An In Vitro Protocol for Propagating Castanea sativa Italian Cultivars
Abstract
1. Introduction
2. Results and Discussion
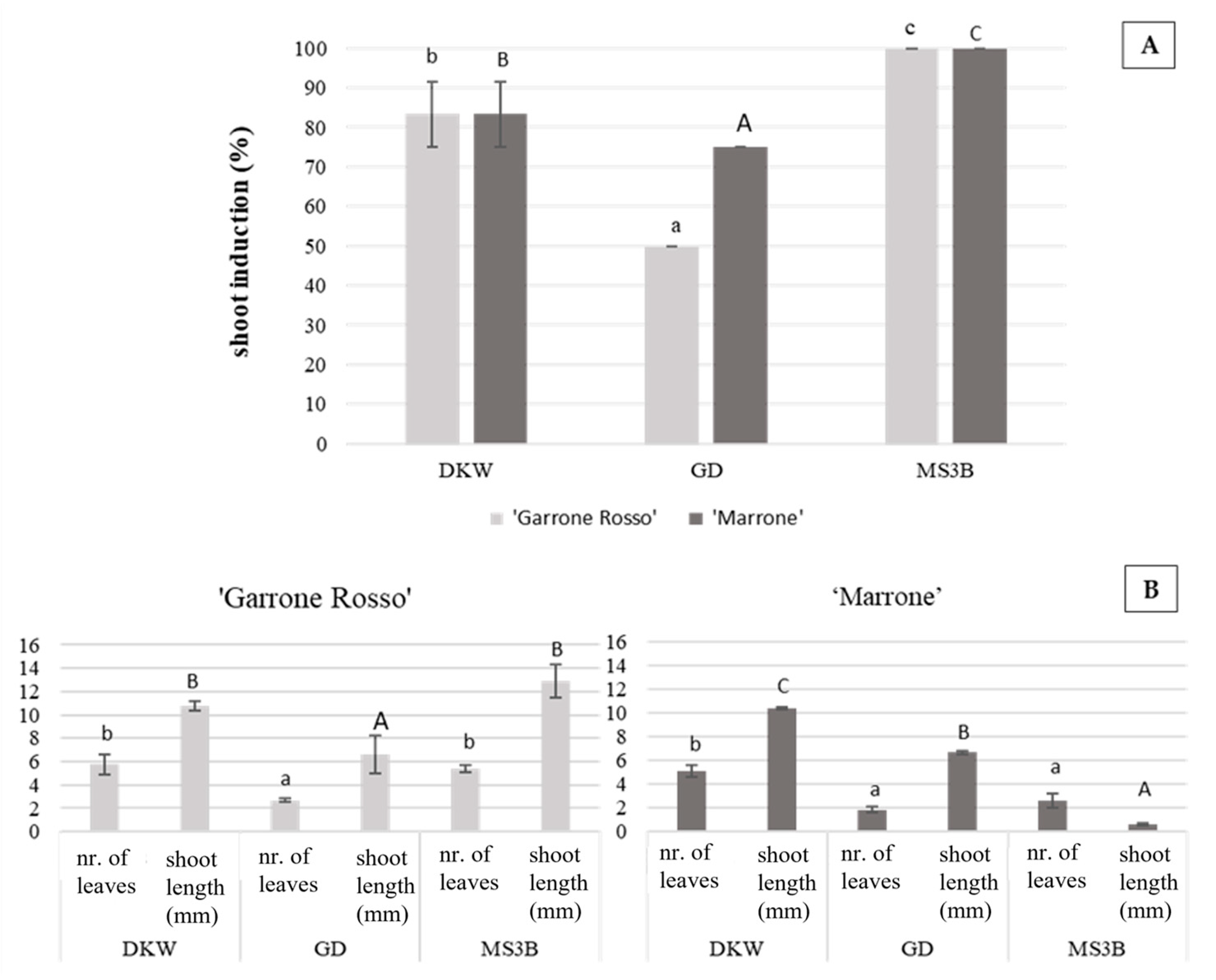
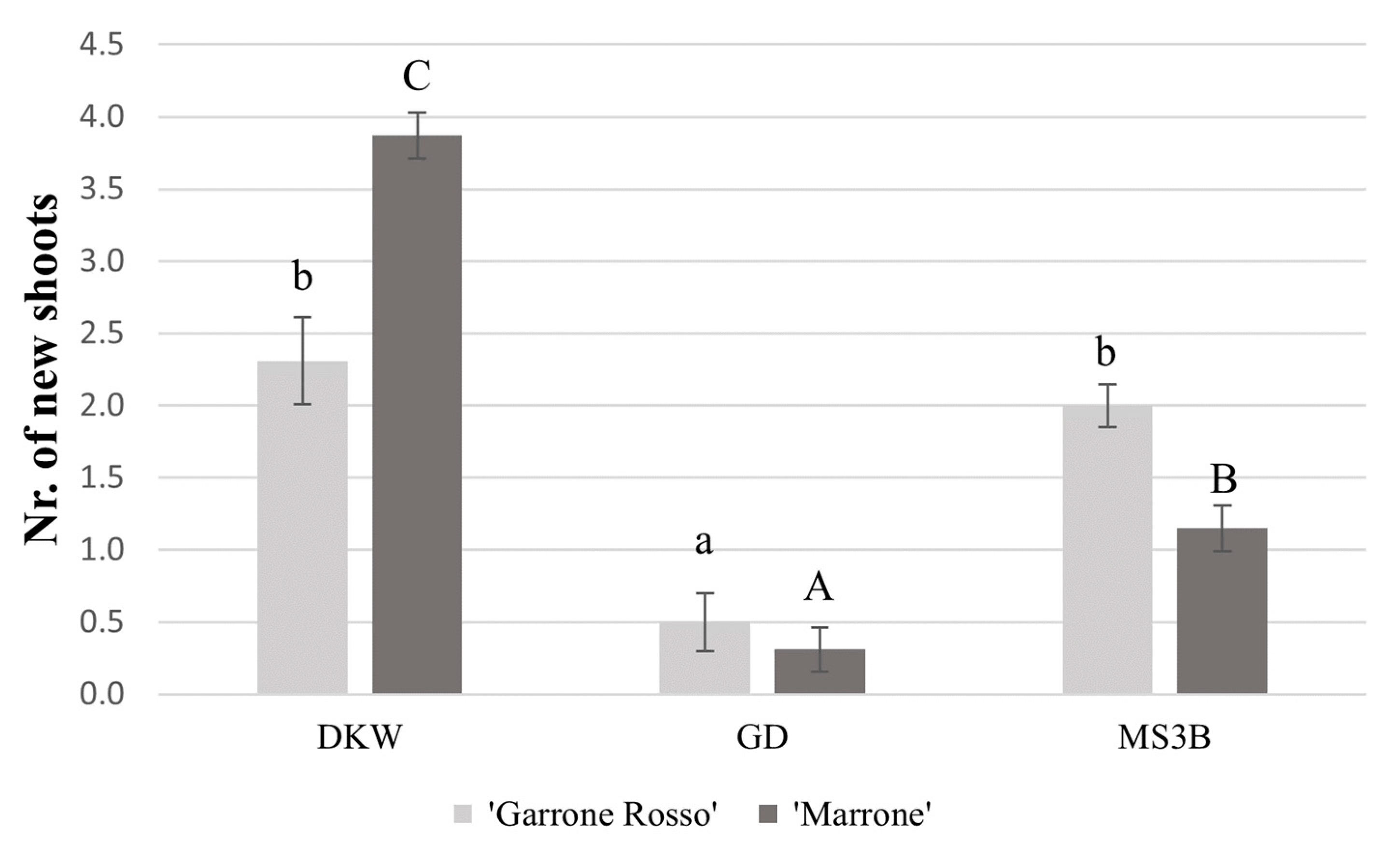
2.1. Culture Establishment
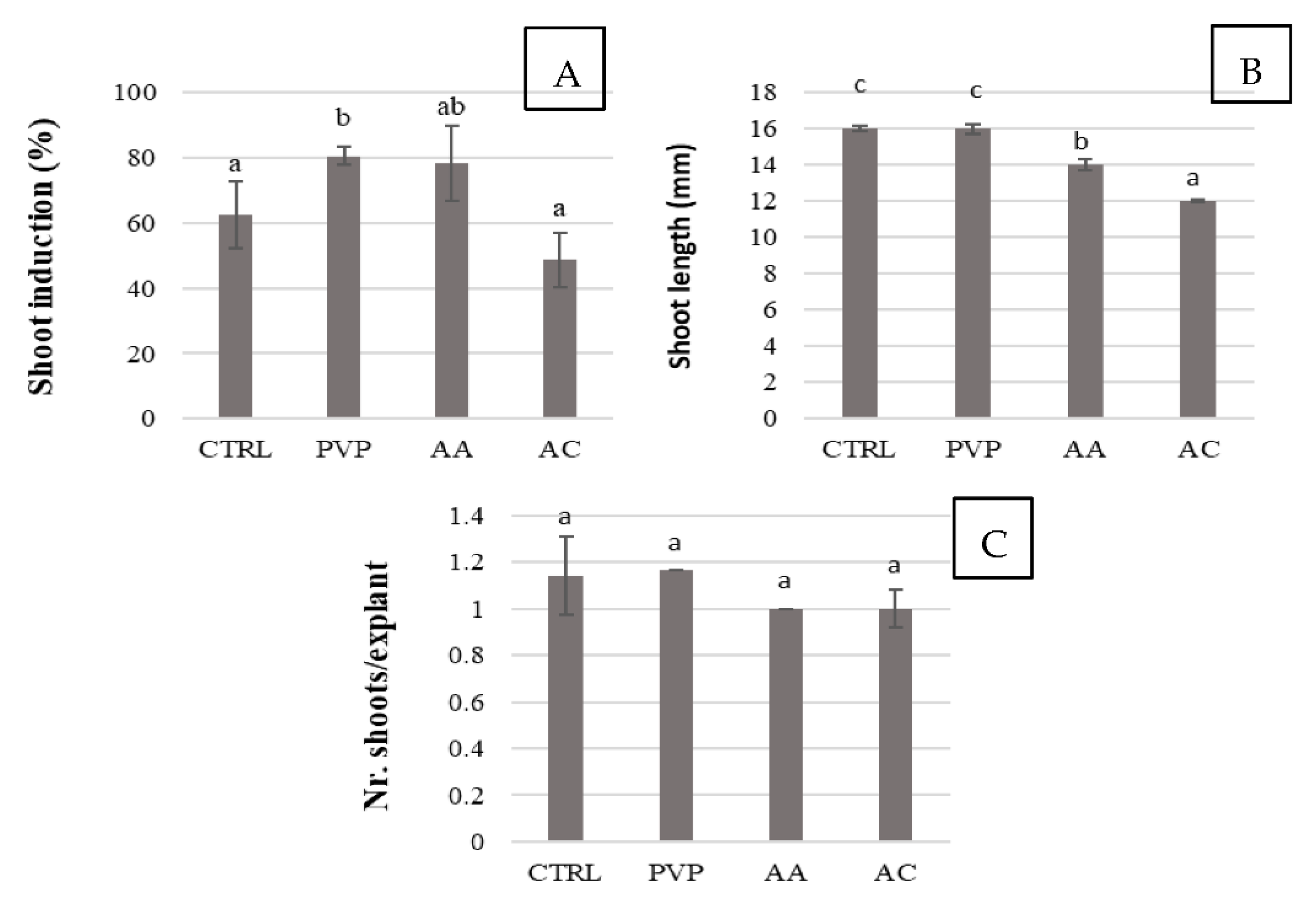
2.2. Evaluation of Polyphenol Controlling Agents to Be Applied in Chesnut In Vitro Culture
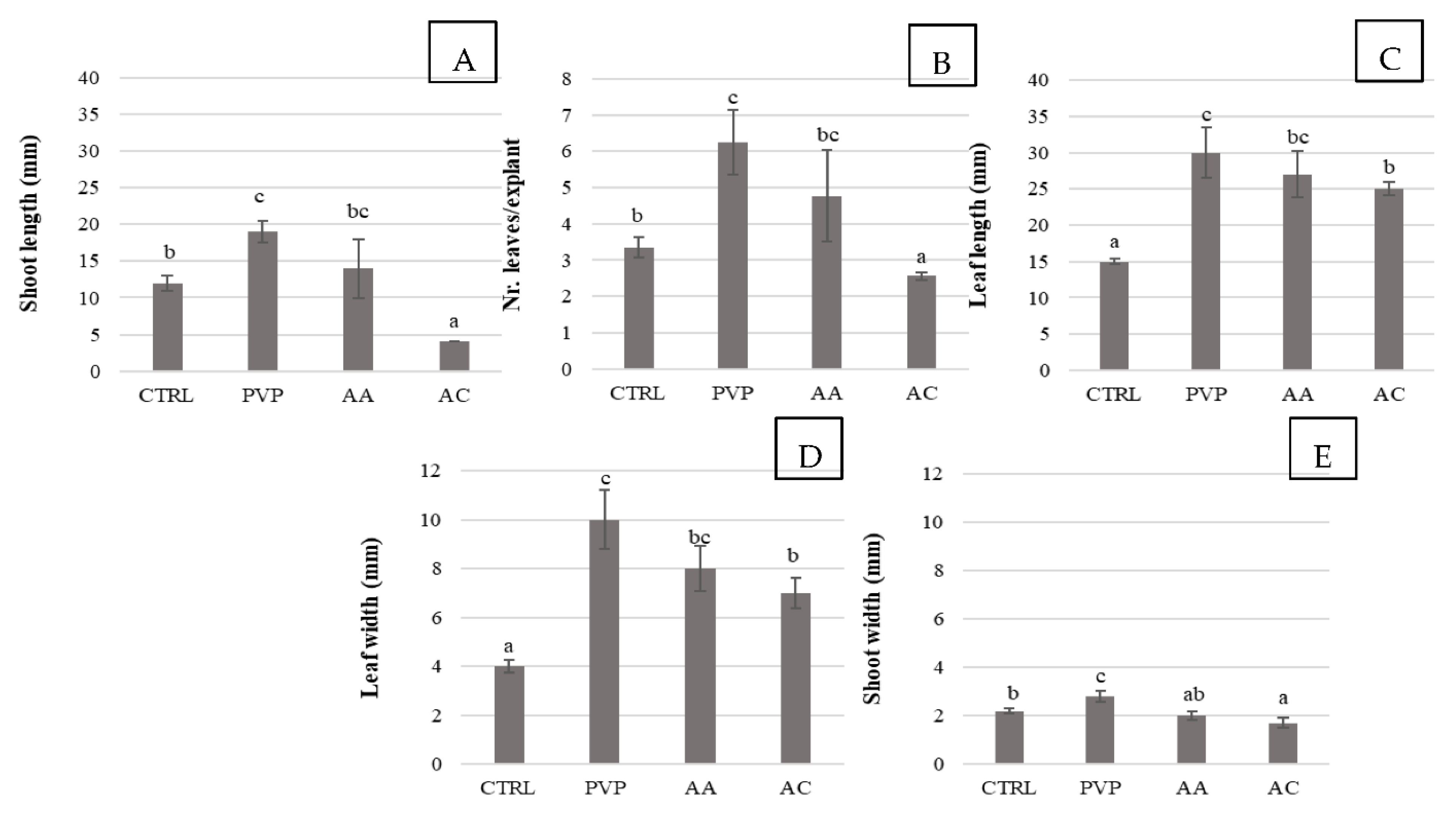
2.3. Shoot Multiplication and Elongation
2.4. Rooting
3. Materials and Methods
3.1. Plant Material
3.2. Culture Establishment and Anti-Phenolic Treatments
3.3. Shoot Multiplication and Elongation
3.4. Rooting
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandes, P.; Tedesco, S.; Vieira da Silva, I.; Santos, C.; Machado, H.; Lourenço Costa, R. A new clonal propagation protocol develops quality root systems in Chestnut. Forests 2020, 11, 826. [Google Scholar] [CrossRef]
- Braga, N.; Rodrigues, F.; Beatriz, M.; Oliveira, P.P. Castanea sativa by-products: A review on added value and sustainable application. Nat. Prod. Res. 2015, 29, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Roces-Díaz, J.V.; Díaz-Varela, E.R.; Barrio-Anta, M.; Álvarez-Álvarez, P. Sweet chestnut agroforestry systems in north-western Spain: Classification, spatial distribution and an ecosystem services assessment. For. Syst. 2018, 27, e03S. [Google Scholar] [CrossRef]
- Gago, D.; Bernal, M.Á.; Sánchez, C.; Aldrey, A.; Cuenca, B.; Christie, C.B.; Vidal, N. Effect of sucrose on growth and stress status of Castanea sativa x C. crenata shoots cultured in liquid medium. Plants 2022, 11, 965. [Google Scholar] [CrossRef]
- Vieitez, A.M.; Sänchez, M.C.; García-Nimo, M.L.; Ballester, A. Protocol for micropropagation of Castanea sativa. In Protocols for Micropropagation of Woody Trees and Fruits; Jain, S.M., Haggman, H., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 299–312. [Google Scholar]
- Pavese, V.; Moglia, A.; Gonthier, P.; Torello Marinoni, D.; Cavalet-Giorsa, E.; Botta, R. Identification of susceptibility genes in Castanea sativa and their transcription dynamics following pathogen infection. Plants 2021, 10, 913. [Google Scholar] [CrossRef]
- Sartor, C.; Dini, F.; Marinoni, D.T.; Mellano, M.G.; Beccaro, G.L.; Alma, A.; Quacchia, A.; Botta, R. Impact of the Asian wasp Dryocosmuskuriphilus (Yasumatsu) on cultivated chestnut: Yield loss and cultivar susceptibility. Sci. Hortic. 2015, 197, 454–460. [Google Scholar] [CrossRef]
- Beccaro, G.; Alma, A.; Bounous, G.; Gomes-Laranjo, J. The Chestnut Handbook: Crop & Forest Management; Beccaro, G., Alma, A., Bounous, G., Gomes-Laranjo, J., Eds.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Bounous, G.; Ertürk, U.; Akyuz, B.; Fulbright, D.W.; Serdar, U. Evaluation of the descriptive characteristics of chestnut. VI Int. Chestnut Symp. 2017, 1220, 35–44. [Google Scholar]
- Torello Marinoni, D.; Akkak, A.; Guaraldo, P.; Boccacci, P.; Ebone, A.; Viotto, E.; Bounous, G.; Ferrara, A.M.; Botta, R. Genetic and morphological characterization of chestnut (Castanea sativa Mill.) germplasm in Piedmont (north-western Italy). Tree Genet. Genomes 2013, 9, 1017–1030. [Google Scholar] [CrossRef]
- Alessandri, S.; Krznar, M.; Ajolfi, D.; Ramos Cabrer, A.M.; Pereira-Lorenzo, S.; Dondini, L. Genetic diversity of Castanea sativa Mill. accessions from the Tuscan-Emilian Apennines and Emilia Romagna Region (Italy). Agronomy 2020, 10, 1319. [Google Scholar] [CrossRef]
- Merkle, S.A.; Viéitez, F.J.; Corredoira, E.; Carlson, J.E. Castanea spp. Chestnut. In Biotechnology of Fruit and Nut Crops; Litz, R.E., Fernando Pliego-Alfaro, A., Hormaza, J.I., Eds.; CABI: Oxfordshire, UK, 2020; pp. 206–237. [Google Scholar]
- Vieitez, A.M.; Vieitez, M.L. Culture of chestnut shoots from buds in vitro. J. Hortic. Sci. 1980, 55, 83–84. [Google Scholar] [CrossRef]
- Vieitez, A.M.; Ballester, A.; Vieitez, M.L.; Vieitez, E. In vitro plantlet regeneration of mature chestnut. J. Hortic. Sci. 1983, 58, 457–463. [Google Scholar] [CrossRef]
- Chevre, A.; Salesses, G. Choice of explants for chestnut micropropagation. In Symposium on In Vitro Problems Related to Mass Propagation of Horticultural Plants; Commission of the European Communities Directorate-General Telecommunications, Information Industries and Innovation: Luxembourg, 1987; Volume 212, pp. 517–524. [Google Scholar]
- Chauvin, J.E.; Salesses, G. The effect of fructose on chestnut micropropagation Castanea sp. ComptesRendus De L’academieDesSci. Ser. 3 Sci. Vie 1988, 306, 207–212. [Google Scholar]
- Liu, Z.; Bi, W.-L.; Shukla, M.R.; Saxena, P.K. In vitro technologies for American chestnut (Castanea dentata (Marshall) Borkh) conservation. Plants 2022, 11, 464. [Google Scholar] [CrossRef]
- Gaidamashvili, M.; Khurtsidze, E.; Kutchava, T.; Lambardi, M.; Benelli, C. Efficientprotocolforimprovingthedevelopmentofcryopreservedembryonicaxesofchestnut (Castanea sativa Mill.) byencapsulation-vitrification. Plants 2021, 10, 231. [Google Scholar] [CrossRef]
- Freitas, T.R.; Santos, J.A.; Silva, A.P.; Fraga, H. Influence of climate change on chestnut trees: A Review. Plants 2021, 10, 1463. [Google Scholar] [CrossRef]
- Fernandes, P.; Amaral, A.; Colavolpe, B.; Balonas, D.; Serra, M.; Pereira, A.; Costa, R.L. Propagation of new chestnut rootstocks with improved resistance to Phytophthora cinnamomi. New Cast Rootstocks. Silva Lusit. 2020, 28, 15–29. [Google Scholar] [CrossRef]
- Corredoira, E.; Martínez, M.T.; Cernadas, M.J.; San José, M.C. Application of biotechnology in the conservation of the genus Castanea. Forest 2017, 8, 394. [Google Scholar] [CrossRef]
- Çölgeçen, H.; Çalișkan, U.K.; Toker, G. Influence of different sterilization methods on callus initiation and production of pigmented callus in ArnebiadensifloraLedeb. Turk. J. Biol. 2011, 35, 513–520. [Google Scholar]
- Ma, M.; Zhao, L.; Tang, S.; Chen, X.; Qin, R. The Effects of Different Disinfection Methods on Seed Germination and Study on the Environmental Bacteria in Safflower (Carthamus tinctorius L.). Crops 2018, 34, 162–167. [Google Scholar]
- Aliyu, O.M.; Awopetu, J.A. In vitro regeneration of hybrid plantlets of cashew (Anacardium occidentale L.) through embryo culture. Afr. J. Biotechnol. 2005, 4, 548–553. [Google Scholar]
- Teixeira da Silva, J.A.; Nezami-Alanagh, E.; Barreal, M.E.; Kher, M.M.; Wicaksono, A.; Gulyás, A.; Hidvégi, N.; Magyar-Tábori, K.; Mendler-Drienyovszki, N.; Márton, L.; et al. Shoot tip necrosis of in vitro plant cultures: A reappraisal of possible causes and solutions. Planta 2020, 252, 47. [Google Scholar] [CrossRef] [PubMed]
- North, J.J.; Ndakidemi, P.A.; Laubscher, C.P. Effects of antioxidants, plant growthregulators and wounding on phenolic compound excretion during micropropagation of Strelitzia reginae. Int. J. Phys. Sci. 2012, 7, 638–646. [Google Scholar] [CrossRef]
- Nishchal, N.; Mir, H.; Rani, R.; Pal, A.K. Effect of antioxidants in controlling phenol exudation in micropropagation of litchi cv. Purbi. Curr. J. Appl. Sci. Technol. 2018, 31, 1–7. [Google Scholar] [CrossRef]
- Ahmed, A.B.A.; Rao, A.S.; Rao, M.V.; Taha, R.M. Effect of picloram, additivesand plant growth regulators on somatic embryogenesis of Phyla nodiflora (L.) Greene. Braz. Arch. Biol. Technol. 2011, 54, 7–13. [Google Scholar] [CrossRef]
- Arif, I.A.; Bakir, M.A.; Khan, H.A.; Ahamed, A.; Al Farhan, A.H.; Al Homaidan, A.A.; Al Sadoon, M.; Bahkali, A.H.; Shobrak, M. A simple method for DNA extraction from mature date palm leaves: Impact of sand grinding and composition of lysis buffer. Int. J. Mol. Sci. 2010, 11, 3149–3157. [Google Scholar] [CrossRef]
- Vieitez, A.M.; Sánchez, C.; San-José, C. Prevention of shoot-tip necrosis in shoot cultures of chestnut and oak. Sci. Hortic. 1989, 41, 151–159. [Google Scholar] [CrossRef]
- Barghchi, M.; Alderson, P.G. In vitro propagation of Pistacia vera L. and thecommercial cultivars Ohadi and Kalleghochi. J. Hortic. Sci. 1985, 60, 423–430. [Google Scholar] [CrossRef]
- Machado, M.P.; da Silva, A.L.L.; Biasi, L.A.; Deschamps, C.; Filho, J.C.B.; Zanette, F. Influence of calcium content of tissue on hyperhydricity and shoot-tip necrosis ofin vitroregenerated shoots of Lavandula angustifolia Mill. Braz. Arch. Biol. Technol. 2014, 57, 636–643. [Google Scholar] [CrossRef]
- Vieitez, A.M.; Corredoira, E.; Ballester, A.; Muñoz, F.; Durán, J.; Ibarra, M. In vitroregeneration of the important North American oak species Quercus alba, Quercus bicolor and Quercus rubra. Plant Cell Tissue Organ Cult. 2009, 98, 135–145. [Google Scholar] [CrossRef]
- Bosela, M.J.; Michler, C.H. Media effects on black walnut (Juglans nigra L.) shoot culture growth in vitro: Evaluation of multiple nutrient formulations and cytokinin types. In Vitro Cell. Dev. Biol. 2008, 44, 316–329. [Google Scholar] [CrossRef]
- Marie Chevre, A.; Gill, S.S.; Mouras, A.; Salesses, G. In vitro vegetative multiplication of chestnut. J. Hortic. Sci. 1983, 58, 23–29. [Google Scholar] [CrossRef]
- Abdelwahd, R.; Hakam, N.; Labhilili, M.; Udupa, S. Use of an adsorbent and antioxidants to reduce the effects of leached phenolics in in vitro plantlet regeneration of faba bean. Afr. J. Biotechnol. 2008, 7, 997–1002. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gresshoff, P.M.; Doy, C.H. Haploid Arabidopsis thaliana callus and plants from anther culture. Aust. J. Biol. Sci. 1972, 25, 259–264. [Google Scholar] [CrossRef]
- Driver, J.A.; Kuniyuki, A.H. In vitro propagation of Paradox walnut rootstock. HortScience 1984, 19, 507–509. [Google Scholar] [CrossRef]
- Silvestri, C.; Rugini, E.; Cristofori, V. The effect of CuSO4 for establishing in vitro culture, and the role nitrogen and iron sources in in vitro multiplication of Corylus avellana L. cv. Tonda Gentile Romana. Plant Biosyst. 2019, 154, 17–23. [Google Scholar] [CrossRef]
- Vieitez, A.M.; Corredoira, E.; Martínez, M.T.; José, M.C.S.; Sánchez, C.; Valladares, S.; Vidal, N.; Ballester, A. Application of biotechnological tools to Quercus improvement. Eur. J. For. Res. 2012, 131, 519–539. [Google Scholar] [CrossRef]
- Gürel, S.; Gülșen, Y. The effects of IBA and BAP on in vitro shoot production of almond (Amygdalus communis L.). Turk. J. Biol. 1998, 22, 375–380. [Google Scholar]
- Bahri, N.B.; Bettaieb, T. In vitro propagation of a forest tree Paulownia tomentosa (Thunb.) Steud.-A valuable medicinal tree species. Albanian J. Agric. Sci. 2013, 12, 37. [Google Scholar]
- Ballester, A.; Bourrain, L.; Corredoira, E.; Gonçalves, J.C.; Lê, C.-L.; Miranda-Fontaíña, E.; San José, M.C.; Sauer, U.; Viéitez Martín, A.M. Improving chestnut micropropagation through axillary shoot development and somatic embryogenesis. For. Snow Landsc. Res. 2001, 76, 460–467. [Google Scholar]
- Miranda, M.; Fernandez, J. Genotypic and environmental variation of Castanea crenata x C. sativa and Castanea sativa clones in aptitude to micropropagation. Silvae Genet. 2001, 50, 153–162. [Google Scholar]
- Clapa, D.; Bunea, C.; Borsai, O.; Pintea, A.; Hârța, M.; Ştefan, R.; Fira, A. The role of Sequestrene 138 in highbush blueberry (Vaccinium corymbosum L.) micropropagation. HortScience 2018, 53, 1487–1493. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially feasible micropropagation of mountain laurel Kalmia latifolia by use of shoot tip culture. Comb. Proc. Int. Plant Prop. Soc. 1980, 30, 421–427. [Google Scholar]
- Martínez, M.T.; Vieitez, F.J.; Solla, A.; Tapias, R.; Ramírez-Martín, N.; Corredoira, E. Vegetative propagation of Phytophthora cinnamomi-tolerant holm oak genotypes by axillary budding and somatic embryogenesis. Forests 2020, 11, 841. [Google Scholar] [CrossRef]
- Martínez, M.T.; Arrillaga, I.; Sales, E.; Pérez-Oliver, M.A.; González-Mas, M.D.C.; Corredoira, E. Micropropagation, characterization, and conservation of Phytophthora cinnamomi-tolerant holm oak mature trees. Forests 2021, 12, 1634. [Google Scholar] [CrossRef]
- Thomas, T.D. The role of activated charcoal in plant tissue culture. Biotechnol. Adv. 2008, 26, 618–631. [Google Scholar] [CrossRef]
- Osterc, G.; Fras, M.Z.; Vonedik, T.; Luthar, Z. The propagation of chestnut (Castanea sativa Mill.) nodal explants. Acta Agric. Slov. 2005, 85, 2. [Google Scholar]



| Sterilization Treatments Tested | |
|---|---|
| I | 30 s in 70% ethanol + 10 min in 33% v/v bleach + 0.2% PPMTM |
| II | 2% PPMTM |
| III | 30 s in 70% ethanol + 10 min in 33% v/v bleach |
| IV | 30 s in 70% ethanol + 20 min in 33% v/v bleach + 0.2% PPMTM |
| V | 25 min in 33% v/v bleach |
| VI | 10 min in 33% v/v bleach |
| VII | 30 s in 70% ethanol + 5 min in 33% v/v bleach |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavese, V.; Ruffa, P.; Abbà, S.; Costa, R.L.; Corredoira, E.; Silvestri, C.; Torello Marinoni, D.; Botta, R. An In Vitro Protocol for Propagating Castanea sativa Italian Cultivars. Plants 2022, 11, 3308. https://doi.org/10.3390/plants11233308
Pavese V, Ruffa P, Abbà S, Costa RL, Corredoira E, Silvestri C, Torello Marinoni D, Botta R. An In Vitro Protocol for Propagating Castanea sativa Italian Cultivars. Plants. 2022; 11(23):3308. https://doi.org/10.3390/plants11233308
Chicago/Turabian StylePavese, Vera, Paola Ruffa, Silvia Abbà, Rita Lourenço Costa, Elena Corredoira, Cristian Silvestri, Daniela Torello Marinoni, and Roberto Botta. 2022. "An In Vitro Protocol for Propagating Castanea sativa Italian Cultivars" Plants 11, no. 23: 3308. https://doi.org/10.3390/plants11233308
APA StylePavese, V., Ruffa, P., Abbà, S., Costa, R. L., Corredoira, E., Silvestri, C., Torello Marinoni, D., & Botta, R. (2022). An In Vitro Protocol for Propagating Castanea sativa Italian Cultivars. Plants, 11(23), 3308. https://doi.org/10.3390/plants11233308











