Iron Status Affects the Zinc Accumulation in the Biomass Plant Szarvasi-1
Abstract
1. Introduction
2. Results
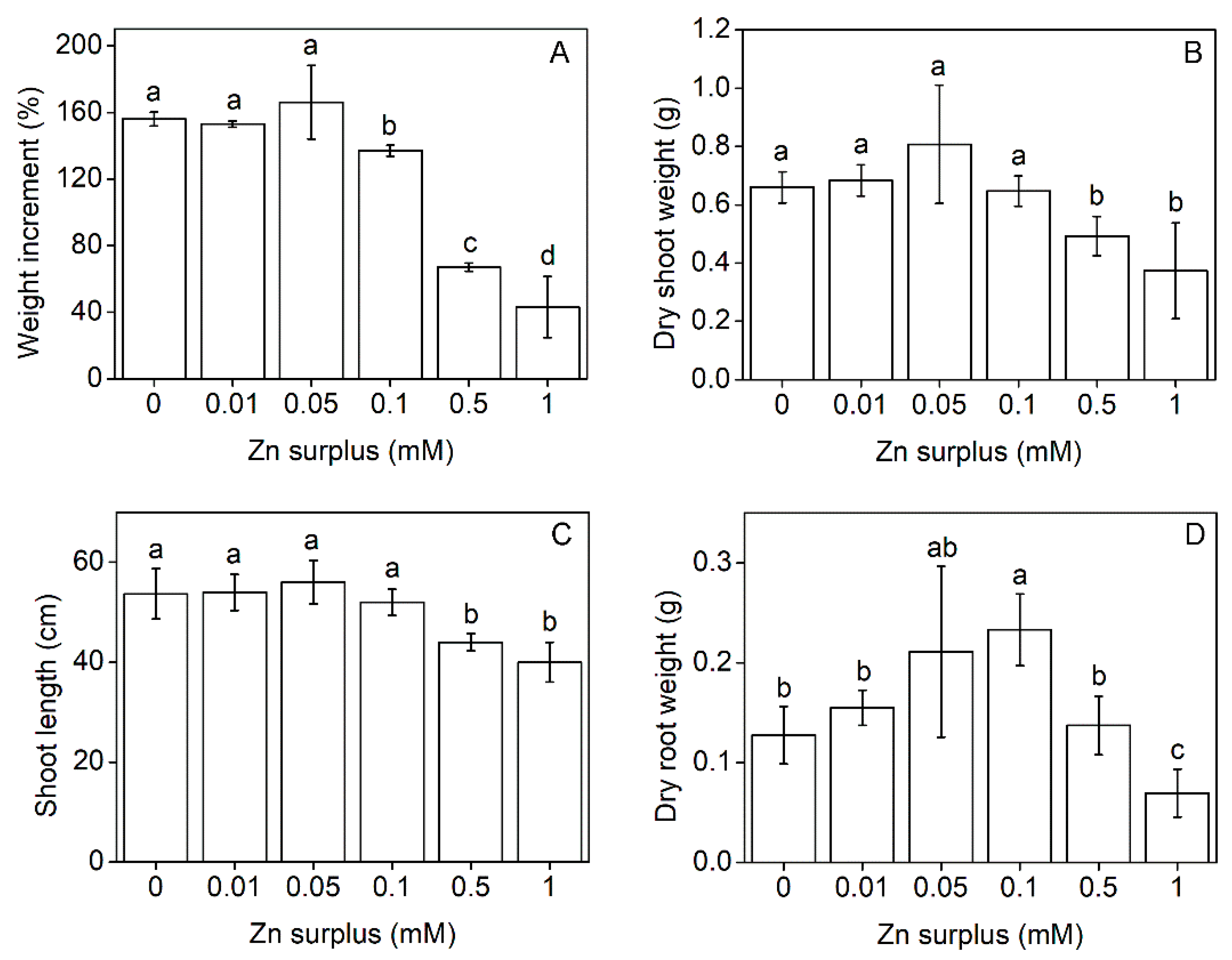
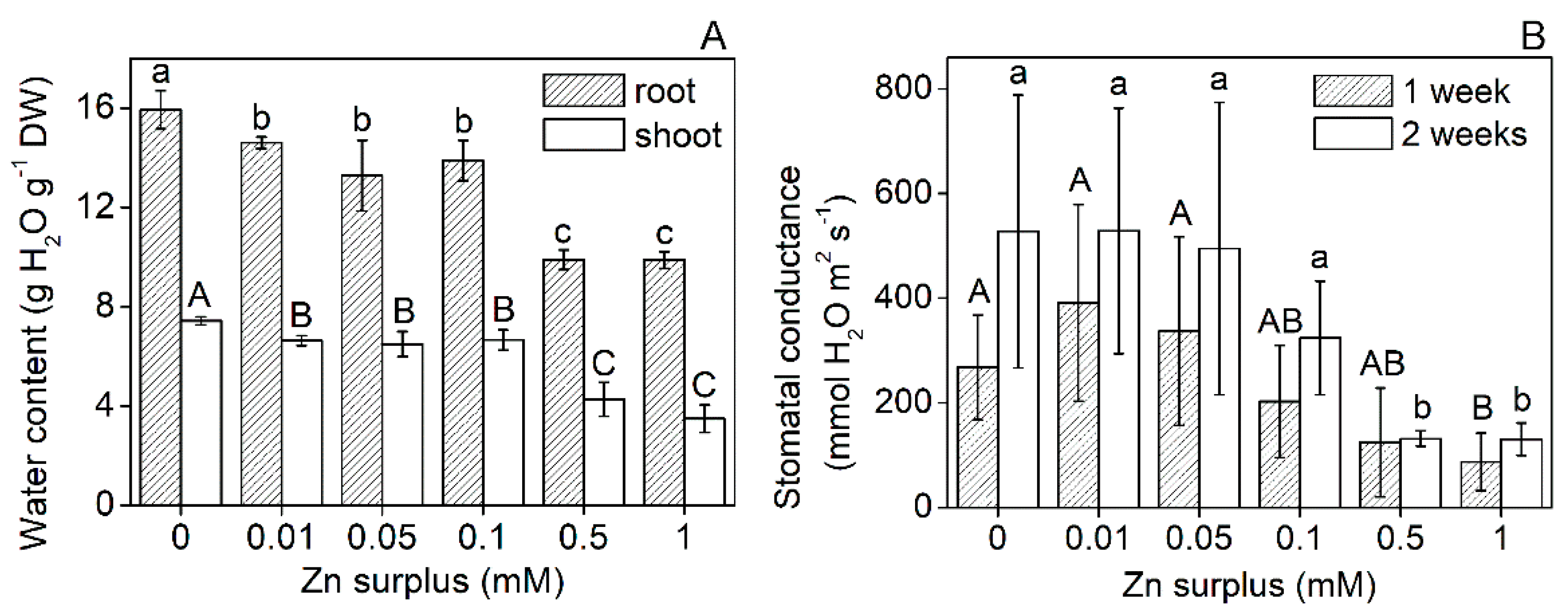
2.1. Plant Growth Parameters
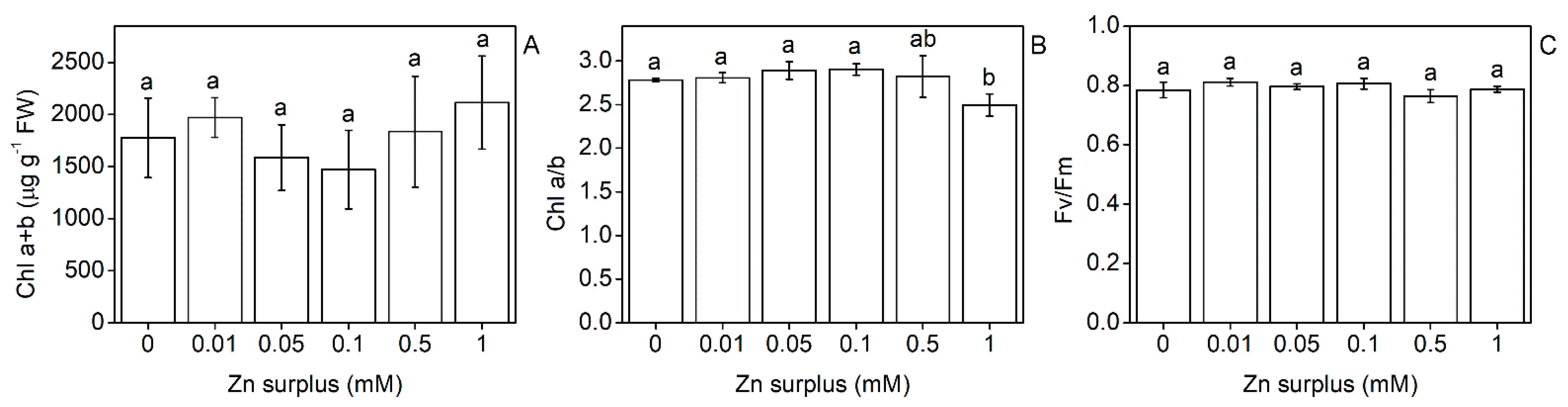
2.2. Physiological Performance of Leaves
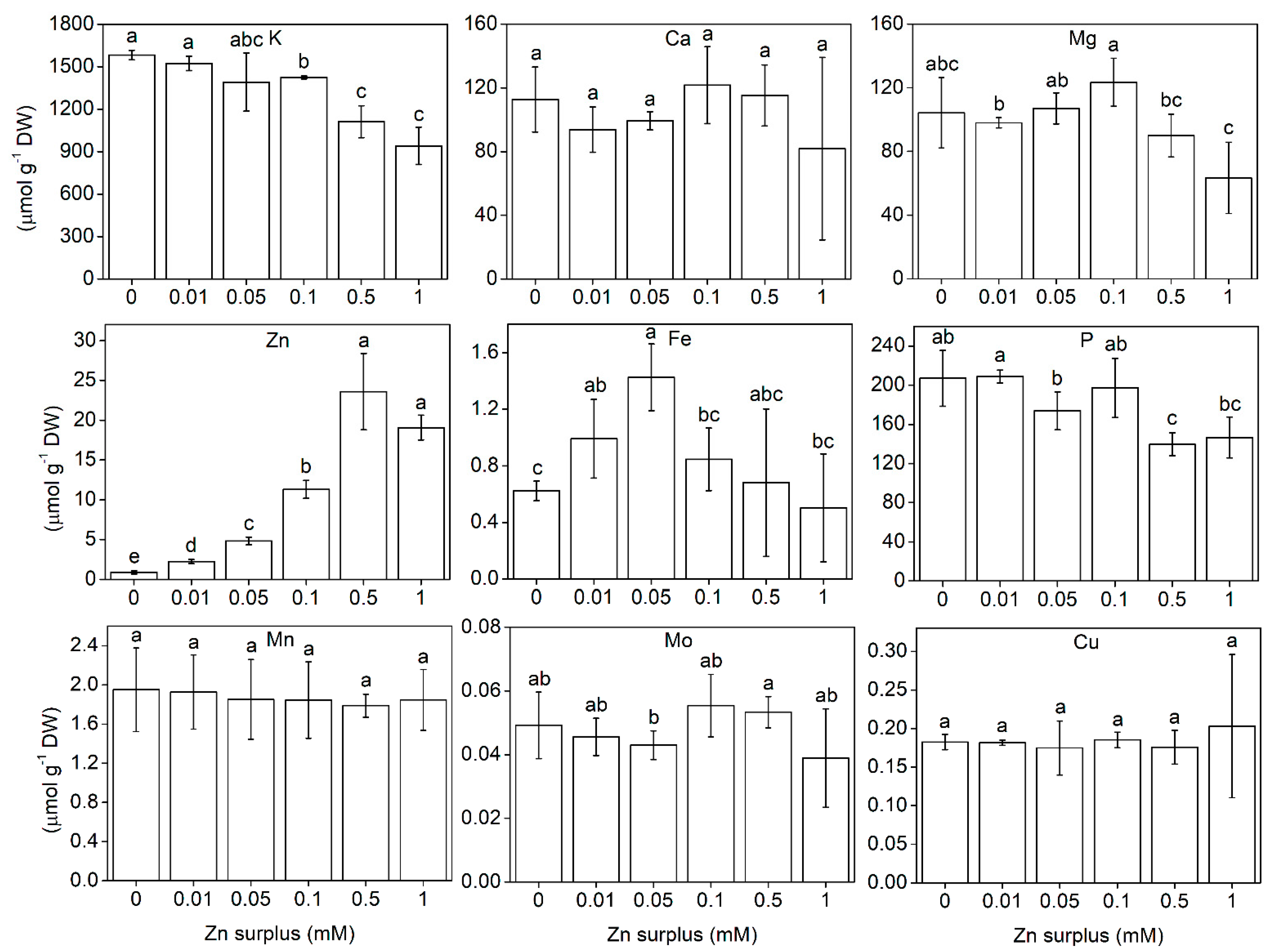
2.3. Element Composition of the Shoots
2.4. Effect of Iron Supply on Zinc Accumulation
2.5. Effect of EDTA on Zinc and Iron Accumulation and Interaction
3. Discussion
3.1. Growth and Physiological Responses of Szarvasi-1 to Surplus Zinc
3.2. Zn Accumulation
3.3. Ionomic Interactions at Zn Exposure
3.4. Zinc and Iron Interaction
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Mass Measurements
4.3. Chlorophyll Concentration
4.4. Stomatal Conductance
4.5. Malondialdehyde Concentration
4.6. Chlorophyll a Fluorescence Induction
4.7. Element Concentration Analysis
4.8. Zn Distribution
4.9. Ferric Chelate Reductase Assay
4.10. Statistical Treatment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomes, H.I. Phytoremediation for bioenergy: Challenges and opportunities. Environ. Technol. Rev. 2012, 1, 59–66. [Google Scholar] [CrossRef]
- Noulas, C.; Tziouvalekas, M.; Karyotis, T. Zinc in soils, water and food crops. J. Trace Elem. Med. Biol. 2018, 49, 252–260. [Google Scholar] [CrossRef]
- Zhu, G.; Xiao, H.; Guo, Q.; Song, B.; Zheng, G.; Zhang, Z.; Zhao, J.; Okoli, C.P. Heavy metal contents and enrichment characteristics of dominant plants in wasteland of the downstream of a lead-zinc mining area in Guangxi, Southwest China. Ecotoxicol. Environ. Saf. 2018, 151, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Li, Q.; Yang, Y.; Yuan, C.Y.; Ouyang, K.; You, P. Risk Assessment of Heavy Metals in Soils of a Lead-Zinc Mining Area in Hunan Province (China). Kem. Ind. 2017, 66, 173–178. [Google Scholar] [CrossRef]
- Gyuricza, V.; Fodor, F.; Szigeti, Z. Phytotoxic effects of heavy metal contaminated soil reveal limitations of extract-based ecotoxicological tests. Water Air Soil Pollut. 2010, 210, 113–122. [Google Scholar] [CrossRef]
- Zeng, H.; Wu, H.; Yan, F.; Yi, K.; Zhu, Y. Molecular regulation of zinc deficiency responses in plant. J. Plant Physiol. 2021, 261, 153419. [Google Scholar] [CrossRef] [PubMed]
- Caldelas, C.; Weiss, D.J. Zinc Homeostasis and isotopic fractionation in plants: A review. Plant Soil 2017, 411, 17–46. [Google Scholar] [CrossRef]
- Chaney, R.L. Zinc Phytotoxicity. In Zinc in Soils and Plants; Robson, A.D., Ed.; Springer Science and Business Media: Dordrecht, The Netherlands, 1993; pp. 135–150. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: London, UK, 2012. [Google Scholar]
- Bonnet, M.; Camares, O.; Veisseire, P. Effects of zinc and influence of Acremonium lolii on growth parameters, chlorophyll a fluorescence and antioxidant enzyme activities of ryegrass (Lolium perenne L. cv Apollo). J. Exp. Bot. 2000, 51, 945–953. [Google Scholar] [CrossRef]
- Masarovič, D.; Slováková, Ľ.; Bokor, B.; Bujdoš, M.; Lux, A. Effect of silicon application on Sorghum bicolor exposed to toxic concentration of zinc. Biologia 2012, 67, 706–712. [Google Scholar] [CrossRef]
- Baker, A.; Brooks, R. Terrestrial Higher Plants Which Hyperaccumulate Metallic Elements. A Review of Their Distribution, Ecology and Phytocehmistry. Biorecovery 1989, 1, 81–126. [Google Scholar]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Krämer, U. Metal hyperaccumulation in plants. Annu. Rev. Plant Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R.D.; Baker, A.J. Metal-accumulating plants. In Phytoremediation of Toxic Metals: Using Plants to Clean Up the Environment; Raskin, I., Ensley, B.D., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2000; pp. 193–229. [Google Scholar]
- Ebbs, S.D.; Kochian, L.V. Phytoextraction of Zinc by Oat (Avena sativa), Barley (Hordeum vulgare), and Indian mustard (Brassica juncea). Environ. Sci. Technol. 1998, 32, 802–806. [Google Scholar] [CrossRef]
- Xu, W.H.; Xiong, Z.T.; Huang, T. Effects of Zn stress on Zn accumulation and anti-oxidant enzymes activity in four varieties of ryegrass. Wuhan Univ. J. Nat. Sci. 2005, 10, 1051–1056. [Google Scholar] [CrossRef]
- Mateos-Naranjo, E.; Castellanos, E.M.; Perez-Martin, A. Zinc tolerance and accumulation in the halophytic species Juncus acutus. Environ. Exp. Bot. 2014, 100, 114–121. [Google Scholar] [CrossRef]
- Van de Mortel, J.E.; Villanueva, L.A.; Schat, H.; Kwekkeboom, J.; Coughlan, S.; Moerland, P.D.; Aarts, M.G. Large Expression Differences in Genes for Iron and Zinc Homeostasis, Stress Response, and Lignin Biosynthesis Distinguish Roots of Arabidopsis thaliana and the Related Metal Hyperaccumulator Thlaspi caerulescens. Plant Physiol. 2006, 142, 1127–1147. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Bashir, K.; Nishizawa, N.K. Zn uptake and translocation in rice plants. Rice 2011, 4, 21–27. [Google Scholar] [CrossRef]
- Bashir, K.; Ishimaru, Y.; Nishizawa, N.K. Molecular mechanisms of zinc uptake and translocation in rice. Plant Soil. 2014, 361, 189–201. [Google Scholar] [CrossRef]
- Tiong, J.; McDonald, G.K.; Genc, Y.; Pedas, P.; Hayes, J.E.; Toubia, J.; Langridge, P.; Huang, C.Y. HvZIP7 mediates zinc accumulation in barley (Hordeum vulgare) at moderately high zinc supply. New Phytol. 2014, 201, 131–143. [Google Scholar] [CrossRef]
- Tiong, J.; McDonald, G.; Genc, Y.; Shirley, N.; Langridge, P.; Huang, C.Y. Increased expression of six ZIP family genes by zinc (Zn) deficiency is associated with enhanced uptake and root-to-shoot translocation of Zn in barley (Hordeum vulgare). New Phytol. 2015, 207, 1097–1109. [Google Scholar] [CrossRef]
- Von Wirén, N.; Marschner, H.; Römheld, V. Roots of Iron-Efficient Maize also Absorb Phytosiderophore-Chelated Zinc. Plant Physiol. 1996, 111, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Neelam, K.; Rawat, N.; Tiwari, V.; Prasad, R.; Tripathi, S.; Randhawa, G.; Dhaliwal, H. Evaluation and identification of wheat-Aegilops addition lines controlling high grain iron and zinc concentration and mugineic acid production. Cereal Res. Commun. 2012, 40, 53–61. [Google Scholar] [CrossRef]
- Suzuki, M.; Takahashi, M.; Tsukamoto, T.; Watanabe, S.; Matsuhashi, S.; Yazaki, J.; Kishimoto, N.; Kikuchi, S.; Nakanishi, H.; Mori, S.; et al. Biosynthesis and secretion of mugineic acid family phytosiderophores in zinc-deficient barley. Plant J. 2006, 48, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Haydon, M.J.; Kawachi, M.; Wirtz, M.; Hillmer, S.; Hell, R.; Krämer, U. Vacuolar Nicotianamine Has Critical and Distinct Roles under Iron Deficiency and for Zinc Sequestration in Arabidopsis. Plant Cell 2012, 24, 724–737. [Google Scholar] [CrossRef]
- Hussain, D.; Haydon, M.J.; Wang, Y.; Wong, E.; Sherson, S.M.; Young, J.; Cobbett, C.S. P-Type ATPase Heavy Metal Transporters with Roles in Essential Zinc Homeostasis in Arabidopsis. Plant Cell 2004, 16, 1327–1339. [Google Scholar] [CrossRef]
- Kawachi, M.; Kobae, Y.; Mimura, T.; Maeshima, M. Deletion of a histidine-rich loop of AtMTP1, a vacuolar Zn2+/H+ antiporter of Arabidopsis thaliana, stimulates the transport activity. J. Biol. Chem. 2008, 283, 8374–8383. [Google Scholar] [CrossRef]
- Csete, S.; Stranczinger, S.; Szalontai, B.; Farkas, A.; Pál, R.W.; Salamon-Albert, E.; Kocsis, M.; Tovari, P.; Vojtela, T.; Dezso, J.; et al. Tall Wheatgrass Cultivar Szarvasi-1 (Elymus elongatus subsp. ponticus cv. Szarvasi-1) as a Potential Energy Crop for Semi-Arid Lands of Eastern Europe. In Sustainable Growth and Applications in Renewable Energy Sources; Nayeripour, M., Ed.; InTech Open: London, UK, 2011; pp. 269–294. [Google Scholar]
- Salamon-Albert, É.; Molnár, H. Environment regulated ecophysiological responses of a tall wheatgrass cultivar. Növénytermelés 2010, 59, 393–396. [Google Scholar]
- Vashegyi, I.; Cseh, E.; Lévai, L.; Fodor, F. Chelator-enhanced lead accumulation in Agropyron elongatum cv. Szarvasi-1 in hydroponic culture. Int. J. Phytoremediat. 2011, 13, 302–315. [Google Scholar] [CrossRef]
- Sipos, G.; Solti, A.; Czech, V.; Vashegyi, I.; Toth, B.; Cseh, E.; Fodor, F. Heavy metal accumulation and tolerance of energy grass (Elymus elongatus subsp. ponticus cv. Szarvasi-1) grown in hydroponic culture. Plant Physiol. Biochem. 2013, 68, 96–103. [Google Scholar] [CrossRef]
- Brune, A.; Urbach, W.; Dietz, K.-J. Compartmentation and transport of zinc in barley primary leaves as basic mechanisms involved in zinc tolerance. Plant Cell Environ. 1994, 17, 153–162. [Google Scholar] [CrossRef]
- Tolrà, R.P.; Poschenrieder, C.; Barceló, J. Zinc hyperaccumulation in Thlaspi caerulescens. I. Influence on growth and mineral nutrition. J. Plant Nutr. 1996, 19, 1531–1540. [Google Scholar] [CrossRef]
- Sagardoy, R.; Vázquez, S.; Florez-Sarasa, I.D.; Albacete, A.; Ribas-Carbó, M.; Flexas, J.; Morales, F. Stomatal and mesophyll conductances to CO2 are the main limitations to photosynthesis in sugar beet (Beta vulgaris) plants grown with excess zinc. New Phytol. 2010, 187, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Nanda, R.; Agrawal, V. Elucidation of zinc and copper induced oxidative stress, DNA damage and activation of defence system during seed germination in Cassia angustifolia Vahl. Environ. Exp. Bot. 2016, 125, 31–41. [Google Scholar] [CrossRef]
- Hamed, S.M.; Zinta, G.; Klöck, G.; Asard, H.; Selim, S.; AbdElgawad, H. Zinc-induced differential oxidative stress and antioxidant responses in Chlorella sorokiniana and Scenedesmus acuminatus. Ecotoxicol. Environ. Saf. 2017, 140, 256–263. [Google Scholar] [CrossRef]
- Kim, C.S.; Jung, J. The susceptibility of mung bean chloroplasts to photoinhibition is increased by an excess supply of iron to plants: A photobiological aspect of iron toxicity in plant. Photochem. Photobiol. 1993, 58, 120–126. [Google Scholar] [CrossRef]
- Sagardoy, R.; Morales, F.; López-Millán, A.F.; Abadía, A.; Abadía, J. Effects of zinc toxicity on sugar beet (Beta vulgaris L.) plants grown in hydroponics. Plant Biol. 2009, 11, 339–350. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Jia, L.; Chen, H.; Wei, X. Zinc-induced oxidative damage, antioxidant enzyme response and proline metabolism in roots and leaves of wheat plants. Ecotoxicol. Environ. Saf. 2013, 89, 150–157. [Google Scholar] [CrossRef]
- Salt, D.E.; Blaylock, M.; Kumar, N.P.B.A.; Dushenkov, V.; Ensley, B.D.; Chet, I.; Raskin, I. Phytoremediation: A Novel Strategy for the Removal of Toxic Metals from the Environment Using Plants. Bio/Technology 1995, 13, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Safaya, N.M. Phosphorus-zinc Interaction in Relation to Absorption Rates of Phosphorus, Zinc, Copper, Manganese, and Iron in Corn. Soil Sci. Soc. Am. J. 1976, 40, 719–722. [Google Scholar] [CrossRef]
- Webb, M.J.; Loneragan, J.F. Zinc translocation to wheat roots and its implications for a phosphorus/zinc interaction in wheat plants. J. Plant Nutr. 1990, 13, 1499–1512. [Google Scholar] [CrossRef]
- Thiébaut, N.; Hanikenne, M. Zinc deficiency responses: Bridging the gap between Arabidopsis and dicotyledonous crops. J Exp. Bot. 2022, 73, 1699–1716. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Yousaf, S.; Sarwar, N.; Hussain, S. Zinc biofortification of cereals—Role of phosphorus and other impediments in alkaline calcareous soils. Environ. Geochem. Health 2019, 41, 2365–2379. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Ram, H.; Kumar, B. Mechanism of Zinc absorption in plants: Uptake, transport, translocation and accumulation. Rev. Environ. Sci. Biotechnol. 2016, 15, 89–109. [Google Scholar] [CrossRef]
- Monnet, F.; Vaillant, N.; Vernay, P.; Coudret, A.; Sallanon, H.; Hitmi, A. Relationship between PSII activity, CO2 fixation, and Zn, Mn and Mg contents of Lolium perenne under zinc stress. J. Plant Physiol. 2001, 158, 1137–1144. [Google Scholar] [CrossRef]
- Nakanishi, H.; Yamaguchi, H.; Sasakuma, T.; Nishizawa, N.K.; Mori, S. Two dioxygenase genes, Ids3 and Ids2, from Hordeum vulgare are involved in the biosynthesis of mugineic acid family phytosiderophores. Plant Mol. Biol. 2000, 44, 199–207. [Google Scholar] [CrossRef]
- Beasley, J.T.; Bonneau, J.P.; Johnson, A.A. Characterisation of the nicotianamine aminotransferase and deoxymugineic acid synthase genes essential to Strategy II iron uptake in bread wheat (Triticum aestivum L.). PLoS ONE 2017, 12, e0177061. [Google Scholar] [CrossRef]
- Briat, J.F.; Rouached, H.; Tissot, N.; Gaymard, F.; Dubos, C. Integration of P, S, Fe, and Zn nutrition signals in Arabidopsis thaliana: Potential involvement of PHOSPHATE STARVATION RESPONSE 1 (PHR1). Front. Plant Sci. 2015, 6, 290. [Google Scholar] [CrossRef]
- López-Bucio, J.S.; Salmerón-Barrera, G.J.; Ravelo-Ortega, G.; Raya-González, J.; León, P.; de la Cruz, H.R.; Guevara-García, Á. Mitogen-activated protein kinase 6 integrates phosphate and iron responses for indeterminate root growth in Arabidopsis thaliana. Planta 2019, 250, 1177–1189. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nagasaka, S.; Senoura, T.; Itai, R.N.; Nakanishi, H.; Nishizawa, N.K. Iron-binding haemerythrin RING ubiquitin ligases regulate plant iron responses and accumulation. Nat. Commun. 2013, 4, 2792. [Google Scholar] [CrossRef]
- Korshunova, Y.O.; Eide, D.; Clark, W.G.; Guerinot, M.L.; Pakrasi, H.B. The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol. Biol. 1999, 40, 37–44. [Google Scholar] [CrossRef]
- Li, S.; Zhou, X.; Chen, J.; Chen, R. Is there a strategy I iron uptake mechanism in maize? Plant Signal. Behav. 2018, 13, e1161877. [Google Scholar] [CrossRef] [PubMed]
- Vatansever, R.; Filiz, E.; Ozyigit, I.I. Genome-wide analysis of iron-regulated transporter 1 (IRT1) genes in plants. Hortic. Environ. Biotechnol. 2015, 56, 516–523. [Google Scholar] [CrossRef]
- Kaznina, N.M.; Titov, A.F.; Repkina, N.S.; Batova, Y.V. Effect of Zinc Excess and Low Temperature on the IRT1 Gene Expression in the Roots and Leaves of Barley. Dokl. Biochem. Biophys. 2019, 487, 264–268. [Google Scholar] [CrossRef]
- Connolly, E.L.; Fett, J.P.; Guerinot, M.L. Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell 2002, 14, 1347–1357. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. BBA Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar] [CrossRef]
- Dhindsa, R.H.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Barócsi, A.; Lenk, S.; Kocsányi, L.; Buschmann, C. Excitation kinetics during induction of chlorophyll a fluorescence. Photosynthetica 2009, 47, 104–111. [Google Scholar] [CrossRef]
- Szalóki, I.; Gerényi, A.; Radócz, G. Confocal macro X-ray fluorescence spectrometer on commercial 3D printer. X-Ray Spectrom. 2017, 46, 497–506. [Google Scholar] [CrossRef]
- Kovács, K.; Kuzmann, E.; Tatár, E.; Vértes, A.; Fodor, F. Investigation of iron pools in cucumber roots by Mössbauer spectroscopy: Direct evidence for the Strategy I iron uptake mechanism. Planta 2009, 229, 271–278. [Google Scholar] [CrossRef] [PubMed]


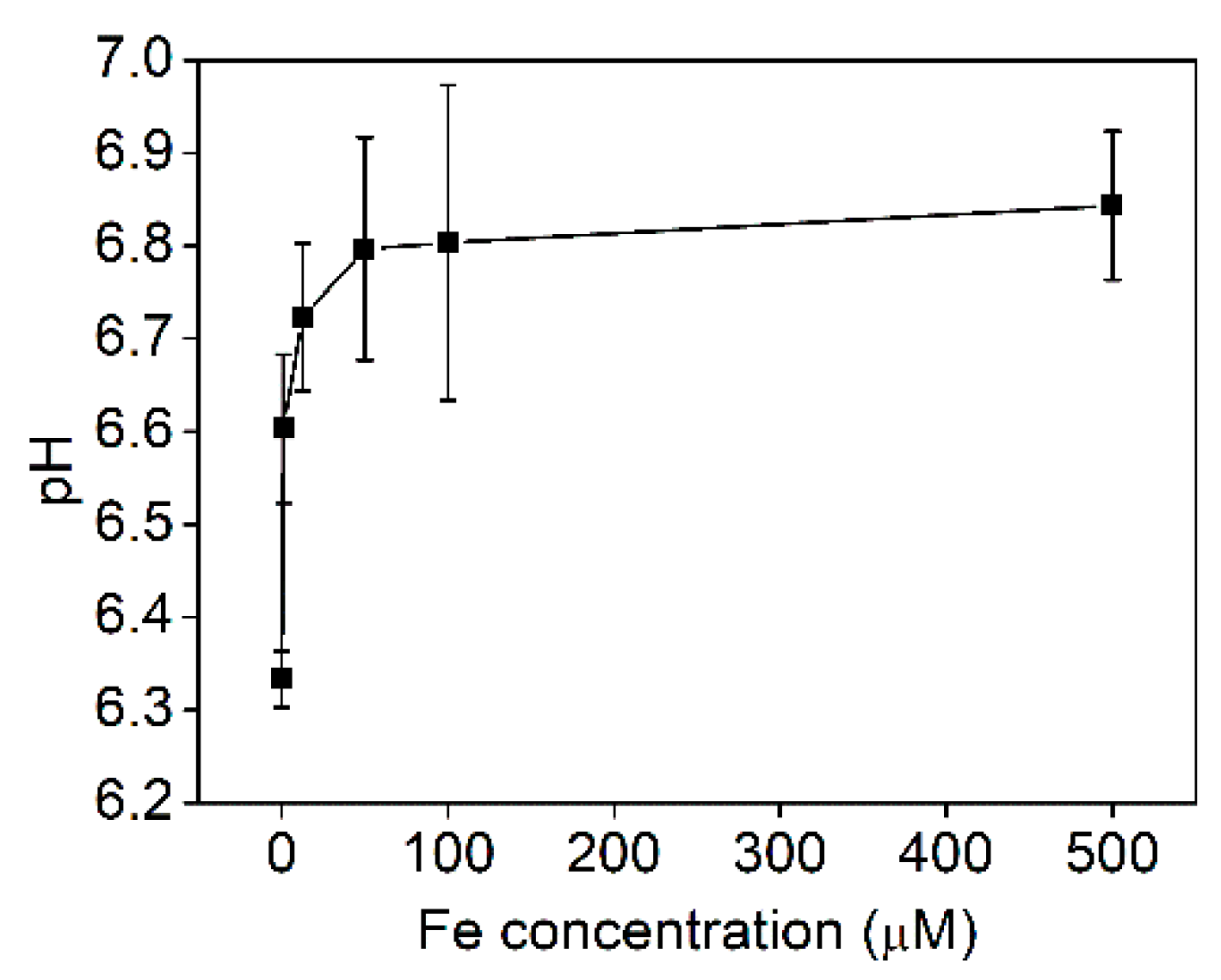

| Surplus Zn (mM) | Initial pH 1 | pH at Harvest |
|---|---|---|
| 0 | 4.07 ± 0.05 e | 7.82 ± 0.09 a |
| 0.01 | 3.99 ± 0.04 e | 7.84 ± 0.02 a |
| 0.05 | 4.05 ± 0.05 e | 7.66 ± 0.19 ab |
| 0.10 | 4.01 ± 0.04 e | 7.63 ± 0.09 b |
| 0.50 | 4.03 ± 0.06 e | 6.71 ± 0.04 c |
| 1.00 | 3.99 ± 0.04 e | 6.21 ± 0.26 d |
| Treatments | Roots | Leaves | |||
|---|---|---|---|---|---|
| Surplus Zn (mM) | Na2EDTA (mM) | Zn (µmol g−1) | Fe (µmol g−1) | Zn (µmol g−1) | Fe (µmol g−1) |
| 0 | 0 | 1.13 ± 0.09 d | 55.02 ± 11.81 a | 0.62 ± 0.11 d | 0.73 ± 0.24 a |
| 0 | 0.1 | 0.46 ± 0.16 d | 2.60 ± 0.62 c | 0.39 ± 0.02 d | 0.74 ± 0.25 a |
| 0 | 0.5 | 0.63 ± 0.12 d | 1.36 ± 0.37 c | 0.43 ± 0.09 d | 1.45 ± 0.53 a |
| 0.01 | 0 | 58.57 ± 6.79 b | 59.30 ± 16.64 a | 5.52 ± 0.38 c | 0.66 ± 0.30 a |
| 0.01 | 0.1 | 11.98 ± 1.40 c | 3.06 ± 0.12 c | 3.46 ± 0.57 c | 0.81 ± 0.14 a |
| 0.01 | 0.5 | 9.47 ± 3.41 c | 1.12 ± 0.91 c | 3.29 ± 0.62 c | 0.54 ± 0.21 a |
| 0.10 | 0 | 178.57 ± 15.27 a | 59.70 ± 10.07 a | 25.96 ± 3.98 a | 0.48 ± 0.09 a |
| 0.10 | 0.1 | 137.96 ± 19.06 a | 20.16 ± 4.01 b | 15.88 ± 3.00 b | 0.73 ± 0.19 a |
| 0.10 | 0.5 | 33.42 ± 7.54 b | 0.82 ± 0.16 c | 16.69 ± 1.01 b | 1.32 ± 0.64 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolberg, F.; Tóth, B.; Rana, D.; Arcoverde Cerveira Sterner, V.; Gerényi, A.; Solti, Á.; Szalóki, I.; Sipos, G.; Fodor, F. Iron Status Affects the Zinc Accumulation in the Biomass Plant Szarvasi-1. Plants 2022, 11, 3227. https://doi.org/10.3390/plants11233227
Kolberg F, Tóth B, Rana D, Arcoverde Cerveira Sterner V, Gerényi A, Solti Á, Szalóki I, Sipos G, Fodor F. Iron Status Affects the Zinc Accumulation in the Biomass Plant Szarvasi-1. Plants. 2022; 11(23):3227. https://doi.org/10.3390/plants11233227
Chicago/Turabian StyleKolberg, Flóra, Brigitta Tóth, Deepali Rana, Vitor Arcoverde Cerveira Sterner, Anita Gerényi, Ádám Solti, Imre Szalóki, Gyula Sipos, and Ferenc Fodor. 2022. "Iron Status Affects the Zinc Accumulation in the Biomass Plant Szarvasi-1" Plants 11, no. 23: 3227. https://doi.org/10.3390/plants11233227
APA StyleKolberg, F., Tóth, B., Rana, D., Arcoverde Cerveira Sterner, V., Gerényi, A., Solti, Á., Szalóki, I., Sipos, G., & Fodor, F. (2022). Iron Status Affects the Zinc Accumulation in the Biomass Plant Szarvasi-1. Plants, 11(23), 3227. https://doi.org/10.3390/plants11233227









