PGP-Bacterium Pseudomonas protegens Improves Bread Wheat Growth and Mitigates Herbicide and Drought Stress
Abstract
1. Introduction
2. Results
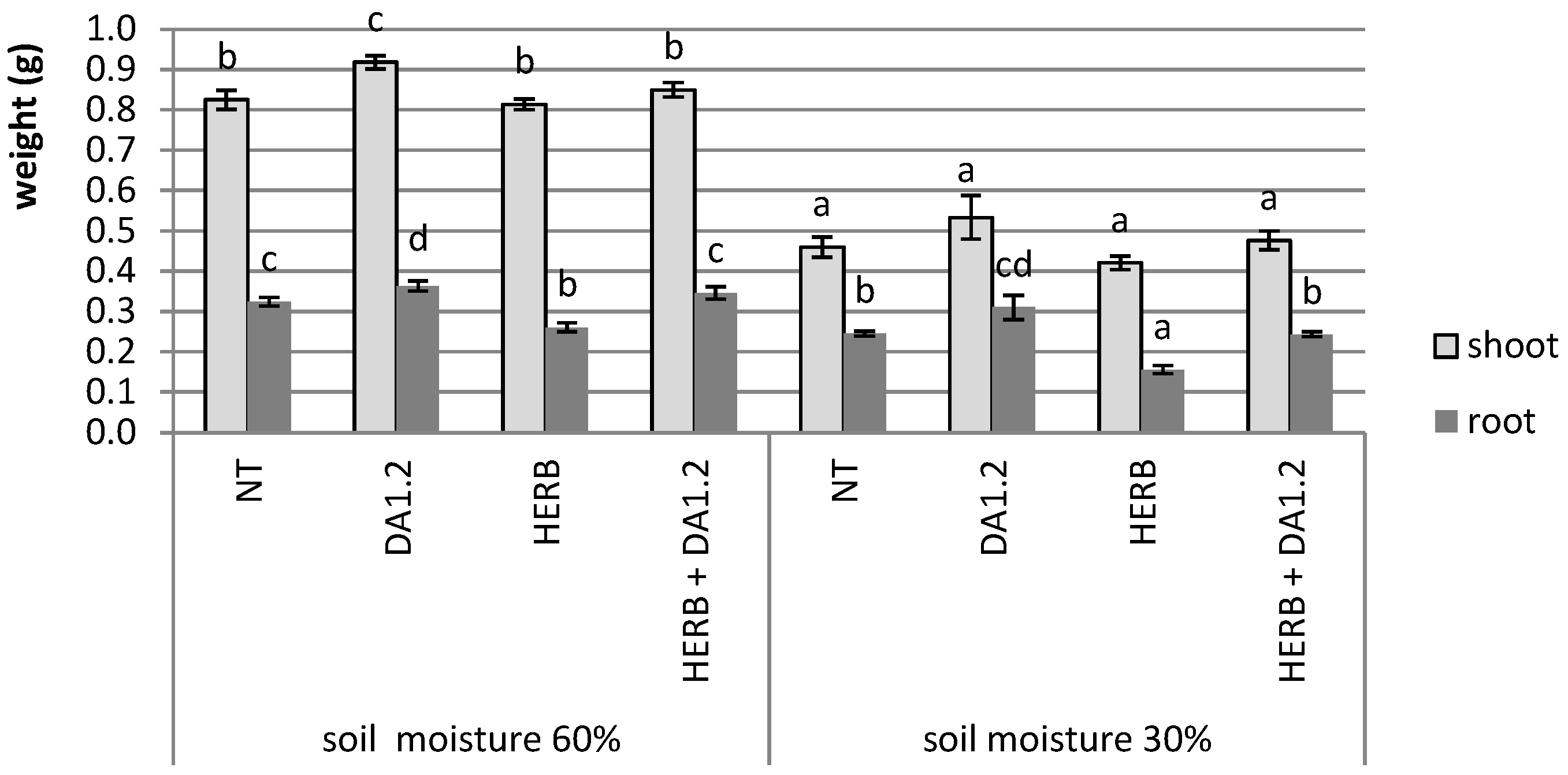
2.1. Plant Weight
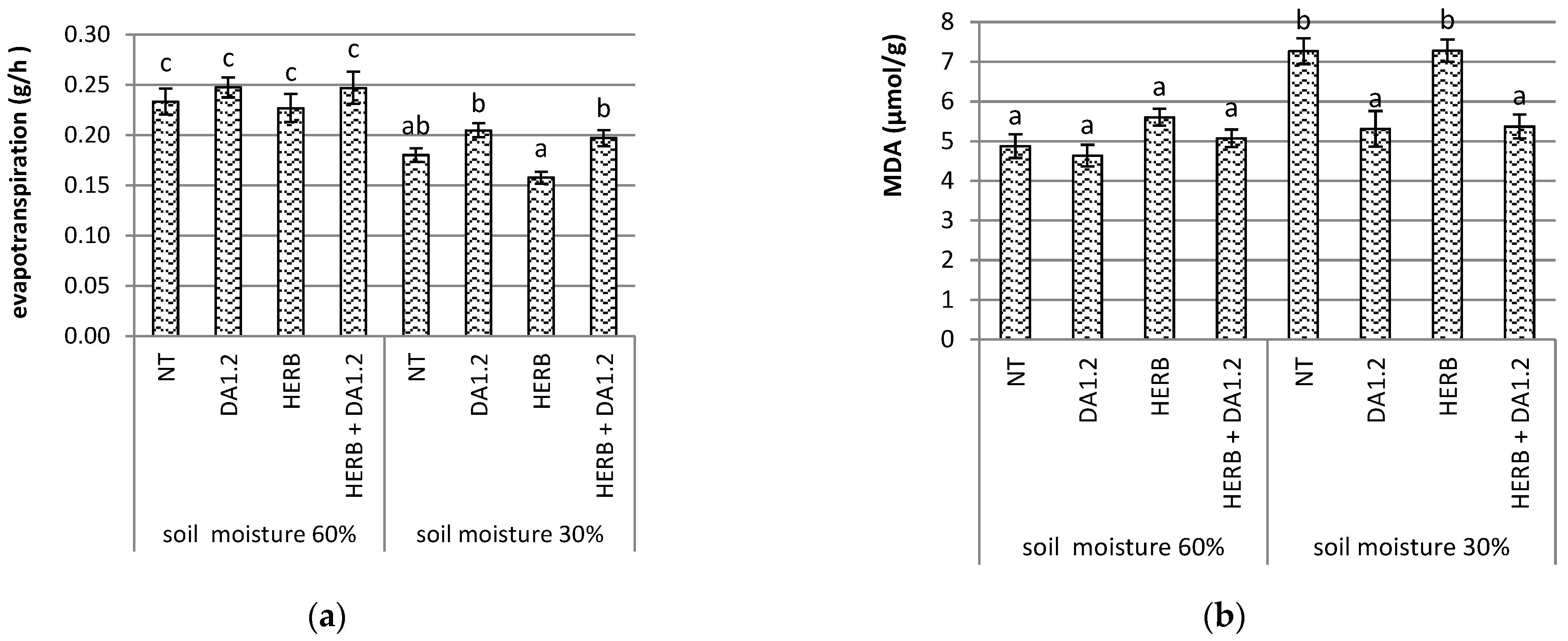
2.2. Characteristics of Water Relations
2.3. Determination of Lipid Peroxidation
2.4. Phytohormones
2.5. The Effect of Treatments on Phytohormones and Yield of Bread Wheat in the Field
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Laboratory Experiment
4.3. Soil-Climate Conditions and Experiment Design in the Field
4.4. Characteristics of Water Relations
4.5. Chemical Analyses
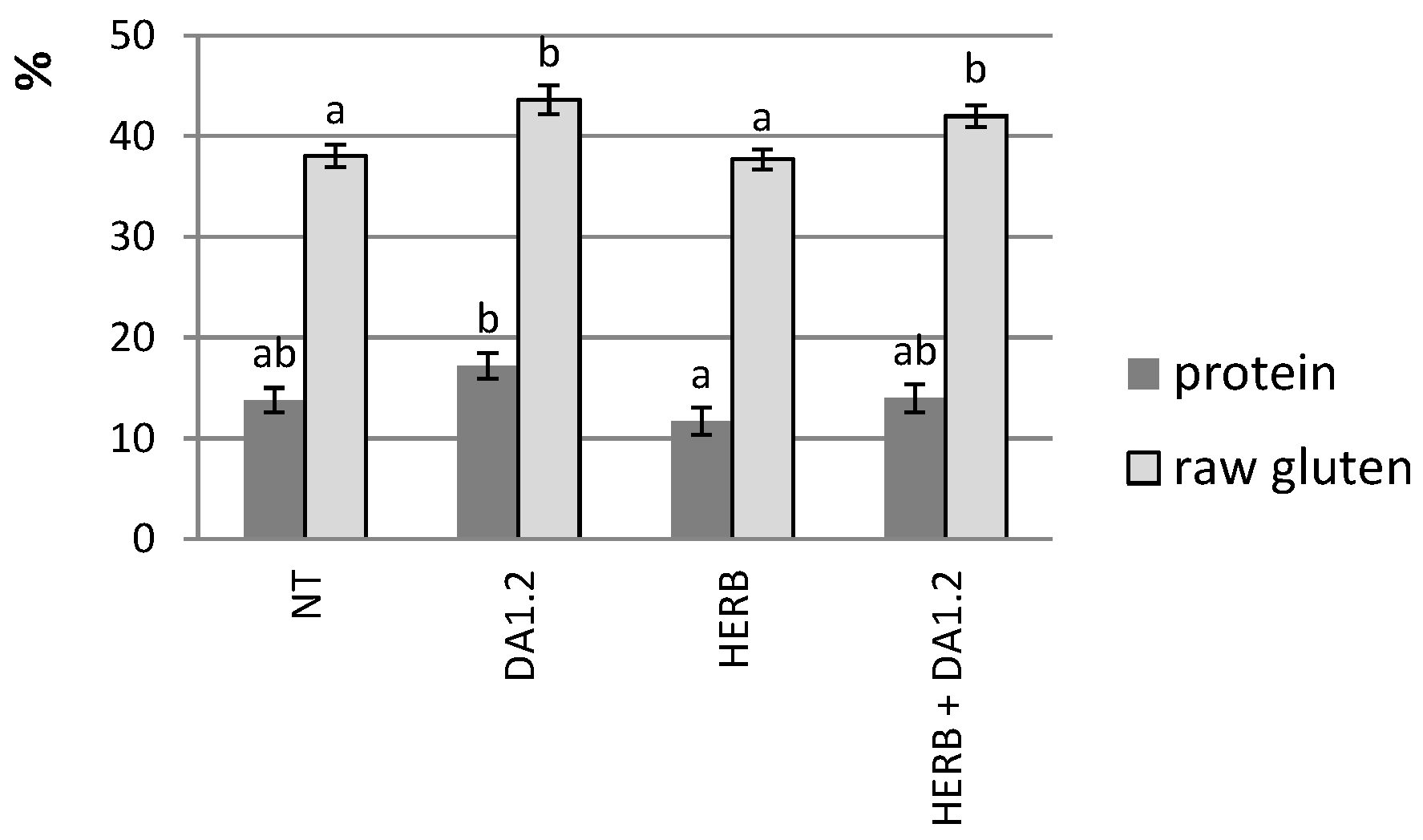
4.6. Grain Properties
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christoffoleti, P.J.; de Figueiredo, M.R.A.; Peres, L.E.P.; Nissen, S.J.; Gaines, T.A. Auxinic herbicides, mechanisms of action, and weed resistance: A look into recent plant science advances. Sci. Agric. 2015, 72, 356–362. [Google Scholar] [CrossRef]
- de Castro Marcato, A.C.; de Souza, C.P.; Fontanetti, C.S. Herbicide 2,4-D: A Review of Toxicity on Non-Target Organisms. Water Air Soil Pollut. 2017, 228, 120. [Google Scholar] [CrossRef]
- Todorova, D.; Sergiev, I.; Katerova, Z.; Shopova, E.; Dimitrova, L.; Brankova, L. Assessment of the Biochemical Responses of Wheat Seedlings to Soil Drought after Application of Selective Herbicide. Plants 2021, 10, 733. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, A.K. A review on herbicide 2, 4-D damage reports in wheat (Triticum aestivum L.). J. Chem. Pharm. Res. 2010, 2, 118–124. [Google Scholar]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Mestre, T.C.; Mittler, R.; Rubio, F.; Garcia-Sanchez, F.; Martinez, V. The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ. 2014, 37, 1059–1073. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R.; Lawson, T. Signal transduction networks during stress combination. J. Exp. Bot. 2020, 71, 1734–1741. [Google Scholar] [CrossRef]
- Zhao, G.; Xu, H.; Zhang, P.; Su, X.; Zhao, H. Effects of 2,4-epibrassinolide on photosynthesis and Rubisco activase gene expression in Triticum aestivum L. seedlings under a combination of drought and heat stress. Plant Growth Regul. 2017, 81, 377–384. [Google Scholar] [CrossRef]
- Lopez-Delacalle, M.; Silva, C.J.; Mestre, T.C.; Martinez, V.; Blanco-Ulate, B.; Rivero, R.M. Synchronization of proline, ascorbate and oxidative stress pathways under the combination of salinity and heat in tomato plants. Environ. Exp. Bot. 2021, 183, 104351. [Google Scholar] [CrossRef]
- Benevenuto, R.F.; Zanatta, C.B.; Guerra, M.P.; Nodari, R.O.; Agapito-Tenfen, S.Z. Proteomic Profile of Glyphosate-Resistant Soybean under Combined Herbicide and Drought Stress Conditions. Plants 2021, 10, 2381. [Google Scholar] [CrossRef]
- Todorova, D.; Aleksandrov, V.; Anev, S.; Sergiev, I. Photosynthesis Alterations in Wheat Plants Induced by Herbicide, Soil Drought or Flooding. Agronomy 2022, 12, 390. [Google Scholar] [CrossRef]
- Singh, I. Plant Growth Promoting Rhizobacteria (PGPR) and Their Various Mechanisms for Plant Growth Enhancement in Stressful Conditions—A Review. Eur. J. Biol. Res. 2018, 8, 191–213. [Google Scholar]
- Chandra, P.; Wunnava, A.; Verma, P.; Chandra, A.; Sharma, R.K. Strategies to Mitigate the Adverse Effect of Drought Stress on Crop Plants—Influences of Soil Bacteria: A Review. Pedosphere 2021, 31, 496–509. [Google Scholar] [CrossRef]
- Karimi, H.; Mahdavi, S.; Asgari Lajayer, B.; Moghiseh, E.; Rajput, V.D.; Minkina, T.; Astatkie, T. Insights on the bioremediation technologies for pesticide-contaminated soils. Environ. Geochem. Health Environ. 2022, 44, 1329–1354. [Google Scholar] [CrossRef] [PubMed]
- Camaille, M.; Fabre, N.; Clément, C.; Ait Barka, E. Advances in Wheat Physiology in Response to Drought and the Role of Plant Growth Promoting Rhizobacteria to Trigger Drought Tolerance. Microorganisms 2021, 9, 687. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Haider, M.Z.; Javed, S.; Saleem, M.H.; Alatawi, A. Drought Stress Amelioration in Maize (Zea mays L.) by Inoculation of Bacillus spp. Strains under Sterile Soil Conditions. Agriculture 2022, 12, 50. [Google Scholar] [CrossRef]
- Chennappa, G.; Sreenivasa, M.Y.; Nagaraja, H. Azotobacter salinestris: A Novel Pesticide-Degrading and Prominent Biocontrol PGPR Bacteria. In Microorganisms for Green Revolution: Microorganisms for Sustainability; Springer: Cham, Switzerland, 2018; Volume 7, pp. 23–43. [Google Scholar] [CrossRef]
- Chetverikov, S.P.; Chetverikova, D.V.; Bakaeva, M.D.; Kenjieva, A.A.; Starikov, S.N.; Sultangazin, Z.R. A promising herbicide resistant bacterial strain of Pseudomonas protegens for stimulation of the growth of agricultural cereal grains. Appl. Biochem. Microbiol. 2021, 57, 110–116. [Google Scholar] [CrossRef]
- Inthama, P.; Pumas, P.; Pekkoh, J.; Pathom-aree, W.; Pumas, C. Plant Growth and Drought Tolerance-Promoting Bacterium for Bioremediation of Paraquat Pesticide Residues in Agriculture Soils. Front. Microbiol. 2021, 12, 604662. [Google Scholar] [CrossRef]
- Motamedi, M.; Zahedi, M.; Karimmojeni, H.; Motamedi, H.; Mastinu, A. Effect of rhizosphere bacteria on antioxidant enzymes and some biochemical characteristics of Medicago sativa L. subjected to herbicide stress. Acta Physiol. Plant. 2022, 44, 84. [Google Scholar] [CrossRef]
- Tétard-Jones, C.; Edwards, R. Potential roles for microbial endophytes in herbicide tolerance in plants. Pest Manag. Sci. 2016, 72, 203–209. [Google Scholar] [CrossRef]
- Suryanarayanan, T.S. Endophytes and weed management: A commentary. Plant Physiol. Rep. 2019, 24, 576–579. [Google Scholar] [CrossRef]
- Ahmad, Z.; Waraich, E.A.; Akhtar, S.; Anjum, S.; Ahmad, T.; Mahboob, W.; Hafeez, O.B.A.; Tapera, T.; Labuschagne, M.; Rizwan, M. Physiological responses of wheat to drought stress and its mitigation approaches. Acta Physiol. Plant 2018, 40, 80. [Google Scholar] [CrossRef]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Plants’ Physio-Biochemical and Phyto-Hormonal Responses to Alleviate the Adverse Effects of Drought Stress: A Comprehensive Review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Martínez-de la Cruz, E.; García-Ramírez, E.; Vázquez-Ramos, J.M.; de la Cruz, H.R.; López-Bucio, J. Auxins differentially regulate root system architecture and cell cycle protein levels in maize seedlings. J. Plant Physiol. 2015, 176, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Bernat, P.; Nykiel-Szymańska, J.; Gajewska, E.; Różalska, S.; Stolarek, P.; Dackowa, J.; Słaba, M. Trichoderma harzianum diminished oxidative stress caused by 2,4- dichlorophenoxyacetic acid (2,4-D) in wheat, with insights from lipidomics. J. Plant Physiol. 2018, 229, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Farooq, M.A.; Gill, R.A.; Wang, J.; Yang, C.; Ali, B.; Wang, G.-X.; Zhou, W. 2,4-D attenuates salinity-induced toxicity by mediating anatomical changes, antioxidant capacity and cation transporters in the roots of rice cultivars. Sci. Rep. 2017, 7, 10443. [Google Scholar] [CrossRef] [PubMed]
- Jalal, A.; de Oliveira, J.C., Jr.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; dos Reis, A.R. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Suzuki, M.; Yamazaki, C.; Mitsui, M.; Kakei, Y.; Mitani, Y.; Nakamura, A.; Ishii, T.; Soeno, K.; Shimada, Y. Transcriptional feedback regulation of YUCCA genes in response to auxin levels in Arabidopsis. Plant Cell Rep. 2015, 34, 1343–1352. [Google Scholar] [CrossRef]
- Ali, B.; Sabri, A.N.; Ljung, K.; Hasnain, S. Auxin production by plant associated bacteria: Impact on endogenous IAA content and growth of Triticum aestivum L. Lett. Appl. Microbiol. 2009, 48, 542–547. [Google Scholar] [CrossRef]
- Ghosh, D.; Gupta, A.; Mohapatra, S. Dynamics of endogenous hormone regulation in plants by phytohormone secreting rhizobacteria under water-stress. Symbiosis 2019, 77, 265–278. [Google Scholar] [CrossRef]
- Muraro, D.; Byrne, H.; King, J.; Bennett, M. The role of auxin and cytokinin signalling in specifying the root architecture of Arabidopsis thaliana. J. Theor. Biol. 2013, 317, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Moore, S.; Chen, C.; Lindsey, K. Crosstalk complexities between auxin, cytokinin, and ethylene in Arabidopsis root development: From experiments to systems modeling, and back again. Mol. Plant 2017, 10, 1480–1496. [Google Scholar] [CrossRef] [PubMed]
- Moubayidin, L.; Di Mambro, R.; Sabatini, S. Cytokinin–auxin crosstalk. Trends Plant Sci. 2009, 14, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Cui, H.; Chen, J.; Chen, P.; Ji, M.; Huang, S.; Li, X. Regulation of 2,4-D Isooctyl Ester on Triticum aestivum and Aegilops tauschii Tillering and Endogenous Phytohormonal Responses. Front. Plant Sci. 2021, 12, 642701. [Google Scholar] [CrossRef]
- Dobbelaere, S.; Vanderleyden, J.; Okon, Y. Plant growth promoting effects of diazotrophs in the rhizosphere. Crit. Rev. Plant Sci. 2003, 22, 107–149. [Google Scholar] [CrossRef]
- Dal Cortivo, C.; Barion, G.; Visioli, G.; Mattarozzi, M.; Mosca, G.; Vamerali, T. Increased root growth and nitrogen accumulation in common wheat following PGPR inoculation: Assessment of plant-microbe interactions by ESEM. Agric. Ecosyst. Environ. 2017, 247, 396–408. [Google Scholar] [CrossRef]
- He, R.; Ni, Y.; Li, J.; Jiao, Z.; Zhu, X.; Jiang, Y.; Li, Q.; Niu, J. Quantitative Changes in the Transcription of Phytohormone-Related Genes: Some Transcription Factors Are Major Causes of the Wheat Mutant dmc Not Tillering. Int. J. Mol. Sci. 2018, 19, 1324. [Google Scholar] [CrossRef]
- Pagnani, G.; Galieni, A.; Stagnari, F.; Pellegrini, M.; Del Gallo, M.; Pisante, M. Open field inoculation with PGPR as a strategy to manage fertilization of ancient Triticum genotypes. Biol. Fertil. Soils 2020, 56, 111–124. [Google Scholar] [CrossRef]
- Askarnejad, M.R.; Soleymani, A.; Javanmard, H.R. Barley (Hordeum vulgare L.) physiology including nutrient uptake affected by plant growth regulators under field drought conditions. J. Plant Nutr. 2021, 44, 2201–2217. [Google Scholar] [CrossRef]
- Dal Cortivo, C.; Ferrari, M.; Visioli, G.; Lauro, M.; Fornasier, F.; Barion, G.; Panozzo, A.; Vamerali, T. Effects of Seed-Applied Biofertilizers on Rhizosphere Biodiversity and Growth of Common Wheat (Triticum aestivum L.) in the Field. Front. Plant Sci. 2020, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescein. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar] [PubMed]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Vysotskaya, L.B.; Arkhipova, T.N.; Kuzmina, L.Y.; Galimsyanova, N.F.; Sidorova, L.V.; Gabbasova, I.M.; Melentiev, A.I.; Veselov, S.Y. Effect of auxin producing and phosphate solubilizing bacteria on mobility of soil phosphorus, growth rate, and P acquisition by wheat plants. Acta Physiol. Plant. 2017, 39, 253. [Google Scholar] [CrossRef]
- Korobova, A.V.; Akhiyarova, G.R.; Veselov, S.Y.; Kudoyarova, G.R.; Fedyaev, V.V.; Farkhutdinov, R.G. Participation of nitrate sensor NRT1.1 in the control of cytokinin level and root elongation under normal conditions and nitrogen deficit. Mosc. Univ. Biol. Sci. Bull. 2019, 74, 221–226. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]

| Without Herbicide | Chistalan | |||
|---|---|---|---|---|
| No Bacteria | Bacteria | No Bacteria | Bacteria | |
| Leaves RWC, % | 87.34 ± 1.36 b1 | 88.05 ± 1.20 b | 78.50 ± 0.78 a | 86.89 ± 1.28 b |
| Leaves ABA, ng/g | 32.36 ± 2.04 d | 27.60 ± 1.97 c | 19.58 ± 2.06 b | 26.39 ± 2.15 c |
| Roots IAA, ng/g | 16.41 ± 1.54 b | 23.07 ± 2.02 b | 10.40 ± 1.76 a | 15.98 ± 1.57 b |
| Productive stems, m−2 | 389.3 ± 19.1 b | 513.7 ± 16.3 c | 357.6 ± 9.6 a | 585.7 ± 24.2 d |
| Weight of 1000 grains, g | 33.4 ± 1.7 a | 32.0 ± 1.5 a | 30.4 ± 1.8 a | 30.5 ± 2.0 a |
| Grains in the spike | 16.9 ± 0.8 a | 17.7 ± 0.9 ab | 19.3 ± 0.7 b | 19.8 ± 0.8 b |
| Yield, kg/ha | 2180 ± 143 a | 2930 ± 127 b | 2090 ± 124 a | 3610 ± 132 c |
| Straw, kg/ha | 2200 ± 38 a | 3650 ± 81 c | 2420 ± 40 b | 4370 ± 33 d |
| May | June | July | August | September | |
|---|---|---|---|---|---|
| Rainfall, mm | |||||
| 2020 | 24.0 | 0 | 32.7 | 15.3 | 0 |
| 2021 | 0.4 | 26.2 | 0.5 | 4.4 | 12.9 |
| Average long-term | 29.1 | 43.9 | 49.4 | 44.1 | 28.0 |
| Average temperature, °C | |||||
| 2020 | +16.0 | +18.0 | +23.1 | +20.1 | +12.3 |
| 2021 | +17.2 | +19.6 | +19.5 | +22.2 | +8.1 |
| Average long-term | +12.5 | +16.8 | +18.5 | +16.4 | +10.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakaeva, M.; Chetverikov, S.; Timergalin, M.; Feoktistova, A.; Rameev, T.; Chetverikova, D.; Kenjieva, A.; Starikov, S.; Sharipov, D.; Hkudaygulov, G. PGP-Bacterium Pseudomonas protegens Improves Bread Wheat Growth and Mitigates Herbicide and Drought Stress. Plants 2022, 11, 3289. https://doi.org/10.3390/plants11233289
Bakaeva M, Chetverikov S, Timergalin M, Feoktistova A, Rameev T, Chetverikova D, Kenjieva A, Starikov S, Sharipov D, Hkudaygulov G. PGP-Bacterium Pseudomonas protegens Improves Bread Wheat Growth and Mitigates Herbicide and Drought Stress. Plants. 2022; 11(23):3289. https://doi.org/10.3390/plants11233289
Chicago/Turabian StyleBakaeva, Margarita, Sergey Chetverikov, Maksim Timergalin, Arina Feoktistova, Timur Rameev, Dar’ya Chetverikova, Aliya Kenjieva, Sergey Starikov, Danil Sharipov, and Gaisar Hkudaygulov. 2022. "PGP-Bacterium Pseudomonas protegens Improves Bread Wheat Growth and Mitigates Herbicide and Drought Stress" Plants 11, no. 23: 3289. https://doi.org/10.3390/plants11233289
APA StyleBakaeva, M., Chetverikov, S., Timergalin, M., Feoktistova, A., Rameev, T., Chetverikova, D., Kenjieva, A., Starikov, S., Sharipov, D., & Hkudaygulov, G. (2022). PGP-Bacterium Pseudomonas protegens Improves Bread Wheat Growth and Mitigates Herbicide and Drought Stress. Plants, 11(23), 3289. https://doi.org/10.3390/plants11233289






