. Food Sci. Nutr. 2004, 44, 253–273. [Google Scholar] [CrossRef]
- Kato da Silva, B.A.; Marques dos Santos, C.H.; Moraes-Gaspar, C.O.; Malzac, C.F.; Rivero-Wendt, C.L.G.; Alves de Souza, C.N.; Bogo, D.; Mesquita-Dourado, D.; Trennepohl-Souza, M.E.; Alencar-Fernandes, F.H.; et al. Effect of Porophyllum ruderale (Jacq.) Cass. in the Liver of the B16-F10 Murine Melanoma Model and Antioxidant Potential. Ensaios Ciência 2021, 25, 309–314. [Google Scholar]
- Jimenez, M.; Guzman, A.P.; Azuara, E.; García, O.; Mendoza, M.R.; Beristain, C.I. Volatile Compounds and Antioxidative Activity of Porophyllum tagetoides Extracts. Plant Foods Hum. Nutr. 2012, 67, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Khan, S.M.; ul Haq, I.; Farzana, F.; Abdullah, A.; Abbasi, A.M.; Alamri, S.; Hashem, M.; Sakhi, S.; Asif, M.; et al. Antioxidant potential in the leaves of grape varieties (Vitis vinifera L.) grown in different soil compositions. Arab. J. Chem. 2021, 14. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Jourdes, M.; Femenia, A.; Simal, S.; Roselló, C.; Teissedre, P.L. Proanthocyanidin composition and antioxidant potential of the stem winemaking byproducts from 10 different grape varieties (Vitis vinifera L.). J. Agric. Food Chem. 2012, 60, 11850–11858. [Google Scholar] [CrossRef] [PubMed]
- Obert, L.A.; Elmore, S.A.; Ennulat, D.; Frazier, K.S. A Review of Specific Biomarkers of Chronic Renal Injury and Their Potential Application in Nonclinical Safety Assessment Studies. Toxicol. Pathol. 2021, 20, 996–1023. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation [NKF]. KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021, 99, S1–S87. [Google Scholar] [CrossRef] [PubMed]
- Gorriz Teruel, J.L.; Navarro-González, J.F.; Mora-Fernández, C.; Martínez-Castelao, A. Factores de progresión de la enfermedad renal crónica en la diabetes mellitus. Diagnóstico y cribado de la enfermedad renal crónica en la diabetes mellitus. Soc. Española Nefrol. 2016, 28, 1–14. [Google Scholar]
- Gutiérrez Vázquez, I.; Domínguez Maza, A.; Acevedo Mariles, J.J. Fisiopatología del síndrome urémico. Rev. Del Hosp. Gen. Dr. Man. Gea González 2003, 6, 13–24. [Google Scholar]
- Molina, P.A. Metabolic syndrome and kidney disease. Rev. Méd. Clín. Condes. 2010, 21, 553–560. [Google Scholar]
- Waters, N.J.; Waterfield, C.J.; Farrant, R.D.; Holmes, E.; Nicholson, J.K. Metabonomic Deconvolution Of Embedded Toxicity: Application To Thioacetamide Hepato- and Nephrotoxicity. Chem. Res. Toxicol. 2005, 18, 639–654. [Google Scholar] [CrossRef]
- García-Maset, R.; Bover, J.; Segura de la Morena, J.; Goicoechea-Diezhandino, M.; Cebollada del Hoyo, J.; Escalada-San Martín, J.; Fácila-Rubio, L.; Gamarra-Ortíz, J.; García-Donaire, J.A.; García-Matarín, L.; et al. Documento de consenso para la detección y manejo de la enfermedad renal crónica. Nefrol. Rev. Soc. Española Nefrol. 2022, 42, 233–264. [Google Scholar] [CrossRef]
- Al-Attar, A.M.; Alrobai, A.A.; Almalki, D.A. Protective effect of olive and juniper leaves extracts on nephrotoxicity induced by thioacetamide in male mice. Saudi J. Biol. Sci. 2017, 24, 15–22. [Google Scholar] [CrossRef]
- Shaikh Omar, A.M. The potential protective influence of flaxseed oil against renal toxicity induced by thioacetamide in rats. Saudi J. Biol. Sci. 2018, 25, 1696–1702. [Google Scholar] [CrossRef]
- Bashandy, S.A.E.; Awdan, S.A.; El Mohamed, S.M.; Abdel, E.; Omara, A. Allium porrum and Bauhinia Variegata Mitigate Acute Liver Failure and Nephrotoxicity Induced by Thioacetamide in Male Rats. Indian J. Clin. Biochem. 2020, 35, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Kadir, F.A.; Kassim, N.M.; Abdulla, M.A.; Yehye, W.A. Effect of oral administration of ethanolic extract of Vitex negundo on thioacetamide-induced nephrotoxicity in rats. BMC Complement. Altern. Med. 2013, 13, 4–8. [Google Scholar]
- De Athayde, A.E.; Salles de Araujo, C.E.; Pergaud Sandjo, L.; Weber Biavatti, M. Metabolomic analysis among ten traditional “Arnica” (Asteraceae) from Brazil. J. Ethnopharmacol. 2021, 265, 113149. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; De León, J.A.; Reyes, S.M.; Miranda, S.Y. Componentes Bioactivos de Diferentes Marcas de Café Comerciales de Panamá. Relación entre Ácidos Clorogénicos y Cafeína. Información Tecnológica 2018, 29, 43–54. [Google Scholar] [CrossRef]
- Suárez-Quiroz, M.L.; Taillefer, W.; López-Méndez, E.M.; González-Ríos, O.; Villeneube, P.; Figueroa-Espinoza, M.C. Antibacterial activity and antifungal and anti-mycotoxigenic activities against Aspergillus flavus and A. ochraceus of green coffee chlorogenic acids and dodecyl chlorogenates. J. Food Saf. 2013, 33, 360–368. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Ou, S.; Kwok, K.-C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Teixeira, J.; Gaspar, A.; Garrido, E.M.; Garrido, J.; Borges, F. Hydroxycinnamic Acid Antioxidants: An Electrochemical Overview. Biomed Res. Int. 2013, 1–11. [Google Scholar] [CrossRef]
- Gladine, C.; Rock, E.; Morand, C.; Bauchart, D.; Durand, D. Bioavailability and antioxidant capacity of plant extracts rich in polyphenols, given as a single acute dose, in sheep made highly susceptible to lipoperoxidation. Br. J. Nutr. 2007, 98, 691–701. [Google Scholar] [CrossRef]
- Muñoz Jáuregui, A.M.; Ramos-Escudero, D.F.; Alvarado-Ortiz Ureta, C.; Castañeda-Castañeda, B. Evaluación de la capacidad antioxidante y contenido de compuestos fenólicos en recursos vegetales promisorios. Revista Sociedad Química Perú 2007, 73, 142–149. [Google Scholar]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.; Boelens, P.G.; van Norren, K.; van Leeuwen, P. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Mamani-Matsuda, M.; Kauss, T.; Al-Kharrat, A.; Rambert, J.; Fawaz, F.; Thiolat, D.; Moynet, D.; Coves, S.; Malvy, D.; Mossalayi, M.D. Therapeutic and preventive properties of quercetin in experimental arthritis correlate with decreased macrophage inflammatory mediators. Biochem. Pharmacol. 2006, 72, 1304–1310. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, P.D.; Lopez-Hernandez, F.J.; Perez-Barriocanal, F.; Morales, A.I.; Lopez-Novoa, J.M. Original Article Quercetin reduces cisplatin nephrotoxicity in rats without compromising its anti-tumour activity. Nephrol. Dial. Transpl. 2011, 26, 3484–3495. [Google Scholar] [CrossRef]
- Morales, A.I.; Vicente-Sánchez, C.; Santiago-Sandoval, J.M.; Egido, J.; Mayoral, P.; Arévalo, M.A.; Fernández-Tagarro, M.; López-Novoa, J.M.; Pérez-Barriocanal, F. Protective effect of quercetin on experimental chronic cadmium nephrotoxicity in rats is based on its antioxidant properties. Food Chem. Toxicol. 2006, 44, 2092–2100. [Google Scholar] [CrossRef]
- Morales, A.I.; Vicente-Sánchez, I.; Jerkic, M.; Santiago, J.M.; Sánchez-González, P.D.; Pérez-Barriocanal f López-Novoa, J.M. Effect of quercetin on metallothionein, nitric oxide synthases and cyclooxygenase-2 expression on experimental chronic cadmium nephrotoxicity in rats. Toxicol. Appl. Pharmacol. 2006, 210, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Al-Majedy, Y.; Al-Amiery, A.; Kadhum, A.A.; BakarMohamad, A. Antioxidant activity of coumarins. Syst. Rev. Pharm. 2017, 8, 24–30. [Google Scholar] [CrossRef]
- Kostova, I.; Bhatia, S.; Grigorov, P.; Balkansky, S.; Parmar, V.S.; Prasad, A.K.; Saso, L. Coumarins as Antioxidants. Curr. Med. Chem. 2012, 18, 3929–3951. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple Coumarins and Analogues in Medicinal Chemistry: Occurrence, Synthesis and Biological Activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.; Huerta, P.; Carrasco, R.; Rodrigo, R. Estrés oxidativo y daño renal. CIMEL Ciencia Investigación Médica Estudiantil Latinoamericana 2003, 8, 44–53. [Google Scholar]
- Ruiz, D.H.; Fernández Caraballo, D.; Rodríguez, J.A.; Ballesteros-Hernández, M. Oxidative stress in hypertension-related renal failure. Revista Cuba. Plantas Med. 2012, 31, 16–25. [Google Scholar]
- Mesa-Vanegas, A.M.; Zapata-Uribe, S.; Arana, L.M.; Zapata, I.C.; Monsalve, Z.; Rojano, B. Actividad antioxidante de extractos de diferente polaridad de Ageratum conyzoides L. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2015, 14, 1–10. [Google Scholar]
- Benzie, I.F.F.; Devaki, M. The Ferric Reducing/Antioxidant Power (FRAP) Assay for Non-Enzymatic Antioxidant Capacity: Concepts, Procedures, Limitations and Applications. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 77–106. ISBN 9781119135388. [Google Scholar]
- FRAP Antioxidant Assay: G-Biosciences. Available online: https://cdn.gbiosciences.com/pdfs/protocol/FRAP_Assay.pdf (accessed on 8 December 2022).
- Folin, O.; Ciocalteu, V. On Tyrosine and Tryptophane determinations in Proteins. J. Biol. Chem. 1927, LXXIII, 627–648. [Google Scholar] [CrossRef]
- OECD. Test No. 423: Acute Oral toxicity - Acute Toxic Class Method. In OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2002; pp. 1–14. [Google Scholar]
- Gutiérrez-Román, A.S.; González-Cortázar, M.; Trejo-Tapia, G.; Herrera-Ruíz, M.; Zamilpa, A.; Sánchez-Mendoza, E.; De la Cruz-Sánchez, N.G.; Jiménez-Ferrer, E. Angiotensin-converting enzyme inhibitors from Salvia elegans Vahl. Nat. Prod. Res. 2021, 35, 5344–5349. [Google Scholar] [CrossRef]


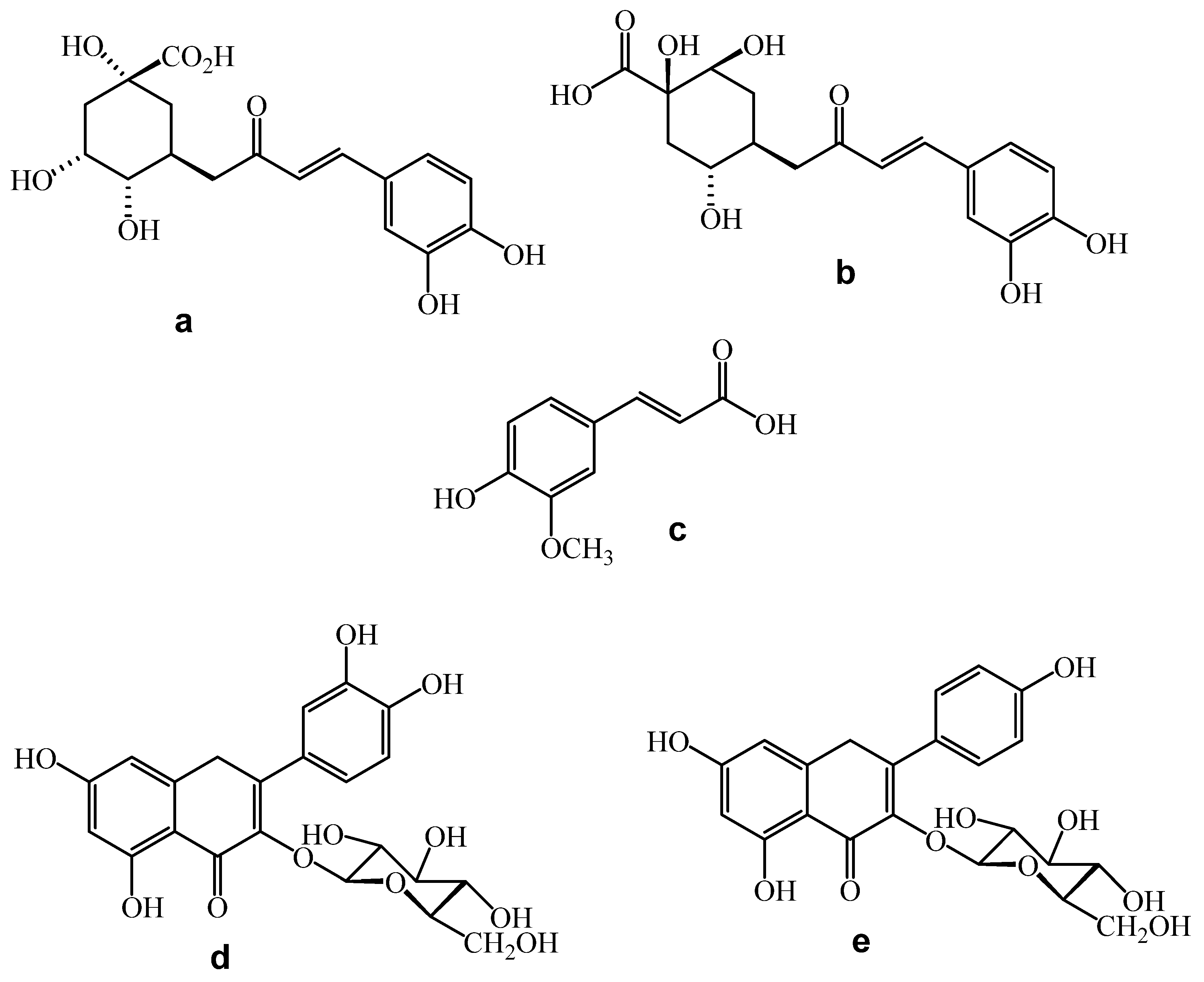
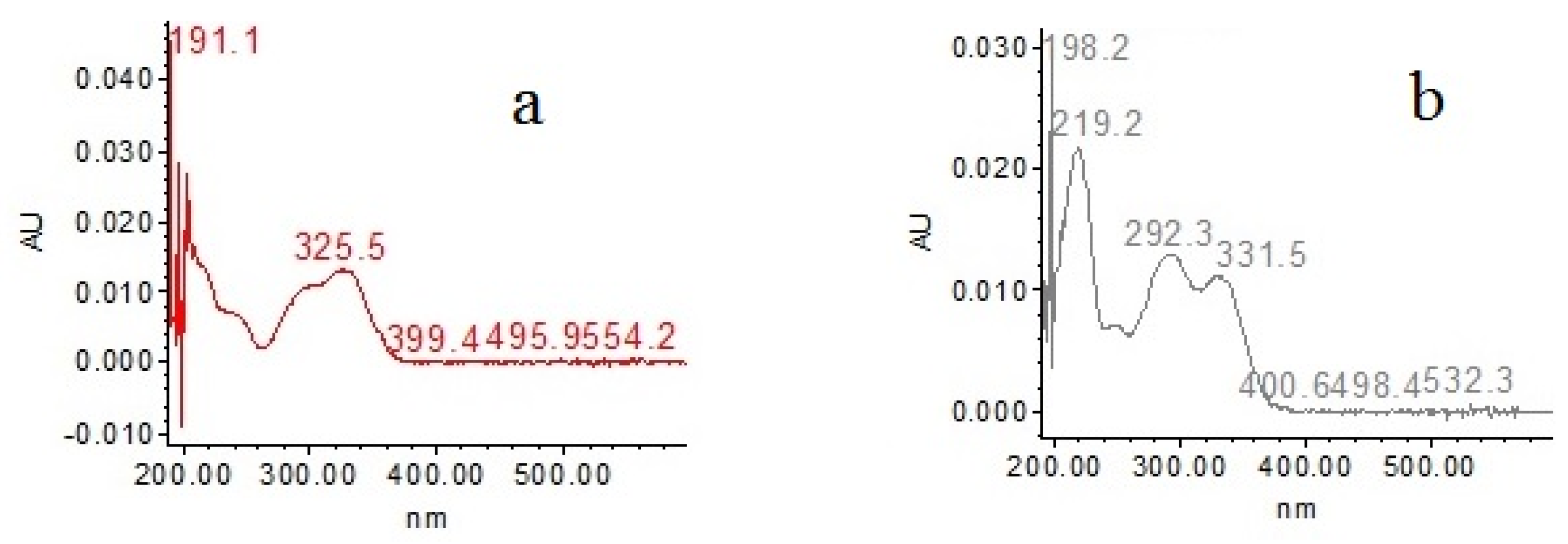
| Sample | Total Phenolics (mgGAE/100 g) | ABTS (µmol TE/100 g) | % Inhibition | DPPH (µmol TE/100 g) | % Inhibition | FRAP (mg FeSO4/100 g) | % Inhibition |
|---|---|---|---|---|---|---|---|
| HEPr | 13993.67 ± 0.016 | 16116.03 ± 0.038 | 32.96 ± 2.496% | 1502.40 ± 0.0407 | 63.06 ± 1.733% | 4836.14 ± 0.072 | 69.04 ± 1.958% |
| Phase | Intragastric Dose (mg/kg) |
|---|---|
| Phase I | 5000 |
| Mortality | 0/5 |
| LD50 | >5000 |
| Compound | mg/g Extract |
|---|---|
| 5-O-caffeoylquinic acid | 310.82 |
| 4-O-caffeoylquinic acid | 340.39 |
| Quercetin-3-O-glucoside | 24.06 |
| Kaempferol-3-O-glucoside | 23.00 |
| Ferulic acid | 137.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Atanacio, M.J.; Bautista, M.; González-Cortazar, M.; Romero-Estrada, A.; De la O-Arciniega, M.; Castañeda-Ovando, A.; Sosa-Gutiérrez, C.G.; Ojeda-Ramírez, D. Nephroprotective Activity of Papaloquelite (Porophyllum ruderale) in Thioacetamide-Induced Injury Model. Plants 2022, 11, 3460. https://doi.org/10.3390/plants11243460
Vázquez-Atanacio MJ, Bautista M, González-Cortazar M, Romero-Estrada A, De la O-Arciniega M, Castañeda-Ovando A, Sosa-Gutiérrez CG, Ojeda-Ramírez D. Nephroprotective Activity of Papaloquelite (Porophyllum ruderale) in Thioacetamide-Induced Injury Model. Plants. 2022; 11(24):3460. https://doi.org/10.3390/plants11243460
Chicago/Turabian StyleVázquez-Atanacio, María José, Mirandeli Bautista, Manasés González-Cortazar, Antonio Romero-Estrada, Minarda De la O-Arciniega, Araceli Castañeda-Ovando, Carolina G. Sosa-Gutiérrez, and Deyanira Ojeda-Ramírez. 2022. "Nephroprotective Activity of Papaloquelite (Porophyllum ruderale) in Thioacetamide-Induced Injury Model" Plants 11, no. 24: 3460. https://doi.org/10.3390/plants11243460
APA StyleVázquez-Atanacio, M. J., Bautista, M., González-Cortazar, M., Romero-Estrada, A., De la O-Arciniega, M., Castañeda-Ovando, A., Sosa-Gutiérrez, C. G., & Ojeda-Ramírez, D. (2022). Nephroprotective Activity of Papaloquelite (Porophyllum ruderale) in Thioacetamide-Induced Injury Model. Plants, 11(24), 3460. https://doi.org/10.3390/plants11243460







