New Chromones from Bouvardia ternifolia (Cav.) Schltdl with Anti-Inflammatory and Immunomodulatory Activity
Abstract
:1. Introduction
2. Results
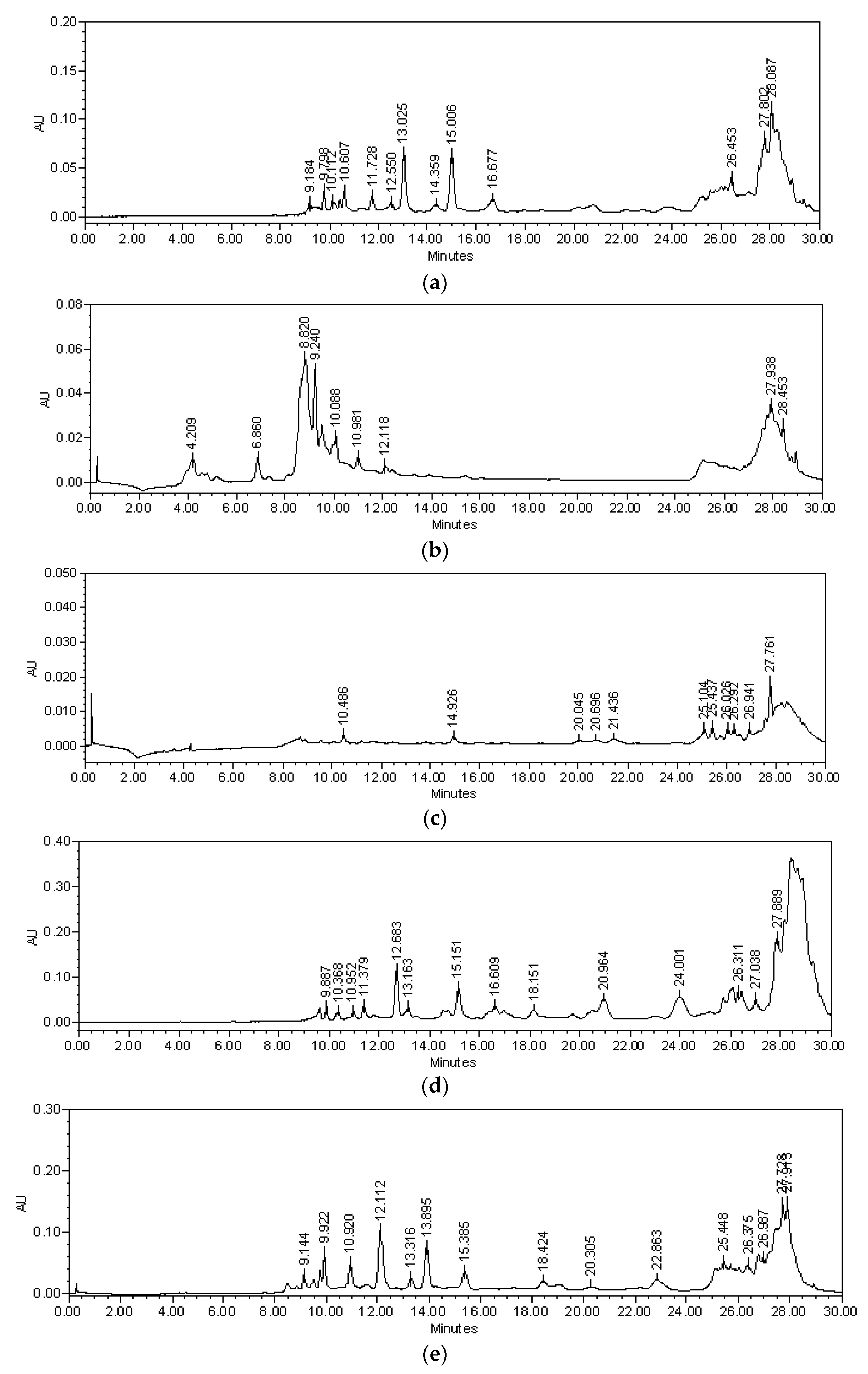
2.1. Chemical Analysis
2.2. Isolation of Compounds 1–4
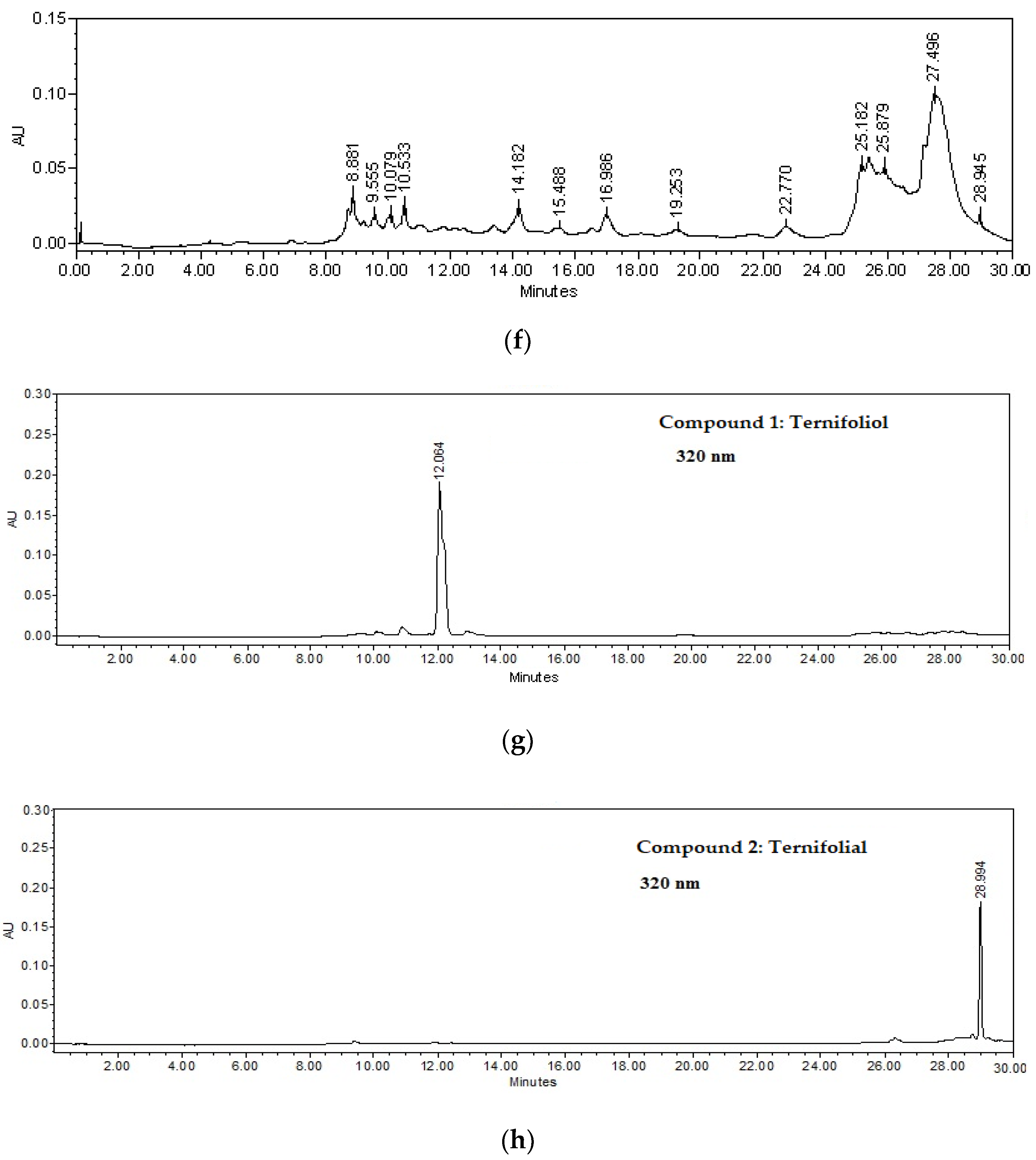
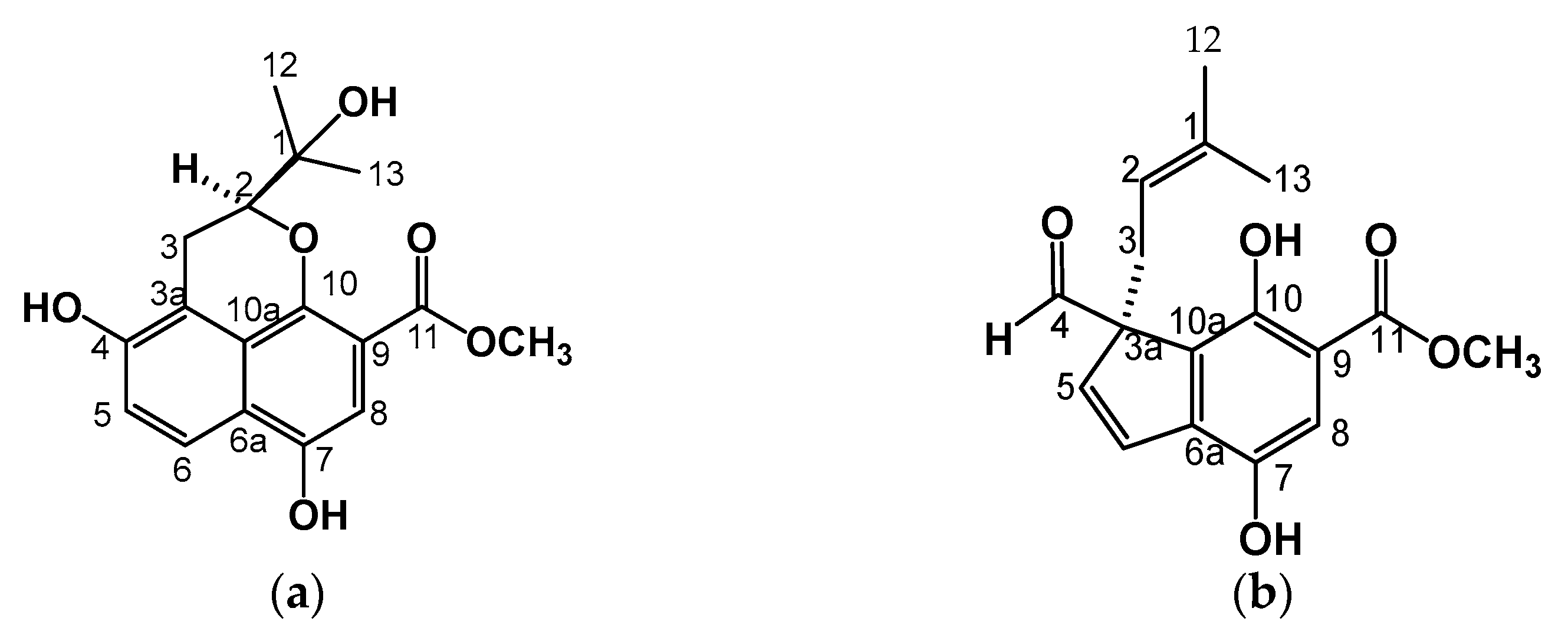
2.2.1. Ternifoliol (1)
2.2.2. Ternifolial (2)
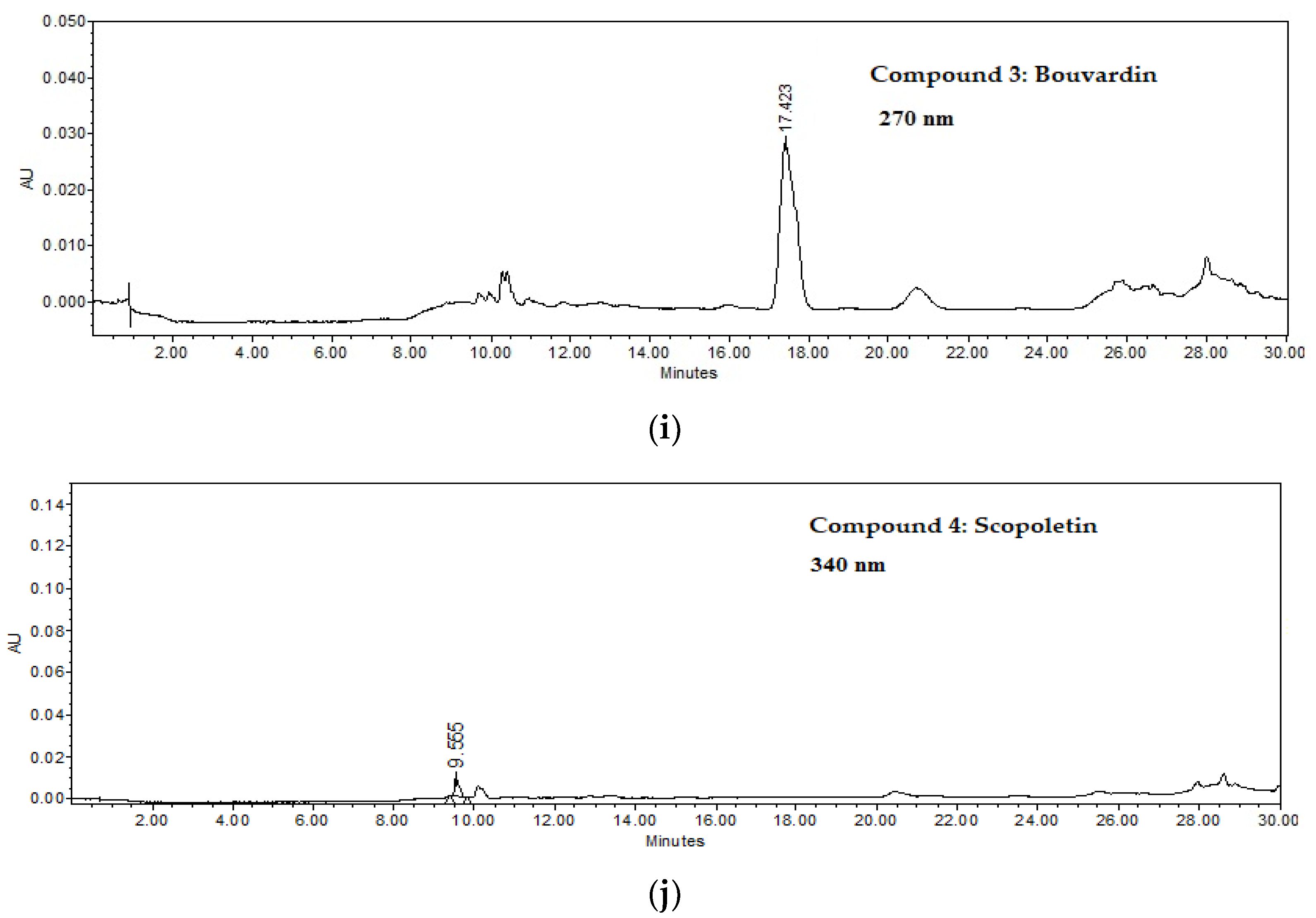
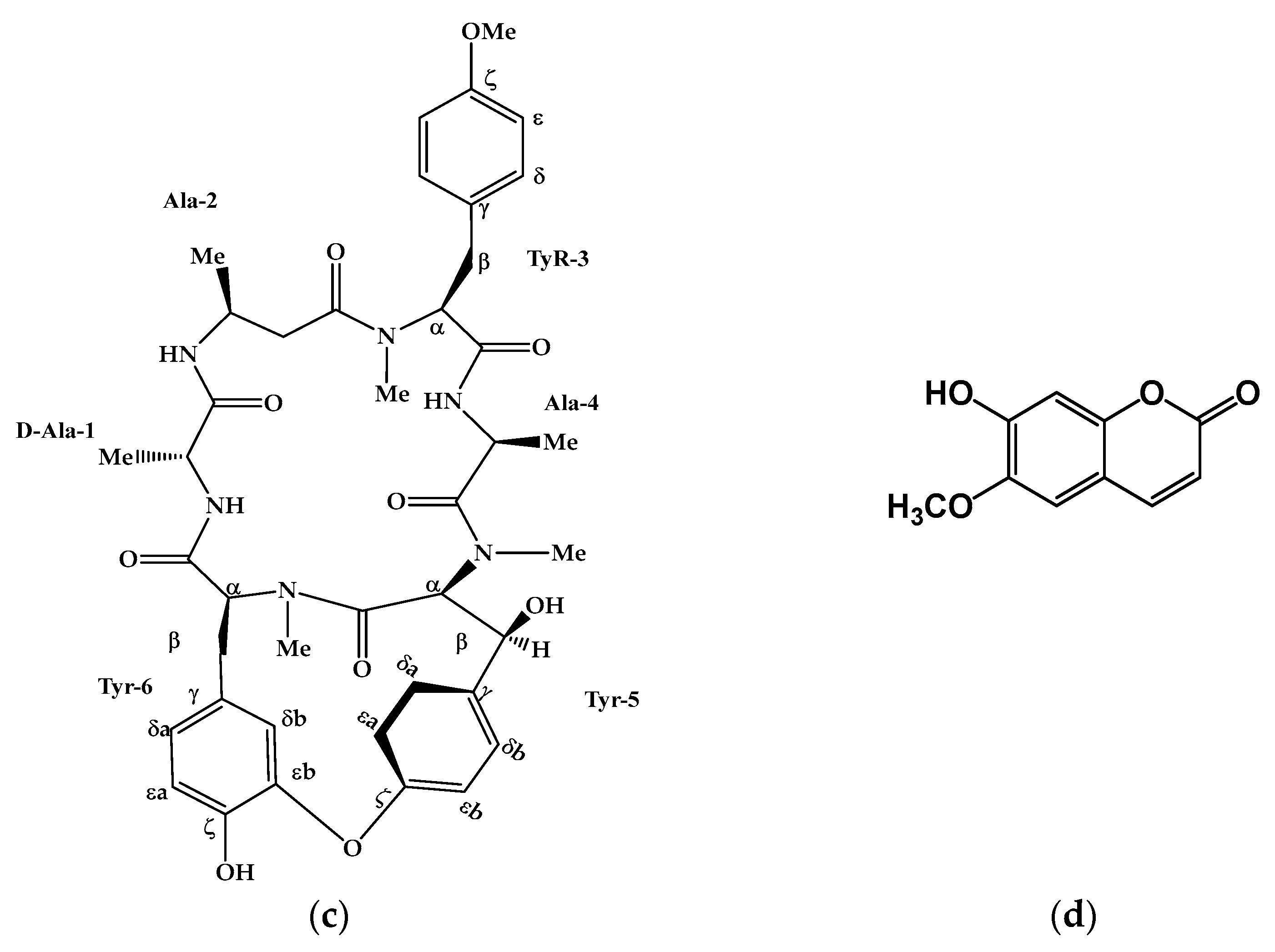
2.2.3. Bouvardin (3)
2.2.4. Scopoletin (4)
2.3. Anti-Inflammatory Activity of B. ternifolia on Joints Edema Induced with Freund’s Adjuvant
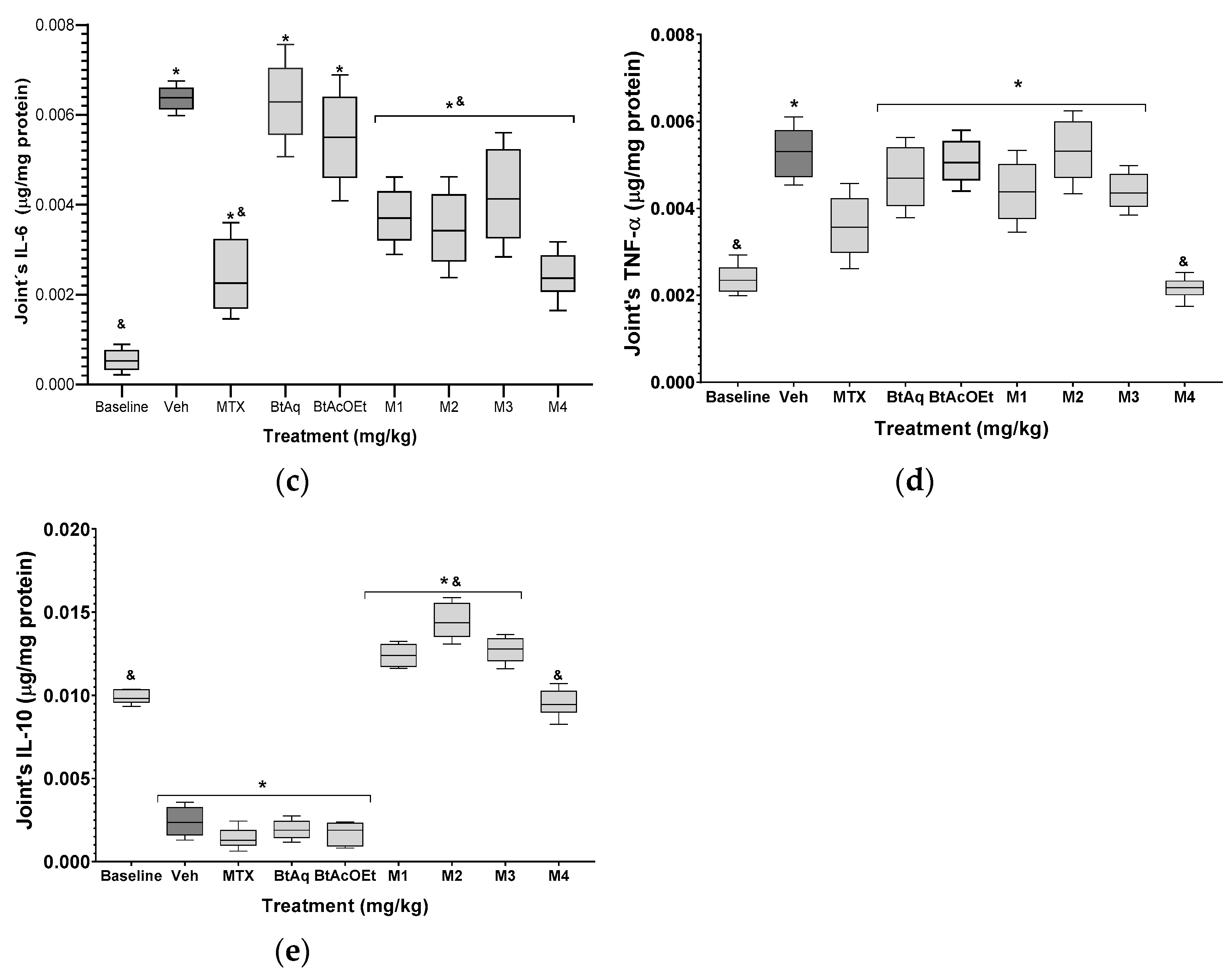
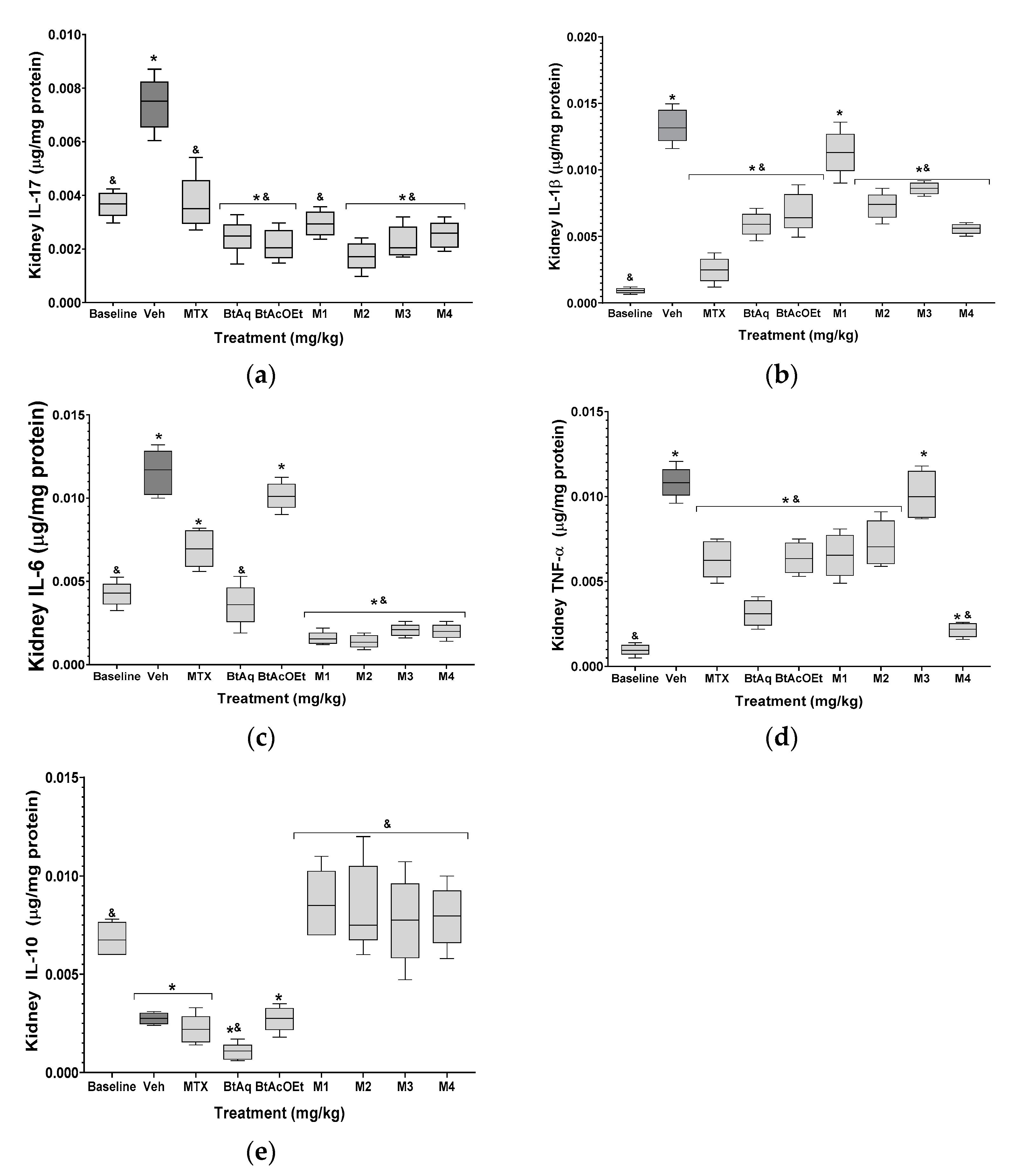
2.4. B. ternifolia on the Cytokine’s Levels in Kidney and Joints of Mice RA-Induced with Freund’s Adjuvant
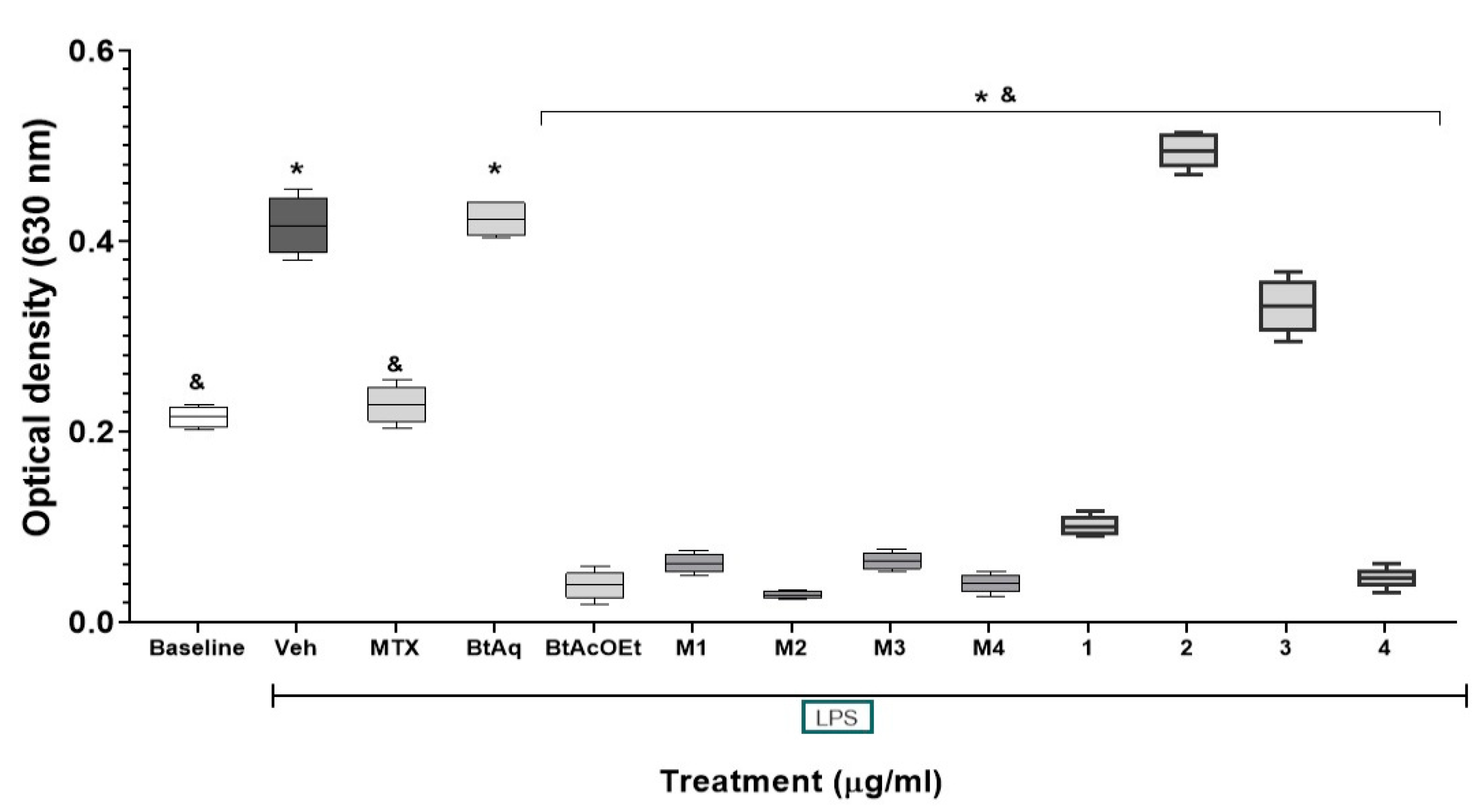
2.5. Effect of Fractions and Compounds from B. ternifolia on the Expression of NF-κB In Vitro
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. General Experimental Procedures
4.3. Extracts and Fractions from B. Ternifolia
4.4. Isolation and Identification of Compounds 1, 2, 3, and 4
4.5. Raw-Blue™ Cells Culture Experiments
4.6. Evaluation of the Antiarthritic Effect In Vivo of Bouvardia Ternifolia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smolen, J.S. Atlas of Rheumatoid Arthritis. In Atlas of Rheumatoid Arthritis; Emery, P., Ed.; Springer Healthcare Ltd.: Cheshire, UK, 2015. [Google Scholar] [CrossRef]
- Sharma, S.; Plant, D.; Bowes, J.; Macgregor, A.; Verstappen, S.; Barton, A.; Viatte, S. HLA-DRB1 haplotypes predict cardiovascular mortality in inflammatory polyarthritis independent of CRP and anti-CCP status. Arthritis. Res. Ther. 2022, 24, 90. [Google Scholar] [CrossRef] [PubMed]
- Radu, A.F.; Bungau, S.G. Management of Rheumatoid Arthritis: An Overview. Cells 2021, 10, 2857. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Ryu, H.J.; Baek, H.M.; Shin, D.M.; Hong, J.H. Dynamic synovial fibroblasts are modulated by NBCn1 as a potential target in rheumatoid arthritis. Exp. Mol. Med. 2022, 54, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Lu, W.; Chen, W.; Wu, Y.; Wang, Q.; Liu, Y. A narrative review of the role of the Notch signaling pathway in rheumatoid arthritis. Ann. Transl. Med. 2022, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Dunkhunthod, B.; Talabnin, C.; Murphy, M.; Thumanu, K.; Sittisart, P.; Eumkeb, G. Gymnema inodorum (Lour.) Decne. Extract Alleviates Oxidative Stress and Inflammatory Mediators Produced by RAW264.7 Macrophages. Oxid. Med. Cell. Longev. 2021, 2021, 8658314. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.N.; Xie, G.P.; Qin, C.H.; Chen, Y.R.; Zhang, K.R.; Li, X.; Yu, B. Aucubin prevents interleukin-1 beta induced inflammation and cartilage matrix degradation via inhibition of NF-κB signaling pathway in rat articular chondrocytes. Int. Immunopharmacol. 2015, 24, 408–415. [Google Scholar] [CrossRef]
- Lee, S.A.; Moon, S.M.; Han, S.H.; Hwang, E.J.; Park, B.R.; Kim, J.-S.; Kim, D.K.; Kim, C.S. Chondroprotective effects of aqueous extract of Anthriscus sylvestris leaves on osteoarthritis In Vitro and In Vivo through MAPKs and NF-κB signaling inhibition. Biomed. Pharmacother. 2018, 103, 1202–1211. [Google Scholar] [CrossRef]
- García-Morales, G.; Huerta-Reyes, M.; González-Cortazar, M.; Zamilpa, A.; Jiménez-Ferrer, E.; Silva-García, R.; Roman-Ramos, R.; Aguilar, R.A. Anti-inflammatory, antioxidant and anti-acetylcholinesterase activities of Bouvardia ternifolia: Potential implications in Alzheimer’s disease. Arch. Pharm. Res. 2015, 38, 1369–1379. [Google Scholar] [CrossRef]
- Herrera-Ruiz, M.; Garcia-Morales, G.; Zamilpa, A.; Gonzalez-Cortazar, M.; Tortoriello, J.; Ventura-Zapata, E.; Jiménez-Ferrer, E. Inhibition of acetylcholinesterase activity by hidroalcoholic extract and their fractions of Bouvardia ternifolia (Cav.) Shcltdl (Rubiaceae). Bol. Latinoam. Plantas. Med. Aromat. 2012, 11, 526–541. [Google Scholar]
- Bates, R.B.; Cole, J.R.; Hoffmann, I.J.J.; Kriek, G.R.; Linz, G.S.; Torrancel, S.J. Solution forms of Bouvardin and relatives from NMR studies. 6-O-Methylbouvardin. Am. Chem. Soc. 1983, 105, 1343–1347. [Google Scholar] [CrossRef]
- Hajizadeh, A.; Abtahi-Froushani, S.M.; Tehrani, A.A.; Azizi, S.; Bani-Hashemi, S.R. Effects of naringenin on experimentally induced rheumatoid arthritis in wistar rats. Arch. Razi. Inst. 2021, 76, 903–912. [Google Scholar] [CrossRef]
- Paul, A.K.; Jahan, R.; Paul, A.; Mahboob, T.; Bondhon, T.A.; Jannat, K.; Hasan, A.; Nissapatorn, V.; Wilairatana, P.; de Lourdes-Pereira, M.; et al. The Role of Medicinal and Aromatic Plants against Obesity and Arthritis: A Review. Nutrients 2022, 14, 985. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Kuroda, T.; Kobayashi, D. Cytokine networks in the pathogenesis of rheumatoid arthritis. Int. J. Mol. Sci. 2021, 22, 10922. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.K.; Li, Y.; Dang, Q.; Yang, F. Monocytes in rheumatoid arthritis: Circulating precursors of macrophages and osteoclasts and, their heterogeneity and plasticity role in RA pathogenesis. Int. Immunopharmacol. 2018, 65, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xu, Z.; Dan, Y.L.; Cheng, J.; Zhao, C.N.; Mao, Y.M.; Xiang, K.; Hu, Y.Q.; He, Y.S.; Pan, H.F. Association between traffic-related air pollution and hospital readmissions for rheumatoid arthritis in Hefei, China: A time-series study. Environ. Pollut. 2021, 268, 115628. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Huang, W.; Zhang, W.; Mei, J.; Zhu, C. A Tale of Two Immune Cells in Rheumatoid Arthritis: The Crosstalk between Macrophages and T Cells in the Synovium. Front. Immunol. 2021, 12, 655477. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.X.; Wu, Y.J.; Zhang, J.; Wei, W. T-cells interact with B cells, dendritic cells, and fibroblast-like synoviocytes as hub-like key cells in rheumatoid arthritis. Int. Immunopharmacol. 2019, 70, 428–434. [Google Scholar] [CrossRef]
- Giles, J.T. Extra-articular manifestations and comorbidity in rheumatoid arthritis: Potential impact of prerheumatoid arthritis prevention. Clin. Ther. 2019, 41, 1246–1255. [Google Scholar] [CrossRef]
- Kapoor, T.; Bathon, J. Renal manifestations of rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 2018, 44, 571–584. [Google Scholar] [CrossRef]
- Kumar, V.; Gupta, M.; Gandhi, S.G.; Bharate, S.S.; Kumar, A.; Vishwakarma, R.A.; Bharate, S.B. Anti-inflammatory chromone alkaloids and glycoside from Dysoxylum binectariferum. Tetrahedron Lett. 2017, 58, 3974–3978. [Google Scholar] [CrossRef]
- Di Stasi, L.C. Coumarin Derivatives in Inflammatory Bowel Disease. Molecules 2021, 26, 422. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.; Saleem, A.; Xaveria, A.; Akhtar, M.F. Daphnetin: A bioactive natural coumarin with diverse therapeutic potentials. Front. Pharmacol. 2022, 13, 993562. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, E.H.M.; Sayed, A.M.; Hussein, O.E.; Mahmoud, A.M. Coumarins as modulators of the Keap1/Nrf2/ARE signaling pathway. Oxid. Med. Cell. Longev. 2020, 2020, 1675957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nygaard, G.; Firestein, G.S. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat. Rev. Rheumatol. 2020, 16, 316–333. [Google Scholar] [CrossRef] [PubMed]
- Navrazhina, K.; Frew, J.W.; Grand, D.; Williams, S.C.; Hur, H.; Gonzalez, J.; Garcet, S.; Krueger, J.G. Interleukin-17RA blockade by brodalumab decreases inflammatory pathways in hidradenitis suppurativa skin and serum. Br. J. Dermatol. 2022, 187, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Yap, H.Y.; Tee, S.Z.Y.; Wong, M.M.T.; Chow, S.K.; Peh, S.C.; Teow, S.Y. Pathogenic role of immune cells in rheumatoid arthritis: Implications in clinical treatment and biomarker development. Cells 2018, 7, 161. [Google Scholar] [CrossRef] [Green Version]
- Mielle, J.; Audo, R.; Hahne, M.; Macia, L.; Combe, B.; Morel, J.; Daien, C. IL-10 Producing B cells ability to induce regulatory T cells is maintained in rheumatoid arthritis. Front. Immunol. 2018, 9, 961. [Google Scholar] [CrossRef] [PubMed]
- Miyagaki, T.; Fujimoto, M.; Sato, S. Regulatory B cells in human inflammatory and autoimmune diseases: From mouse models to clinical research. Int. Immunol. 2015, 27, 495–504. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Rui, K.; Wang, S.; Tian, J. Advances of Regulatory B Cells in Autoimmune Diseases. Front. Immunol. 2021, 12, 592914. [Google Scholar] [CrossRef]
- Stadhouders, R.; Lubberts, E.; Hendriks, R.W. A cellular and molecular view of T helper 17 cell plasticity in autoimmunity. J. Autoimmun. 2018, 87, 1–15. [Google Scholar] [CrossRef]
- Shi, L.; Hu, F.; Zhu, L.; Xu, C.; Zhu, H.; Li, Y.; Liu, H.; Li, C.; Liu, N.; Xu, L.; et al. CD70-mediated CD27 expression downregulation contributed to the regulatory B10 cell impairment in rheumatoid arthritis. Mol. Immunol. 2020, 119, 92–100. [Google Scholar] [CrossRef]
- Zha, B.; Wang, L.; Liu, X.; Liu, J.; Chen, Z.; Xu, J.; Morel, J. Decrease in proportion of CD19 + CD24(hi) CD27 + B cells and impairment of their suppressive function in Graves’ disease. PLoS ONE 2012, 7, e49835. [Google Scholar] [CrossRef]
- Guo, X.; Xu, T.; Zheng, J.; Cui, X.; Li, M.; Wang, K.; Su, M.; Zhang, H.; Zheng, K.; Sun, C. Accumulation of synovial fluid CD19 + CD24hiCD27 + B cells was associated with bone destruction in rheumatoid arthritis. Sci. Rep. 2020, 10, 14386. [Google Scholar] [CrossRef]
- Jiang, J.; Qin, T.; Zhang, L.; Liu, Q.; Wu, J.; Dai, R.; Zhou, L.; Zhao, Q.; Luo, X.; Wang, H.; et al. IL-21 Rescues the Defect of IL-10-Producing Regulatory B Cells and Improves Allergic Asthma in DOCK8 Deficient Mice. Front. Immunol. 2021, 12, 695596. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal. Transd. Target. Ther. 2017, 2, e17023. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, J.P.; Carmody, R.J. NF-κB and the Transcriptional Control of Inflammation. Int. Rev. Cell. Mol. Biol. 2018, 335, 41–84. [Google Scholar] [CrossRef]
- Chitnis, M.P.; Alate, A.D.; Menon, R.S. Effect of bouvardin (NSC 259968) on the growth characteristics a nucleic acids and protein syntheses profiles of P388 leukemia cells. Chemother 1981, 27, 126–130. [Google Scholar] [CrossRef]
- Campos-Vidal, Y.; Herrera-Ruiz, M.; Trejo-Tapia, G.; Gonzalez-Cortazar, M.; Jiménez Aparicio, A.; Zamilpa, A. Gastroprotective activity of kaempferol glycosides from Malvaviscus arboreus Cav. J. Ethnopharmacol. 2021, 268, 113633. [Google Scholar] [CrossRef]



| Position | 1H (J in Hz) 1 | 13C | 1H (J in Hz) 2 | 13C |
|---|---|---|---|---|
| 1 | 72.9 | 133.6 | ||
| 2 | 3.81 (1H, s, br) | 83.9 | 4.56 (1H, dd, 7.3, 8.8) | 119.3 |
| 3 | a) 3.20 (1H, dd, 3.6, 13.5) | 23.6 | a) 3.41 (1H, dd, 7.3, 13.9) | 37.2 |
| 3a | b) 2.72 (1H, dd, 3.6, 13.5) | 115.9 | b) 2.74 (1H, dd, 7.3, 13.9) | 56.7 |
| 4 | 147.9 | 204.9 | ||
| 5 | 120.6 | 132.7 | ||
| 6 | 7.86 (H dd, 0.7, 9.1) | 123.9 | 137.6 | |
| 6a | 7.13 (1H, d, 9.1) | 125.2 | 127.57 | |
| 7 | 149.4 | 6.21 (1H, d, 10.27) | 154.1 | |
| 8 | 105.7 | 113.4 | ||
| 9 | 6.90 (1H, s) | 112.7 | 7.28 (1H, s) | 112.2 |
| 10 | 152.6 | 144.7 | ||
| 10a | 122.7 | 127.53 | ||
| 11 | 169.2 | 6.21 (1H, d, 10.27) | 170.6 | |
| 12 | 25.9 | 25.6 | ||
| 13 | 1.38 (3H, s) | 26.4 | 1.44 (1H, d, 16.14) | 17.7 |
| OCH3 | 1.44 (3H, s) | 52.42 | 1.44 (1H, d, 16.14) | 133.6 |
| Group | Oral Treatment | Doses |
|---|---|---|
| Baseline | Water + tween 1% | 100 µL/10 g |
| Veh | Water + tween 1% + collagen type II + CFA * | 1 mg/mL |
| Experimental | Methotrexate | 25 mg/kg every 5 days |
| BtAq | 25 mg /kg for 15 days | |
| BtAcOEt | ||
| M1 | ||
| M2 | ||
| M4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapata Lopera, Y.M.; Jiménez-Ferrer, E.; Herrera-Ruiz, M.; Zamilpa, A.; González-Cortazar, M.; Rosas-Salgado, G.; Santillán-Urquiza, M.A.; Trejo-Tapia, G.; Jiménez-Aparicio, A.R. New Chromones from Bouvardia ternifolia (Cav.) Schltdl with Anti-Inflammatory and Immunomodulatory Activity. Plants 2023, 12, 1. https://doi.org/10.3390/plants12010001
Zapata Lopera YM, Jiménez-Ferrer E, Herrera-Ruiz M, Zamilpa A, González-Cortazar M, Rosas-Salgado G, Santillán-Urquiza MA, Trejo-Tapia G, Jiménez-Aparicio AR. New Chromones from Bouvardia ternifolia (Cav.) Schltdl with Anti-Inflammatory and Immunomodulatory Activity. Plants. 2023; 12(1):1. https://doi.org/10.3390/plants12010001
Chicago/Turabian StyleZapata Lopera, Yury Maritza, Enrique Jiménez-Ferrer, Maribel Herrera-Ruiz, Alejandro Zamilpa, Manasés González-Cortazar, Gabriela Rosas-Salgado, Mayra Alejandra Santillán-Urquiza, Gabriela Trejo-Tapia, and Antonio Ruperto Jiménez-Aparicio. 2023. "New Chromones from Bouvardia ternifolia (Cav.) Schltdl with Anti-Inflammatory and Immunomodulatory Activity" Plants 12, no. 1: 1. https://doi.org/10.3390/plants12010001
APA StyleZapata Lopera, Y. M., Jiménez-Ferrer, E., Herrera-Ruiz, M., Zamilpa, A., González-Cortazar, M., Rosas-Salgado, G., Santillán-Urquiza, M. A., Trejo-Tapia, G., & Jiménez-Aparicio, A. R. (2023). New Chromones from Bouvardia ternifolia (Cav.) Schltdl with Anti-Inflammatory and Immunomodulatory Activity. Plants, 12(1), 1. https://doi.org/10.3390/plants12010001









