Steroidal Saponins with Plant Growth Stimulation Effects; Yucca schidigera as a Commercial Source
Abstract
1. Introduction
2. Results
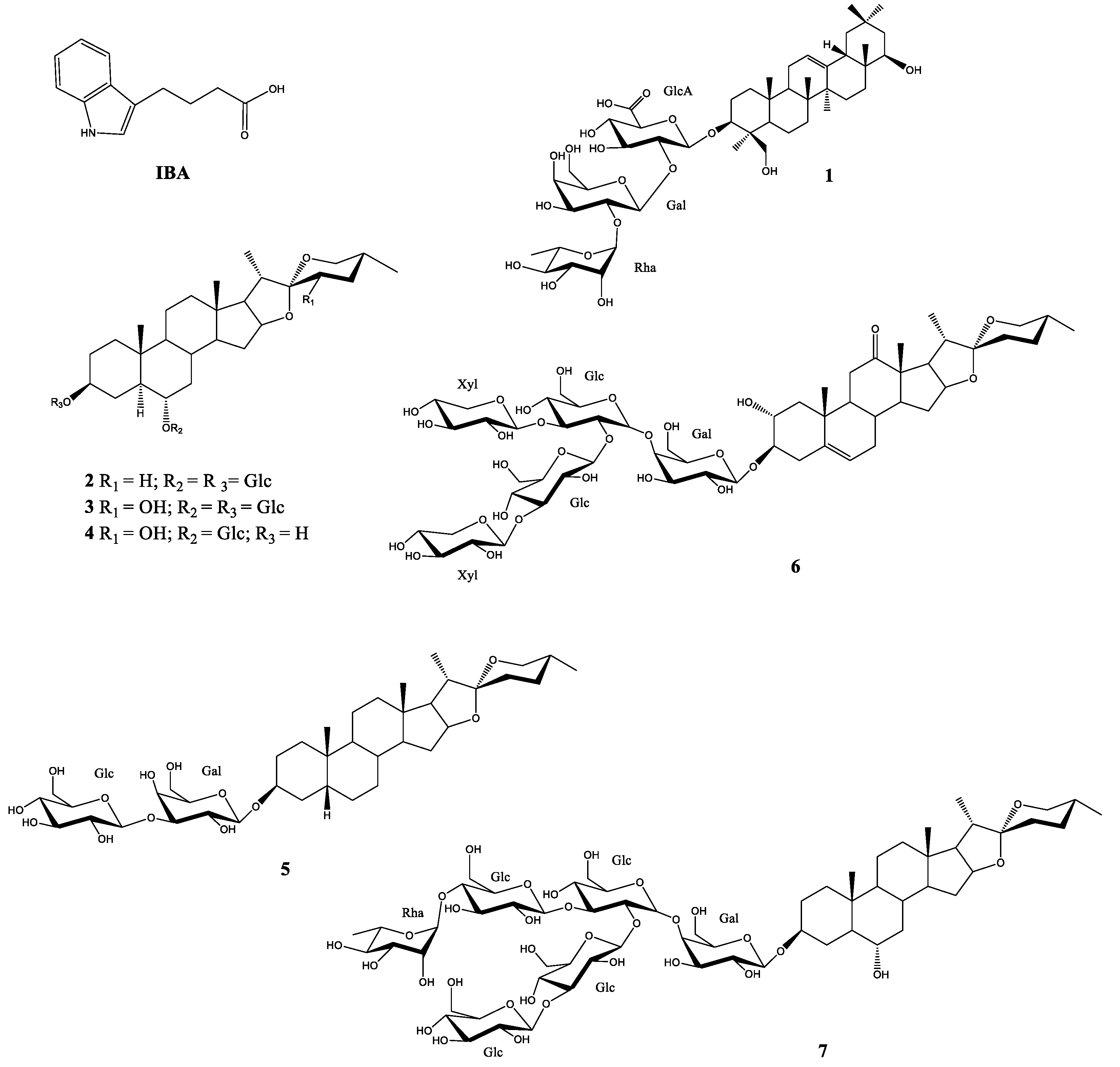
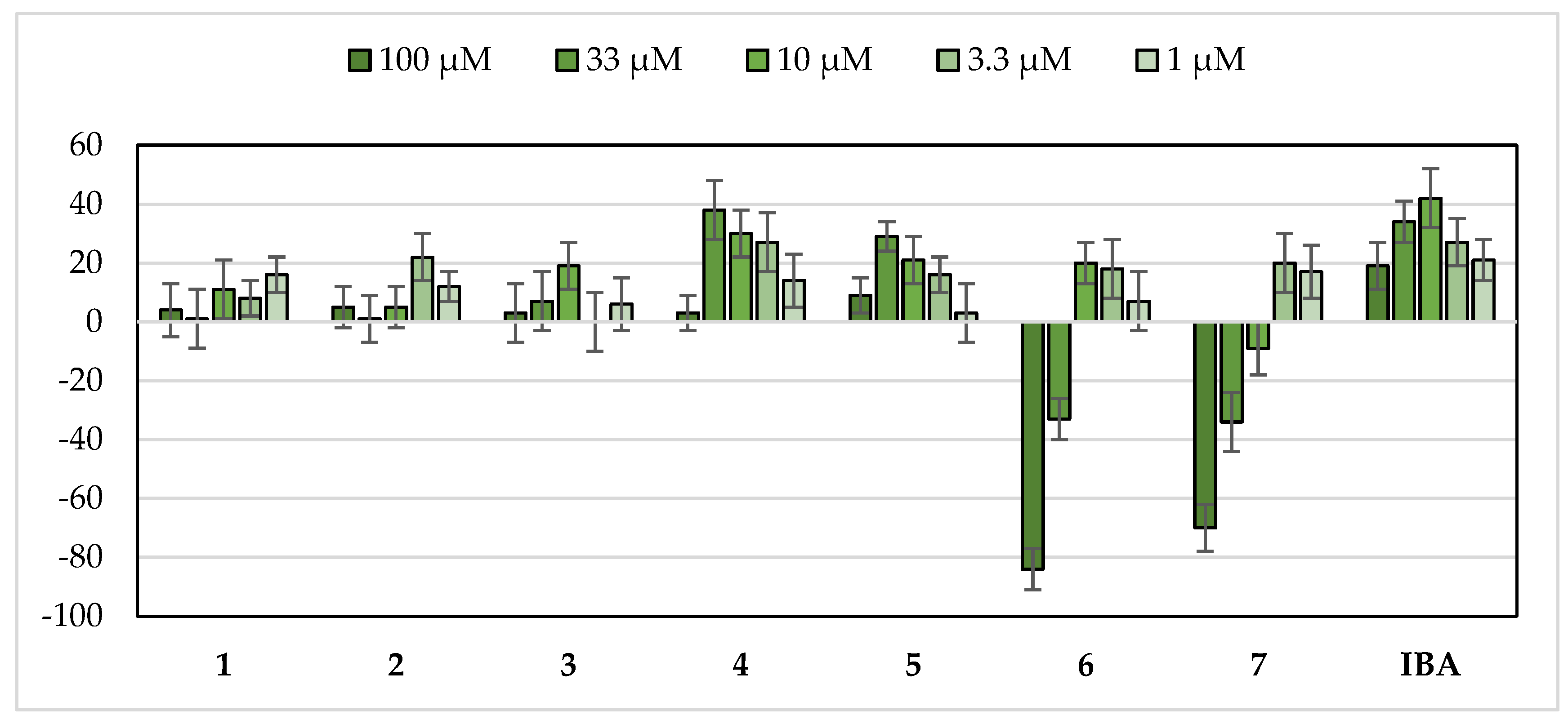
2.1. Stimulation Activity of Steroidal Saponins
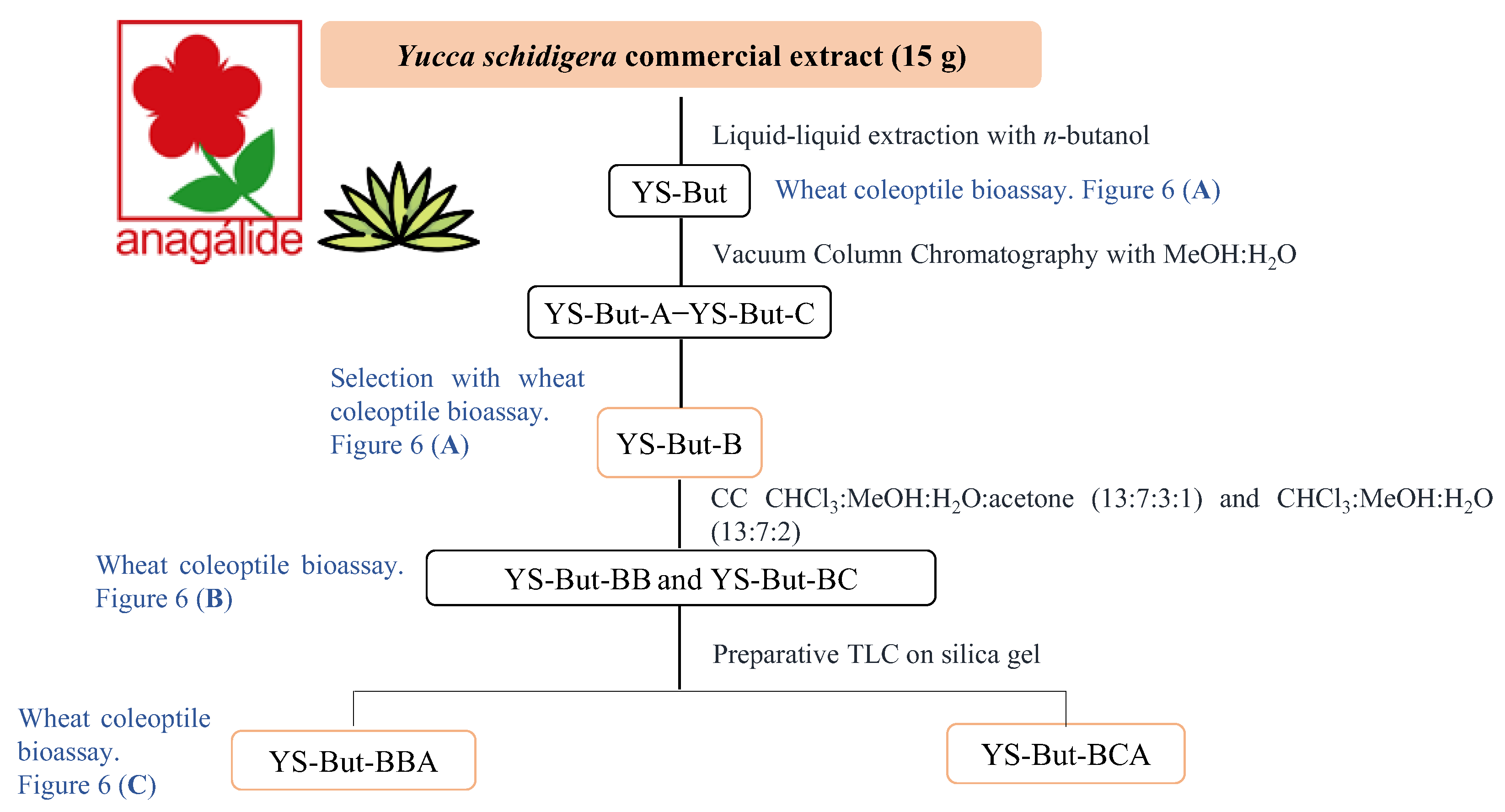
2.2. Bio-Guided Fractionation of Yucca Schidigera
2.3. Characterization of YS-But-BBA and YS-But-BCA Fractions
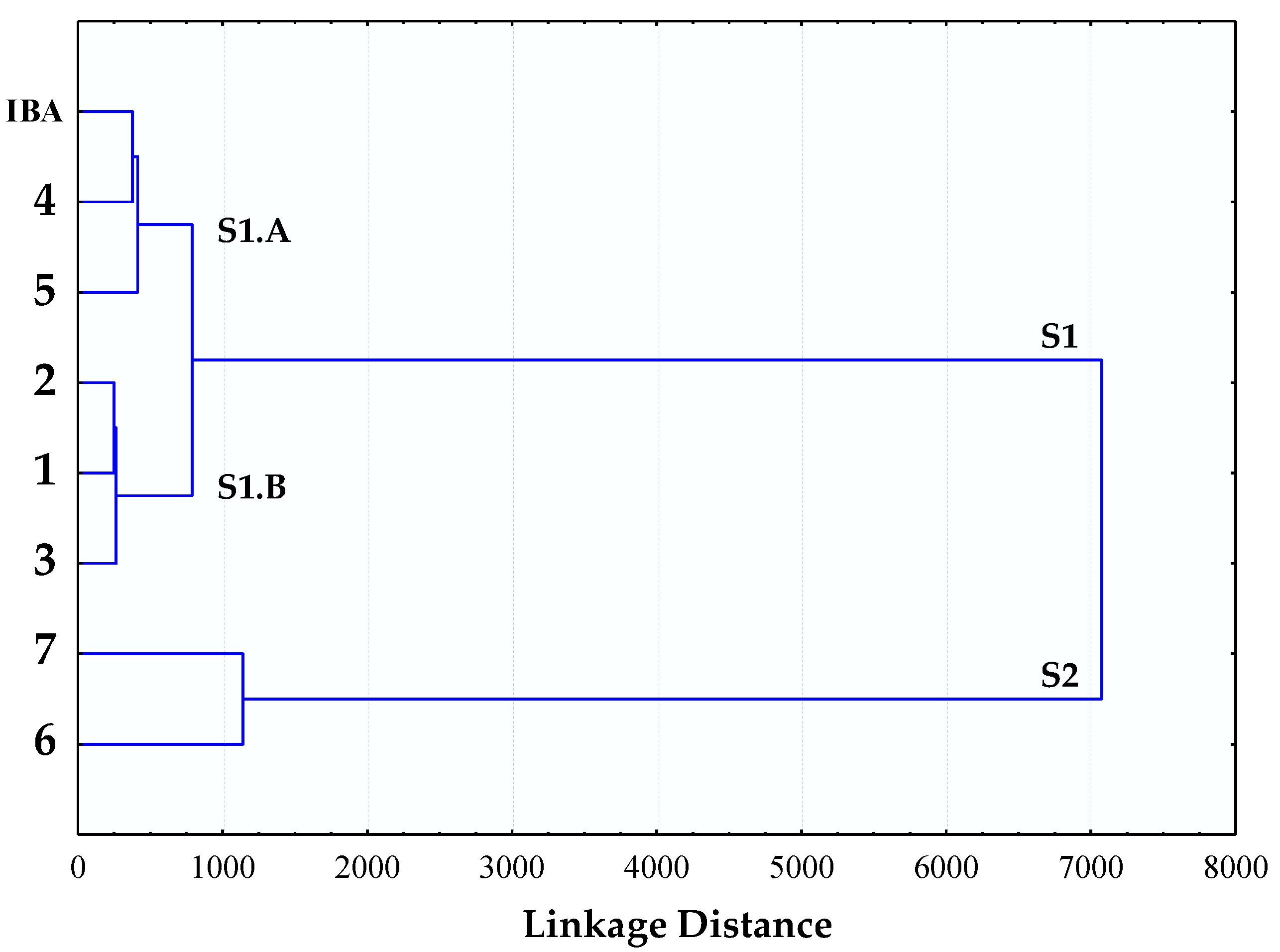
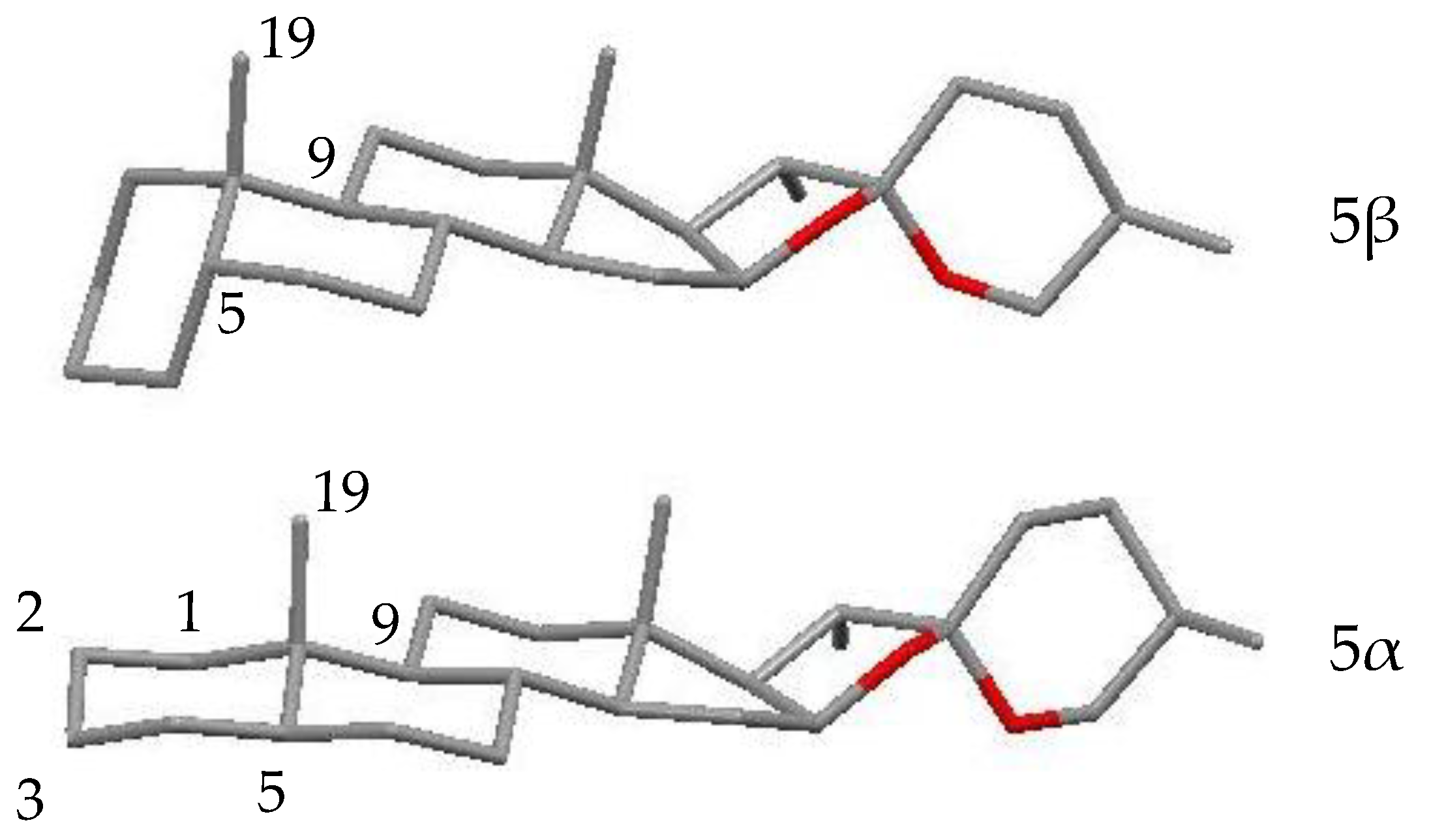
2.4. Active Saponins in Yucca Schidigera Extract
3. Discussion
3.1. Stimulation Activity of Steroidal Saponins
3.2. Yucca schidigera as a Commercial Source of Growth Stimulating Saponins
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.4. UPLC-QToF/MSE Analysis
4.5. Etiolated Wheat Coleoptile Bioassay
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Helmkamp, G.; Bonner, J. Some Relationships of Sterols to Plant Growth. Plant Physiol. 1953, 28, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Vendrig, J. Growth-Regulating Activity of Some Saponins. Nature 1964, 203, 1301–1302. [Google Scholar] [CrossRef]
- Oakenfull, D. Aggregation of Saponins and Bile Acids in Aqueous Solution. Aust. J. Chem. 1986, 39, 1671–1683. [Google Scholar] [CrossRef]
- Waller, G.R.; Yang, C.; Chen, L.; Su, L.; Liou, R.; Wu, S.; Young, C.; Lee, M. Mungean Saponins as Allelochemical. In Recent Advances in Allelopathy; Macías, F.A., Molinillo, J.M.G., Galindo, J.C., Cutler, H.G., Eds.; Servicio de Publicaciones-Universidad de Cádiz: Cádiz, Spain, 1999; Volume 1, pp. 91–108. [Google Scholar]
- Yamauchi, Y.; Kihata, K.; Horie, H. Growth Regulators Containing Tea Seed Saponin for Vegetable Plants 2000. JP 2000119118A, 25 April 2000. [Google Scholar]
- Yu, X.-L.; He, Y. Tea Saponins: Effective Natural Surfactants Beneficial for Soil Remediation, from Preparation to Application. RSC Adv. 2018, 8, 24312–24321. [Google Scholar] [CrossRef]
- Chou, C.-H.; Waller, G.R.; Cheng, C.S.; Yang, C.F.; Kim, D. Allelochemical Activity of Naturally Occurring Compounds from Mugbean (Vigna Radiata L.) Plants and Their Surronding Soil. Bot. Bull. Acad. Sin. 1995, 36, 9–18. [Google Scholar]
- Tsurumi, S.; Tsujino, Y. Chromosaponin I Stimulates the Growth of Lettuce Roots. Physiol. Plant. 1995, 93, 785–789. [Google Scholar] [CrossRef]
- Tsurumi, S.; Wada, S. Chromosaponin I Stimulates the Elongation of Cortical Cells in Lettuce Roots. Plant Cell Physiol. 1995, 36, 925–929. [Google Scholar] [CrossRef]
- Tsurumi, S.; Ishizawa, K.; Rahman, A.; Soga, K.; Hoson, T.; Goto, N.; Kamisaka, S. Effects of Chromosaponin I and Brassinolide on the Growth of Roots in Etiolated Arabidopsis Seedlings. J. Plant Physiol. 2000, 156, 60–67. [Google Scholar] [CrossRef]
- Rahman, A.; Tsurumi, S.; Amakawa, T.; Soga, K.; Hoson, T.; Goto, N.; Kamisaka, S. Involvement of Ethylene and Gibberellin Signalings in Chromosaponin I-Induced Cell Division and Cell Elongation in the Roots of Arabidopsis Seedlings. Plant Cell Physiol. 2000, 41, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Ahamed, A.; Amakawa, T.; Goto, N.; Tsurumi, S. Chromosaponin I Specifically Interacts with AUX1 Protein in Regulating the Gravitropic Response of Arabidopsis Roots. Plant Physiol. 2001, 125, 990–1000. [Google Scholar] [CrossRef]
- Hernández-Carlos, B.; González-Coloma, A.; Orozco-Valencia, Á.U.; Ramírez-Mares, M.V.; Andrés-Yeves, M.F.; Joseph-Nathan, P. Bioactive Saponins from Microsechium helleri and Sicyos bulbosus. Phytochemistry 2011, 72, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Calle, J.M.; Pérez, A.J.; Simonet, A.M.; Guerra, J.O.; Macías, F.A. Steroidal Saponins from Furcraea Hexapetala Leaves and Their Phytotoxic Activity. J. Nat. Prod. 2016, 79, 2903–2911. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.J.; Simonet, A.M.; Calle, J.M.; Pecio, Ł.; Guerra, J.O.; Stochmal, A.; Macías, F.A. Phytotoxic Steroidal Saponins from Agave Offoyana Leaves. Phytochemistry 2014, 105, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.J.; Calle, J.M.; Simonet, A.M.; Guerra, J.O.; Stochmal, A.; Macías, F.A. Bioactive Steroidal Saponins from Agave Offoyana Flowers. Phytochemistry 2013, 95, 298–307. [Google Scholar] [CrossRef]
- Durán, A.G.; Benito, J.; Macías, F.A.; Simonet, A.M. Agave Steroidal Saponins as Potential Bioherbicides. Agronomy 2021, 11, 2404. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Defining Hormesis. Hum. Exp. Toxicol. 2002, 21, 91–97. [Google Scholar] [CrossRef]
- Jalal, A.; de Oliveira Junior, J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; Dos Reis, A.R. Hormesis in Plants: Physiological and Biochemical Responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef]
- Nitsch, J.P.; Nitsch, C. Studies on the Growth of Coleoptile and First Internode Sections. A New, Sensitive, Straight-Growth Test for Auxins. Plant Physiol. 1956, 31, 94–111. [Google Scholar] [CrossRef]
- Hancock, C.R.; Barlow, H.W.B.; Lacey, H.J. The East Malling Coleoptile Straight Growth Test Method. J. Exp. Bot. 1964, 15, 166–176. [Google Scholar] [CrossRef]
- Frick, E.M.; Strader, L.C. Roles for IBA-Derived Auxin in Plant Development. J. Exp. Bot. 2018, 69, 169–177. [Google Scholar] [CrossRef]
- Durán, A.G.; Celaj, O.; Macías, F.A.; Simonet, A.M. Dereplication of Bioactive Spirostane Saponins from Agave macroacantha. J. Nat. Prod. 2021, 84, 2904–2913. [Google Scholar] [CrossRef]
- Simonet, A.M.; Durán, A.G.; Pérez, A.J.; Macías, F.A. Features in the NMR Spectra of the Aglycones of Agave Spp. Saponins. HMBC Method for Aglycone Identification (HMAI). Phytochem. Anal. 2021, 32, 38–61. [Google Scholar] [CrossRef]
- Macías, F.A.; Guerra, J.O.; Simonet, A.M.; Nogueiras, C.M. Characterization of the Fraction Components Using 1D TOCSY and 1D ROESY Experiments. Four New Spirostane Saponins from Agave brittoniana Trel. Spp. Brachypus. Magn. Reson. Chem. 2007, 45, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Wang, J.; Ruan, J.; Yao, X.; Huang, P.; Wang, Y.; Yu, H.; Han, L.; Zhang, Y.; Wang, T. Spirostane-Type Saponins Obtained from Yucca schidigera. Molecules 2018, 23, 167. [Google Scholar] [CrossRef] [PubMed]
- Sastre, F.; Ferreira, F.; Pedreschi, F. A Systematic Approach for the Chromatographic Fractionation and Purification of Major Steroid Saponins in Commercial Extracts of Yucca Schidigera Roezl. J. Chromatogr. B 2017, 1046, 235–242. [Google Scholar] [CrossRef]
- Skhirtladze, A.; Perrone, A.; Montoro, P.; Benidze, M.; Kemertelidze, E.; Pizza, C.; Piacente, S. Steroidal Saponins from Yucca Gloriosa L. Rhizomes: LC–MS Profiling, Isolation and Quantitative Determination. Phytochemistry 2011, 72, 126–135. [Google Scholar] [CrossRef]
- Oleszek, W.; Sitek, M.; Stochmal, A.; Piacente, S.; Pizza, C.; Cheeke, P. Steroidal Saponins of Yucca Schidigera Roezl. J. Agric. Food Chem. 2001, 49, 4392–4396. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Ruan, J.; Wu, S.; Huang, P.; Yan, J.; Yu, H.; Zhang, Y.; Wang, T. Separation and Bioactive Assay of 25R/S-Spirostanol Saponin Diastereomers from Yucca Schidigera Roezl (Mojave) Stems. Molecules 2018, 23, 2562. [Google Scholar] [CrossRef]
- Zong, G.; Liang, X.; Zhang, J.; Duan, L.; Tan, W.; Wang, D. Synthesis and Plant Growth Regulation Activity of α-d-ManpNAc-(1→2)-[α-l-Rhap-(1→3)-]α-l-Rhap-(1→4)-β-d-GlupNAc-(1→3)-α-l-Rhap, the Repeating Unit of O-Antigen of Rhizobium Trifolii 4s. Carbohydr. Res. 2014, 388, 87–93. [Google Scholar] [CrossRef]
- Holland, J.J.; Roberts, D.; Liscum, E. Understanding Phototropism: From Darwin to Today. J. Exp. Bot. 2009, 60, 1969–1978. [Google Scholar] [CrossRef]
- Boysen-Jensen, P. Über Die Leitung Des Phototropischen Reizes in Der Avenakoleoptile. Ber. Dtsch. Bot. Ges. 2013, 31, 559–566. [Google Scholar]
- Went, F. On Growth Accelerating Substances in the Coleoptile of Avena sativa. Proc. Sect. Sci. K. Akad. Van Wet. Te Amst. 1926, 30, 10–19. [Google Scholar]
- Vargas-Hernandez, M.; Macias-Bobadilla, I.; Guevara-Gonzalez, R.G.; Romero-Gomez, S.D.J.; Rico-Garcia, E.; Ocampo-Velazquez, R.V.; Alvarez-Arquieta, L.D.L.; Torres-Pacheco, I. Plant Hormesis Management with Biostimulants of Biotic Origin in Agriculture. Front. Plant Sci. 2017, 8, 1762. [Google Scholar] [CrossRef]
- Korbei, B.; Luschnig, C. Plants on (Brassino)Steroids. Nat. Plants 2021, 7, 548–549. [Google Scholar] [CrossRef]
- Romero-Avila, M.; de Dios-Bravo, G.; Mendez-Stivalet, J.M.; Rodríguez-Sotres, R.; Iglesias-Arteaga, M.A. Synthesis and Biological Activity of Furostanic Analogues of Brassinosteroids Bearing the 5α-Hydroxy-6-Oxo Moiety. Steroids 2007, 72, 955–959. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, J.; Liu, X.; Gong, W.; Zhang, C. Synthesis of Brassinosteroids Analogues from Laxogenin and Their Plant Growth Promotion. Nat. Prod. Res. 2015, 29, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Arteaga, M.A.; Gil, R.P.; Martínez, C.S.P.; Manchado, F.C. Spirostanic Analogues of Teasterone. Synthesis, Characterisation and Biological Activity of Laxogenin, (23S)-Hydroxylaxogenin and 23-Ketolaxogenin (23-Oxolaxogenin). J. Chem. Soc. Perkin Trans. 1 2001, 261–266. [Google Scholar] [CrossRef]
- Vukašinović, N.; Wang, Y.; Vanhoutte, I.; Fendrych, M.; Guo, B.; Kvasnica, M.; Jiroutová, P.; Oklestkova, J.; Strnad, M.; Russinova, E. Local Brassinosteroid Biosynthesis Enables Optimal Root Growth. Nat. Plants 2021, 7, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, G.G.; Durán, A.G.; Macías, F.A.; Simonet, A.M. Structure, Bioactivity and Analytical Methods for the Determination of Yucca Saponins. Molecules 2021, 26, 5251. [Google Scholar] [CrossRef] [PubMed]
- Sidana, J.; Singh, B.; Sharma, O.P. Saponins of Agave: Chemistry and Bioactivity. Phytochemistry 2016, 130, 22–46. [Google Scholar] [CrossRef]
- Cheeke, P.R. Actual and Potential Applications of Yucca Schidigera and Quillaja Saponaria Saponins in Human and Animal Nutrition. In Saponins in Food, Feedstuffs and Medicinal Plants; Oleszek, W., Marston, A., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 241–254. [Google Scholar]
- Andresen, M.; Cedergreen, N. Plant Growth Is Stimulated by Tea-Seed Extract: A New Natural Growth Regulator? HortScience 2010, 45, 1848–1853. [Google Scholar] [CrossRef]
- Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Bautista, E.; Hernández, A.O.; Macías, F.A. Phytotoxicity Evaluation of Sesquiterpene Lactones and Diterpenes from Species of the Decachaeta, Salvia and Podachaenium Genera. Phytochem. Lett. 2016, 18, 68–76. [Google Scholar] [CrossRef]


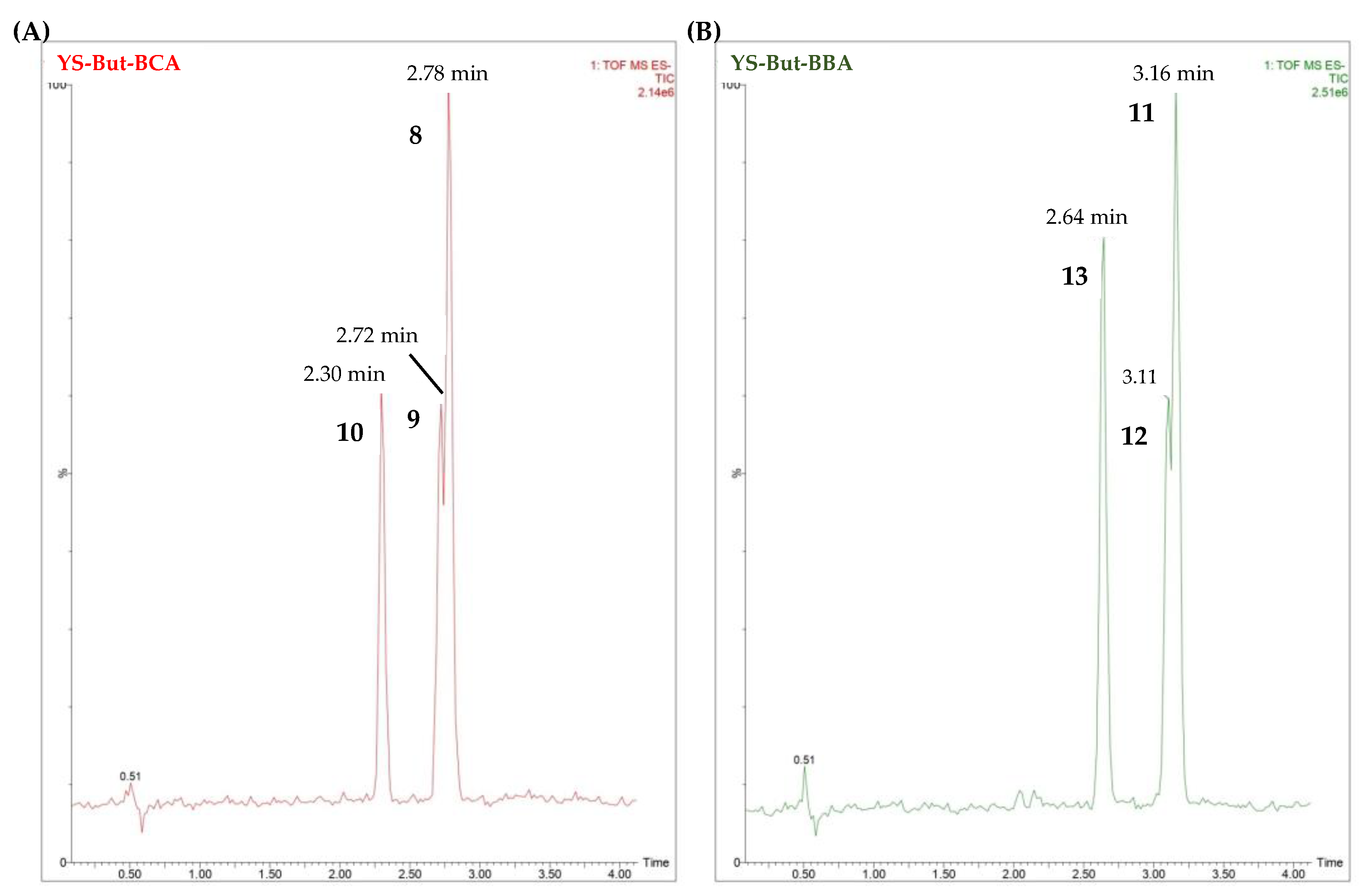
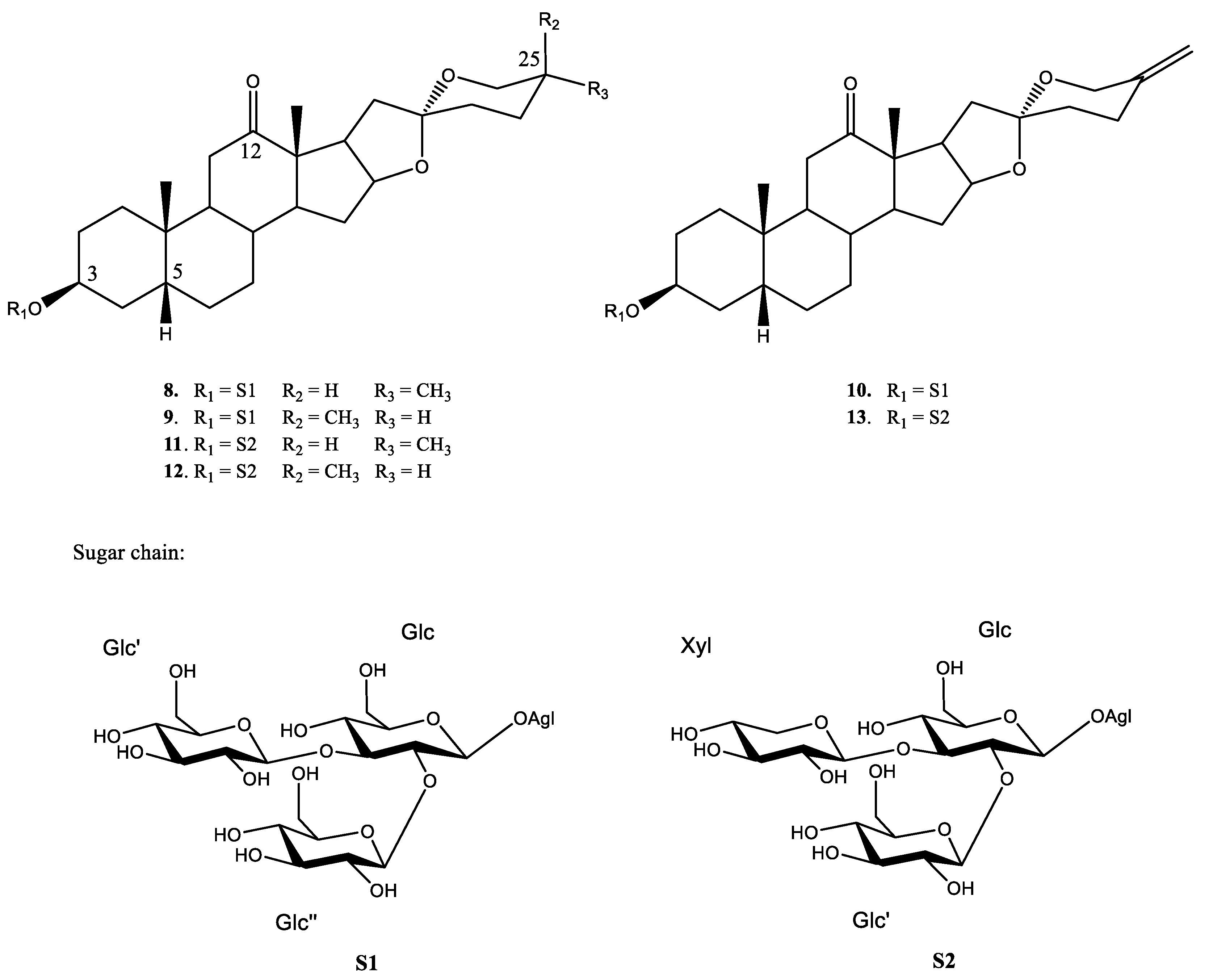
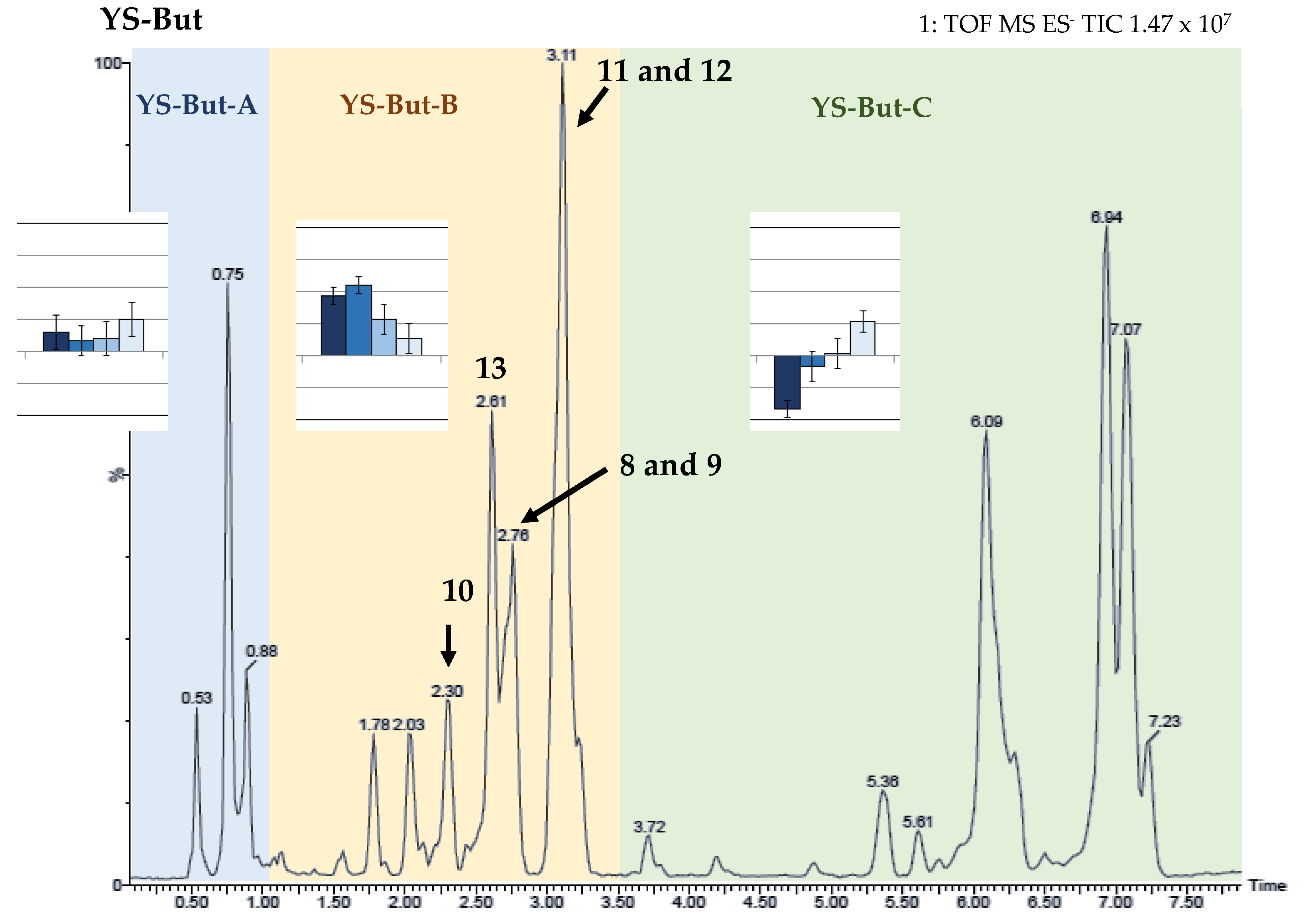
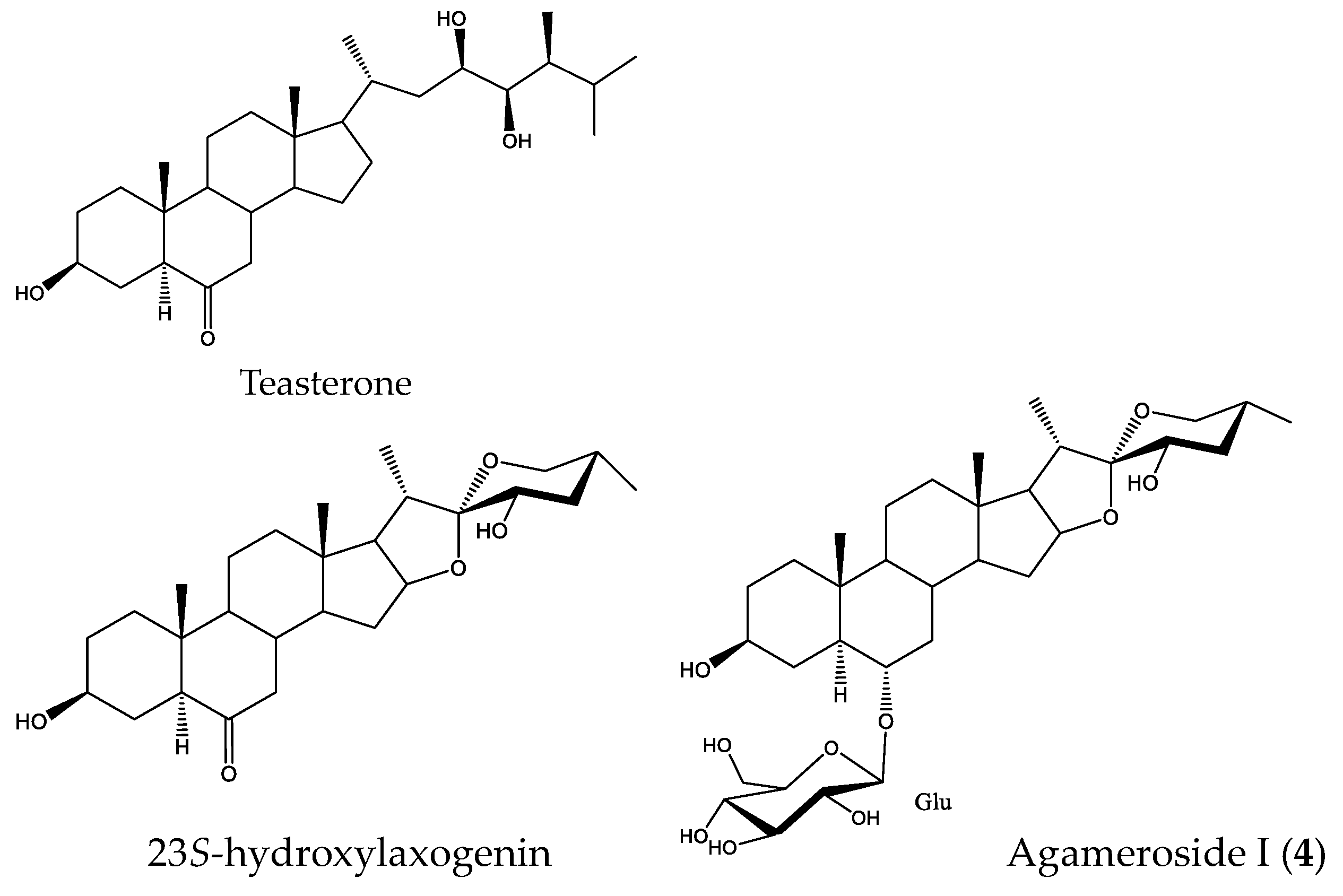
| Saponin-Enriched Fraction | Compounds | Retention Time (min.) | [M–H]– | Fragmentation (m/z) | Molecular Formula [M–H]– | mDa |
|---|---|---|---|---|---|---|
| YS-But-BCA | 8 | 2.78 | 915.4567 | 753.4037, 591.3527 | C45H71O19 | −0.6 |
| 9 | 2.72 | 915.4586 | 753.4008, 591.3475 | C45H71O19 | −2.3 | |
| 10 | 2.30 | 913.4427 | 751.3886, 589.3322 | C45H69O19 | −0.6 | |
| YS-But-BBA | 11 | 3.16 | 885.4481 | 753.4009, 591.3557 | C44H69O18 | −0.8 |
| 12 | 3.11 | 885.4537 | 723.3862, 591.3455 | C44H69O18 | −0.3 | |
| 13 | 2.64 | 883.4299 | 751.3875, 589.3236 | C44H67O18 | −2.8 |
| 1 H NMR Signal | HMBC Correlations | Methyl Assig. | Flowchart Information | ||
|---|---|---|---|---|---|
| 4.77 and 4.80 (D14) | SP C25 DB | ||||
| 1.35 d | 42.9 | 54.4 (D6) | 109.5 (D5) | C-21 | SP C12 CO |
| 1.31 d | 42.6 | 54.4 (D6) | 109.5 (D5) | C-21 | SP C12 CO |
| 1.04 d (D2) | 26.2 (D1) | 27.6 | 65.2 (D1) | C-27 | SP C25 S |
| 0.67 d (D2) | 29.1 (D1) | 30.6 | 66.9 (D1) | C-27 | SP C25 R |
| 1.06 s (S2) | 54.2 | 55.8 | 213.1 (S1) | C-18 | SP C12 CO |
| 0.93 s | 30.4 (S10) | 36.0 | 41.9 (S9) | C-19 | H-5β |
| Sugar Chain | Compounds | Retention Time (min) | [M + HCOO]– | Relative Abundance 1 |
|---|---|---|---|---|
| S1 | 8/9 | 2.76 | 961.4646/961.4651 | 8.5 |
| 10 | 2.30 | 959.4451 | 3.0 | |
| S2 | 11/12 | 3.11 | 931.4529/931.4554 | 23.4 |
| 13 | 2.61 | 929.4365 | 8.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durán, A.G.; Calle, J.M.; Butrón, D.; Pérez, A.J.; Macías, F.A.; Simonet, A.M. Steroidal Saponins with Plant Growth Stimulation Effects; Yucca schidigera as a Commercial Source. Plants 2022, 11, 3378. https://doi.org/10.3390/plants11233378
Durán AG, Calle JM, Butrón D, Pérez AJ, Macías FA, Simonet AM. Steroidal Saponins with Plant Growth Stimulation Effects; Yucca schidigera as a Commercial Source. Plants. 2022; 11(23):3378. https://doi.org/10.3390/plants11233378
Chicago/Turabian StyleDurán, Alexandra G., Juan M. Calle, Davinia Butrón, Andy J. Pérez, Francisco A. Macías, and Ana M. Simonet. 2022. "Steroidal Saponins with Plant Growth Stimulation Effects; Yucca schidigera as a Commercial Source" Plants 11, no. 23: 3378. https://doi.org/10.3390/plants11233378
APA StyleDurán, A. G., Calle, J. M., Butrón, D., Pérez, A. J., Macías, F. A., & Simonet, A. M. (2022). Steroidal Saponins with Plant Growth Stimulation Effects; Yucca schidigera as a Commercial Source. Plants, 11(23), 3378. https://doi.org/10.3390/plants11233378











