Methanolic Extracts of Chiococca alba in Aedes aegypti Biorational Management: Larvicidal and Repellent Potential, and Selectivity against Non-Target Organisms
Abstract
1. Introduction
2. Material and Methods
2.1. Plant and Root Extracts
2.2. Chemical Characterization of Chiococca alba Root Extract
2.3. Insect Populations
2.3.1. Aedes aegypti
2.3.2. Belostoma anurum
2.4. Toxicity of Chiococca alba Root Extract against Aedes aegypti Larvae and Selectivity against Its Predator, Belostoma anurum
2.5. Repellency of the Pure and Formulated Extract of Chiococca alba and Cytotoxic Effects on Mammalian Cell Lines
2.5.1. The Gel and Cream Formulations of the Chiococca alba Root Extract
2.5.2. Repellency Bioassays
2.5.3. In Silico Studies of the Interaction between Chiococca alba Ligand Molecules and the AaegOBP1 Receptor
2.5.4. Cytotoxicity of the Root Extract of Chiococca alba and Rutin
2.6. Statistical Analysis
3. Results
3.1. HPLC Fingerprinting and GC-MS Analysis
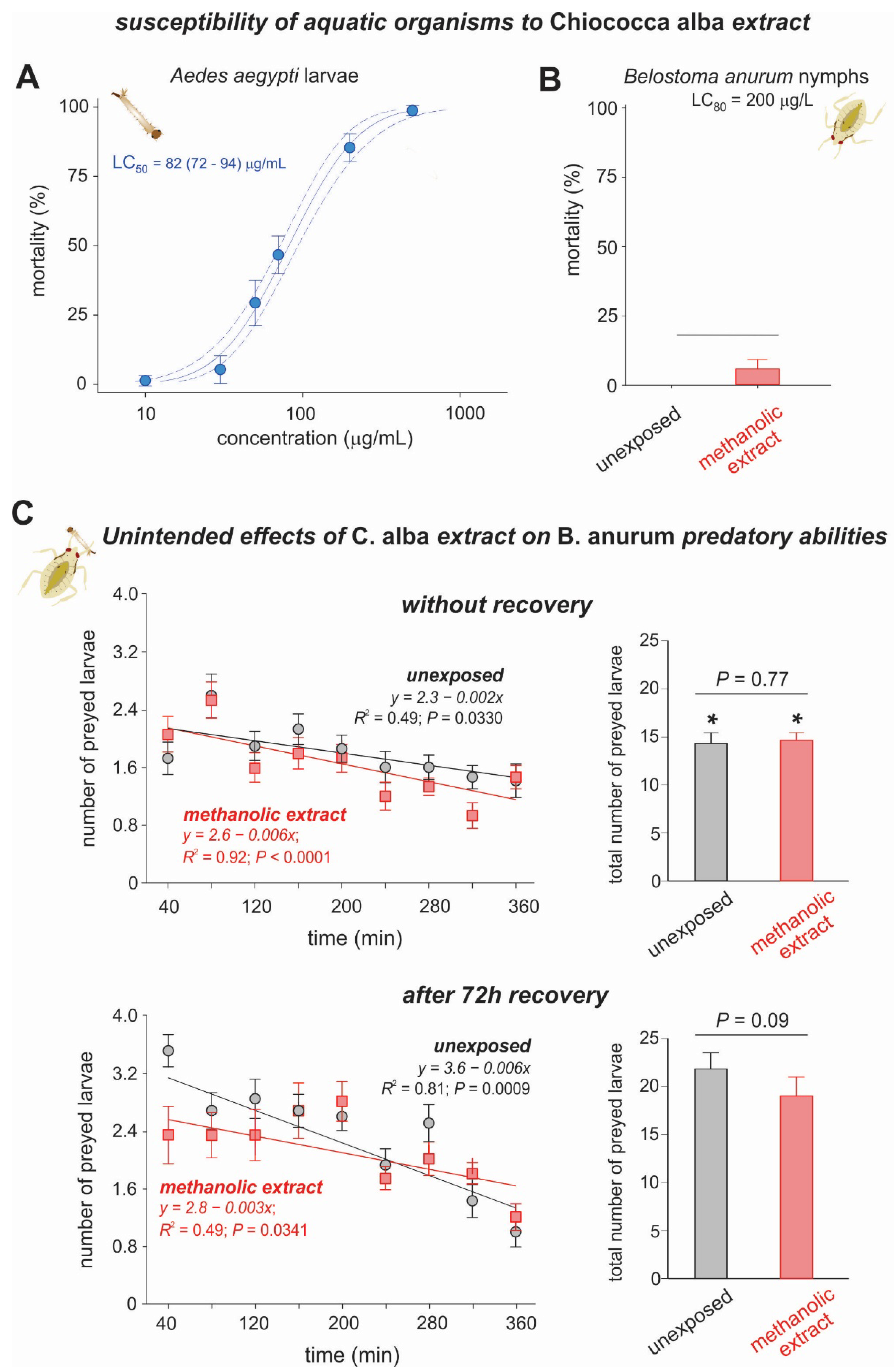
3.2. Toxicity of Chiococca alba Extracts to Aedes aegypti Larvae and Belostoma anurum Nymphs
Effects of Chiococca alba Extract on the Predatory Abilities of Belostoma anurum
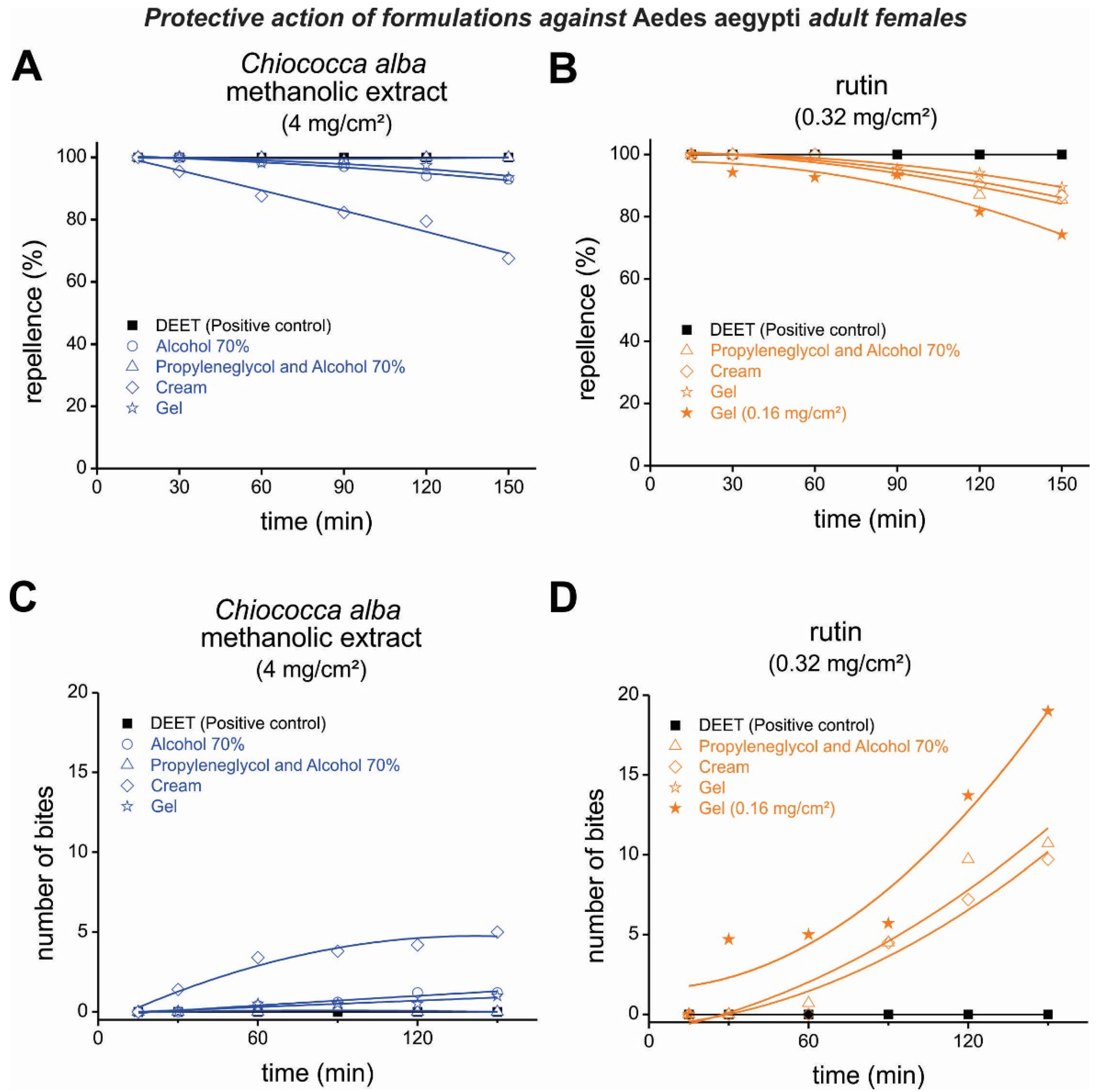
3.3. Repellent Activity of Formulations Containing an Extract of Chiococca alba and Rutin
Interaction between the Molecules of Chiococca alba and the AeagOBP1 Receptor of Aedes aegypti
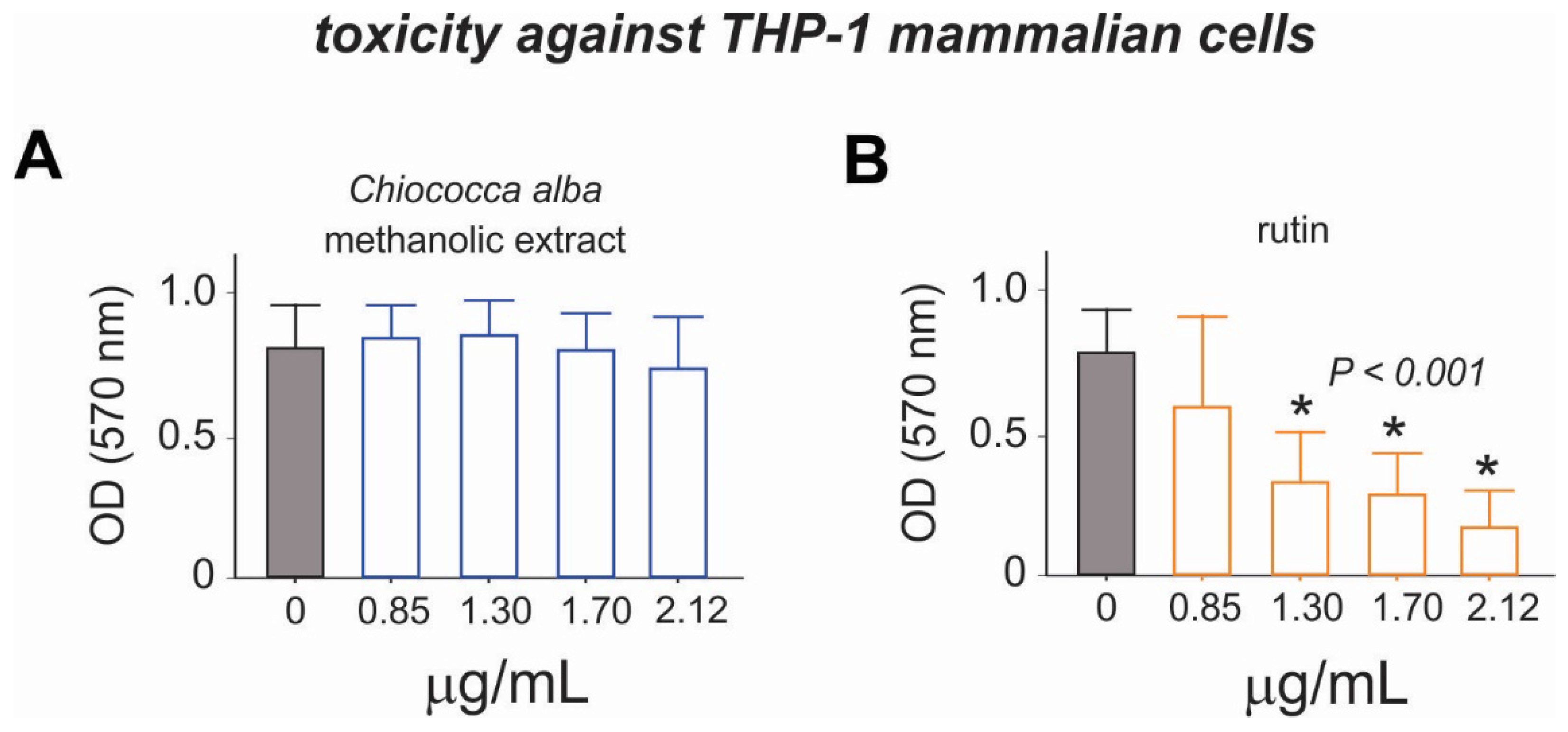
3.4. Cytotoxicity of the Root Extract of Chiococca alba and Rutin to THP-1 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fernando, I.P.S.; Nah, J.-W.; Jeon, Y.-J. Potential Anti-Inflammatory Natural Products from Marine Algae. Environ. Toxicol. Pharmacol. 2016, 48, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. A Renaissance for Botanical Insecticides? Pest Manag. Sci. 2015, 71, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- Soares, I.M.; Ribeiro, M.F.; da Costa, O.J.; de Souza, É.E.; Aguiar, A.A.; Barbosa, R.S.; Alvim, T.C.; Ascêncio, S.D.; Aguiar, R.W.S. Application of a Degreasing Process and Sequential Ultrasound-Assisted Extraction to Obtain Phenolic Compounds and Elucidate of the Potential Antioxidant of Siparuna guianensis Aublet. J. Med. Plants Res. 2017, 11, 357–366. [Google Scholar] [CrossRef]
- Mendes, L.A.; Martins, G.F.; Valbon, W.R.; Souza, T.S.; Menini, L.; Ferreira, A.; Silva Ferreira, M.F. Larvicidal Effect of Essential Oils from Brazilian Cultivars of Guava on Aedes aegypti L. Ind. Crop. Prod. 2017, 108, 684–689. [Google Scholar] [CrossRef]
- Sousa, R.M.O.F.; Cunha, A.C.; Fernandes-Ferreira, M. The Potential of Apiaceae Species as Sources of Singular Phytochemicals and Plant-Based Pesticides. Phytochemistry 2021, 187, 112714. [Google Scholar] [PubMed]
- Borges, J.C.M.; Haddi, K.; Oliveira, E.E.; Andrade, B.S.; Nascimento, V.L.; Melo, T.S.; Didonet, J.; Carvalho, J.C.T.; Cangussu, A.S.R.; Soares, I.M. Mosquiticidal and Repellent Potential of Formulations Containing Wood Residue Extracts of a Neotropical Plant, Tabebuia heptaphylla. Ind. Crop. Prod. 2019, 129, 424–433. [Google Scholar] [CrossRef]
- Ferreira, T.P.; Haddi, K.; Corrêa, R.F.T.; Zapata, V.L.B.; Piau, T.B.; Souza, L.F.N.; Santos, S.M.G.; Oliveira, E.E.; Jumbo, L.O.V.; Ribeiro, B.M.; et al. Prolonged Mosquitocidal Activity of Siparuna guianensis Essential Oil Encapsulated in Chitosan Nanoparticles. PLoS Negl. Trop. Dis. 2019, 13, e0007624. [Google Scholar] [CrossRef]
- Maia, J.D.; La Corte, R.; Martinez, J.; Ubbink, J.; Prata, A.S. Improved Activity of Thyme Essential Oil (Thymus vulgaris) against Aedes aegypti Larvae Using a Biodegradable Controlled Release System. Ind. Crop. Prod. 2019, 136, 110–120. [Google Scholar] [CrossRef]
- Ntalli, N.; Koliopoulos, G.; Giatropoulos, A.; Menkissoglu-Spiroudi, U. Plant secondary metabolites against arthropods of medical importance. Phytochem. Rev. 2019, 18, 1255–1275. [Google Scholar] [CrossRef]
- Giatropoulos, A.; Kimbaris, A.; Michaelakis, A.; Papachristos, D.P.; Polissiou, M.G.; Emmanouel, N. Chemical composition and assessment of larvicidal and repellent capacity of 14 Lamiaceae essential oils against Aedes albopictus. Parasitol. Res. 2018, 117, 1953–1964. [Google Scholar] [CrossRef]
- WHO. WHO Model List of Essential Medicines—21st List; WHO: Geneva, Switzerland, 2019.
- WHO. Global Programme to Eliminate Lymphatic Filariasis: Progress Report 2020; WHO: Geneva, Switzerland, 2021.
- Haddi, K.; Tomé, H.V.V.; Du, Y.; Valbon, W.R.; Nomura, Y.; Martins, G.F.; Dong, K.; Oliveira, E.E. Detection of a New Pyrethroid Resistance Mutation (V410L) in the Sodium Channel of Aedes aegypti: A Potential Challenge for Mosquito Control. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, X.; Wang, D.; Zou, Z.; Liang, Z. Effect of Drought Stress on Growth and Accumulation of Active Constituents in Salvia Miltiorrhiza Bunge. Ind. Crop. Prod. 2011, 33, 84–88. [Google Scholar] [CrossRef]
- Valbon; Haddi, K.; Souza, R.A.; Carvalho, G.A.; Guedes, R.N.C.; Martins, G.F.; Oliveira, E.E. “Armed to the Teeth”: The Multiple Ways to Survive Insecticidal and Predatory Challenges in Aedes aegypti Larvae. Pestic. Biochem. Physiol. 2019, 156, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Moura, W.S.; Oliveira, E.E.; Haddi, K.; Correa, R.F.T.; Piau, T.B.; Moura, D.S.; Santos, S.F.; Grisolia, C.K.; Ribeiro, B.M.; Aguiar, R.W.S. Cassava Starch-Based Essential Oil Microparticles Preparations: Functionalities in Mosquito Control and Selectivity against Non-Target Organisms. Ind. Crop. Prod. 2021, 162, 113289. [Google Scholar] [CrossRef]
- Roy, D.N.; Goswami, R.; Pal, A. The Insect Repellents: A Silent Environmental Chemical Toxicant to the Health. Environ. Toxicol. Pharmacol. 2017, 50, 91–102. [Google Scholar] [CrossRef]
- Tavares, M.; Silva, M.R.M.; Siqueira, L.B.O.; Rodrigues, R.A.S.; Bodjolle-d’Almeida, L.; Santos, E.P.; Ricci-Júnior, E. Trends in Insect Repellent Formulations: A Review. Int. J. Pharm. 2018, 539, 190–209. [Google Scholar] [CrossRef]
- Ogoma, S.B.; Ngonyani, H.; Simfukwe, E.T.; Mseka, A.; Moore, J.; Killeen, G.F. Spatial Repellency of Transfluthrin-Treated Hessian Strips against Laboratory-Reared Anopheles arabiensis Mosquitoes in a Semi-Field Tunnel Cage. Parasit. Vectors 2012, 5, 1–5. [Google Scholar] [CrossRef]
- Ogoma, S.B.; Ngonyani, H.; Simfukwe, E.T.; Mseka, A.; Moore, J.; Maia, M.F.; Moore, S.J.; Lorenz, L.M. The Mode of Action of Spatial Repellents and Their Impact on Vectorial Capacity of Anopheles Gambiae Sensu Stricto. PLoS ONE 2014, 9, e110433. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Q.; Xu, P.; Andreazza, F.; Valbon, W.R.; Bandason, E.; Chen, M.; Yan, R.; Feng, B.; Smith, L.; et al. A Dual-Target Molecular Mechanism of Pyrethrum Repellency against Mosquitoes. Nat. Commun. 2021, 12, 2553. [Google Scholar] [CrossRef]
- Fradin, M.S. Mosquitoes and Mosquito Repellents: A Clinician’s Guide. Ann. Intern. Med. 1998, 128, 931–940. [Google Scholar] [CrossRef]
- Li, M.X.; Ma, Y.P.; Zhang, H.X.; Sun, H.Z.; Su, H.H.; Pei, S.J.; Du, Z.Z. Repellent, Larvicidal and Adulticidal Activities of Essential Oil from Dai Medicinal Plant Zingiber cassumunar against Aedes albopictus. Plant Divers. 2021, 43, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kumar, P.; Singh, H.; Agrawal, V. Biocontrol of Mosquito Vectors through Herbal-Derived Silver Nanoparticles: Prospects and Challenges. Environ. Sci. Pollut. Res. 2020, 27, 25987–26024. [Google Scholar]
- Bhattacharyya, J.; Cunha, E.V.L. A Triterpenoid from the Root-Bark of Chiococca Alba. Phytochemistry 1992, 31, 2546–2547. [Google Scholar] [CrossRef]
- Gazda, V.E.; Gomes-Carneiro, M.R.; Barbi, N.S.; Paumgartten, F.J.R. Toxicological Evaluation of an Ethanolic Extract from Chiococca alba Roots. J. Ethnopharmacol. 2006, 105, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, R.W.S.; Borges, J.C.M.; Cangussu, A.S.R.; Moraes, C.B. Uso de Extrato de Chiococca alba (L.) Contra Vírus Da Dengue. Patent INPI BR 10 2018 071298-5, 6 October 2018. [Google Scholar]
- Leal, M.O.L.; Nascimento, L.B.; Coutinho, A.J.; Tamaio, N.; Brandes, A.F.N. Development of External Vascular Cylinders (Neoformations) in Stems and Roots of Chiococca alba (L.) Hitchc. (Rubiaceae). Flora Morphol. Distrib. Funct. Ecol. Plants 2020, 264, 151569. [Google Scholar] [CrossRef]
- WHO. Guidelines for Efficacy Testing of Mosquito Repellents for Human Skin Control of Neglected Tropical Diseases WHO Pesticide Evaluation Scheme; WHO: Geneva, Switzerland, 2009.
- Valbon; Haddi, K.; Gutiérrez, Y.; Cruz, F.M.; Azevedo, K.E.X.; Campos, J.S.P.; Salaro, A.L.; Oliveira, E.E. Life History Traits and Predatory Performance of Belostoma anurum (Hemiptera: Belostomatidae), a Biological Control Agent of Disease Vector Mosquitoes. Neotrop. Entomol. 2019, 48, 899–908. [Google Scholar] [CrossRef]
- Luís, Â.; Neiva, D.M.; Pereira, H.; Gominho, J.; Domingues, F.; Duarte, A.P. Bioassay-Guided Fractionation, GC–MS Identification and in Vitro Evaluation of Antioxidant and Antimicrobial Activities of Bioactive Compounds from Eucalyptus Globulus Stump Wood Methanolic Extract. Ind. Crop. Prod. 2016, 91, 97–103. [Google Scholar] [CrossRef]
- Ishaaya, I.; Nauen, R.; Horowitz, A.R. Insecticides Design Using Advanced Technologies; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 3540469044. [Google Scholar]
- Abd El-Hafiz, M.A.; Weniger, B.; Quirion, J.C.; Anton, R. Ketoalcohols, Lignans and Coumarins from Chiococca alba. Phytochemistry 1991, 30, 2029–2031. [Google Scholar] [CrossRef]
- Carbonezi, C.A.; Martins, D.; Young, M.C.M.; Lopes, M.N.; Furlan, M.; Rodrigues Filho, E.; Bolzani, V.D.S. Iridoid and Seco-Iridoid Glucosides from Chioccoca alba (Rubiaceae). Phytochemistry 1999, 51, 781–785. [Google Scholar] [CrossRef]
- Borges-Argáez, R.; Medina-Baizabál, L.; May-Pat, F.; Waterman, P.G.; Peña-Rodríguez, L.M. Merilactone, an Unusual C19 Metabolite from the Root Extract of Chiococca alba. J. Nat. Prod. 2001, 64, 228–231. [Google Scholar] [CrossRef]
- Adjibade, Y.; Kuballa, B.; Cabalion, P.; Jung, M.L.; Beck, J.P.; Anton, R. Cytotoxicity on Human Leukemic and Rat Hepatoma Cell Lines of Alkaloid Extracts of Psychotria Forsteriana. Planta Med. 1989, 55, 567–568. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.; Nozawa, C.; Linhares, R.E.C. Características Gerais e Epidemiologia Dos Arbovírus Emergentes No Brasil. Rev. Pan-Amaz. Saúde 2014, 5, 10. [Google Scholar] [CrossRef]
- Afolabi, O.J.; Simon-Oke, I.A.; Elufisan, O.O.; Oniya, M.O. Adulticidal and Repellent Activities of Some Botanical Oils against Malaria Mosquito: Anopheles gambiae (Diptera: Culicidae). Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 135–138. [Google Scholar] [CrossRef]
- Rajkumar, S.; Jebanesan, A. Bioactivity of Flavonoid Compounds from Poncirus trifoliata L. (Family: Rutaceae) against the Dengue Vector, Aedes aegypti L. (Diptera: Culicidae). Parasitol. Res. 2008, 104, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Vandock, K.P.; Mitchell, M.J.; Fioravanti, C.F. Effects of Plant Flavonoids on Manduca sexta (Tobacco Hornworm) Fifth Larval Instar Midgut and Fat Body Mitochondrial Transhydrogenase. Arch. Insect Biochem. Physiol. 2012, 80, 15–25. [Google Scholar] [CrossRef]
- Engida, A.M.; Kasim, N.S.; Tsigie, Y.A.; Ismadji, S.; Huynh, L.H.; Ju, Y.-H. Extraction, Identification and Quantitative HPLC Analysis of Flavonoids from Sarang Semut (Myrmecodia pendan). Ind. Crop. Prod. 2013, 41, 392–396. [Google Scholar] [CrossRef]
- Al Muqarrabun, L.M.R.; Ahmat, N.; Aris, S.R.S. A Review of the Medicinal Uses, Phytochemistry and Pharmacology of the Genus Sapium. J. Ethnopharmacol. 2014, 155, 9–20. [Google Scholar] [CrossRef]
- Pessoa, L.Z.S.; Duarte, J.L.; Ferreira, R.M.A.; Oliveira, A.E.M.F.M.; Cruz, R.A.S.; Faustino, S.M.M.; Carvalho, J.C.T.; Fernandes, C.P.; Souto, R.N.P.; Araújo, R.S. Nanosuspension of Quercetin: Preparation, Characterization and Effects against Aedes aegypti Larvae. Rev. Bras. Farmacogn. 2018, 28, 618–625. [Google Scholar] [CrossRef]
- Govindarajan, M.; Mathivanan, T.; Elumalai, K.; Krishnappa, K.; Anandan, A. Ovicidal and Repellent Activities of Botanical Extracts against Culex Quinquefasciatus, Aedes aegypti and Anopheles stephensi (Diptera: Culicidae). Asian Pac. J. Trop. Biomed. 2011, 1, 43–48. [Google Scholar] [CrossRef]
- Choochote, W.; Chaithong, U.; Kamsuk, K.; Jitpakdi, A.; Tippawangkosol, P.; Tuetun, B.; Champakaew, D.; Pitasawat, B. Repellent Activity of Selected Essential Oils against Aedes aegypti. Fitoterapia 2007, 78, 359–364. [Google Scholar] [CrossRef]
- Casteli, V.C.; Mendonça, C.C.; de Campos, M.A.L.; Ferrari, M.; Machado, S.R.P. Desenvolvimento e Estudos de Estabilidade Preliminares de Emulsões O/A Contendo Cetoconazol 2.0%. Acta Sci. Health Sci. 2008, 30, 121–128. [Google Scholar]
- Thireou, T.; Kythreoti, G.; Tsitsanou, K.E.; Koussis, K.; Drakou, C.E.; Kinnersley, J.; Kröber, T.; Guerin, P.M.; Zhou, J.-J.; Iatrou, K. Identification of Novel Bioinspired Synthetic Mosquito Repellents by Combined Ligand-Based Screening and OBP-Structure-Based Molecular Docking. Insect Biochem. Mol. Biol. 2018, 98, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Tsitsanou, K.E.; Thireou, T.; Drakou, C.E.; Koussis, K.; Keramioti, M.V.; Leonidas, D.D.; Eliopoulos, E.; Iatrou, K.; Zographos, S.E. Anopheles Gambiae Odorant Binding Protein Crystal Complex with the Synthetic Repellent DEET: Implications for Structure-Based Design of Novel Mosquito Repellents. Cell. Mol. Life Sci. 2012, 69, 283–297. [Google Scholar] [CrossRef]
- Venthur, H.; Zhou, J.-J. Odorant Receptors and Odorant-Binding Proteins as Insect Pest Control Targets: A Comparative Analysis. Front. Physiol. 2018, 9, 1163. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.; Liu, S. The Behavioral Response of Lasioderma serricorne (Coleoptera: Anobiidae) to Citronellal, Citral, and Rutin. Springerplus 2016, 5, 1–7. [Google Scholar] [CrossRef]
- Li, K.; Wang, S.; Zhang, K.; Ren, L.; Ali, A.; Zhang, Y.; Zhou, J.; Guo, Y. Odorant Binding Characteristics of Three Recombinant Odorant Binding Proteins in Microplitis mediator (Hymenoptera: Braconidae). J. Chem. Ecol. 2014, 40, 541–548. [Google Scholar] [CrossRef]
- Leal, W.S. Odorant Reception in Insects: Roles of Receptors, Binding Proteins, and Degrading Enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef]
- Valbon, W.; Andreazza, F.; Oliveira, E.E.; Liu, F.; Feng, B.; Hall, M.; Klimavicz, J.; Coats, J.; Dong, K. Bioallethrin Activates Specific Olfactory Sensory Neurons and Elicits Spatial Repellency in Aedes aegypti. Pest Manag. Sci. 2022, 78, 438–445. [Google Scholar] [CrossRef]
- Melo, N.; Capek, M.; Arenas, O.M.; Afify, A.; Yilmaz, A.; Potter, C.J.; Laminette, P.J.; Para, A.; Gallio, M.; Stensmyr, M.C. The Irritant Receptor TRPA1 Mediates the Mosquito Repellent Effect of Catnip. Curr. Biol. 2021, 31, 1988–1994. [Google Scholar] [CrossRef]
- Andreazza, F.; Valbon, W.R.; Wang, Q.; Liu, F.; Xu, P.; Bandason, E.; Chen, M.; Wu, S.; Smith, L.B.; Scott, J.G.; et al. Sodium Channel Activation Underlies Transfluthrin Repellency in Aedes aegypti. PLoS Negl. Trop. Dis. 2021, 15, e0009546. [Google Scholar] [CrossRef]
- Keziah, E.A.; Nukenine, E.N.; Danga, S.P.Y.; Younoussa, L.; Esimone, C.O. Creams Formulated with Ocimum gratissimum L. and Lantana camara L. Crude Extracts and Fractions as Mosquito Repellents against Aedes aegypti L. (Diptera: Culicidae). J. Insect Sci. 2015, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Dickens, J.C.; Bohbot, J.D. Mini Review: Mode of Action of Mosquito Repellents. Pestic. Biochem. Physiol. 2013, 106, 149–155. [Google Scholar] [CrossRef]
- Ditzen, M.; Pellegrino, M.; Vosshall, L.B. Insect Odorant Receptors Are Molecular Targets of the Insect Repellent DEET. Science 2008, 319, 1838–1842. [Google Scholar] [CrossRef]
- Limenew, A.; Tadesse, M. Phytochemical Screening and Peroxide Value Determination of Methanolic Extract of Four Traditional Medicinal Plants from Debre Tabor Town, Ethiopia. J. Med. Plants Res. 2018, 12, 203–208. [Google Scholar] [CrossRef][Green Version]
- Ferreira, T.P.; Kuckelhaus, I.G.P.; Tschoeke, P.H.; Cangussu, A.S.R.; Borges, J.C.M.; Moura, W.S.; Aguiar, R.W. Influence of Seasonality on the Yield and Composition of the Essential Oil of Siparuna guianensis Aublet. Afr. J. Biotechnol. 2017, 16, 1611–1618. [Google Scholar]
- Aguiar, R.W.S.; Santos, S.F.; Morgado, F.S.; Ascencio, S.D.; Lopes, M.M.; Viana, K.F.; Didonet, J.; Ribeiro, B.M. Insecticidal and Repellent Activity of Siparuna guianensis Aubl. (Negramina) against Aedes aegypti and Culex Quinquefasciatus. PLoS ONE 2015, 10, e0116765. [Google Scholar] [CrossRef]
- Valbon, W.R.; Cruz, F.M.; Ramos, G.S.; Tomé, H.V.V.; Oliveira, E.E. Sublethal Exposure to Deltamethrin Reduces the Abilities of Giant Water Bugs to Prey upon Aedes aegypti Larvae. Chemosphere 2018, 191, 350–356. [Google Scholar] [CrossRef]
- Holling, C.S. Some Characteristics of Simple Types of Predations and Parasitism1. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Gutiérrez, Y.; Ramos, G.S.; Tomé, H.V.V.; Oliveira, E.E.; Salaro, A.L. Bti-Based Insecticide Enhances the Predatory Abilities of the Backswimmer Buenoa tarsalis (Hemiptera: Notonectidae). Ecotoxicology 2017, 26, 1147–1155. [Google Scholar] [CrossRef]
- Valbon, W.R.; Hatano, E.; Oliveira, N.R.X.; Ataíde, Á.D.; Corrêa, M.J.M.; Gomes, S.F.; Martins, G.F.; Haddi, K.; Alvarenga, E.S.; Oliveira, E.E. Detrimental Effects of Pyriproxyfen on the Detoxification and Abilities of Belostoma anurum to Prey upon Aedes aegypti Larvae. Environ. Pollut. 2021, 284, 117130. [Google Scholar] [CrossRef]
- Valbon, W.; Araújo, S.H.C.; Nery, R.S.; Barbosa, J.F.; Newland, P.L.; Oliveira, E.E. Sublethal Exposure to Pyriproxyfen Does Not Impair the Abilities of the Backswimmer Buenoa amnigenus to Prey upon Aedes aegypti Larvae. Ecotoxicology 2022, 31, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Batistuzzo, J.A.O.; Itaya, M.; Eto, Y. Formulário Médico-Farmacêutico, 5th ed.; Editora Atheneu: São Paulo, Brazil, 2015; ISBN 8574541095. [Google Scholar]
- Leite, N.R.; Krogh, R.; Xu, W.; Ishida, Y.; Iulek, J.; Leal, W.S.; Oliva, G. Structure of an Odorant-Binding Protein from the Mosquito Aedes aegypti Suggests a Binding Pocket Covered by a PH-Sensitive “Lid”. PLoS ONE 2009, 4, e8006. [Google Scholar] [CrossRef] [PubMed]
- Sanner, M.F. Python: A Programming Language for Software Integration and Development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar] [PubMed]
- Schrödinger, L.; DeLano, W. The PyMOL Molecular Graphics System, Version 2.0; Schrodinger, LLC.: San Carlos, CA, USA, 2022.
- BIOVIA, Dassault Systèmes. Discovery Studio, Version 4.5; BIOVIA: San Diego, CA, USA, 2022.
- Moura, W.S.; Souza, S.R.; Campos, F.S.; Cangussu, A.S.R.; Santos, E.M.S.; Andrade, B.S.; Gomes, C.H.B.; Viana, K.F.; Haddi, K.; Oliveira, E.E.; et al. Antibacterial Activity of Siparuna guianensis Essential Oil Mediated by Impairment of Membrane Permeability and Replication of Pathogenic Bacteria. Ind. Crop. Prod. 2020, 146, 112142. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT® 9.1 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2004; p. 5136. [Google Scholar]


| Peak | Constituent | Chemical Structure | Retention Time | Molecular Formula | Chemical Composition (%) |
|---|---|---|---|---|---|
| 01 | non-identified | - | 3.069 | C14H22O | 4.43 |
| 02 | 1-Deoxy-2,4-O-methylene-D-xylitol | 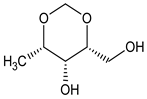 | 5.710 | C6HC12O4 | 1.93 |
| 03 | 2-fenilpropanal |  | 6.670 | C9H10O | 4.21 |
| 04 | 1-(2-hydroxyphenyl) ethanone | 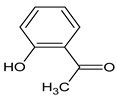 | 6.914 | C8H8O2 | 2.61 |
| 05 | 2-isopropoxifenol | - | 7.375 | C9 H12O2 | 1.54 |
| 06 | non-identified | 9.026 | - | 6.87 | |
| 07 | 2,6-dimethoxyphenol |  | 9.519 | C8H10O3 | 2.32 |
| 08 | 3-nitrobenzyl iodide | 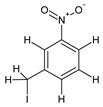 | 10.655 | C7H6INO2 | 6.01 |
| 09 | 1,6-Anhydro-beta-D-glucopyranose | 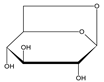 | 11.142 | C6H10O5 | 3.56 |
| 10 | non-identified | - | 12.212 | - | 2.47 |
| 11 | non-identified | - | 12.646 | - | 10.15 |
| 12 | (E)-4-Methoxycinnamic acid | 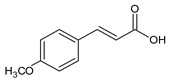 | 13.776 | C10H10O3 | 5. 05 |
| 13 | Coniferol | 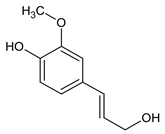 | 14.186 | C10H12O3 | 2.98 |
| 14 | Tetradecanoic acid | 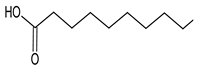 | 14.298 | C14H28O2 | 1.81 |
| 15 | 3,5-Dimethoxycinnamic acid | 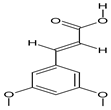 | 15.899 | C11H12O4 | 5.49 |
| 16 | Oleyl alcohol, trifluoroacetate |  | 16.150 | C20H35F3O2 | 1.55 |
| 17 | Palmitic acid | 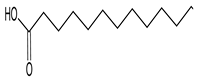 | 16.347 | C16H32O2 | 10.83 |
| 18 | non-identified | - | 23.613 | - | 2.97 |
| 19 | non-identified | - | 30.302 | - | 11.19 |
| 20 | Tris (2,4-di-terc-butilfenil) |  | 33.683 | C42H63O4P | 12.5 |
| Sources of Variation | df | F | p | ||
|---|---|---|---|---|---|
| Between factors | |||||
| Extract (Ex) | 1 | 0.06 | 0.80 | ||
| Error | 28 | - | - | ||
| dfnum | dfden | Wilks’ lambda | F | P | |
| Within each factor | |||||
| Time (T) | 8 | 21 | 0.309 | 5.87 | 0.0050 * |
| T × Ex | 8 | 21 | 0.436 | 3.40 | 0.0116 * |
| Sources of Variation | df | F | p | ||
|---|---|---|---|---|---|
| Between factors | |||||
| Extract (Ex) | 1 | 3.06 | 0.09 | ||
| Error | 28 | - | - | ||
| dfnum | dfden | Wilks’ lambda | F | P | |
| Within each factor | |||||
| Time (T) | 8 | 21 | 0.145 | 15.42 | <0.0001 * |
| T × Ex | 8 | 21 | 0.617 | 1.63 | 0.17 |
| Binding | Compounds | Affinity Energy (kcal/mol) |
|---|---|---|
| 14 | Rutin | −8.1 |
| 12 | Oleyl alcohol, trifluoroacetate | −7.4 |
| 11 | 3,5-Dimethoxycinnamic acid | −7 |
| 15 | Morin | −7 |
| 8 | (E)-4-Methoxycinnamic acid | −6.9 |
| 9 | Coniferol | −6.8 |
| 10 | Tetradecanoic acid | −6.5 |
| 6 | 3-nitrobenzyl iodide | −6.4 |
| 13 | Palmitic Acid | −6.4 |
| 2 | 2-phenylpropanal | −6.1 |
| 3 | 1- (2-Hydroxyphenyl) ethanone | −6 |
| 4 | 2-isopropoxyphenol | −5.9 |
| 5 | 2,6-dimethoxyphenol | −5.7 |
| 7 | 1,6-Anhydro-beta-D-glucopyranose | −5.2 |
| 1 | 1-Deoxy-2,4-O-methylene-D-xylitol | −5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, J.C.M.; Haddi, K.; Valbon, W.R.; Costa, L.T.M.; Ascêncio, S.D.; Santos, G.R.; Soares, I.M.; Barbosa, R.S.; Viana, K.F.; Silva, E.A.P.; et al. Methanolic Extracts of Chiococca alba in Aedes aegypti Biorational Management: Larvicidal and Repellent Potential, and Selectivity against Non-Target Organisms. Plants 2022, 11, 3298. https://doi.org/10.3390/plants11233298
Borges JCM, Haddi K, Valbon WR, Costa LTM, Ascêncio SD, Santos GR, Soares IM, Barbosa RS, Viana KF, Silva EAP, et al. Methanolic Extracts of Chiococca alba in Aedes aegypti Biorational Management: Larvicidal and Repellent Potential, and Selectivity against Non-Target Organisms. Plants. 2022; 11(23):3298. https://doi.org/10.3390/plants11233298
Chicago/Turabian StyleBorges, Jaqueline C. M., Khalid Haddi, Wilson R. Valbon, Lara T. M. Costa, Sérgio D. Ascêncio, Gil R. Santos, Ilsamar M. Soares, Robson S. Barbosa, Kelvinson F. Viana, Eder A. P. Silva, and et al. 2022. "Methanolic Extracts of Chiococca alba in Aedes aegypti Biorational Management: Larvicidal and Repellent Potential, and Selectivity against Non-Target Organisms" Plants 11, no. 23: 3298. https://doi.org/10.3390/plants11233298
APA StyleBorges, J. C. M., Haddi, K., Valbon, W. R., Costa, L. T. M., Ascêncio, S. D., Santos, G. R., Soares, I. M., Barbosa, R. S., Viana, K. F., Silva, E. A. P., Moura, W. S., Andrade, B. S., Oliveira, E. E., & Aguiar, R. W. S. (2022). Methanolic Extracts of Chiococca alba in Aedes aegypti Biorational Management: Larvicidal and Repellent Potential, and Selectivity against Non-Target Organisms. Plants, 11(23), 3298. https://doi.org/10.3390/plants11233298











