Mitigation of Negative Effects of Chromium (VI) Toxicity in Faba Bean (Vicia faba) Plants through the Supplementation of Kinetin (KN) and Gibberellic Acid (GA3)
Abstract
1. Introduction
2. Results
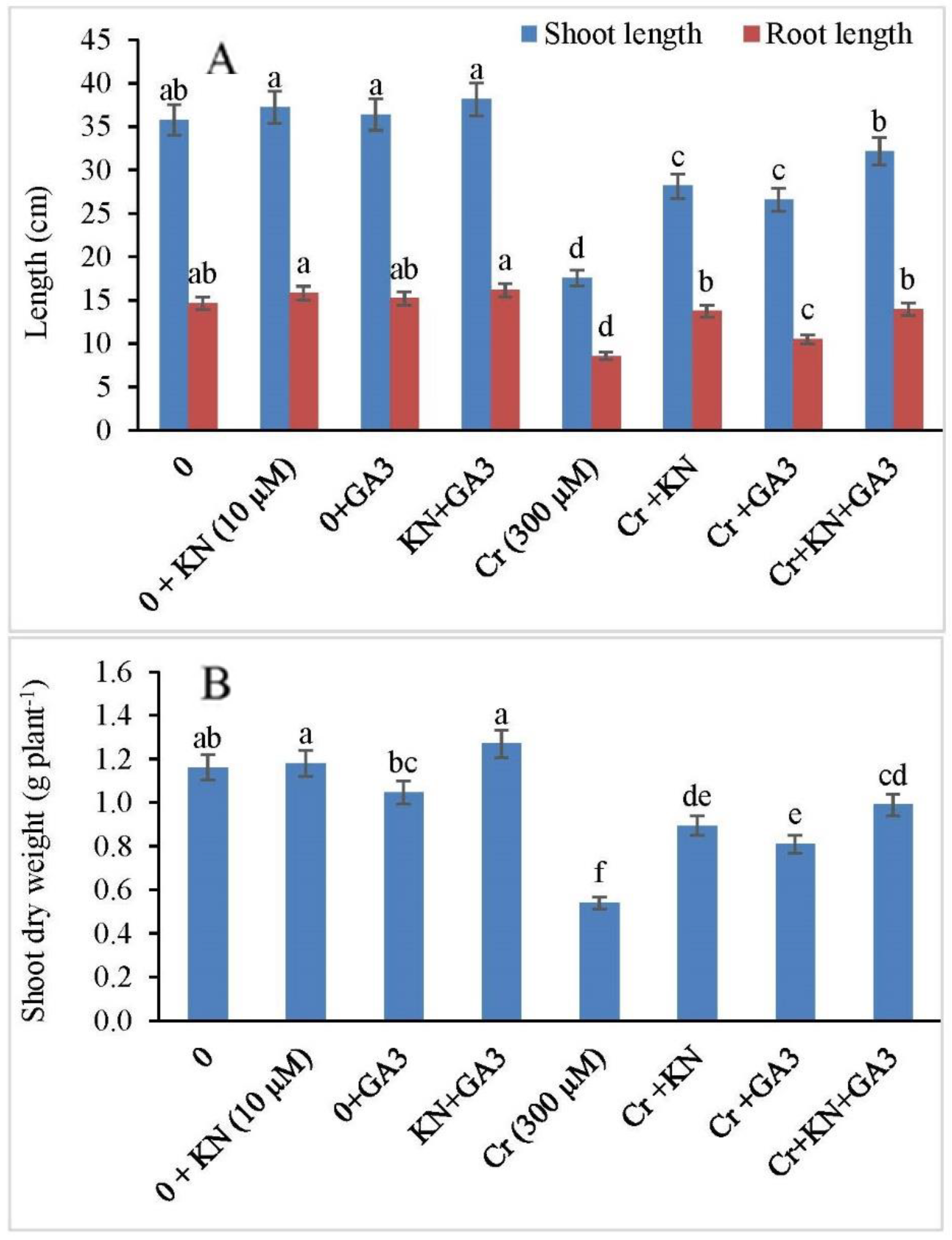
2.1. Growth and Biomass Yield
2.2. Chromium Accumulation and Translocation Factor
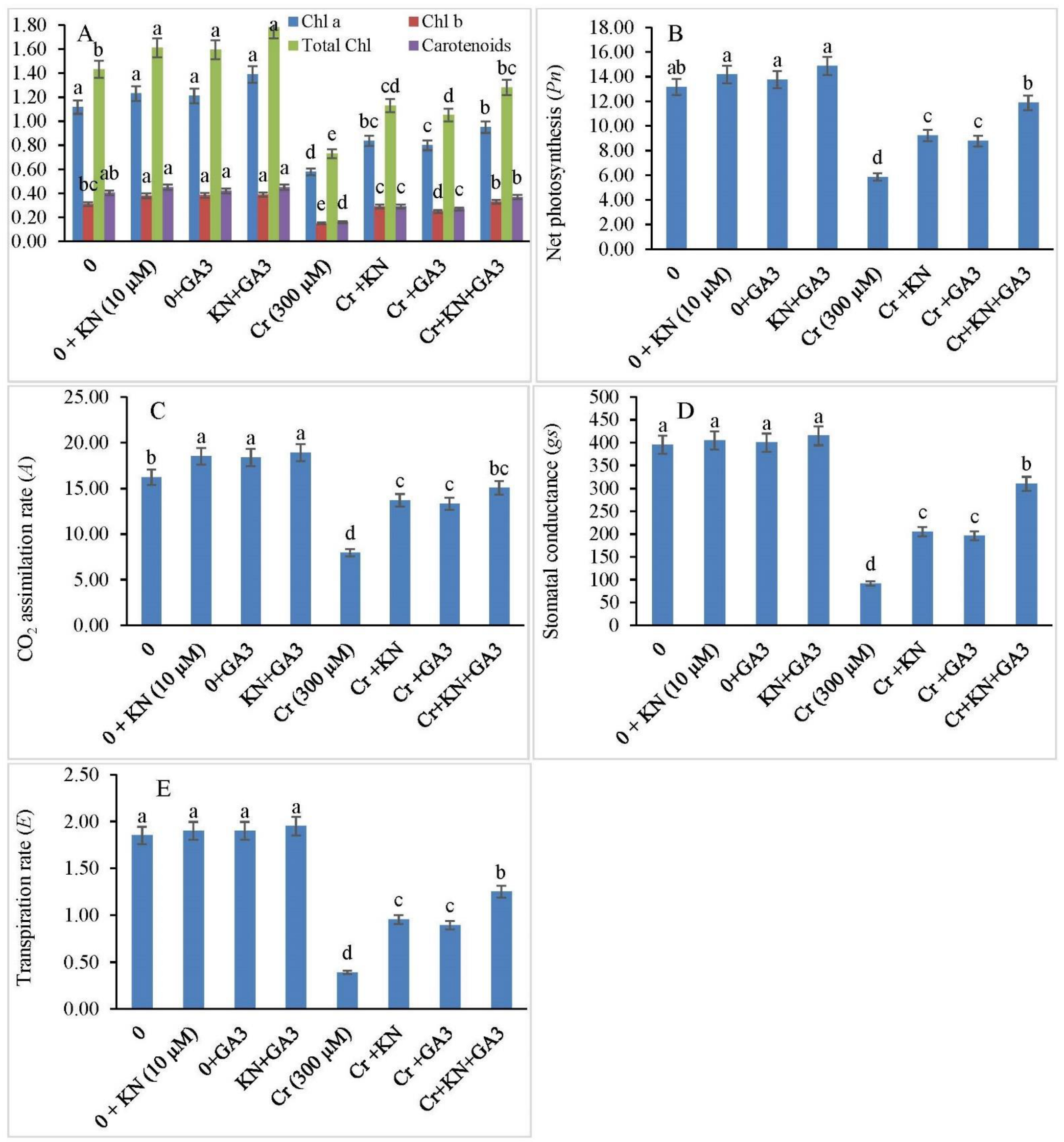
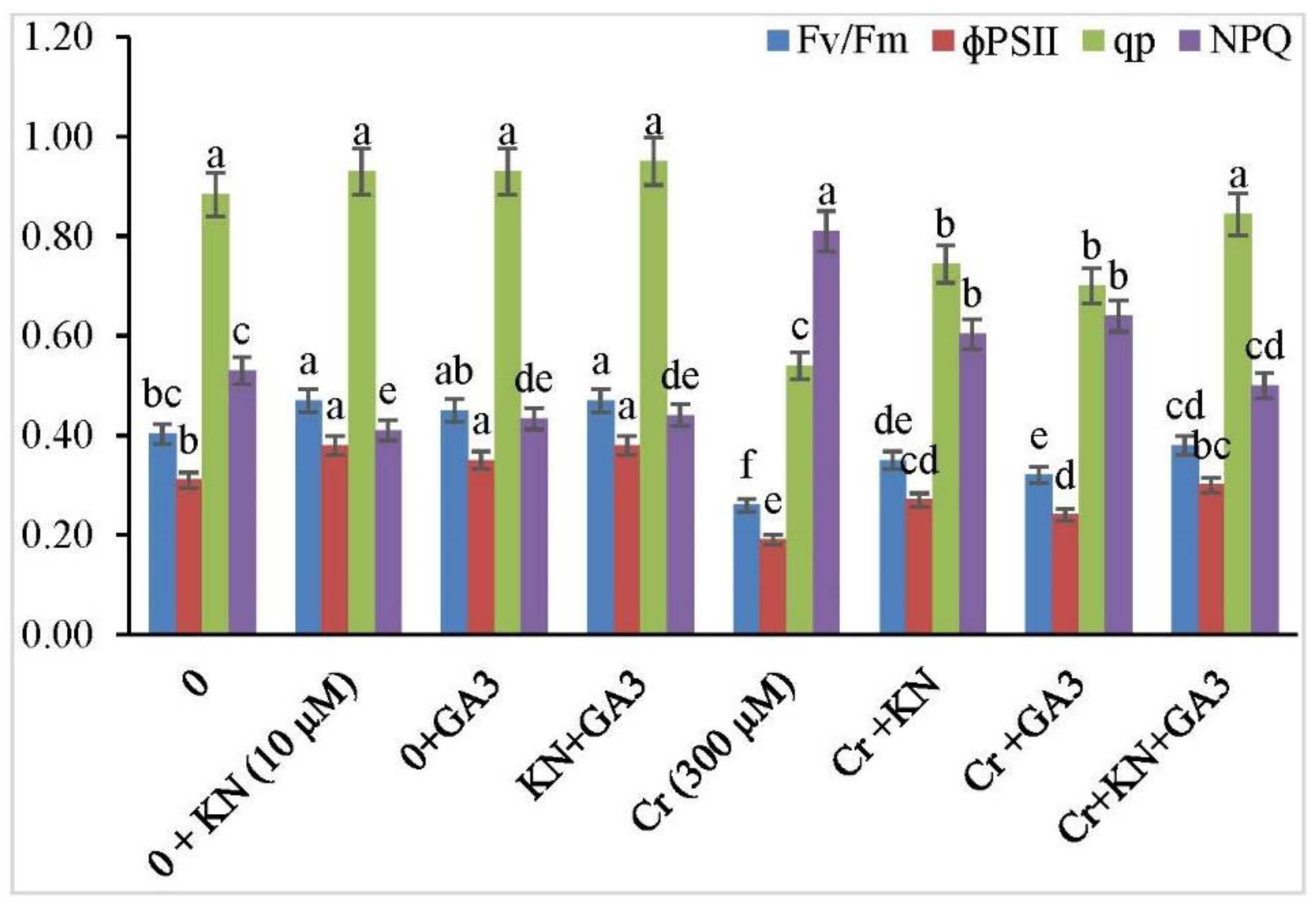
2.3. Pigment Systems, Photosynthesis, and Gas Exchange Parameters
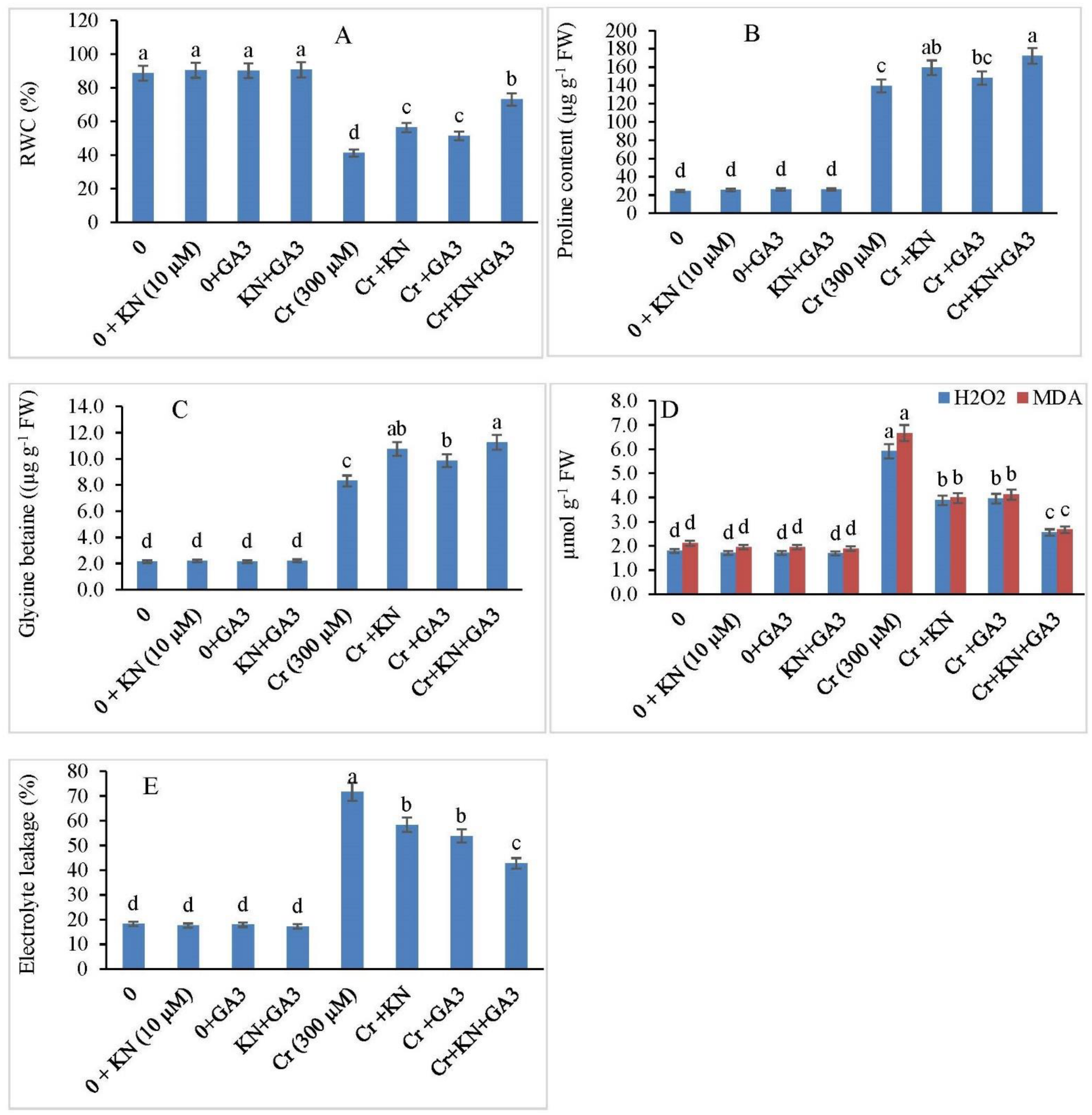
2.4. Relative Water Content, and Proline and GB Content
2.5. H2O2, MDA Content, and EL
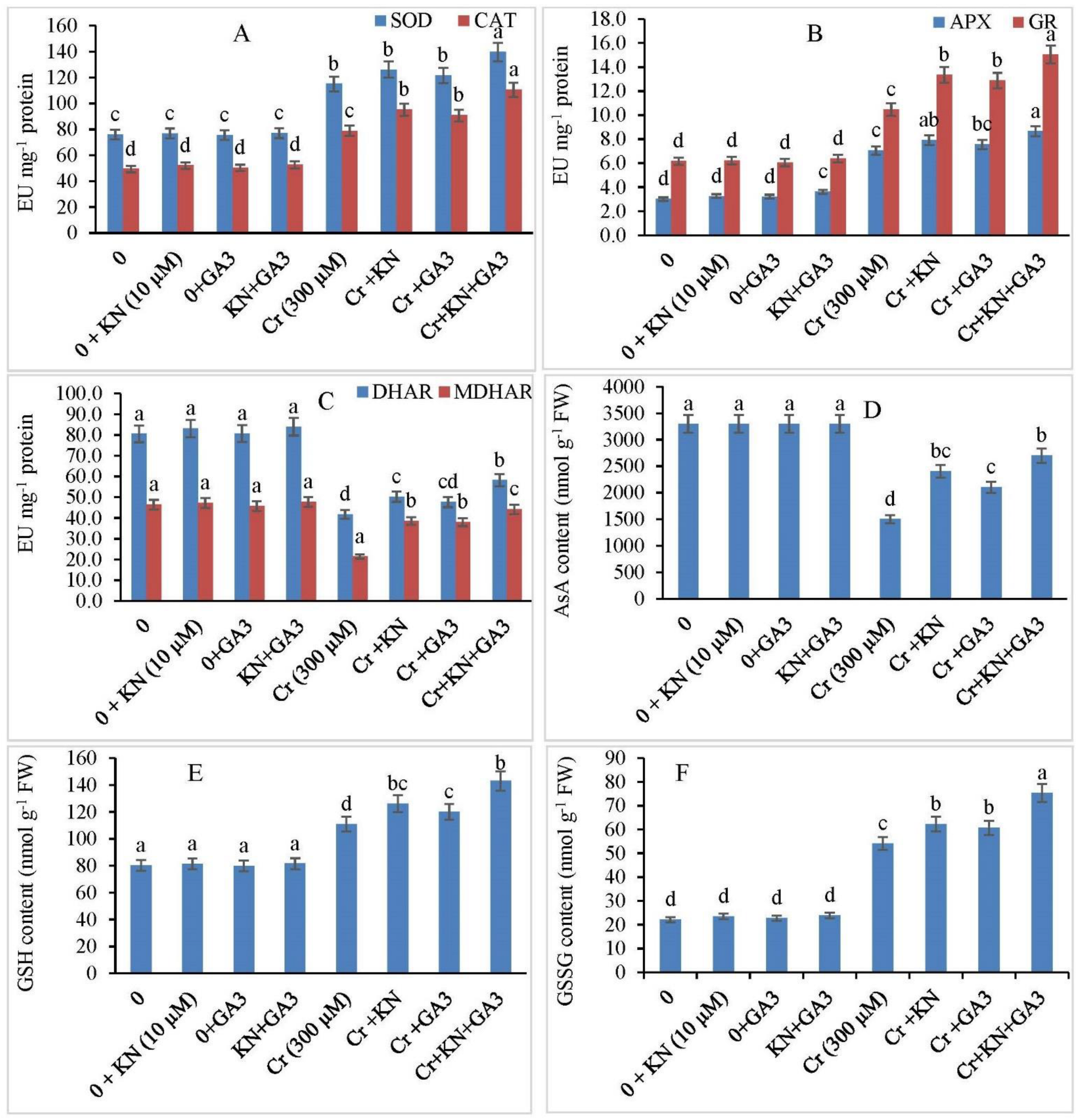
2.6. Antioxidants Enzyme Activity
2.7. Kinetin and GA3 Application Maintains Ascorbic Acid (AsA), GSH, and GSSG Ratio
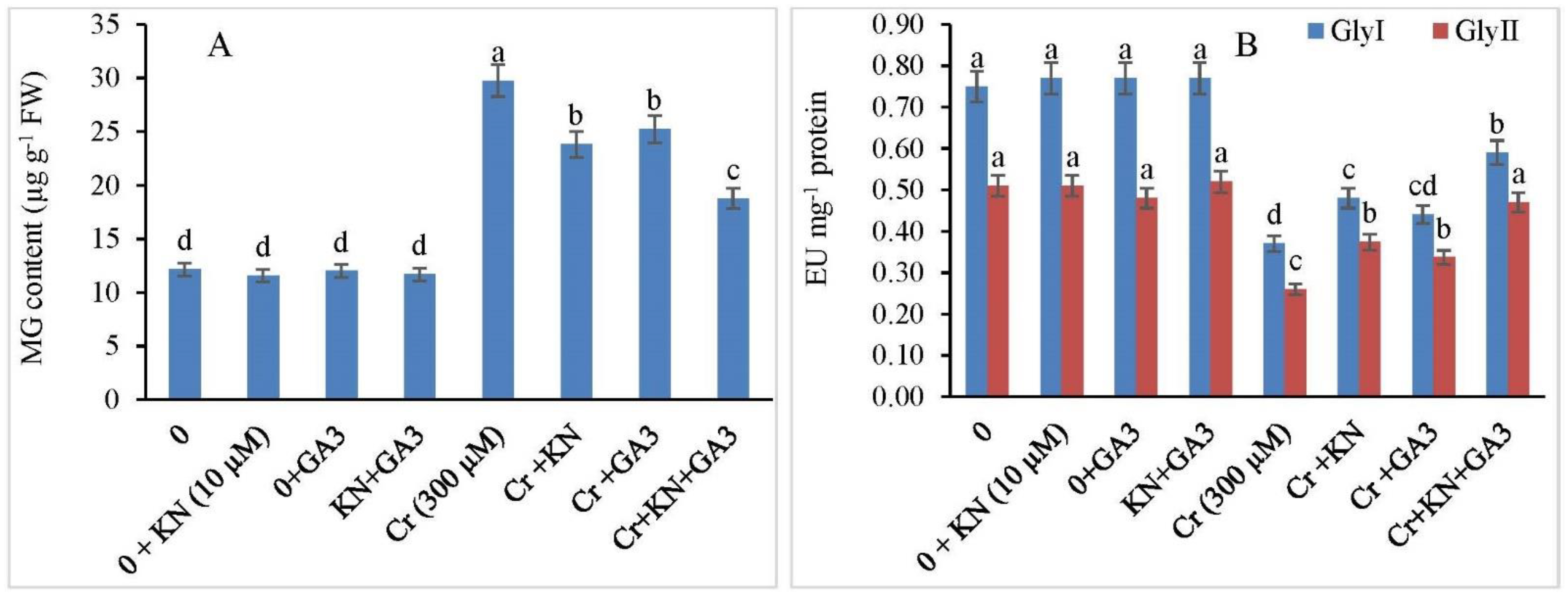
2.8. Methylglyoxal (MG) and Glyoxalase System
3. Discussion
3.1. Plant Growth and Biomass
3.2. Photosynthetic Efficiency
3.3. Relative Water Content, and Proline and GB Contents
4. Materials and Methods
4.1. Plant Growth and Treatments
- 0 mM (Control);
- 0 mM + Kinetin (10 µM);
- 0 mM + GA3 (10−6 M);
- 0 mM + Kinetin (10 µM) + GA3 (10−6 M);
- Cr (300 μM);
- Cr (300 μM) + Kinetin (10 µM);
- Cr (300 μM) + GA3 (10−6 M);
- Cr (300 μM) + Kinetin (10 µM) + GA3 (10−6 M).
| Parameters | |
|---|---|
| Texture | Sandy loam |
| pH | 8.83 |
| Cr | ND |
| Organic matter | 1.12 |
4.2. Plant Growth Parameters
4.3. Estimation of Chromium
4.4. Pigment Content, Photosynthesis, and Gas Exchange Parameters
4.5. Analysis of LRWC and Proline and Glycine Betaine Content
4.6. Estimation of Hydrogen Peroxide (H2O2), Lipid Peroxidation (Malondialdehyde), and Electrolyte Leakage
4.7. Enzyme Extraction and Assays
4.8. Nonenzymatic Antioxidants
4.9. MG Content Estimation
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barakat, A.; Ennaji, W.; Krimissa, S.; Bouzaid, M. Heavy metal contamination and ecological-health risk evaluation in peri-urban wastewater-irrigated soils of Beni-Mellal city (Morocco). Int. J. Environ. Health Res. 2020, 30, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Khalilzadeh, R.; Pirzad, A.; Sepehr, E.; Khan, S.; Anwar, S. Long-Term Effect of Heavy Metal–Polluted Wastewater Irrigation on Physiological and Ecological Parameters of Salicornia europaea L. J. Soil Sci. Plant Nutr. 2020, 20, 1574–1587. [Google Scholar] [CrossRef]
- Usman, K.; Al Jabri, H.; Abu-Dieyeh, M.H.; Alsafran, M.H. Comparative assessment of toxic metals bioaccumulation and the mechanisms of chromium (Cr) tolerance and uptake in Calotropis procera. Front. Plant Sci. 2020, 11, 883. [Google Scholar] [CrossRef] [PubMed]
- Nemat, H.; Shah, A.A.; Akram, W.; Ramzan, M.; Yasin, N.A. Ameliorative effect of co-application of Bradyrhizobium japonicum EI09 and Se to mitigate chromium stress in Capsicum annum L. Int. J. Phytoremediat. 2020, 22, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, C.; Wang, X.; Qing, X.; Wang, P.; Zhang, Y.; Zhang, X.; Zhao, X. Selenium alleviated chromium stress in Chinese cabbage (Brassica campestris L. ssp. Pekinensis) by regulating root morphology and metal element uptake. Ecotoxicol. Environ. Saf. 2019, 173, 314–321. [Google Scholar] [CrossRef]
- Stambulska, U.Y.; Bayliak, M.M.; Lushchak, V.I. Chromium (VI) toxicity in legume plants: Modulation effects of rhizobial symbiosis. BioMed Res. Int. 2018, 2018, 8031213. [Google Scholar] [CrossRef] [PubMed]
- da Conceicao Gomes, M.A.; Hauser-Davis, R.A.; Suzuki, M.S.; Vitoria, A.P. Plant chromium uptake and transport, physiological effects and recent advances in molecular investigations. Ecotoxicol. Environ. Saf. 2017, 140, 55–64. [Google Scholar] [CrossRef]
- Singh, H.P.; Mahajan, P.; Kaur, S.; Batish, D.R.; Kohli, R.K. Chromium toxicity and tolerance in plants. Environ. Chem. Lett. 2013, 11, 229–254. [Google Scholar] [CrossRef]
- Dai, H.; Shan, C. Effects of lanthanum on the antioxidant capacity of chloroplasts and chlorophyll fluorescence parameters of maize seedlings under chromium stress. Photosynthetica 2019, 57, 27–31. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Arslan Ashraf, M.; Ali, Q.; Abbas, Z.; Rizwan, M.; El-Sheikh, M.A.; Alyemeni, M.N.; Wijaya, L. Zinc-lysine supplementation mitigates oxidative stress in rapeseed (Brassica napus L.) by preventing phytotoxicity of chromium, when irrigated with tannery wastewater. Plants 2020, 9, 1145. [Google Scholar] [CrossRef]
- Fan, W.-J.; Feng, Y.-X.; Li, Y.-H.; Lin, Y.-J.; Yu, X.-Z. Unraveling genes promoting ROS metabolism in subcellular organelles of Oryza sativa in response to trivalent and hexavalent chromium. Sci. Total Environ. 2020, 744, 140951. [Google Scholar] [CrossRef] [PubMed]
- Habib, N.; Ali, Q.; Ali, S.; Javed, M.T.; Zulqurnain Haider, M.; Perveen, R.; Shahid, M.R.; Rizwan, M.; Abdel-Daim, M.M.; Elkelish, A. Use of nitric oxide and hydrogen peroxide for better yield of wheat (Triticum aestivum L.) under water deficit conditions: Growth, osmoregulation, and antioxidative defense mechanism. Plants 2020, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Jannat, A.; Munir, F.; Fatima, N.; Amir, R. Biochemical and molecular mechanisms of abiotic stress tolerance. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives II; Springer: Berlin/Heidelberg, Germany, 2020; pp. 187–230. [Google Scholar]
- Nazir, Y.; Halim, H.; Prabhakaran, P.; Ren, X.; Naz, T.; Mohamed, H.; Nosheen, S.; Mustafa, K.; Yang, W.; Abdul Hamid, A. Different classes of phytohormones act synergistically to enhance the growth, lipid and DHA biosynthetic capacity of Aurantiochytrium sp. SW1. Biomolecules 2020, 10, 755. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Bashri, G.; Prasad, S.M.; Singh, V.P. Kinetin alleviates UV-B-induced damage in Solanum lycopersicum: Implications of phenolics and antioxidants. J. Plant Growth Regul. 2019, 38, 831–841. [Google Scholar] [CrossRef]
- Gangwar, S.; Singh, V.P.; Tripathi, D.K.; Chauhan, D.K.; Prasad, S.M.; Maurya, J.N. Plant responses to metal stress: The emerging role of plant growth hormones in toxicity alleviation. In Emerging Technologies and Management of Crop Stress Tolerance; Elsevier: Amsterdam, The Netherlands, 2014; pp. 215–248. [Google Scholar]
- Tiwari, S.; Prasad, S.M. Regulation of insecticide toxicity by kinetin in two paddy field cyanobacteria: Physiological and biochemical assessment. Environ. Pollut. 2020, 259, 113806. [Google Scholar] [CrossRef]
- Acidri, R.; Sawai, Y.; Sugimoto, Y.; Handa, T.; Sasagawa, D.; Masunaga, T.; Yamamoto, S.; Nishihara, E. Exogenous kinetin promotes the nonenzymatic antioxidant system and photosynthetic activity of coffee (Coffea arabica L.) plants under cold stress conditions. Plants 2020, 9, 281. [Google Scholar] [CrossRef]
- Abeed, A.H.; Eissa, M.A.; Abdel-Wahab, D.A. Effect of exogenously applied jasmonic acid and kinetin on drought tolerance of wheat cultivars based on morpho-physiological evaluation. J. Soil Sci. Plant Nutr. 2021, 21, 131–144. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alamri, S.A.; Alam, P.; Ashraf, M.; Ahmad, P. Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate-glutathione cycle and glyoxalase system. PLoS ONE 2018, 13, e0202175. [Google Scholar] [CrossRef]
- Khan, A.; Bilal, S.; Khan, A.L.; Imran, M.; Shahzad, R.; Al-Harrasi, A.; Al-Rawahi, A.; Al-Azhri, M.; Mohanta, T.K.; Lee, I.-J. Silicon and gibberellins: Synergistic function in harnessing ABA signaling and heat stress tolerance in date palm (Phoenix dactylifera) L. Plants 2020, 9, 620. [Google Scholar] [CrossRef]
- Zhu, G.; An, L.; Jiao, X.; Chen, X.; Zhou, G.; McLaughlin, N. Effects of gibberellic acid on water uptake and germination of sweet sorghum seeds under salinity stress. Chil. J. Agric. Res. 2019, 79, 415–424. [Google Scholar] [CrossRef]
- Hasan, S.; Sehar, Z.; Khan, N.A. Gibberellic Acid and sulfur-mediated reversal of cadmium-inhibited photosynthetic performance in mungbean (Vigna radiata L.) involves nitric oxide. J. Plant Growth Regul. 2020, 39, 1605–1615. [Google Scholar] [CrossRef]
- Iftikhar, A.; Rizwan, M.; Adrees, M.; Ali, S.; Qayyum, M.F.; Hussain, A. Effect of gibberellic acid on growth, biomass, and antioxidant defense system of wheat (Triticum aestivum L.) under cerium oxide nanoparticle stress. Environ. Sci. Pollut. Res. 2020, 27, 33809–33820. [Google Scholar] [CrossRef] [PubMed]
- Vishal, B.; Kumar, P.P. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, A.; Ali, S.; Yasmeen, T.; Arif, M.S.; Zubair, M.; Rizwan, M.; Alhaithloul, H.A.S.; Alayafi, A.A.; Soliman, M.H. Effect of gibberellic acid on growth, photosynthesis and antioxidant defense system of wheat under zinc oxide nanoparticle stress. Environ. Pollut. 2019, 254, 113109. [Google Scholar] [CrossRef]
- Saijo, Y.; Loo, E.P.I. Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol. 2020, 225, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Elshafei, A.A.M.; Amer, M.A.E.; Elenany, M.A.M.; Helal, A.G.A.E. Evaluation of the genetic variability of faba bean (Vicia faba L.) genotypes using agronomic traits and molecular markers. Bull. Natl. Res. Cent. 2019, 43, 106. [Google Scholar] [CrossRef]
- Gupta, K.; Srivastava, A.; Srivastava, S.; Kumar, A. Phyto-genotoxicity of arsenic contaminated soil from Lakhimpur Kheri, India on Vicia faba L. Chemosphere 2020, 241, 125063. [Google Scholar] [CrossRef]
- Chen, H.-C.; Zhang, S.-L.; Wu, K.-J.; Li, R.; He, X.-R.; He, D.-N.; Huang, C.; Wei, H. The effects of exogenous organic acids on the growth, photosynthesis and cellular ultrastructure of Salix variegata Franch. Under Cd stress. Ecotoxicol. Environ. Saf. 2020, 187, 109790. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan, M.; Ali, Q.; Ali, S. Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains. Environ. Sci. Pollut. Res. 2019, 26, 7579–7588. [Google Scholar] [CrossRef]
- Bhat, J.A.; Shivaraj, S.; Singh, P.; Navadagi, D.B.; Tripathi, D.K.; Dash, P.K.; Solanke, A.U.; Sonah, H.; Deshmukh, R. Role of silicon in mitigation of heavy metal stresses in crop plants. Plants 2019, 8, 71. [Google Scholar] [CrossRef]
- Ahmad, R.; Ali, S.; Rizwan, M.; Dawood, M.; Farid, M.; Hussain, A.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol. Plant. 2020, 168, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Shreya, D.; Jinal, H.N.; Kartik, V.P.; Amaresan, N. Amelioration effect of chromium-tolerant bacteria on growth, physiological properties and chromium mobilization in chickpea (Cicer arietinum) under chromium stress. Arch. Microbiol. 2020, 202, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, R.; Ilyas, N.; Arshad, M.; Khalid, A. Amelioration potential of biochar for chromium stress in wheat. Pak. J. Bot. 2020, 52, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Ulhassan, Z.; Gill, R.A.; Huang, H.; Ali, S.; Mwamba, T.M.; Ali, B.; Huang, Q.; Hamid, Y.; Khan, A.R.; Wang, J. Selenium mitigates the chromium toxicity in Brassicca napus L. by ameliorating nutrients uptake, amino acids metabolism and antioxidant defense system. Plant Physiol. Biochem. 2019, 145, 142–152. [Google Scholar] [CrossRef]
- Wakeel, A.; Xu, M. Chromium morpho-phytotoxicity. Plants 2020, 9, 564. [Google Scholar] [CrossRef]
- Alamri, S.; Ali, H.M.; Khan, M.I.R.; Singh, V.P.; Siddiqui, M.H. Exogenous nitric oxide requires endogenous hydrogen sulfide to induce the resilience through sulfur assimilation in tomato seedlings under hexavalent chromium toxicity. Plant Physiol. Biochem. 2020, 155, 20–34. [Google Scholar] [CrossRef]
- Ayyaz, A.; Farooq, M.A.; Kanwal, A.; Aslam, M.; Iqbal, M.; Manzoor, A.; Khalid, A.; Umer, S.; Bano, H.; Rasool, B. Differential responses of exogenous melatonin on growth, photosynthesis and antioxidant defence system in two Brassica napus L. cultivars under chromium stress. Int. J. Environ. Agric. Biotechnol. 2020, 5, 397–411. [Google Scholar]
- do Nascimento, J.L.; de Almeida, A.-A.F.; Barroso, J.P.; Mangabeira, P.A.; Ahnert, D.; Sousa, A.G.; Silva, J.V.S.; Baligar, V.C. Physiological, ultrastructural, biochemical and molecular responses of young cocoa plants to the toxicity of Cr (III) in soil. Ecotoxicol. Environ. Saf. 2018, 159, 272–283. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Sabatino, L.; Vetrano, F. Effect of gibberellic acid on growth, yield, and quality of leaf lettuce and rocket grown in a floating system. Agronomy 2019, 9, 382. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F. The role of salicylic acid and gibberellin signaling in plant responses to abiotic stress with an emphasis on heavy metals. Plant Signal. Behav. 2020, 15, 1777372. [Google Scholar] [CrossRef]
- Jusoh, M.; Loh, S.H.; Aziz, A.; Cha, T.S. Gibberellin promotes cell growth and induces changes in fatty acid biosynthesis and upregulates fatty acid biosynthetic genes in Chlorella vulgaris UMT-M1. Appl. Biochem. Biotechnol. 2019, 188, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone priming: Regulator for heavy metal stress in plants. J. Plant Growth Regul. 2019, 38, 739–752. [Google Scholar] [CrossRef]
- Aldesuquy, H.; Baka, Z.; Mickky, B. Kinetin and spermine mediated induction of salt tolerance in wheat plants: Leaf area, photosynthesis and chloroplast ultrastructure of flag leaf at ear emergence. Egypt. J. Basic Appl. Sci. 2014, 1, 77–87. [Google Scholar] [CrossRef]
- Kamboj, S.; Mathpal, B. Improving rice grain quality by foliar application of plant growth regulators under various mode of Zn application. Plant Arch. 2019, 19, 2181–2184. [Google Scholar]
- Ansari, S.; Pandey, A.K.; Singh, U.P. Effect of kinetin on growth parameters of cowpea (Vigna unguiculata) L. J. Pharmacogn. Phytochem. 2020, 9, 623–628. [Google Scholar]
- Kaur, S.; Gupta, A.K.; Kaur, N. Gibberellic acid and kinetin partially reverse the effect of water stress on germination and seedling growth in chickpea. Plant Growth Regul. 1998, 25, 29–33. [Google Scholar] [CrossRef]
- Kabar, K.; Baltepe, S. Effects of kinetin and gibberellic acid in overcoming high temperature and salinity (NaCl) stresses on the germination of barley and lettuce seeds. In Proceedings of Phyton: Annales Rei Botanicae; Berger and Sohne: Horn, Austria.
- Terzi, I.; Kocaçalişkan, İ. The effects of gibberellic acid and kinetin on overcoming the effects of juglone stress on seed germination and seedling growth. Turk. J. Bot. 2010, 34, 67–72. [Google Scholar] [CrossRef]
- EL, M. Effect of kinetin and GA3 treatments on growth and flowering of Dendranthema grandiflorium cv. Art Queen plants. Middle East J. 2018, 7, 801–815. [Google Scholar]
- Munir, M.A.M.; Liu, G.; Yousaf, B.; Ali, M.U.; Abbas, Q.; Ullah, H. Synergistic effects of biochar and processed fly ash on bioavailability, transformation and accumulation of heavy metals by maize (Zea mays L.) in coal-mining contaminated soil. Chemosphere 2020, 240, 124845. [Google Scholar] [CrossRef]
- Saleem, M.H.; Fahad, S.; Khan, S.U.; Din, M.; Ullah, A.; Sabagh, A.E.; Hossain, A.; Llanes, A.; Liu, L. Copper-induced oxidative stress, initiation of antioxidants and phytoremediation potential of flax (Linum usitatissimum L.) seedlings grown under the mixing of two different soils of China. Environ. Sci. Pollut. Res. 2020, 27, 5211–5221. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Zinta, G.; Hamed, B.A.; Selim, S.; Beemster, G.; Hozzein, W.N.; Wadaan, M.A.; Asard, H.; Abuelsoud, W. Maize roots and shoots show distinct profiles of oxidative stress and antioxidant defense under heavy metal toxicity. Environ. Pollut. 2020, 258, 113705. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Tokas, J.; Singal, H. Amelioration of chromium VI toxicity in Sorghum (Sorghum bicolor L.) using glycine betaine. Sci. Rep. 2019, 9, 16020. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.; Chocobar-Ponce, S.; Pagano, E.; Prado, F.; Rosa, M. Differential effects of Zn concentrations on Cr (VI) uptake by two Salvinia species: Involvement of thiol compounds. Int. J. Phytoremediat. 2021, 23, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Ali, S.; Refay, Y.; Rizwan, M.; Alhammad, B.A.; El-Hendawy, S.E. Chromium resistant microbes and melatonin reduced Cr uptake and toxicity, improved physio-biochemical traits and yield of wheat in contaminated soil. Chemosphere 2020, 250, 126239. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Soares, C.; Sousa, B.; Martins, M.; Kumar, V.; Shahzad, B.; Sidhu, G.P.; Bali, A.S.; Asgher, M.; Bhardwaj, R. Nitric oxide-mediated regulation of oxidative stress in plants under metal stress: A review on molecular and biochemical aspects. Physiol. Plant. 2020, 168, 318–344. [Google Scholar] [CrossRef]
- Rai, V.; Vajpayee, P.; Singh, S.N.; Mehrotra, S. Effect of chromium accumulation on photosynthetic pigments, oxidative stress defense system, nitrate reduction, proline level and eugenol content of Ocimum tenuiflorum L. Plant Sci. 2004, 167, 1159–1169. [Google Scholar] [CrossRef]
- Ali, S.; Farooq, M.A.; Yasmeen, T.; Hussain, S.; Arif, M.S.; Abbas, F.; Bharwana, S.A.; Zhang, G. The influence of silicon on barley growth, photosynthesis and ultra-structure under chromium stress. Ecotoxicol. Environ. Saf. 2013, 89, 66–72. [Google Scholar] [CrossRef]
- Nabi, A.; Naeem, M.; Aftab, T.; Khan, M. Alterations in photosynthetic pigments, antioxidant machinery, essential oil constituents and growth of menthol mint (Mentha arvensis L.) upon nickel exposure. Braz. J. Bot. 2020, 43, 721–731. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Guo, K.; Huo, Y.; He, G.; Sun, H.; Guan, Y.; Xu, N.; Yang, W.; Sun, G. Toxic effects of heavy metal Cd and Zn on chlorophyll, carotenoid metabolism and photosynthetic function in tobacco leaves revealed by physiological and proteomics analysis. Ecotoxicol. Environ. Saf. 2020, 202, 110856. [Google Scholar] [CrossRef]
- Falkowska, M.; Pietryczuk, A.; Piotrowska, A.; Bajguz, A.; Grygoruk, A.; Czerpak, R. The effect of gibberellic acid (GA3) on growth, metal biosorption and metabolism of the green algae Chlorella vulgaris (Chlorophyceae) Beijerinck exposed to cadmium and lead stress. Pol. J. Environ. Stud. 2011, 20, 53–59. [Google Scholar]
- Tiwari, S.; Patel, A.; Prasad, S.M. Kinetin alleviates chromium toxicity on growth and PS II photochemistry in Nostoc muscorum by regulating antioxidant system. Ecotoxicol. Environ. Saf. 2018, 161, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, X.; Liu, Y.; Wei, J.; Shen, W.; Shen, Z.; Cui, J. Hemin-mediated alleviation of zinc, lead and chromium toxicity is associated with elevated photosynthesis, antioxidative capacity; suppressed metal uptake and oxidative stress in rice seedlings. Plant Growth Regul. 2017, 81, 253–264. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Saeed, R.; Rizwan, M.; Bukhari, S.A.H.; Abbasi, G.H.; Hussain, A.; Ali, B.; Zamir, M.S.I.; Ahmad, I. Combined application of citric acid and 5-aminolevulinic acid improved biomass, photosynthesis and gas exchange attributes of sunflower (Helianthus annuus L.) grown on chromium contaminated soil. Int. J. Phytoremediat. 2019, 21, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Ali, S.; Rehman, M.; Rana, M.S.; Rizwan, M.; Kamran, M.; Imran, M.; Riaz, M.; Soliman, M.H.; Elkelish, A. Influence of phosphorus on copper phytoextraction via modulating cellular organelles in two jute (Corchorus capsularis L.) varieties grown in a copper mining soil of Hubei Province, China. Chemosphere 2020, 248, 126032. [Google Scholar] [CrossRef]
- Teszlák, P.; Kocsis, M.; Gaál, K.; Nikfardjam, M.P. Regulatory effects of exogenous gibberellic acid (GA3) on water relations and CO2 assimilation among grapevine (Vitis vinifera L.) cultivars. Sci. Hortic. 2013, 159, 41–51. [Google Scholar] [CrossRef]
- Shah, S. Effects of salt stress on mustard as affected by gibberellic acid application. Gen. Appl. Plant Physiol. 2007, 33, 97–106. [Google Scholar]
- Kamran, M.; Danish, M.; Saleem, M.H.; Malik, Z.; Parveen, A.; Abbasi, G.H.; Jamil, M.; Ali, S.; Afzal, S.; Riaz, M. Application of abscisic acid and 6-benzylaminopurine modulated morpho-physiological and antioxidative defense responses of tomato (Solanum lycopersicum L.) by minimizing cobalt uptake. Chemosphere 2021, 263, 128169. [Google Scholar] [CrossRef]
- Khalil, R.R.; Moustafa, A.N.; Bassuony, F.M.; Haroun, S.A. Kinetin and/or calcium affect growth of Phaseolus vulgaris L. plant grown under heavy metals stress. J. Environ. Sci 2017, 46, 103–120. [Google Scholar]
- Choi, H.G.; Moon, B.Y.; Kang, N.J. Correlation between strawberry (Fragaria ananassa Duch.) productivity and photosynthesis-related parameters under various growth conditions. Front. Plant Sci. 2016, 7, 1607. [Google Scholar] [CrossRef]
- Mathur, S.; Kalaji, H.; Jajoo, A. Investigation of deleterious effects of chromium phytotoxicity and photosynthesis in wheat plant. Photosynthetica 2016, 54, 185–192. [Google Scholar] [CrossRef]
- Sunil, B.; Saini, D.; Bapatla, R.B.; Aswani, V.; Raghavendra, A.S. Photorespiration is complemented by cyclic electron flow and the alternative oxidase pathway to optimize photosynthesis and protect against abiotic stress. Photosynth. Res. 2019, 139, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, F.; Mu, S.; Zhang, D.; Pan, X.; Lee, D.-J. Simultaneous analysis of photosystem responses of Microcystis aeruginoga under chromium stress. Ecotoxicol. Environ. Saf. 2013, 88, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Farouk, S.; Al-Amri, S. Ameliorative roles of melatonin and/or zeolite on chromium-induced leaf senescence in marjoram plants by activating antioxidant defense, osmolyte accumulation, and ultrastructural modification. Ind. Crops Prod. 2019, 142, 111823. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, Z.; Li, X.; Zha, D. Effects of cytokinin on photosynthetic gas exchange, chlorophyll fluorescence parameters and antioxidative system in seedlings of eggplant (Solanum melongena L.) under salinity stress. Acta Physiol. Plant. 2012, 34, 2105–2114. [Google Scholar] [CrossRef]
- Ahmad, B.; Jaleel, H.; Shabbir, A.; Khan, M.M.A.; Sadiq, Y. Concomitant application of depolymerized chitosan and GA3 modulates photosynthesis, essential oil and menthol production in peppermint (Mentha piperita) L. Sci. Hortic. 2019, 246, 371–379. [Google Scholar] [CrossRef]
- Anwar, A.; Xianchang, Y.; Yansu, L. Seed priming as a promising technique to improve growth, chlorophyll, photosynthesis and nutrient contents in cucumber seedlings. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 116–127. [Google Scholar] [CrossRef]
- Bashir, M.A.; Naveed, M.; Ahmad, Z.; Gao, B.; Mustafa, A.; Núñez-Delgado, A. Combined application of biochar and sulfur regulated growth, physiological, antioxidant responses and Cr removal capacity of maize (Zea mays L.) in tannery polluted soils. J. Environ. Manag. 2020, 259, 110051. [Google Scholar] [CrossRef]
- Pandian, S.; Rakkammal, K.; Rathinapriya, P.; Rency, A.S.; Satish, L.; Ramesh, M. Physiological and biochemical changes in sorghum under combined heavy metal stress: An adaptive defence against oxidative stress. Biocatal. Agric. Biotechnol. 2020, 29, 101830. [Google Scholar] [CrossRef]
- Pandey, A.; Gautam, A.; Pandey, P.; Dubey, R. Alleviation of chromium toxicity in rice seedling using Phyllanthus emblica aqueous extract in relation to metal uptake and modulation of antioxidative defense. South Afr. J. Bot. 2019, 121, 306–316. [Google Scholar] [CrossRef]
- Stanton, K.M.; Mickelbart, M.V. Maintenance of water uptake and reduced water loss contribute to water stress tolerance of Spiraea alba Du Roi and Spiraea tomentosa L. Hortic. Res. 2014, 1, 14033. [Google Scholar] [CrossRef][Green Version]
- Zhu, X.F.; Jiang, T.; Wang, Z.W.; Lei, G.J.; Shi, Y.Z.; Li, G.X.; Zheng, S.J. Gibberellic acid alleviates cadmium toxicity by reducing nitric oxide accumulation and expression of IRT1 in Arabidopsis thaliana. J. Hazard. Mater. 2012, 239, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Siddique, A.; Kandpal, G.; Kumar, P. Proline accumulation and its defensive role under diverse stress condition in plants: An Overview. J. Pure Appl. Microbiol. 2018, 12, 1655–1659. [Google Scholar] [CrossRef]
- Forlani, G.; Trovato, M.; Funck, D.; Signorelli, S. Regulation of proline accumulation and its molecular and physiological functions in stress defence. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 73–97. [Google Scholar]
- Mansour, M.M.F.; Salama, K.H.A. Proline and abiotic stresses: Responses and adaptation. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives II; Springer: Berlin/Heidelberg, Germany, 2020; pp. 357–397. [Google Scholar]
- Shafi, A.; Zahoor, I.; Mushtaq, U. Proline accumulation and oxidative stress: Diverse roles and mechanism of tolerance and adaptation under salinity stress. In Salt Stress, Microbes, and Plant Interactions: Mechanisms and Molecular Approaches; Springer: Berlin/Heidelberg, Germany, 2019; pp. 269–300. [Google Scholar]
- Zouari, M.; Hassena, A.B.; Trabelsi, L.; Rouina, B.B.; Decou, R.; Labrousse, P. Exogenous proline-mediated abiotic stress tolerance in plants: Possible mechanisms. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 99–121. [Google Scholar]
- Kumar, V.; Shriram, V.; Hoque, T.S.; Hasan, M.; Burritt, D.J.; Hossain, M.A. Glycinebetaine-mediated abiotic oxidative-stress tolerance in plants: Physiological and biochemical mechanisms. In Stress Signaling In Plants: Genomics and Proteomics Perspective, Volume 2; Springer: Berlin/Heidelberg, Germany, 2017; pp. 111–133. [Google Scholar]
- Kurepin, L.V.; Ivanov, A.G.; Zaman, M.; Pharis, R.P.; Hurry, V.; Hüner, N. Interaction of glycine betaine and plant hormones: Protection of the photosynthetic apparatus during abiotic stress. In Photosynthesis: Structures, Mechanisms, and Applications; Springer: Berlin/Heidelberg, Germany, 2017; pp. 185–202. [Google Scholar]
- Siddiqui, S.A.; Kumari, A.; Rathore, M.S. Glycine Betaine as a Major Osmolyte under Abiotic Stress in Halophytes. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Springer: Berlin, Germany, 2021; pp. 2069–2087. [Google Scholar]
- Adhikari, A.; Adhikari, S.; Ghosh, S.; Azahar, I.; Shaw, A.K.; Roy, D.; Roy, S.; Saha, S.; Hossain, Z. Imbalance of redox homeostasis and antioxidant defense status in maize under chromium (VI) stress. Environ. Exp. Bot. 2020, 169, 103873. [Google Scholar] [CrossRef]
- Christou, A.; Georgiadou, E.C.; Zissimos, A.M.; Christoforou, I.C.; Christofi, C.; Neocleous, D.; Dalias, P.; Torrado, S.O.; Argyraki, A.; Fotopoulos, V. Hexavalent chromium leads to differential hormetic or damaging effects in alfalfa (Medicago sativa L.) plants in a concentration-dependent manner by regulating nitro-oxidative and proline metabolism. Environ. Pollut. 2020, 267, 115379. [Google Scholar] [CrossRef] [PubMed]
- Guarino, F.; Ruiz, K.B.; Castiglione, S.; Cicatelli, A.; Biondi, S. The combined effect of Cr (III) and NaCl determines changes in metal uptake, nutrient content, and gene expression in quinoa (Chenopodium quinoa Willd.). Ecotoxicol. Environ. Saf. 2020, 193, 110345. [Google Scholar] [CrossRef] [PubMed]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Abd_Allah, E.F.; Alqarawi, A.A.; Ahmad, P. Selenium modulates dynamics of antioxidative defence expression, photosynthetic attributes and secondary metabolites to mitigate chromium toxicity in Brassica juncea L. plants. Environmental and Experimental Botany 2019, 161, 180–192. [Google Scholar] [CrossRef]
- Bruno, L.B.; Karthik, C.; Ma, Y.; Kadirvelu, K.; Freitas, H.; Rajkumar, M. Amelioration of chromium and heat stresses in Sorghum bicolor by Cr6+ reducing-thermotolerant plant growth promoting bacteria. Chemosphere 2020, 244, 125521. [Google Scholar] [CrossRef]
- Qadir, M.; Hussain, A.; Hamayun, M.; Shah, M.; Iqbal, A.; Murad, W. Phytohormones producing rhizobacterium alleviates chromium toxicity in Helianthus annuus L. by reducing chromate uptake and strengthening antioxidant system. Chemosphere 2020, 258, 127386. [Google Scholar] [CrossRef]
- Zaid, A.; Mohammad, F.; Fariduddin, Q. Plant growth regulators improve growth, photosynthesis, mineral nutrient and antioxidant system under cadmium stress in menthol mint (Mentha arvensis) L. Physiol. Mol. Biol. Plants 2020, 26, 25–39. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Hasanuzzaman, M. Exogenous kinetin and putrescine synergistically mitigate salt stress in Luffa acutangula by modulating physiology and antioxidant defense. Physiol. Mol. Biol. Plants 2020, 26, 2125–2137. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Mir, R.A.; Alyemeni, M.N.; Ahmad, P. Combined effects of brassinosteroid and kinetin mitigates salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant Physiol. Biochem. 2020, 147, 31–42. [Google Scholar] [CrossRef] [PubMed]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How does proline treatment promote salt stress tolerance during crop plant development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef] [PubMed]
- Wakeel, A.; Xu, M.; Gan, Y. Chromium-induced reactive oxygen species accumulation by altering the enzymatic antioxidant system and associated cytotoxic, genotoxic, ultrastructural, and photosynthetic changes in plants. Int. J. Mol. Sci. 2020, 21, 728. [Google Scholar] [CrossRef]
- Liu, T.; Ye, X.; Li, M.; Li, J.; Qi, H.; Hu, X. H2O2 and NO are involved in trehalose-regulated oxidative stress tolerance in cold-stressed tomato plants. Environ. Exp. Bot. 2020, 171, 103961. [Google Scholar] [CrossRef]
- Nazir, F.; Hussain, A.; Fariduddin, Q. Hydrogen peroxide modulate photosynthesis and antioxidant systems in tomato (Solanum lycopersicum L.) plants under copper stress. Chemosphere 2019, 230, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Ackin, A.; Ackin, A.; Yildirim, C. Application of fulvic acid modulates photosynthetic pigments and malondialdehyde content in bread wheat (Triticum aestivum cv. Ekiz) to increase resistance to chromium stress. Int. J. Agric. Biol 2020, 23, 142–148. [Google Scholar]
- Rawat, N.; Singla-Pareek, S.L.; Pareek, A. Membrane dynamics during individual and combined abiotic stresses in plants and tools to study the same. Physiol. Plant. 2021, 171, 653–676. [Google Scholar] [CrossRef]
- Sadžak, A.; Mravljak, J.; Maltar-Strmečki, N.; Arsov, Z.; Baranović, G.; Erceg, I.; Kriechbaum, M.; Strasser, V.; Přibyl, J.; Šegota, S. The structural integrity of the model lipid membrane during induced lipid peroxidation: The role of flavonols in the inhibition of lipid peroxidation. Antioxidants 2020, 9, 430. [Google Scholar] [CrossRef]
- Ghori, N.-H.; Ghori, T.; Hayat, M.; Imadi, S.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Kumbar, M.; Mirajkar, K.K.; Arvind, K. Phytochemical response in rice (Oryza sativa L.) genotype during the vegetative and reproductive stage under drought stress and non-stress conditions. J. Plant Biochem. Biotechnol. 2021, 30, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Zhang, G.; Chen, Y.; Gao, J.; Sun, Y.-R.; Sun, M.-F.; Chen, J.-P. Exogenous application of gibberellic acid and ascorbic acid improved tolerance of okra seedlings to NaCl stress. Acta Physiol. Plant. 2019, 41, 93. [Google Scholar] [CrossRef]
- Jakovljević, D.; Stanković, M. Adaptive strategies of plants under adverse environment: Mitigating effects of antioxidant system. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives II; Springer: Berlin/Heidelberg, Germany, 2020; pp. 163–186. [Google Scholar]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Gangwar, S.; Singh, V.P.; Garg, S.K.; Prasad, S.M.; Maurya, J.N. Kinetin supplementation modifies chromium (VI) induced alterations in growth and ammonium assimilation in pea seedlings. Biol. Trace Elem. Res. 2011, 144, 1327–1343. [Google Scholar] [CrossRef]
- Hassan, M.; Mansoor, S. Priming seeds with phytohormones alleviates cadmium toxicity in mung bean (Vigna radiata L. Wilczek) seedlings. Pak. J. Bot. 2017, 49, 2071–2078. [Google Scholar]
- Morgenstern, J.; Campos Campos, M.; Nawroth, P.; Fleming, T. The glyoxalase system—New insights into an ancient metabolism. Antioxidants 2020, 9, 939. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Murillo-Amador, B.; Ashraf, M. Involvement of L-cysteine desulfhydrase and hydrogen sulfide in glutathione-induced tolerance to salinity by accelerating ascorbate-glutathione cycle and glyoxalase system in capsicum. Antioxidants 2020, 9, 603. [Google Scholar] [CrossRef]
- Sawan, Z.; Mohamed, A.; Sakr, R.; Tarrad, A. Effect of kinetin concentration and methods of application on seed germination, yield components, yield and fiber properties of the Egyptian cotton (Gossypium barbadense). Environ. Exp. Bot. 2000, 44, 59–68. [Google Scholar] [CrossRef]
- Alsahli, A.A.; Bhat, J.A.; Alyemeni, M.N.; Ashraf, M.; Ahmad, P. Hydrogen sulfide (H2S) mitigates arsenic (As)-Induced toxicity in pea (Pisum sativum L.) plants by regulating osmoregulation, antioxidant defense system, ascorbate glutathione cycle and glyoxalase system. J. Plant Growth Regul. 2021, 40, 2515–2531. [Google Scholar] [CrossRef]
- Gupta, A.; Sinha, S. Chromium levels in vegetables and grains grown on tannery effluent irrigated area of Jajmau, Kanpur, India: Influence on dietary intake. Bull. Environ. Contam. Toxicol. 2006, 77, 658–664. [Google Scholar] [CrossRef]
- Duman, F.; Urey, E.; Koca, F.D. Temporal variation of heavy metal accumulation and translocation characteristics of narrow-leaved cattail (Typha angustifolia) L. Environ. Sci. Pollut. Res. 2015, 22, 17886–17896. [Google Scholar] [CrossRef]
- Lichtenthaler, H.; Wellburn, A. Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Barrs, H.; Weatherley, P. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Avestan, S.; Ghasemnezhad, M.; Esfahani, M.; Byrt, C.S. Application of nano-silicon dioxide improves salt stress tolerance in strawberry plants. Agronomy 2019, 9, 246. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.; Grattan, S. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Matowe, W. Drought Tolerance in Two Mosses: Correlated with Enzymatic Defence against Lipid Peroxidation. J. Exp. Bot. 1981, 32, 79–91. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Colowick, S., Kaplan, N., Eds.; Elsevier: Gainesville, FL, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Foster, J.G.; Hess, J.L. Responses of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol. 1980, 66, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Miyake, C.; Asada, K. Thylakoid-bound ascorbate peroxidase in spinach chloroplasts and photoreduction of its primary oxidation product monodehydroascorbate radicals in thylakoids. Plant Cell Physiol. 1992, 33, 541–553. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; He, W.; Guo, J.; Chang, X.; Su, P.; Zhang, L. Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J. Exp. Bot. 2005, 56, 3041–3049. [Google Scholar] [CrossRef]
- Yu, C.-W.; Murphy, T.M.; Lin, C.-H. Hydrogen peroxide-induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct. Plant Biol. 2003, 30, 955. [Google Scholar] [CrossRef]
- Wild, R.; Ooi, L.; Srikanth, V.; Münch, G. A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: The N-acetyl-L-cysteine assay. Anal. Bioanal. Chem. 2012, 403, 2577–2581. [Google Scholar] [CrossRef]
| Treatments | Shoot Cr (mg kg−1 DW) | Root Cr (mg kg−1 DW) | Translocation Factor (TF) |
|---|---|---|---|
| 0 | ND | ND | ND |
| 0 + KN (10 µM) | ND | ND | ND |
| 0 + GA3 | ND | ND | ND |
| KN + GA3 | ND | ND | ND |
| Cr (300 µM) | 31.43 ± 0.727 a | 57.48 ± 1.32 a | 0.546 ± 0.014 a |
| Cr + Kn | 19.25 ± 0.424 c | 42.15 ± 0.97 b | 0.456 ± 0.008 c |
| Cr + GA3 | 22.71 ± 0.565 b | 45.67 ± 1.13 b | 0.497 ± 0.011 b |
| Cr + Kn + GA3 | 15.48 ± 0.311 d | 32.72 ± 0.65 c | 0.473 ± 0.008 bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, P.; Azzam, M.A.; Balawi, T.A.; Raja, V.; Bhat, J.A.; Ahmad, P. Mitigation of Negative Effects of Chromium (VI) Toxicity in Faba Bean (Vicia faba) Plants through the Supplementation of Kinetin (KN) and Gibberellic Acid (GA3). Plants 2022, 11, 3302. https://doi.org/10.3390/plants11233302
Alam P, Azzam MA, Balawi TA, Raja V, Bhat JA, Ahmad P. Mitigation of Negative Effects of Chromium (VI) Toxicity in Faba Bean (Vicia faba) Plants through the Supplementation of Kinetin (KN) and Gibberellic Acid (GA3). Plants. 2022; 11(23):3302. https://doi.org/10.3390/plants11233302
Chicago/Turabian StyleAlam, Pravej, Maged A. Azzam, Thamer Al Balawi, Vaseem Raja, Javaid Akhter Bhat, and Parvaiz Ahmad. 2022. "Mitigation of Negative Effects of Chromium (VI) Toxicity in Faba Bean (Vicia faba) Plants through the Supplementation of Kinetin (KN) and Gibberellic Acid (GA3)" Plants 11, no. 23: 3302. https://doi.org/10.3390/plants11233302
APA StyleAlam, P., Azzam, M. A., Balawi, T. A., Raja, V., Bhat, J. A., & Ahmad, P. (2022). Mitigation of Negative Effects of Chromium (VI) Toxicity in Faba Bean (Vicia faba) Plants through the Supplementation of Kinetin (KN) and Gibberellic Acid (GA3). Plants, 11(23), 3302. https://doi.org/10.3390/plants11233302










