A Modeled High-Density Fed-Batch Culture Improves Biomass Growth and β-Glucans Accumulation in Microchloropsis salina
Abstract
1. Introduction
2. Results
2.1. Kinetic Parameters in an Optimal Environment
2.2. Fed-Batch Culture Modeling
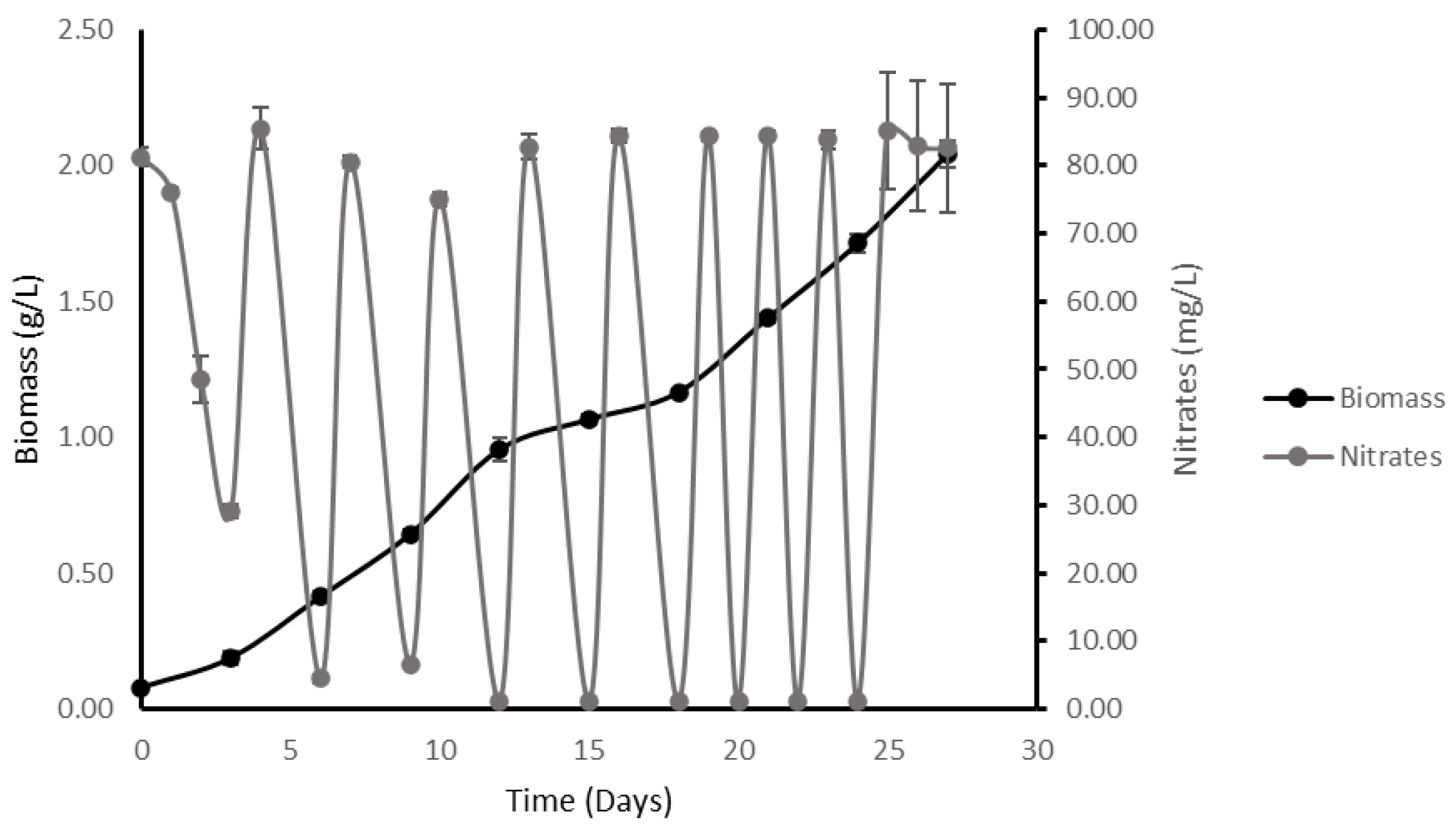
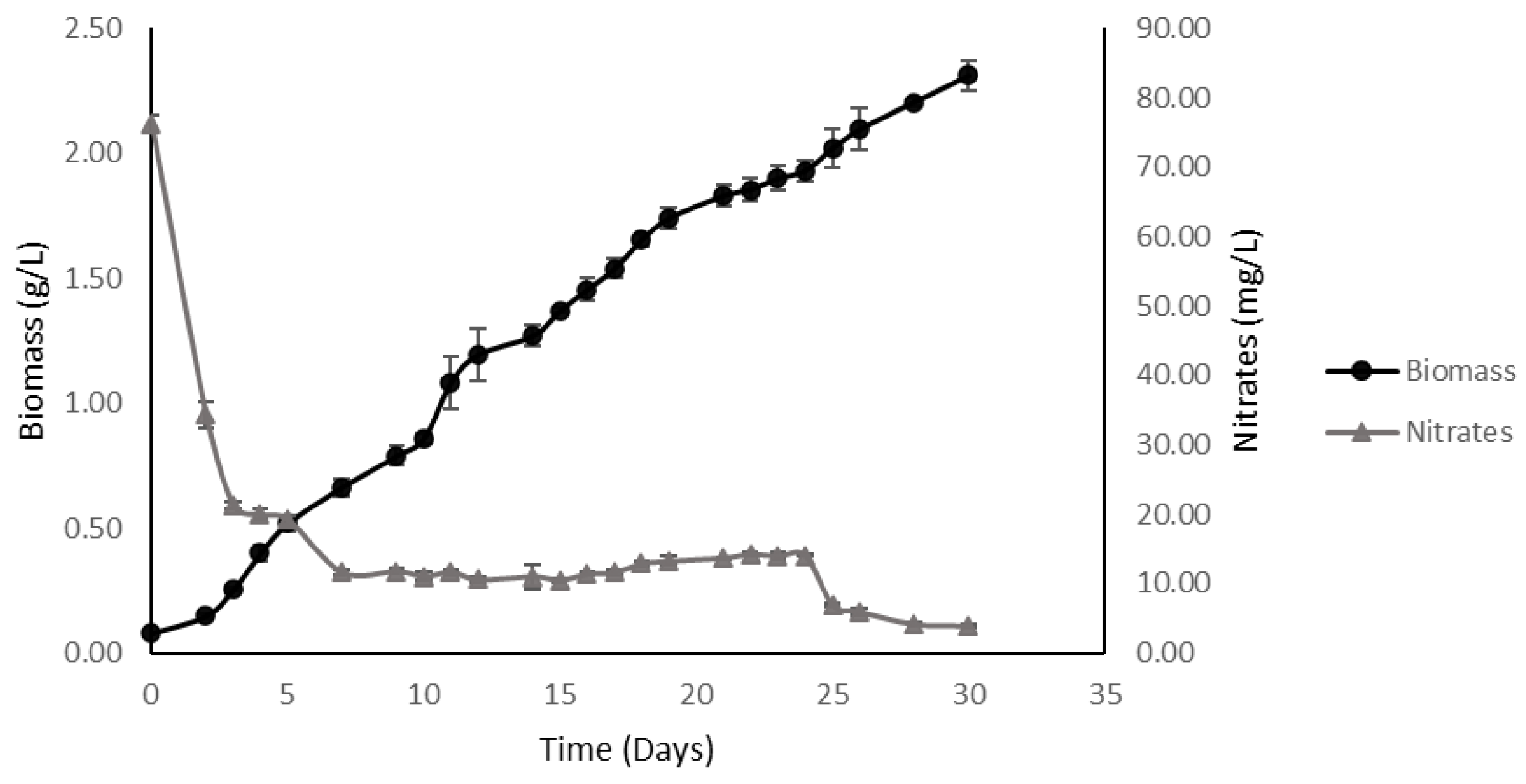
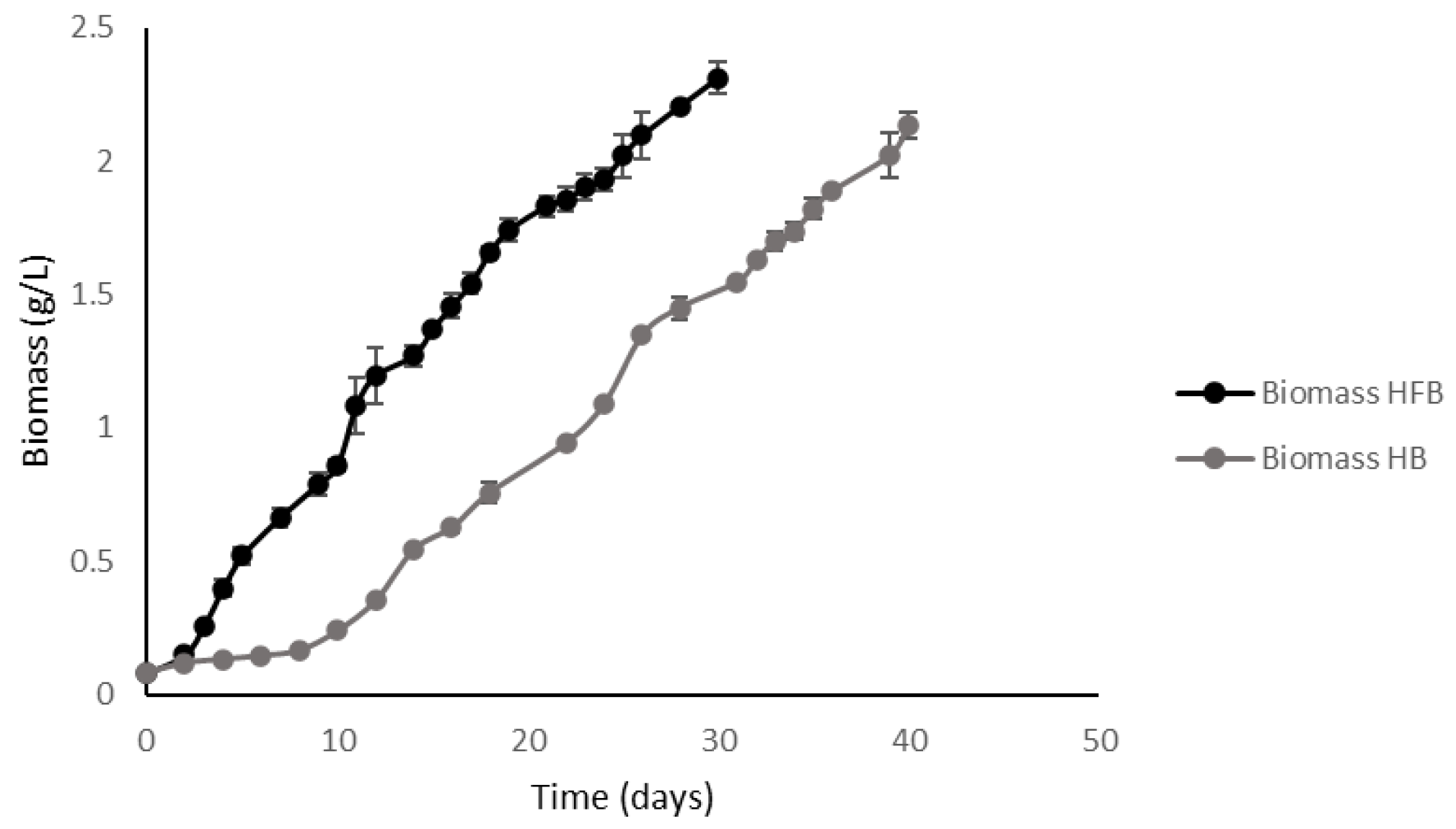
2.3. High-Density Batch vs. High-Density Fed-Batch
2.3.1. High-Density Batch
2.3.2. High-Density Fed-Batch
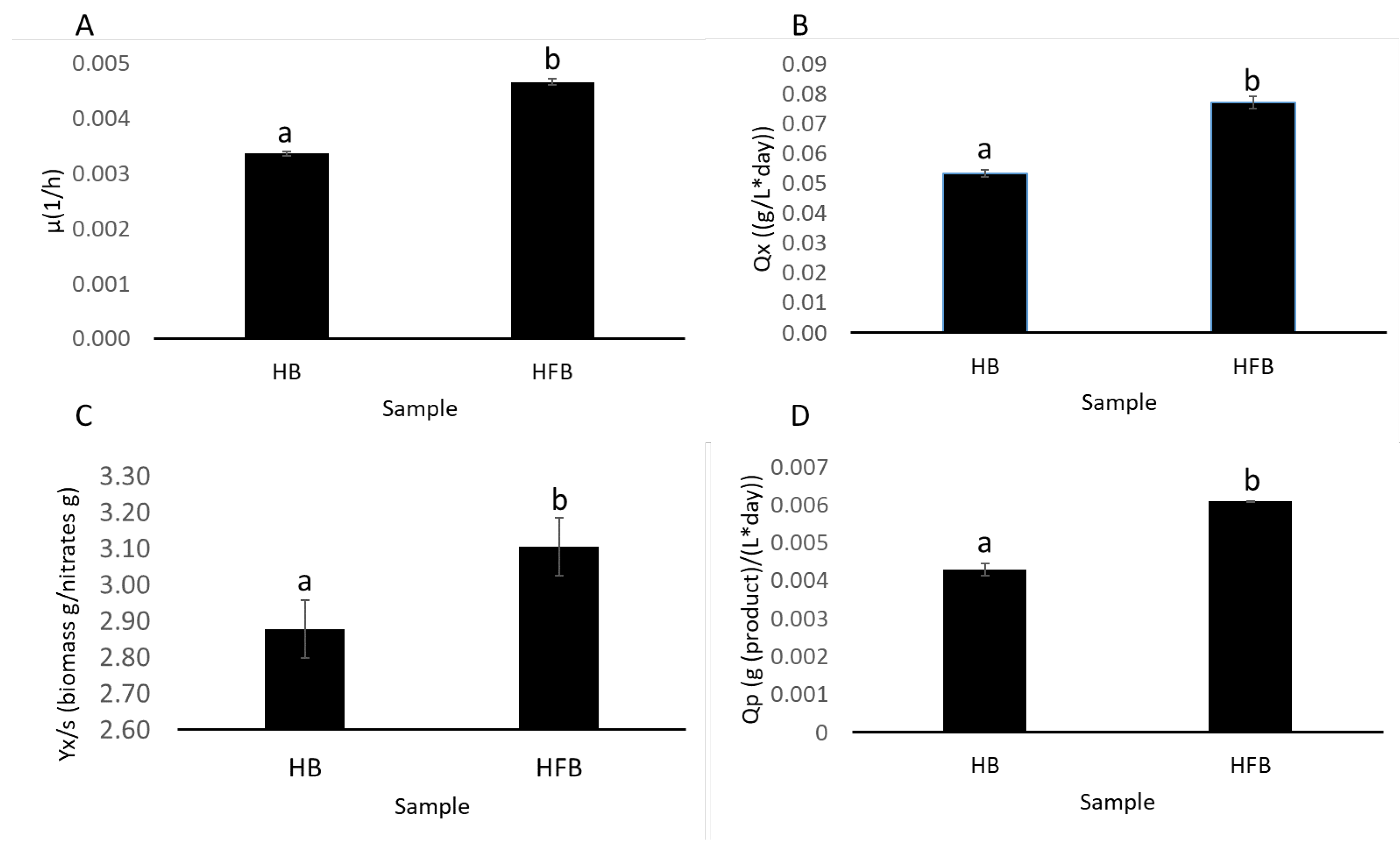
2.3.3. Comparative Analysis of Kinetic Parameters
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Microalgal Strain and Seed Culture
4.3. Experimental Methodology
4.4. Analytical Methods
4.4.1. Microalgal Growth and Biomass Measurements
4.4.2. β-Glucans Measurements
4.4.3. Lipid Measurements
4.4.4. Protein Measurements
4.4.5. Total Sugar Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moreira, J.B.; Vaz, B.d.S.; Cardias, B.B.; Cruz, C.G.; Almeida, A.C.A.d.; Costa, J.A.V.; Morais, M.G.d. Microalgae Polysaccharides: An Alternative Source for Food Production and Sustainable Agriculture. Polysaccharides 2022, 3, 441–457. [Google Scholar] [CrossRef]
- Stier, H.; Ebbeskotte, V.; Gruenwald, J. Immune-modulatory effects of dietary Yeast Beta-1,3/1,6-D-glucan. Nutr. J. 2014, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.; Koppula, S.; Lee, K. The effects of β-glucans on Cancer Metastasis. Anti-Cancer Agents Med. Chem. 2013, 13, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Qiu, W.; Wang, X.; Liu, J. Recent Advancements and Future Perspectives of Microalgae Derived Pharmaceuticals. Mar. Drugs 2021, 19, 703. [Google Scholar] [CrossRef] [PubMed]
- Reis, B.; Gonçalves, A.T.; Santos, P.; Sardinha, M.; Conceição, L.E.C.; Serradeiro, R.; Pérez-Sánchez, J.; Calduch-Giner, J.; Schmid-Staiger, U.; Frick, K.; et al. Immune Status and Hepatic Antioxidant Capacity of Gilthead Seabream Sparus aurata Juveniles Fed Yeast and Microalga Derived β-glucans. Mar. Drugs 2021, 19, 653. [Google Scholar] [CrossRef]
- Murphy, E.J.; Rezoagli, E.; Major, I.; Rowan, N.; Laffey, J.G. β-Glucans. Encyclopedia 2021, 1, 831–847. [Google Scholar] [CrossRef]
- Chan, G.C.; Chan, W.K.; Sze, D.M. The effects of beta-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2, 2–25. [Google Scholar] [CrossRef]
- Hadiuzzaman, M.; Moniruzzaman, M.; Shahjahan, M.; Bai, S.C.; Min, T.; Hossain, Z. β-Glucan: Mode of Action and Its Uses in Fish Immunomodulation. Front. Mar. Sci. 2022, 9, 905986. [Google Scholar] [CrossRef]
- Soltanian, S.; Stuyven, E.; Cox, E.; Sorgeloos, P.; Bossier, P. Beta-glucans as immunostimulant in vertebrates and invertebrates. Crit. Rev. Microbiol. 2009, 35, 109–138. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L.; Sima, P. The effects of β–glucan on fish immunity. N. Am. J. Med. Sci. 2013, 5, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Zanuzzo, F.; Koch, J.; de Oliveira, C.; Sima, P.; Vetvicka, V. Development of Fish Immunity and the Role of β-Glucan Immune Responses. Mol. 2020, 25, 5378. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, D.; Contreras, L.; Ehrenfeld, N. ß-glucans, production and properties in microalgae with emphasis on Nannochloropsis genus (Ochrophyta, Eustigmatales). Rev. Biol. Mar. Oceanogr. 2016, 52, 33–49. [Google Scholar] [CrossRef]
- Rodriguez, E.; Steffolani, M.; Martinez, M.; León, A. The use of Nannochloropsis sp. as a source of omega-3 fatty acids in dry pasta: Chemical, technological and sensory evaluation. Int. J. Food Sci. Technol. 2017, 53, 499–507. [Google Scholar] [CrossRef]
- Prüser, T.F.; Braun, P.G.; Wiacek, C. Microalgae as a novel food. Potential and legal framework. Ernahr. Umsch. 2021, 68, 78–85. [Google Scholar]
- Rojo-Cebreros, A.H.; Ibarra-Castro, L.; Martínez-Brown, J.M.; Velasco-Blanco, G.; Martínez-Téllez, M.A.; Medina-Jasso, M.A.; Nieves-Soto, M.; Quintana-Zavala, D. Potential of Nannochloropsis in beta glucan production. In Nannochloropsis: Biology, Biotechnological, Potential and Challenges; Jan, M., Kazik, P., Eds.; Nova Sciences Publishers Inc.: New York, NY, USA, 2017; pp. 181–225. [Google Scholar]
- Vieler, A.; Wu, G.; Tsai, C.H.; Bullard, B.; Cornish, A.J.; Harvey, C.; Reca, I.B.; Thornburg, C.; Achawanantakun, R.; Buehl, C.J.; et al. Genome, functional gene annotation, and nuclear transformation of the heterokont oleaginous alga Nannochloropsis oceanica CCMP1779. PLoS Genet. 2012, 8, e1003064. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ning, K.; Li, J.; Hu, J.; Han, D.; Wang, H.; Zeng, X.; Jing, X.; Zhou, Q.; Su, X.; et al. Nannochloropsis genomes reveal evolution of microalgal oleaginous traits. PLoS Genet. 2014, 10, e1004094. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.A.; Genard, B.; Zito, F.; Tremblay, R.; Warschawski, D.E.; Marcotte, I. Identification of lipid and saccharide constituents of whole microalgal cells by 13C solidstate NMR. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2015, 1848, 369–377. [Google Scholar] [CrossRef]
- Fleurence, J. Perspectives on the Use of Algae in Agriculture and Animal Production. Phycology 2021, 1, 79–82. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, J.; Cui, J.; Feng, Y.; Cui, Q. Metabolic profiles of Nannochloropsis oceanica IMET1 under nitrogen-deficiency stress. Bioresour. Technol. 2013, 130, 731–738. [Google Scholar] [CrossRef]
- Schädler, T.; Thurn, A.-L.; Brück, T.; Weuster-Botz, D. Continuous Production of Lipids with Microchloropsis salina in Open Thin-Layer Cascade Photobioreactors on a Pilot Scale. Energies 2021, 14, 500. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, F.; Sun, Z.; Zhang, Q.; Xue, S.; Cong, W. On-line modeling intracellular carbon and energy metabolism of Nannochloropsis sp. in nitrogen-repletion and nitrogen limitation cultures. Bioresour. Technol. 2014, 164, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Yanguez, K.; Carlos, L.; Contreras-Porcia, L.; Ehrenfeld, N. Response to oxidative stress induced by high light and carbon dioxide (CO2) in the biodiesel producer model Nannochloropsis salina (Ochrophyta, Eustigmatales). Rev. Biol. Mar. Oceanogr. 2015, 50, 163–175. [Google Scholar] [CrossRef]
- Kryvenda, A.; Tischner, R.; Steudel, B.; Griehl, C.; Armon, R.; Friedl, T. Testing for terrestrial microalgae productivity under elevated CO2 conditions and nutrient limitation. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Kao, C.Y.; Tsai, M.T.; Ong, S.C.; Chen, C.H.; Lin, C.S. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour. Technol. 2009, 100, 833–838. [Google Scholar] [CrossRef]
- Fu, L.; Li, Q.; Yan, G.; Zhou, D. Hormesis effects of phosphorus on the viability of Chlorella regularis cells under nitrogen limitation. Biotechnol. Biofuels 2019, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Cheirsilp, B.; Torpee, S. Enhanced growth and lipid production of microalgae under mixotrophic culture condition: Effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour. Technol. 2012, 110, 510–516. [Google Scholar] [CrossRef]
- Araujo, G.; Silva, J.; Viana, C.; Fernandes, F. Effect of sodium nitrate concentration on biomass and oil production of four microalgae species. Int. J. Sustain. Energy 2019, 39, 41–50. [Google Scholar] [CrossRef]
- Nouri, H.; Mohammadi Roushandeh, J.; Hallajisani, A.; Golzary, A.; Daliry, S. The effects of glucose, nitrate, and pH on cultivation of Chlorella sp. Microalgae. Glob. J. Environ. Sci. Manag. 2021, 7, 103–116. [Google Scholar]
- Wan, C.; Bai, F.W.; Zhao, X.Q. Effects of nitrogen concentration and media replacement on cell growth and lipid production of oleaginous marine microalga Nannochloropsis oceanica DUT01. Biochem. Eng. J. 2013, 78, 32–38. [Google Scholar] [CrossRef]
- Dean, A.P.; Nicholson, J.M.; Sigee, D.C. Impact of phosphorus quota and growth phase on carbon allocation in Chlamydomonas reinhardtii: An FTIR microspectroscopy study. Eur. J. Phycol. 2008, 43, 345–354. [Google Scholar] [CrossRef]
- Traller, J.C.; Hildebrand, M. High throughput imaging to the diatom Cyclotella cryptica demonstrates substantial cell-to-cell variability in the rate and extent of triacylglycerol accumulation. Algal Res. 2013, 2, 244–252. [Google Scholar] [CrossRef]
- Cecchin, M.; Berteotti, S.; Paltrinieri, S.; Vigliante, I.; Iadarola, B.; Giovannone, B.; Maffei, M.E.; Delledonne, M.; Ballottari, M. Improved lipid productivity in Nannochloropsis gaditana in nitrogen-replete conditions by selection of pale green mutants. Biotechnol. Biofuels 2020, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.-Y.; Jiang, J.-P.; Wang, H.-T.; Cao, X.-P.; Xue, S.; Yang, Q.; Wang, W.-L. The characteristics of TAG and EPA accumulation in Nannochloropsis oceanica IMET1 under different nitrogen supply regimes. Bioresour. Technol. 2015, 179, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Hanc, D.; Gerkenc, H.; Lid, Y.; Sommerfeld, M.; Hue, Q.; Xu, J. Molecular mechanisms for photosynthetic carbon partitioning into storage neutral lipids in Nannochloropsis oceanica under nitrogen-depletion conditions. Algal Res. 2015, 7, 66–77. [Google Scholar] [CrossRef]
- Kawano, T.; Naito, J.; Nishioka, M.; Nishida, N.; Takahashi, M.; Kashiwagi, S.; Sugino, T.; Watanabe, Y. Effect of Food Containing Paramylon Derived from Euglena gracilis EOD-1 on Fatigue in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Trial. Nutrients 2020, 12, 3098. [Google Scholar] [CrossRef]
- Granum, E.; Kirkvold, S.; Myklestad, S. Cellular and extracellular production of carbohydrates and amino acids by the marine diatom Skeletonema costatum: Diel variations and effects of nitrogen depletion. Mar. Ecol. Prog. Ser. 2002, 242, 83–94. [Google Scholar] [CrossRef]
- Rojo, A.; Ibarra, L.; Martínez, M.; Medina, M.; Nieves, M.; Quintana, D. Potential of Nannochloropsis in Beta glucan production Nannochloropsis. In Nannochloropsis: Biology, Biotechnological, Potential and Challenges; Jan, M., Kazik, P., Eds.; Nova Sciences Publishers Inc.: New York, NY, USA, 2017; pp. 181–225. [Google Scholar]
- Wang, T.; Chu, S.; Tsai, Y.; Lin, F.; Lee, W. Influence of inoculum cell density and carbón dioxide concentration on fed-batch cultivation of Nannochloropsis oculata. Biomass Bioenergy 2015, 77, 9–15. [Google Scholar] [CrossRef]
- Fawley, M.W.; Jameson, I.; Fawley, K.P. The phylogeny of the genus Nannochloropsis (Monodopsidaceae, Eustigmatophyceae), with descriptions of N. australis sp. nov. and Microchloropsis gen. nov. Phycologia 2015, 54, 545–552. [Google Scholar] [CrossRef]
- Acevedo, F.; Gentina, J.; Illanes, A. Cinética de fermentaciones. In Fundamentos de Ingeniería Bioquímica; Ediciones Universitarias de Valparaiso: Valparaiso, Chile, 2002; pp. 162–163. [Google Scholar]
- Liu, J.; Sun, Z.; Gerken, H.; Liu, Z.; Jiang, Y.; Chen, F. Chlorella zofingiensis as an alternative microalgal producer of astaxanthin: Biology and industrial potential. Mar. Drugs 2014, 12, 3487–3515. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 26–60. [Google Scholar]
- Carvalho, A.P.; Meireles, L.A.; Malcata, F.X. Rapid spectrophotometric determination of nitrates and nitrites in marine aqueous culture media. Analusis 1998, 26, 347–351. [Google Scholar] [CrossRef]
- Cook, J.R. Quantitative measurement of paramylum in Euglena gracilis. J. Protozool. 1967, 14, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Slocombe, S.P.; Ross, M.; Thomas, N.; McNeill, S.; Stanley, M.S. A rapid and general method for measurement of protein in micro-algal biomass. Bioresour. Technol. 2013, 129, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Richmond, A.; Hu, Q. Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Magnitude |
|---|---|
| Yx/s | 6.2000 |
| si [g/L] | 0.7040 |
| s [g/L] | 0.0400 |
| b | 0.2429 |
| μ [h−1] | 0.0052 |
| Vo [L] | 0.5000 |
| V [L] | 1.5000 |
| Xo [g/L] | 0.3200 |
| t [h] | 631.8820 |
| Compound | High-Density Batch (% Dry Weight) | High-Density Fed-Batch (% Dry Weight) |
|---|---|---|
| Lipids | 43.72 ± 1.33 | 40.20 ± 2.20 |
| Carbohydrates | 17.23 ± 0.15 | 16.23 ± 1.78 |
| Proteins | 10.23 ± 0.68 | 12.53 ± 0.92 |
| β-glucans | 8.04 ± 0.14 | 7.71 ± 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocaranza, D.; Balic, I.; Bruna, T.; Moreno, I.; Díaz, O.; Moreno, A.A.; Caro, N. A Modeled High-Density Fed-Batch Culture Improves Biomass Growth and β-Glucans Accumulation in Microchloropsis salina. Plants 2022, 11, 3229. https://doi.org/10.3390/plants11233229
Ocaranza D, Balic I, Bruna T, Moreno I, Díaz O, Moreno AA, Caro N. A Modeled High-Density Fed-Batch Culture Improves Biomass Growth and β-Glucans Accumulation in Microchloropsis salina. Plants. 2022; 11(23):3229. https://doi.org/10.3390/plants11233229
Chicago/Turabian StyleOcaranza, Darío, Iván Balic, Tamara Bruna, Ignacio Moreno, Oscar Díaz, Adrián A. Moreno, and Nelson Caro. 2022. "A Modeled High-Density Fed-Batch Culture Improves Biomass Growth and β-Glucans Accumulation in Microchloropsis salina" Plants 11, no. 23: 3229. https://doi.org/10.3390/plants11233229
APA StyleOcaranza, D., Balic, I., Bruna, T., Moreno, I., Díaz, O., Moreno, A. A., & Caro, N. (2022). A Modeled High-Density Fed-Batch Culture Improves Biomass Growth and β-Glucans Accumulation in Microchloropsis salina. Plants, 11(23), 3229. https://doi.org/10.3390/plants11233229






