Abstract
Dryopteris sp. is known for its various pharmacological effects and is used as a traditional medicine in Asia. The present study investigated the chemical composition and antimicrobial activity of Dryopteris sp. distributed in Korea. The chemical compounds in the ethanolic extracts of Dryopteris lacera and Dryopteris bissetiana were investigated by ultra-high performance liquid chromatography–quadrupole time-of-flight–mass spectrometry analysis and identified by exploring the UNIFI traditional medicine library. Flavonoids such as juglanin, 6-hydroxyluteolin 7-O-laminaribioside, peltatoside, kaempferitrin, hyperoside, and astragalin were identified in both D. lacera and D. bissetiana. Neochlorogenic acid was identified as a caffeoylquinic acid in D. bissetiana. Both extracts of D. lacera and D. bissetiana exhibited antibacterial activity against Gram-positive pathogens, Staphylococcus aureus and Streptococcus mutans. The minimum inhibitory concentration of D. bissetiana against S. aureus was less than 625 ppm. The antibacterial activity was attributed to the identified phenolic compounds, juglanin, 6-hydroxyluteolin 7-O-laminaribioside, kaempferitrin, astragalin, and neochlorogenic acid. Therefore, D. lacera and D. bissetiana can be used as Gram-positive selective antibiotics for further investigation.
1. Introduction
Flavonoids are an important group of natural products with polyphenolic structures. They belong to a class of plant secondary metabolites widely found in fruits, vegetables, grains, flowers, tea, and wine. Flavonoids demonstrate various biological activities in plants, animals, and bacteria. These natural compounds protect plants from different biotic and abiotic stresses and play functional roles as signal molecules, antimicrobial defensive compounds, and resistance to environmental conditions that are harmful to growth [1]. In particular, flavonoids have beneficial effects on human and animal health. They are considered essential components in several nutraceutical, pharmaceutical, medicinal, and cosmetic applications due to their anti-oxidative, anti-inflammatory, anti-mutagenic, and anti-cancer properties, combined with their ability to modulate critical cellular enzyme functions [2]. Recently, antibiotic resistance has emerged as a major global problem, and new therapeutic agents are urgently required. Among many studies, flavonoids in the field of anti-infective compounds have been reported to exhibit antibacterial, antiviral, and antifungal properties [3,4]. Furthermore, flavonoids are natural compounds extracted from plants and have potentially available activities such as direct antibacterial activity, synergistic effects with antibiotics, and inhibition of bacterial toxicity. Therefore, flavonoids can be effective in the treatment and prevention of various infectious and toxic-mediated diseases.
Terpenoids, also known as isoprenoids or terpenes, are generally considered plant or fungal metabolites and are a large class of natural products ubiquitous in nature. Terpenoids have ring structures and exhibit a wide range of biological activities. The biological activity of certain terpenoids is often assumed to interact with their membrane-binding proteins or to be lipophilic. Aromatic plants and wood resins, such as turpentine, produce terpenoids that are the main ingredient of essential oils. Moreover, many plants containing terpenoids are used in traditional medicine because of their anti-inflammatory and pain-relieving properties [5]. Several species belonging to the Asteraceae family have been traditionally used to treat inflammatory conditions. These plants produce sesquiterpene lactones that contribute to their therapeutic activities [6]. Many essential oils have been tested for their anti-inflammatory and analgesic activities in various cellular and in vivo animal models. A recent study has also reported the anti-diabetic properties of terpenoids because of their ability to lower blood glucose levels by regulating glucose metabolism in humans and animals. In addition, they have a range of biological activities, including antimalarial and antimicrobial activities [7,8,9]. There are approximately 200 types of triterpenoids with different structural characteristics, a majority of which have antiviral, antitumor, antioxidant, and anti-inflammatory activities [10,11].
Dryopteris is a perennial herb distributed worldwide, which is estimated to contain 300 species with the highest diversity in eastern Asia [12,13,14]. Dryopteris lacera (Thunb.) Kuntze and Dryopteris bissetiana (Baker) C. Chr. are among the plants that belong to the Dryopteris genus of the Dryopteridaceae family. The extract of Dryopteris sp. has been reported to have antimicrobial [15], cytoprotective [16], antioxidant [17], and anticancer effects [18], and has been used as a traditional medicine in Asia [19]. Moreover, the rhizome of Dryopteris sp. has been reported to contain various chemical compounds such as phloroglucinols, flavonoids, and triterpenes [20].
In this study, we investigated the chemical compounds, flavonoids and terpenoids, of D. lacera and D. bissetiana through ultra-high-performance liquid chromatography– quadrupole time-of-flight–mass spectrometry (UPLC–QTOF–MS) analysis. The components were identified the UNIFI traditional medicine library. Additionally, the antimicrobial activity of the ethanolic extracts of D. lacera and D. bissetiana against Gram-positive and Gram-negative bacteria and fungal pathogens was performed.
2. Results and Discussion
The bioactive components of D. lacera and D. bissetiana were identified by UPLC–QTOF–MS analysis. The natural product analysis was performed using the LC–QTOF MSE mode. In this mode, the main precursor ion was detected using a low collision energy value, which was then exposed to stronger collision energy to analyze the pattern of the product ions. Figure 1 and Figure 2 shows the UPLC-QTOF-MS base peak ion (BPI) chromatogram and component confirmed plot chromatogram. The relevant data, including retention time (min), experimental m/z [M−H]− or [M+HCOO]−, theoretical mass, predicted chemical formula, and tentatively identified compound are listed in Table 1 and Table 2. The retention times, mass spectra, and fragment information were compared, and compounds were identified by exploring the UNIFI traditional medicine library containing over 600 herbs and over 6000 compounds. Therefore, it was possible to identify flavonoids and terpenoids compounds containing glucose.
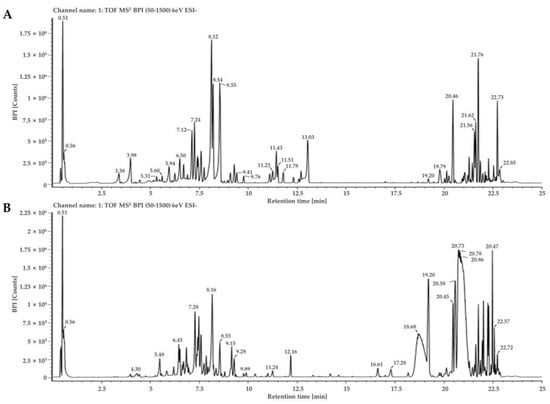
Figure 1.
Base peak ion(BPI) chromatogram of D. lacera (A), and D. bissetiana (B) extracts by UPLC-QTOF-MS analysis.
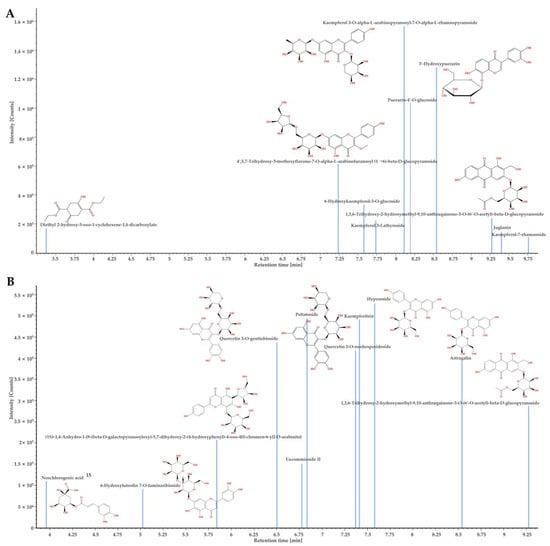
Figure 2.
The confirmed component plot of UPLC-QTOF-MS analysis of D. lacera (A), and D. bissetiana (B) extracts.

Table 1.
The bioactive components of D. lacera based on the UPLC-QTOF-MS analysis.

Table 2.
The bioactive components of D. bissetiane based on the UPLC-QTOF-MS analysis.
The chemical compound determined from the D. lacera UPLC–QTOF–MS analysis at a retention time of 9.4 min was identified as juglanin, which presented [M−H]− ion at m/z 417. The product ions were formed at m/z 284 and m/z 255, with the main peak at m/z 284 attributed to the loss of glucose moiety (Figure 3A). Juglanin is a natural product found in many plants. It has various biological activities, including antimicrobial, anticancer, antioxidant, and anti-inflammatory effects, as well as protecting skin from ultraviolet B exposure [21,22,23,24,25].
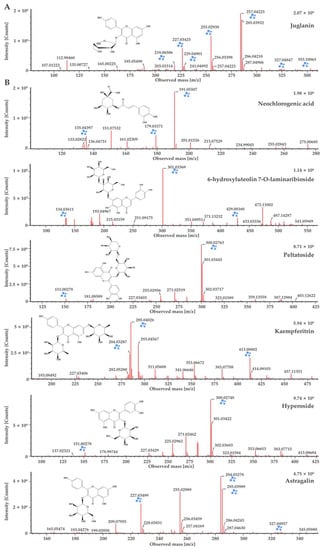
Figure 3.
UPLC-QTOF-MS analysis of the major components in terms of matching scores of D. lacera (A), and D. bissetiana (B) extracts.
Flavonoids, 6-hydroxyluteolin 7-O-laminaribioside, peltatoside, kaempferitrin, hyperoside, and astragalin from D. bissetianai were detected by UPLC–QTOF–MS analysis (Table 2). The [M−H]− ion at m/z 625 with two glucose moieties attached was identified as 6-hydroxyluteolin 7-O-laminaribioside. The fragment ions were formed at m/z 429 without glucose moieties and at m/z 301 with only one glucose moiety. The main peak at m/z 301 indicated the loss of two glucose moieties. Peltatoside was identified at m/z 595 [M−H]−, with its fragment ions at m/z 300 and m/z 151. The main peak at m/z 300 was suggested the structure without the glucose moiety. Kaempferitrin confirmed at m/z 577 [M−H]−, with its fragment ions at m/z 413 and m/z 285. The main peak at m/z 285 was formed due to the loss of the glucose moiety. Hyperoside was detected at m/z 463 [M−H]−, with fragment ions at m/z 300 and m/z 151. The main peak at m/z 300 indicated the loss of glucose moiety. Astragalin was determined at m/z 447 [M−H]−, and the fragment ions were confirmed at m/z 284 and m/z 227. The main peak at m/z 284 suggested the loss of glucose moiety (Figure 3B). Phenolic compounds, including 6-hydroxyluteolin 7-O-laminaribioside of the medicinal plant Globularia alypum, exhibits antioxidant and antimicrobial activities [26]. Peltatoside isolated from Annona crassilora, used in folk medicine, is known to inhibit acute local inflammation and demonstrate analgesic activity [27,28]. Kaempferitrin from many plants shows antimicrobial, antitumor, and antioxidant activities [29,30,31]. It has also been suggested as a putative therapeutic agent for diabetic nephropathy by suppressing the mitochondrial/cytochrome c-mediated apoptosis pathway [32] and rheumatoid arthritis (RA) by ameliorating inflammation in RA fibroblast-like synoviocytes [33]. Hyperoside is a quercetin 3-d-galactoside derived from plants that is known to have anti-cancer effects. It inhibits the cervical cancer HeLa and C-33A cell proliferation [34], A549 and H1975 lung cancer cell proliferation [35], and induces apoptosis in HT-29 colon [36], SKOV3 and HO-8910 ovarian [37], and MCF-7 and 4T1 breast cancer cells [38]. Astragalin is kaempferol-3-O-ß-D-glucoside, a bioactive natural flavonoid that is known for its medicinal importance such as antimicrobial, antioxidant, anti-inflammatory, anti-cancer, and neuroprotective activities [39,40,41,42,43,44]. It is also applied in cosmetics to inhibit melanin secretion and protect against UV damage [45,46]. Neochlorogenic acid was identified as a caffeoylquinic acid in D. bissetiana (Table 2) at m/z 353 [M−H]−. The fragments ions were confirmed at m/z 191 and m/z 179. The main peak at m/z 191 suggested the loss of the caffeic acid group, while the peak at m/z 179 indicated the loss of the quinic acid group (Figure 3B). Neochlorogenic acid is a natural polyphenolic compound found in plants, known for its antioxidant, antibacterial, antitumor, and anti-inflammatory properties [47,48]. Furthermore, neochlorogenic acid shows anti-photoaging effects and is an effective agent for skin wrinkle formation and dehydration [49].
The antibacterial assay revealed that D. lacera and D. bissetiana exhibits antibacterial activity against the selected indicator pathogenic strains, as summarized in Table 3. A clear zone of inhibition of S. aureus NCCP14560 and S. mutans KCTC3065 was observed using D. lacera and D. bissetiana extracts. The MIC of D. lacera extract was 5 mg/mL against S. aureus and S. mutans, and the MIC of D. bissetiana extract was 0.625 mg/mL and 5 mg/mL against each pathogen, respectively (Table 3). Conversely, D. lacera and D. bissetiana extracts were inactive against E. coli KCTC2617, S. Enteritidis NCCP14546, and C. albicans NCCP31077. Phenolic compounds identified in D. lacera and D. bissetiana are known for their antimicrobial activity. It has been reported that ethanol extracts of plants contain various chemical compounds that affect multiple sites of bacterial cell walls [50]. However, the ethanolic extracts of D. lacera and D. bissetiana only had antimicrobial effects on S. aureus and S. mutans, which are Gram-positive bacterial pathogens among the tested microorganisms. Moreover, the D. bissetiana extract showed antibacterial activity against S. aureus at very low concentrations of less than 0.625 mg/mL suggesting further study of its clinical use. In particular, the emergence of antibiotic-resistant strains of S. aureus, such as methicillin-resistant S. aureus and vancomycin-resistant S. aureus, is a global risk in clinical medicine.

Table 3.
Antibacterial activity of the plant extract against the indicator strains.
3. Materials and Methods
3.1. Plant Materials and Preparation of Plant Extract
The leaves of D. lacera (Thunb.) Kuntze and D. bissetiana (Baker) C. Chr were collected from Mt. Jiri of Gyeongsang-do, South Korea, and identified by an experienced taxonomist. The collected specimens of D. lacera Kuntze and D. bissetiana C. Chr were deposited in the Natural Products Bank, Wildlife Genetic Resources Center at the National Institute of Biological Resources. The collected leaves were dried in a drying oven at 40 °C for 2 d for preparing the crude extract. Approximately 500 g of dried leaves were extracted from 70% ethanol (analytical grade, Sigma-Aldrich, St. Louis, MI, USA), four times the volume of the leaves, for 48 h. The ethanol crude extracts from the leaves were centrifuged at 4000× g for 5 min, filtered using a Bückner funnel, and all ethanol was removed using a rotary evaporator. The extract was stored at −80 °C in a freezer until further use.
3.2. UPLC-QTOF-MS Analysis of Dryopteris Plants
The chemical profile of the 70 % EtOH extract of plants was analyzed using a Waters ACQUITY UPLC I-Class PLUS equipped with a Waters ACQUITY UPCL HSS T3 column (100 mm × 2.1 mm, 1.8 µM), maintained at an isothermal temperature of 40 °C. A binary pump delivered the mobile phase at flow rate of 0.2 mL/min under a gradient elution using two mobile phases, water containing 0.1 % (v/v) formic acid (solvent A) and acetonitrile containing 0.1 % (v/v) formic acid (solvent B). The following were the gradient conditions: 0–1 min, 95 % solvent A; 1–17 min, 60 % solvent A; 17–21 min, 40 % solvent A; 11–22 min, 0 % solvent A; 22.9–25 min, 95 % solvent A. The auto-sampler was set to an injection volume of 5 µL. Mass spectrometric analysis was performed using a Waters Xevo-G2-XS QTOF LCMS equipped with an electrospray ionization source. The analysis was conducted in negative ion mode, set for detecting mass-to-charge ratio (m/z) in the range of 50–1500. The source temperature was set at 120 °C with a capillary voltage 2.5 kV. Ar (g) was used as the collision gas. Data acquisition and analysis were controlled using the traditional medicine library of Waters’ UNIFI software 1.9 version. In addition, the identification of the components was performed by checking the quality allowable error range ± 5 ppm fragmentation pattern. If the fragment ion pattern did not match, it was finally identified using a Chemspider (http://www.chemspider.com/, accessed on 5 November 2021) online database.
3.3. Antibacterial Analysis
The antibacterial activities of plant extracts against well-known pathogenic microorganisms were evaluated using a previously described disk diffusion method [51], with slight modifications. The following five microbial pathogens were used as indicators of antibacterial activities: E. coli KCTC2617, Salmonella enterica serovar Enteritidis NCCP14546, Staphylococcus aureus NCCP14560, Streptococcus mutans KCTC3065, and Candida albicans NCCP31077. Initially, these pathogenic strains were grown on suitable media at 30–37 °C for 20 h. E. coli and S. aureus were grown on nutrient agar, S. Enteritidis on tryptic soy agar, S. mutans on brain heart infusion agar, and C. albicans on Sabouraud dextrose agar. Diffusion disks with a diameter of 8 mm were placed on the agar, and the plant extract with a concentration of 20 mg/mL using a diluent solvent (DMSO:EtOH = 1:1, v/v) was dispensed onto the disks. The plates were incubated at 30–37 °C for 24 h, and the diameters of the inhibition zones around each disk were measured for positive and negative controls, gentamycin (2.5 mg/mL), hygromycin (10 mg/mL), and a diluent solvent were used as, respectively. Further, different concentrations of plant extract (20, 5, 2.5, 1.25, and 0.625 mg/mL were prepared by five-fold serial dilution to determine the minimum inhibitory concentrations (MIC). MIC is regarded as the lowest concentration that inhibits the microbial pathogen growth. Each prepared extract was applied to pathogen-inoculated agar media and then incubated under the required growth conditions [52]. All antimicrobial assays were conducted in triplicates.
3.4. Statistical Analysis
Data were analyzed using One-way ANOVA Tukey post hoc test for multiple comparisons by IBS SPSS Statistics 20 software. All experiments were conducted in three replicates per treatment.
4. Conclusions
In conclusion, we identified the chemical compounds of D. lacera and D. bissetiana by UPLC–QTOF–MS and presented various biological phenolic compounds, such as juglanin, 6-hydroxyluteolin 7-O-laminaribioside, peltatoside, kaempferitrin, hyperoside, astragalin, and neochlorogenic acid. Both Dryopteris plants in this study showed antibacterial activity against the Gram-positive pathogens. However, the extracts of D. lacera and D. bissetiana did not exhibit antimicrobial activity against the Gram-negative. Therefore, D. lacera and D. bissetiana can be used as Gram-positive selective antibiotics for further investigation.
Author Contributions
Conceptualization, Y.K. and Y.K.S.; methodology, Y.K., J.-S.S., J.-A.K. and B.-H.L.; formal analysis, Y.K., J.-S.S. and J.-A.K.; investigation, Y.K. and Y.K.S.; resources, Y.K. and Y.K.S.; data curation, Y.K., D.J.L., J.-S.S. and J.-A.K.; writing—original draft preparation, Y.K. and Y.K.S.; writing—review and editing, Y.K., D.J.L. and Y.K.S.; visualization, Y.K. and D.J.L.; supervision, Y.K. and Y.K.S.; project administration, Y.K. and Y.K.S.; funding acquisition, Y.K. and Y.K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the National Institute of Biological Resources (NIBR), funded by the Ministry of Environment (MOE) of the Republic of Korea (NIBR202219101).
Data Availability Statement
Not applicable.
Acknowledgments
We thank the anonymous reviewers for their careful reviewing of this manuscript and providing insightful comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Panche, A.N.; ADiwan, D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2007, 51, 116–134. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Morton, D.W. The Current and Potential Therapeutic Uses of Parthenolide; Elsevier: Amsterdam, The Netherlands, 2018; pp. 61–91. [Google Scholar]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef]
- Habtemariam, S. Antidiabetic Potential of Monoterpenes: A Case of Small Molecules Punching above Their Weight. Int. J. Mol. Sci. 2017, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Onguéné, P.A.; Ntie-Kang, F.; Lifongo, L.L.; Ndom, J.C.; Sippl, W.; Mbaze, L.M. The potential of anti-malarial compounds derived from African medicinal plants, part I: A pharmacological evaluation of alkaloids and terpenoids. Malar. J. 2013, 12, 449. [Google Scholar] [CrossRef]
- Silva, F.S.; Oliveira, P.J.; Duarte, M.F. Oleanolic, Ursolic, and Betulinic Acids as Food Supplements or Pharmaceutical Agents for Type 2 Diabetes: Promise or Illusion? J. Agric. Food Chem. 2016, 64, 2991–3008. [Google Scholar] [CrossRef]
- Ghani, U. Terpenoids and steroids. In Alpha-Glucosidase Inhibitors; Elsevier: Amsterdam, The Netherlands, 2020; pp. 101–117. [Google Scholar]
- Graßmann, J. Terpenoids as Plant Antioxidants. In Plant Hormones; Gulf Professional Publishing: Houston, TX, USA, 2005; pp. 505–535. [Google Scholar]
- Schuettpelz, E.; Schneider, H. A community-derived classification for extant lycophytes and ferns. J. Syst. Evol. 2016, 54, 563–603. [Google Scholar]
- Sessa, E.B.; Zhang, L.B.; Väre, H.; Juslén, A. What We Do (and Don’t) Know About Ferns: Dryopteris (Dryopteridaceae) as a Case Study. Syst. Bot. 2015, 40, 387–399. [Google Scholar] [CrossRef]
- Zhang, L.B.; Zhang, L.; Dong, S.Y.; Sessa, E.B.; Gao, X.F.; Ebihara, A. Molecular circumscription and major evolutionary lineages of the fern genus Dryopteris (Dryopteridaceae). BMC Evol. Biol. 2012, 12, 180. [Google Scholar] [CrossRef] [PubMed]
- Min, B.S.; Tomiyama, M.; Ma, C.-M.; Nakamura, N.; Hattori, M. Kaempferol Acetylrhamnosides from the Rhizome of Dryopteris crassirhizoma and Their Inhibitory Effects on Three Different Activities of Human Immunodeficiency Virus-1 Reverse Transcriptase. Chem. Pharm. Bull. 2001, 49, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Lee, K.H.; Chae, S.; Zhang, R.; Jung, M.S.; Ham, Y.M.; Baik, J.S.; Lee, N.H.; Hyun, J.W. Cytoprotective Effect of Phloroglucinol on Oxidative Stress Induced Cell Damage via Catalase Activation. J. Cell. Biochem. 2006, 97, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Na, M.K.; An, R.B.; Min, B.S.; Lee, H.K. Antioxidant activity of two phloroglucinol derivatives from Dryopteris crassirhizoma. Biol. Pharm. Bull. 2003, 26, 1354–1356. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.D.; Zhao, D.D.; Jiang, S.; Xue, B.; Zhang, Y.L.; Yan, X.F. Anticancer Phenolics from Dryopteris fragrans (L.) Schott. Molecules 2018, 23, 680. [Google Scholar] [CrossRef]
- Namba, T. The Encyclopedia of Wakan-Yaku (Traditional Sino-Japanese Medicines) with Color Pictures; New completely ed.; Hoikusha Publishing: Osaka, Japan, 1994. [Google Scholar]
- Yang, Y.; Lee, G.J.; Yoon, D.H.; Yu, T.; Oh, J.; Jeong, D.; Lee, J.; Kim, S.H.; Kim, T.W.; Cho, J.Y. ERK1- and TBK1-targeted anti-inflammatory activity of an ethanol extract of Dryopteris crassirhizoma. J. Ethnopharmacol. 2013, 145, 499–508. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, Y.Q.; Xu, J.; Wang, J.P.; Meng, Z.L.; Hong, Y.Q. Juglanin inhibits lung cancer by regulation of apoptosis, ROS and autophagy induction. Oncotarget 2017, 8, 93878–93898. [Google Scholar] [CrossRef]
- Da Silva Sa, F.A.; De Paula, J.A.M.; Dos Santos, P.A.; De Almeida Ribeiro Oliveira, L.; De Almeida Ribeiro Oliveira, G.; Liao, L.M.; De Paula, J.R.; Do Rosario Rodrigues Silva, M. Phytochemical Analysis and Antimicrobial Activity of Myrcia tomentosa (Aubl.) DC. Leaves. Molecules 2017, 22, 1100. [Google Scholar] [CrossRef]
- Sun, Z.L.; Dong, J.L.; Wu, J. Juglanin induces apoptosis and autophagy in human breast cancer progression via ROS/JNK promotion. Biomed. Pharmacother. 2017, 85, 303–312. [Google Scholar] [CrossRef]
- Wen, Z.M.; Jie, J.; Zhang, Y.; Liu, H.; Peng, L.P. A self-assembled polyjuglanin nanoparticle loaded with doxorubicin and anti-Kras siRNA for attenuating multidrug resistance in human lung cancer. Biochem. Biophys. Res. Commun. 2017, 493, 1430–1437. [Google Scholar] [CrossRef]
- Zhang, F.X.; Xu, R.S. Juglanin ameliorates LPS-induced neuroinflammation in animal models of Parkinson’s disease and cell culture via inactivating TLR4/NF-kappaB pathway. Biomed. Pharmacother. 2018, 97, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Taghzooti, O.K.; Balouirib, M.; Ouedrhiric, W.; Echchahad, A.; Romanea, A. In vitro evaluation of the antioxidant and antimicrobial effects of Globularia alypum L. extracts. J. Mater. Environ. Sci. 2016, 7, 1988–1995. [Google Scholar]
- Oliveira, C.D.; Veloso, C.C.; Ferreira, R.C.; Lage, G.A.; Pimenta, L.P.; Duarte, I.D.; Romero, T.R.; Perez, A.C. Peltatoside Isolated from Annona crassiflora Induces Peripheral Antinociception by Activation of the Cannabinoid System. Planta Med. 2017, 83, 261–267. [Google Scholar] [CrossRef]
- Prata, M.N.L.; Charlie-Silva, I.; Gomes, J.M.M.; Barra, A.; Berg, B.B.; Paiva, I.R.; Melo, D.C.; Klein, A.; Romero, M.G.M.C.; Oliveira, C.C.; et al. Anti-inflammatory and immune properties of the peltatoside, isolated from the leaves of Annona crassiflora Mart., in a new experimental model zebrafish. Fish Shellfish Immunol. 2020, 101, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghani, N.T.; Shoukry, A.F.; el Nashar, R.M. Flow injection potentiometric determination of pipazethate hydrochloride. Analyst 2001, 126, 79–85. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Ortiz-Sánchez, E.; García-Regalado, A.; Ruiz, G.; Núñez-Martínez, J.M.; González-Sánchez, I.; Quintanar-Jurado, V.; Morales-Sánchez, E.; Dominguez, F.; López-Toledo, G.; et al. Kaempferitrin induces apoptosis via intrinsic pathway in HeLa cells and exerts antitumor effects. J. Ethnopharmacol. 2013, 145, 476–489. [Google Scholar] [CrossRef]
- Vellosa, J.C.; Regasini, L.O.; Belló, C.; Schemberger, J.A.; Khalil, N.M.; De Araujo Morandim-Giannetti, A.; Da Silva Bolzani, V.; Brunetti, I.L.; De Faria Oliberia, O.M. Preliminary in vitro and ex vivo evaluation of afzelin, kaempferitrin and pterogynoside action over free radicals and reactive oxygen species. Arch. Pharm. Res. 2015, 38, 1168–1177. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, R.; Liu, D.; Zuo, M.; Zhao, C.; Zhang, T.; Li, W. Protective Effects of Kaempferitrin on Advanced Glycation End Products Induce Mesangial Cell Apoptosis and Oxidative Stress. Int. J. Mol. Sci. 2018, 19, 3334. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Q. Kaempferitrin inhibits proliferation, induces apoptosis, and ameliorates inflammation in human rheumatoid arthritis fibroblast-like synoviocytes. Phytother. Res. 2019, 33, 1726–1735. [Google Scholar] [CrossRef]
- Guo, W.; Yu, H.; Zhang, L.; Chen, X.; Liu, Y.; Wang, Y.; Zhang, Y. Effect of hyperoside on cervical cancer cells and transcriptome analysis of differentially expressed genes. Cancer Cell Int. 2019, 19, 235. [Google Scholar] [CrossRef]
- Liu, Y.H.; Liu, G.H.; Mei, J.J.; Wang, J. The preventive effects of hyperoside on lung cancer in vitro by inducing apoptosis and inhibiting proliferation through Caspase-3 and P53 signaling pathway. Biomed. Pharmacother. 2016, 83, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Guon, T.E.; Chung, H.S. Hyperoside and rutin of Nelumbo nucifera induce mitochondrial apoptosis through a caspase-dependent mechanism in HT-29 human colon cancer cells. Oncol. Lett. 2016, 11, 2463–2470. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ji, M.; Han, Y.; Guo, Y.; Zhu, W.; Gao, F.; Yang, X.; Zhang, C. PGRMC1-dependent autophagy by hyperoside induces apoptosis and sensitizes ovarian cancer cells to cisplatin treatment. Int. J. Oncol. 2017, 50, 835–846. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, T.; Zhu, X.; Yang, C.; Wang, Y.; Zhou, N.; Ju, B.; Zhou, T.; Deng, G.; Qiu, C. Hyperoside Induces Breast Cancer Cells Apoptosis via ROS-Mediated NF-kappaB Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 131. [Google Scholar] [CrossRef] [PubMed]
- Bainey, K.R.; Armstrong, P.W. Clinical perspectives on reperfusion injury in acute myocardial infarction. Am. Heart J. 2014, 167, 637–645. [Google Scholar] [CrossRef]
- Bitis, L.; Kultur, S.; Melıkoglu, G.; Ozsoy, N.; Can, A. Flavonoids and antioxidant activity of Rosa agrestis leaves. Nat. Prod. Res. 2010, 24, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Burmistrova, O.; Quintana, J.; Díaz, J.G.; Estévez, F. Astragalin heptaacetate-induced cell death in human leukemia cells is dependent on caspases and activates the MAPK pathway. Cancer Lett. 2011, 309, 71–77. [Google Scholar] [CrossRef]
- Ivanova, A.; Mikhova, B.; Najdenski, H.; Tsvetkova, I.; Kostova, I. Chemical composition and antimicrobial activity of wild garlic Allium ursinum of Bulgarian origin. Nat. Prod. Commun. 2009, 4, 1059–1062. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Choi, Y.-J.; Kang, M.-K.; Park, S.-H.; Antika, L.D.; Lee, E.-J.; Kim, D.Y.; Kang, Y.-H. Astragalin Inhibits Allergic Inflammation and Airway Thickening in Ovalbumin-Challenged Mice. J. Agric. Food Chem. 2017, 65, 836–845. [Google Scholar] [CrossRef]
- Kotani, M.; Matsumoto, M.; Fujita, A.; Higa, S.; Wang, W.; Suemura, M.; Kishimoto, T.; Tanaka, T. Persimmon leaf extract and astragalin inhibit development of dermatitis and IgE elevation in NC/Nga mice. J. Allergy Clin. Immunol. 2000, 106, 159–166. [Google Scholar] [CrossRef]
- Kim, G.-E.; Kang, H.-K.; Seo, E.-S.; Jung, S.-H.; Park, J.-S.; Kim, D.-H.; Kim, D.-W.; Ahn, S.-A.; Sunwoo, C.; Kim, D. Glucosylation of the flavonoid, astragalin by Leuconostoc mesenteroides B-512FMCM dextransucrase acceptor reactions and characterization of the products. Enzyme Microb. Technol. 2012, 50, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Svobodová, A.; Psotová, J.; Walterová, D. Natural phenolics in the prevention of uv-induced skin damage. A review. Biomed. Papers 2003, 147, 137–145. [Google Scholar] [CrossRef]
- Che, J.; Zhao, T.; Liu, W.; Chen, S.; Yang, G.; Li, X.; Liu, D. Neochlorogenic acid enhances the antitumor effects of pingyangmycin via regulating TOP2A. Mol. Med. Rep. 2021, 23, 158. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.H.; Zhang, S.-D.; Wang, L.-T.; Yu, L.; Zhao, X.-L.; Ni, H.-Y.; Wang, Y.-Q.; Wang, J.-D.; Shan, C.-H.; Fu, Y.-J. Anti-Inflammatory Effects of Neochlorogenic Acid Extract from Mulberry Leaf (Morus alba L.) Against LPS-Stimulated Inflammatory Response through Mediating the AMPK/Nrf2 Signaling Pathway in A549 Cells. Molecules 2020, 25, 1385. [Google Scholar] [CrossRef]
- Ahn, H.S.; Kim, H.J.; Na, C.; Jang, D.S.; Shin, Y.-K.; Lee, S.H. The Protective Effect of Adenocaulon himalaicum Edgew. and Its Bioactive Compound Neochlorogenic Acid against UVB-Induced Skin Damage in Human Dermal Fibroblasts and Epidermal Keratinocytes. Plants 2021, 10, 1669. [Google Scholar] [CrossRef]
- Oonmetta-aree, J.; Suzuki, T.; Gasaluck, P.; Eumkeb, G. Antimicrobial properties and action of galangal (Alpinia galanga Linn.) on Staphylococcus aureus. LWT Food Sci. Technol. 2006, 39, 1214–1220. [Google Scholar] [CrossRef]
- Tagg, J.; McGiven, A. Assay system for bacteriocins. Appl. Microbiol. 1971, 21, 943. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).