Grapevine Leafroll-Associated Virus 3 Replication in Grapevine Hosts Changes through the Dormancy Stage
Abstract
1. Introduction
2. Results
2.1. Sanitary Status of Imported Cuttings and Inoculation Success
2.2. Reference Gene Selection
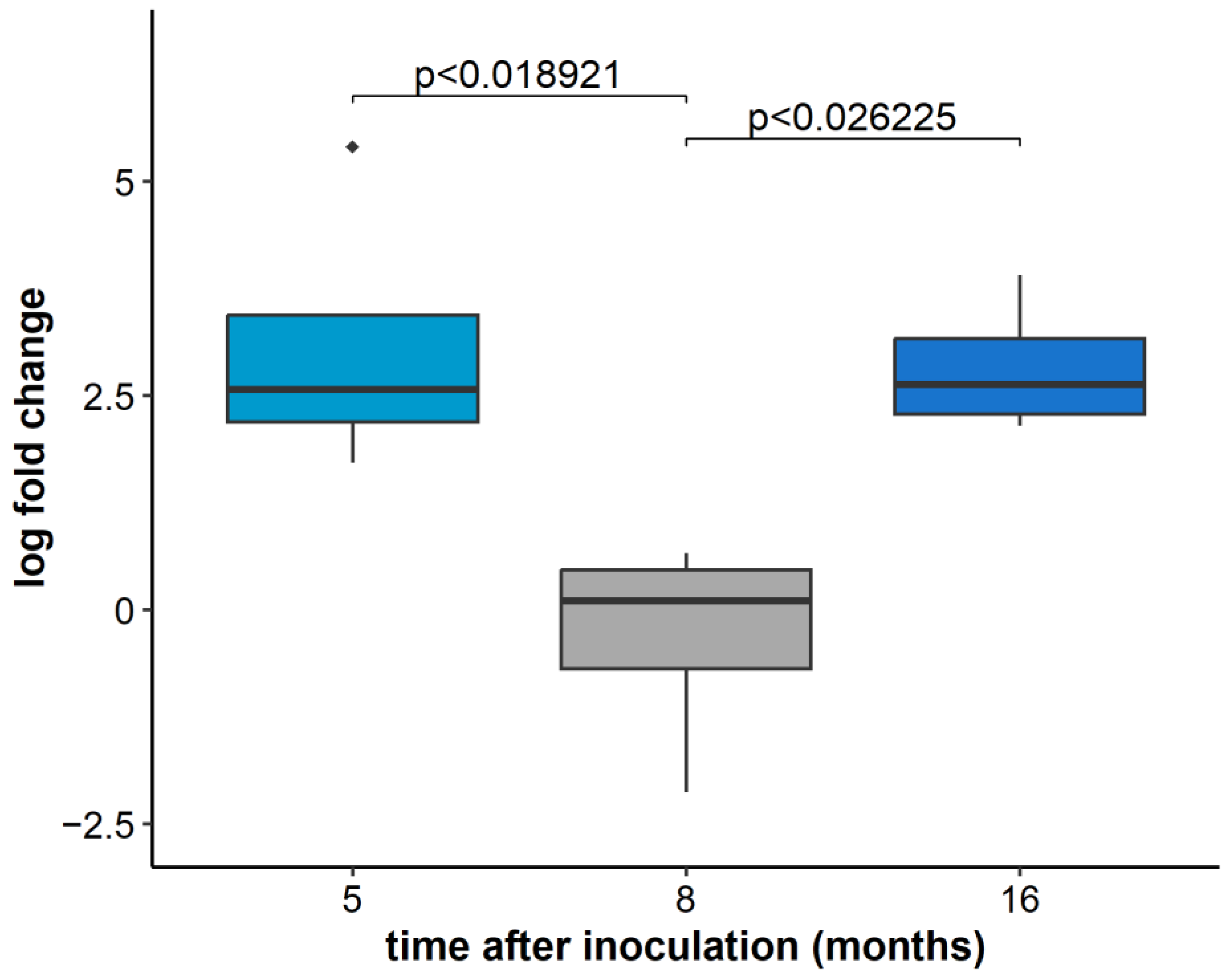
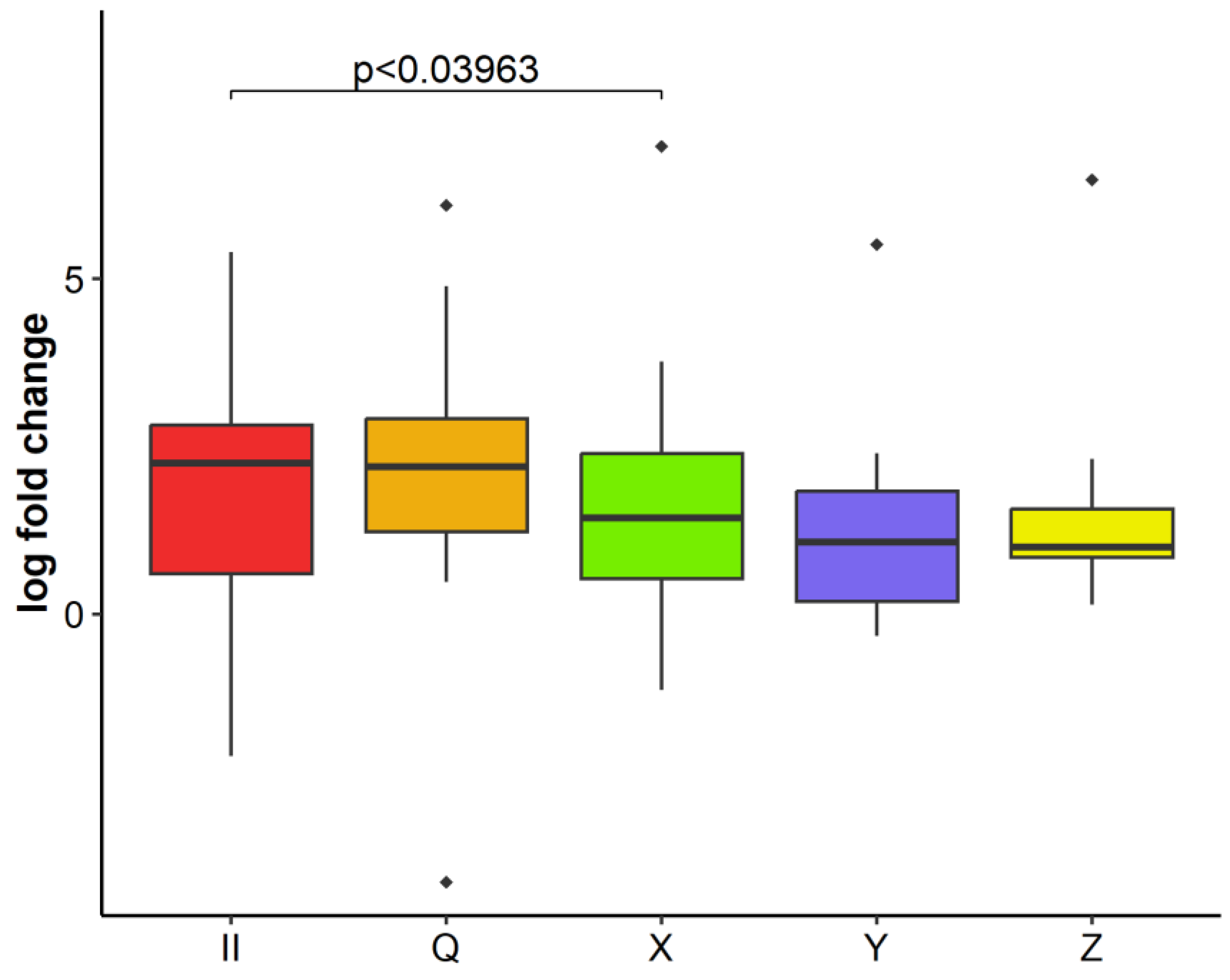
2.3. GLRaV-3 Load in Infected Plants over Time
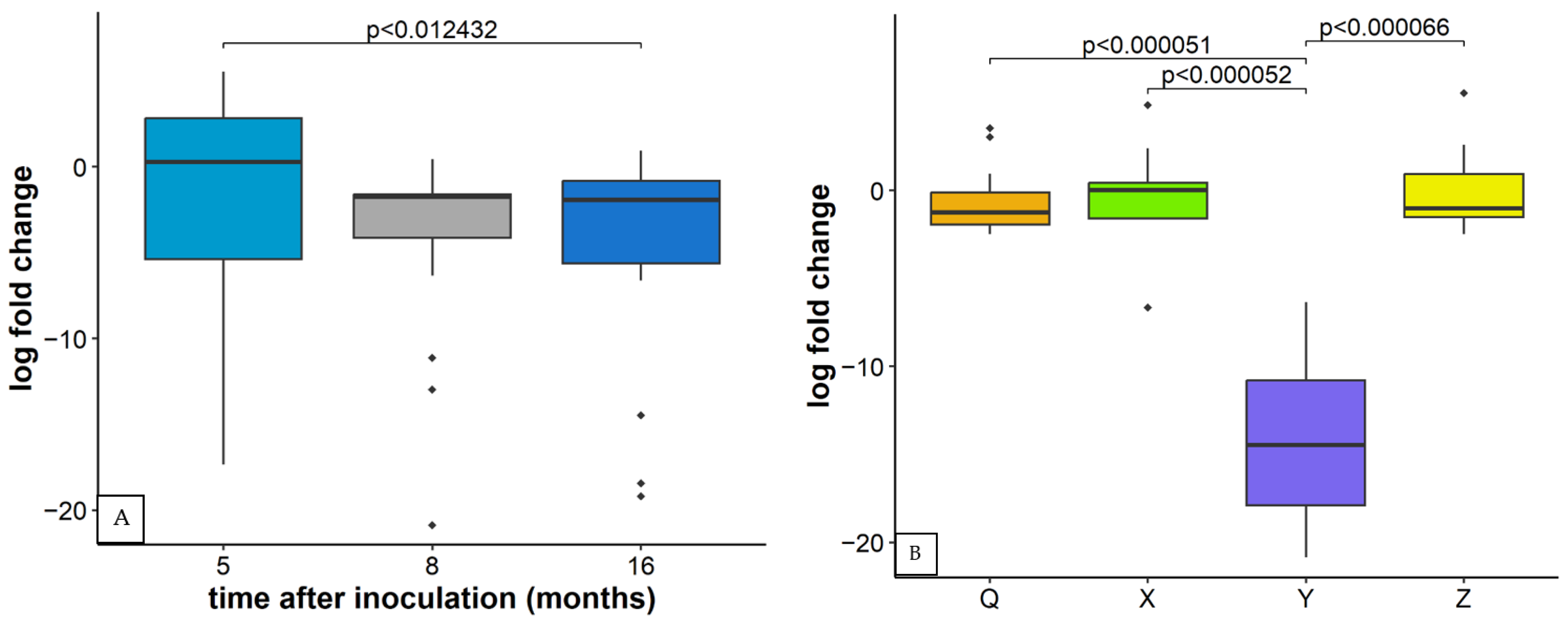
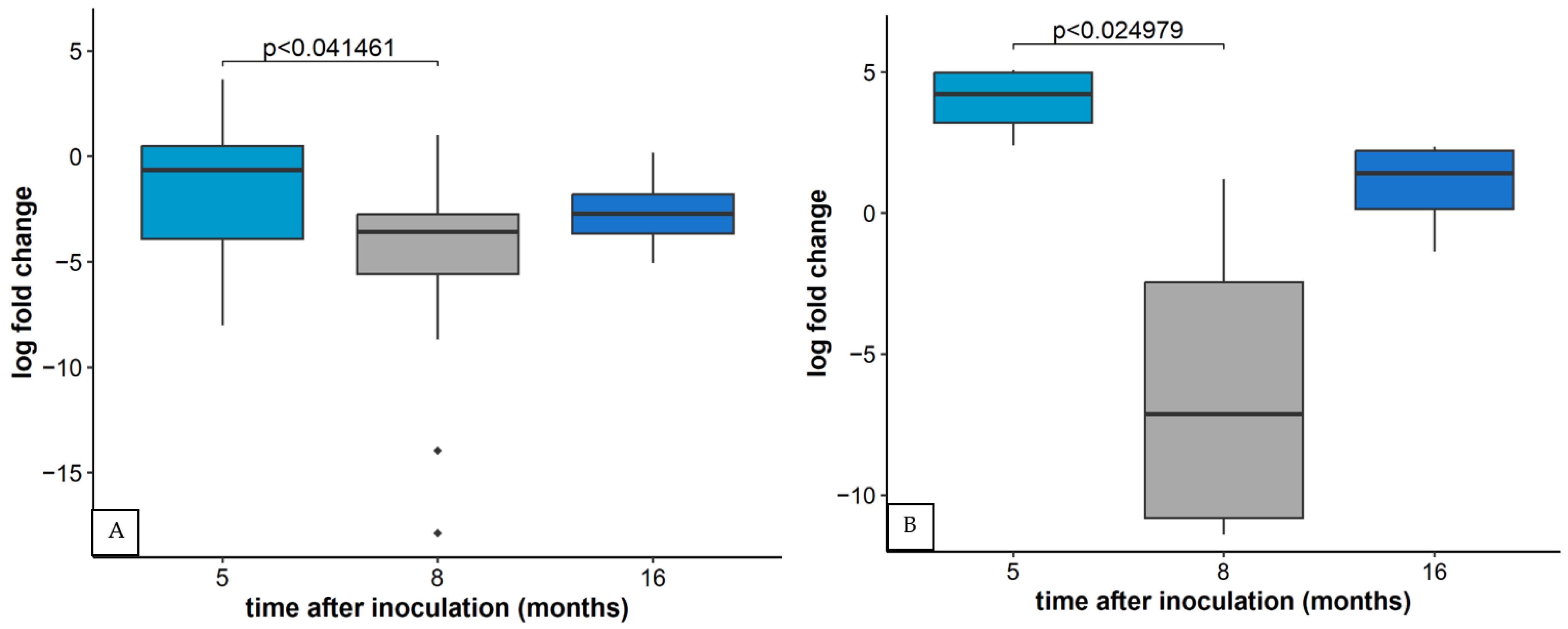
2.4. Relative Concentration of Other Viruses
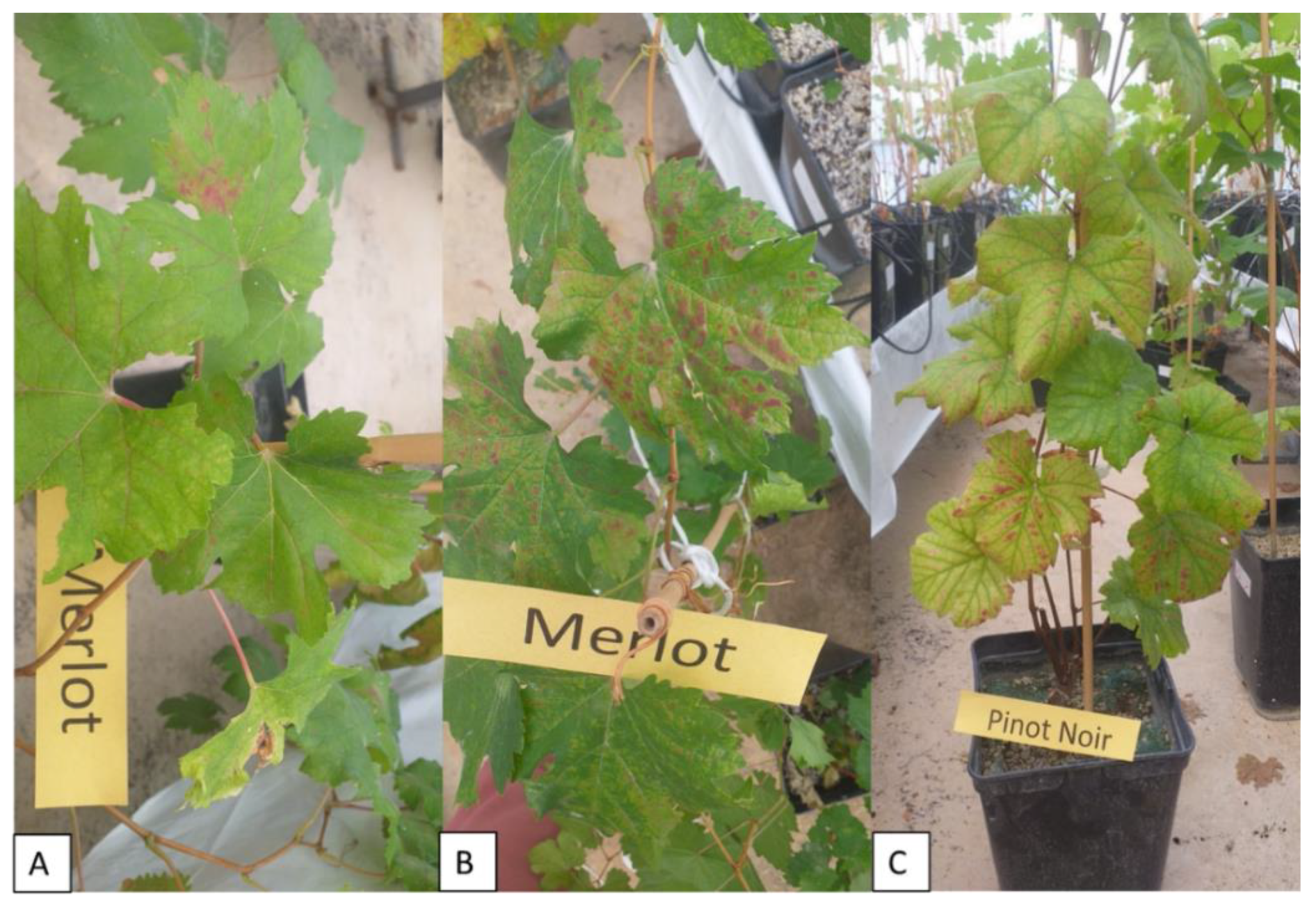
2.5. Symptom Expression of Grapevine Leafroll Disease
3. Discussion
4. Materials and Methods
4.1. Plant Material and Virus Inoculation
4.2. Symptom Assessment
4.3. Virus Detection in Imported Cuttings and Inoculated Grapevine Plants
4.4. Viral Load Quantification in Grapevine Plants
4.5. Primer Selection and Reaction Conditions
4.6. Reference Gene Candidate Selection and Relative Concentration of Virus mRNA
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martelli, G.P. An Overview on Grapevine Viruses, Viroids and the Diseases They Cause. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 31–46. ISBN 9783319577067. [Google Scholar]
- Cieniewicz, E.J.; Qiu, W.; Saldarelli, P.; Fuchs, M. Believing is seeing: Lessons from emerging viruses in grapevine. J. Plant Pathol. 2020, 102, 619–632. [Google Scholar] [CrossRef]
- Dolja, V.V.; Meng, B.; Martelli, G.P. Evolutionary aspects of grapevine virology. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Springer: Cham, Switzerland, 2017; pp. 659–688. [Google Scholar]
- Wang, M.B.; Masuta, C.; Smith, N.A.; Shimura, H. RNA silencing and plant viral diseases. Mol. Plant-Microb. Interact. 2012, 25, 1275–1285. [Google Scholar] [CrossRef]
- Martelli, G.P. Grapevine virology highlights 2006–2009. In Proceedings of the Extended Abstracts of the 16th Meeting of ICVG, Dijon Le Progrès Agricole et Viticole, Hors Série—Spécial Congrés ICVG, Dijon, France, 31 August–4 September 2009; pp. 15–24. [Google Scholar]
- Commission Directive 2005/43/EC of 23 June 2005 Amending the Annexes to Council Directive 68/193/EEC on the Marketing of Material for the Vegetative Propagation of the Vine. 2005. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32005L0043 (accessed on 16 November 2022).
- Almeida, R.P.P.; Daane, K.M.; Bell, V.A.; Blaisdell, G.K.; Cooper, M.L.; Herrbach, E.; Pietersen, G. Ecology and management of grapevine leafroll disease. Front. Microbiol. 2013, 4, 94. [Google Scholar] [CrossRef]
- Maree, H.J.; Almeida, R.P.P.; Bester, R.; Chooi, K.M.; Cohen, D.; Dolja, V.V.; Fuchs, M.F.; Golino, D.A.; Jooste, A.E.C.; Martelli, G.P.; et al. Grapevine leafroll-associated virus 3. Front. Microbiol. 2013, 4, 82. [Google Scholar] [CrossRef]
- Naidu, R.; Rowhani, A.; Fuchs, M.; Golino, D.; Martelli, G.P. Grapevine Leafroll: A complex viral disease affecting a high-value fruit crop. Plant Dis. 2014, 98, 1172–1185. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.T.; Maree, H.J.; Gouveia, P.; Naidu, R.A. Grapevine leafroll-associated virus 3. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Springer: Cham, Switzerland, 2017; pp. 167–194. ISBN 9783319577067. [Google Scholar]
- Dolja, V.V.; Kreuze, J.F.; Valkonen, J.P.T. Comparative and functional genomics of closteroviruses. Virus Res. 2006, 117, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.A.; Maree, H.J.; Burger, J.T. Grapevine Leafroll Disease and Associated Viruses: A Unique Pathosystem. Annu. Rev. Phytopathol. 2015, 53, 613–634. [Google Scholar] [CrossRef]
- Pacifico, D.; Caciagli, P.; Palmano, S.; Mannini, F.; Marzachì, C. Quantitation of Grapevine leafroll associated virus-1 and -3, Grapevine virus A, Grapevine fanleaf virus and Grapevine fleck virus in field-collected Vitis vinifera L. ‘Nebbiolo’ by real-time reverse transcription-PCR. J. Virol. Methods 2011, 172, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bester, R.; Pepler, P.T.; Burger, J.T.; Maree, H.J. Relative quantitation goes viral: An RT-qPCR assay for a grapevine virus. J. Virol. Methods 2014, 210, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.W.; Daugherty, M.P.; Almeida, R.P.P. Seasonal dynamics and virus translocation of Grapevine leafroll-associated virus 3 in grapevine cultivars. Plant Pathol. 2012, 61, 977–985. [Google Scholar] [CrossRef]
- Song, Y.; Hanner, R.H.; Meng, B. Probing into the effects of grapevine leafroll-associated viruses on the physiology, fruit quality and gene expression of grapes. Viruses 2021, 13, 593. [Google Scholar] [CrossRef] [PubMed]
- Hančević, K.; Saldarelli, P.; Čarija, M.; Černi, S.; Zdunić, G.; Mucalo, A.; Radić, T. Predominance and Diversity of GLRaV-3 in Native Vines of Mediterranean Croatia. Plants 2021, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Lara, A.; Brisbane, R.S.; Aram, K.; Golino, D.; Al Rwahnih, M. Detection of new vitiviruses infecting grapevine in California. Arch. Virol. 2019, 164, 2573–2580. [Google Scholar] [CrossRef] [PubMed]
- Čarija, M.; Radić, T.; Černi, S.; Mucalo, A.; Zdunić, G.; Vončina, D.; Jagunić, M.; Hančević, K. Prevalence of Virus Infections and GLRaV-3 Genetic Diversity in Selected Clones of Croatian Indigenous Grapevine Cultivar Plavac Mali. Pathogens 2022, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Rowhani, A.; Daubert, S.; Arnold, K.; Al Rwahnih, M.; Klaassen, V.; Golino, D.; Uyemoto, J.K. Synergy between grapevine vitiviruses and grapevine leafroll viruses. Eur. J. Plant Pathol. 2018, 151, 919–925. [Google Scholar] [CrossRef]
- Komar, V.; Vigne, E.; Demangeat, G.; Lemaire, O.; Fuchs, M. Comparative performance of virus-infected vitis vinifera cv. savagnin rose grafted onto three rootstocks. Am. J. Enol. Vitic. 2010, 61, 68–73. [Google Scholar]
- Monis, J.; Bestwick, R.K. Detection and localization of grapevine leafroll associated closteroviruses in greenhouse and tissue culture grown plants. Am. J. Enol. Vitic. 1996, 47, 199–205. [Google Scholar]
- Rohde, A.; Bhalerao, R.P. Plant dormancy in the perennial context. Trends Plant Sci. 2007, 12, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Velasco, L.; Bota, J.; Montero, R.; Cretazzo, E. Differences of three ampeloviruses’ multiplication in plant may explain their incidences in vineyards. Plant Dis. 2014, 98, 395–400. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Engelbrecht, D.J.; Kasdorf, G.G.F. Transmission of grapevine leafroll disease and and associated closteroviruses by the vine mealybug, Plannococcus ficus. Phytoparasitica 1990, 22, 341–436. [Google Scholar]
- Luo, M.; Gao, Z.; Li, H.; Li, Q.; Zhang, C.; Xu, W.; Song, S.; Ma, C.; Wang, S. Selection of reference genes for miRNA qRT-PCR under abiotic stress in grapevine. Sci. Rep. 2018, 8, 4444. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.E.; Olsson, N.; Schlosser, J.; Peng, F.; Lund, S.T. An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol. 2006, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Gutha, L.R.; Casassa, L.F.; Harbertson, J.F.; Naidu, R.A. Modulation of flavonoid biosynthetic pathway genes and anthocyanins due to virus infection in grapevine (Vitis vinifera L.) leaves. BMC Plant Biol. 2010, 10, 187. [Google Scholar] [CrossRef]
- Bertazzon, N.; Forte, V.; Filippin, L.; Causin, R.; Maixner, M.; Angelini, E. Association between genetic variability and titre of Grapevine Pinot gris virus with disease symptoms. Plant Pathol. 2017, 66, 949–959. [Google Scholar] [CrossRef]
- Martelli, G.P. Leafroll. In Graft-Transmissible Diseases of Grapevines Handbook for Detection and Diagnosis; Food & Agriculture Organization: Rome, Italy, 1993; pp. 37–45. ISBN 9251032459. [Google Scholar]
- Naidu, R.A.; O’Neil, S.; Walsh, D. Grapevine Leafroll Disease; WSU Extension Bulletin EB2027E; Washington State University: Washington, DC, USA, 2008; 20p. [Google Scholar]
- Constable, F.E.; Connellan, J.; Nicholas, P.; Rodoni, B.C. The reliability of woody indexing for detection of grapevine virus-associated diseases in three different climatic conditions in Australia. Aust. J. Grape Wine Res. 2013, 19, 74–80. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 1–32. [Google Scholar]
- Lahogue, F.; Boulard, G. Schneider Comparison de differentes techniques de greffage vis-a-vis de leur efficacite de transmission virale sur vigne. Vitis 1995, 34, 177–183. [Google Scholar]
- Clark, M.F.; Adams, A.N. Characteristics of the microplate method of enzyme linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, D.J.; McLean, M.A.; Mukerji, S.; Green, M. Improved RNA extraction from woody plants for the detection of viral pathogens by reverse transcription-polymerase chain reaction. Plant Dis. 1997, 81, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Gambino, G.; Gribaudo, I. Simultaneous detection of nine grapevine viruses by multiplex reverse transcription-polymerase chain reaction with coamplification of a plant RNA as internal control. Phytopathology 2006, 96, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Saldarelli, P.; Giampetruzzi, A.; Morelli, M.; Malossini, U.; Pirolo, C.; Bianchedi, P.; Gualandri, V. Genetic variability of Grapevine Pinot gris virus and its association with Grapevine leaf mottling and deformation. Phytopathology 2015, 105, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, D.H.; Eichhorn, K.W.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Phänologische Entwicklungsstadien der Weinrebe (Vitis vinifera L. ssp. vinifera). Codierung und Beschreibung nach der erweiterten BBCH-Skala. Wein-Wissenschaft 1994, 49, 66–70. [Google Scholar]
- Faggioli, F.; Anaclerio, F.; Angelini, E.; Antonelli, M.G.; Bertazzon, E.; Bianchi, G.; Bianchedi, P.; Bianco, P.A.; Botti, S.; Bragagna, P.; et al. Protocollo diagnostico per i virus della vite coperti da norme fitosanitarie: GLRaV 1, GLRaV 2, GLRaV 3, GVA, GVB, ArMV, GFLV, GFkV. Petria 2013, 23, 567–616. [Google Scholar]
- Thornton, B.; Basu, C. Real-time PCR (qPCR) primer design using free online software. Biochem. Mol. Biol. Educ. 2011, 39, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- TIBCO Statistica, v. 14.0.1; TIBCO Software Inc.: Palo Alto, CA, USA, 2022.


| Name of the Inoculum | Viral Composition of the Inoculum | Number of Plants Grafted per Each Grapevine Variety | |||
|---|---|---|---|---|---|
| TR | PN | CF | M | ||
| II | GLRaV-3 * | 6 | 2 | 3 | 1 |
| X | GLRaV-3, GVA, GPGV, GRSPaV | 5 | 2 | 1 | 2 |
| Y | GLRaV-3, GVA, GLRaV-1, GPGV, GRSPaV | 5 | 2 | 1 | 2 |
| Z | GLRaV-3, GVA | 5 | 2 | / ** | 2 |
| Q | GLRaV-3, GVA, GLRaV-2, GFkV, GPGV, GRSPaV | 5 | 3 | 1 | 2 |
| Reference Gene Candidate | Normfinder Stability Value | geNorm Stability Value |
|---|---|---|
| Actin | 0.28 | 0.454 |
| α-tubulin | 0.14 | 0.454 |
| GAPDH | 0.70 | 1.336 |
| Inoculum | Virus Composition | Indicator | GLD Symptom Development |
|---|---|---|---|
| II | GLRaV-3 * | TR | AS *, AS, AS, AS, AS |
| M | MO | ||
| PN | MO, AS | ||
| CF | MI, MI, MI | ||
| X | GLRaV-3, GVA, GRSPaV, GPGV | TR | AS, AS, AS, AS, AS |
| M | MI, S | ||
| PN | AS, AS | ||
| CF | AS | ||
| Y | GLRaV-3, GLRaV-1, GVA, GRSPaV, GPGV | TR | S, AS, S, MO, MO |
| M | S, AS | ||
| PN | AS, S | ||
| CF | MI | ||
| Q | GLRaV-3, GLRaV-2, GVA, GFkV, GRSPaV, GPGV | TR | AS, AS, AS, AS, AS |
| M | MO, MI | ||
| PN | S, S | ||
| CF | AS | ||
| Z | GLRaV-3, GVA | TR | AS, AS, AS, AS, AS |
| M | MI, AS | ||
| PN | MO, MI |
| Primer | Primer Sequence (5′–3′) | Primer Concentration |
|---|---|---|
| Vv actin | F: CTTGCATCCCTCAGCACCTT | 0.4 µM |
| R: TCCTGTGGACAATGGATGGA | ||
| Vv tubulin | F: CAGCCAGATCTTCACGAGCTT | 0.4 µM |
| R: GTTCTCGCGCATTGACCATA | ||
| Vv GAPDH * | F: TTCTCGTTGAGGGCTATTCCA | 0.4 µM |
| R:CCACAGACTTCATCGGTGACA | ||
| GLRaV-3_ORF1a | F: GGGRACGGARAAGTGTTACC | 0.4 µM |
| R: TCCAAYTGGGTCATRCACAA | ||
| GLRaV-3_CP | F: ATGAAYGARAARGTYATGGC | 0.48 µM |
| R: CTAAACGCYTGYTGYCTAG | ||
| GLRaV-2 | F: CAAATAGTTTCGGAGAGAGGAATG | 0.33 µM |
| R: GCGATACAAAAGTCAACGTAAGC | ||
| GLRaV-1 | F: TCTTTACCAACCCCGAGATGAA | 0.33 µM |
| R: GTGTCTGGTGACGTGCTAAACG | ||
| GVA | F: GACAAATGGCACACTACG | 0.33 µM |
| R: AAGCCTGACCTAGTCATCTTGG | ||
| GFkV | F: CTAGCTCTCGCTCTGACTCT | 0.33 µM |
| R: TCATACCACAGGAACTGGAA | ||
| GRSPaV | F: AATAATTCCCCGATTTCAAGGC | 0.33 µM |
| R: AGGATTTAGCATRGAAAGGGAATAC | ||
| GPGV | F: TGTCGATTCGTCAGGAGTTG | 0.33 µM |
| R: GGGTAAATTGTCTCCCCTGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čarija, M.; Černi, S.; Stupin-Polančec, D.; Radić, T.; Gaši, E.; Hančević, K. Grapevine Leafroll-Associated Virus 3 Replication in Grapevine Hosts Changes through the Dormancy Stage. Plants 2022, 11, 3250. https://doi.org/10.3390/plants11233250
Čarija M, Černi S, Stupin-Polančec D, Radić T, Gaši E, Hančević K. Grapevine Leafroll-Associated Virus 3 Replication in Grapevine Hosts Changes through the Dormancy Stage. Plants. 2022; 11(23):3250. https://doi.org/10.3390/plants11233250
Chicago/Turabian StyleČarija, Mate, Silvija Černi, Darija Stupin-Polančec, Tomislav Radić, Emanuel Gaši, and Katarina Hančević. 2022. "Grapevine Leafroll-Associated Virus 3 Replication in Grapevine Hosts Changes through the Dormancy Stage" Plants 11, no. 23: 3250. https://doi.org/10.3390/plants11233250
APA StyleČarija, M., Černi, S., Stupin-Polančec, D., Radić, T., Gaši, E., & Hančević, K. (2022). Grapevine Leafroll-Associated Virus 3 Replication in Grapevine Hosts Changes through the Dormancy Stage. Plants, 11(23), 3250. https://doi.org/10.3390/plants11233250








