Antidiabetic and Toxicological Effects of the Tea Infusion of Summer Collection from Annona cherimola Miller Leaves
Abstract
:1. Introduction
2. Results
2.1. Effect of the Tea Infusion Extract from A. cherimola Miller in STID Mice
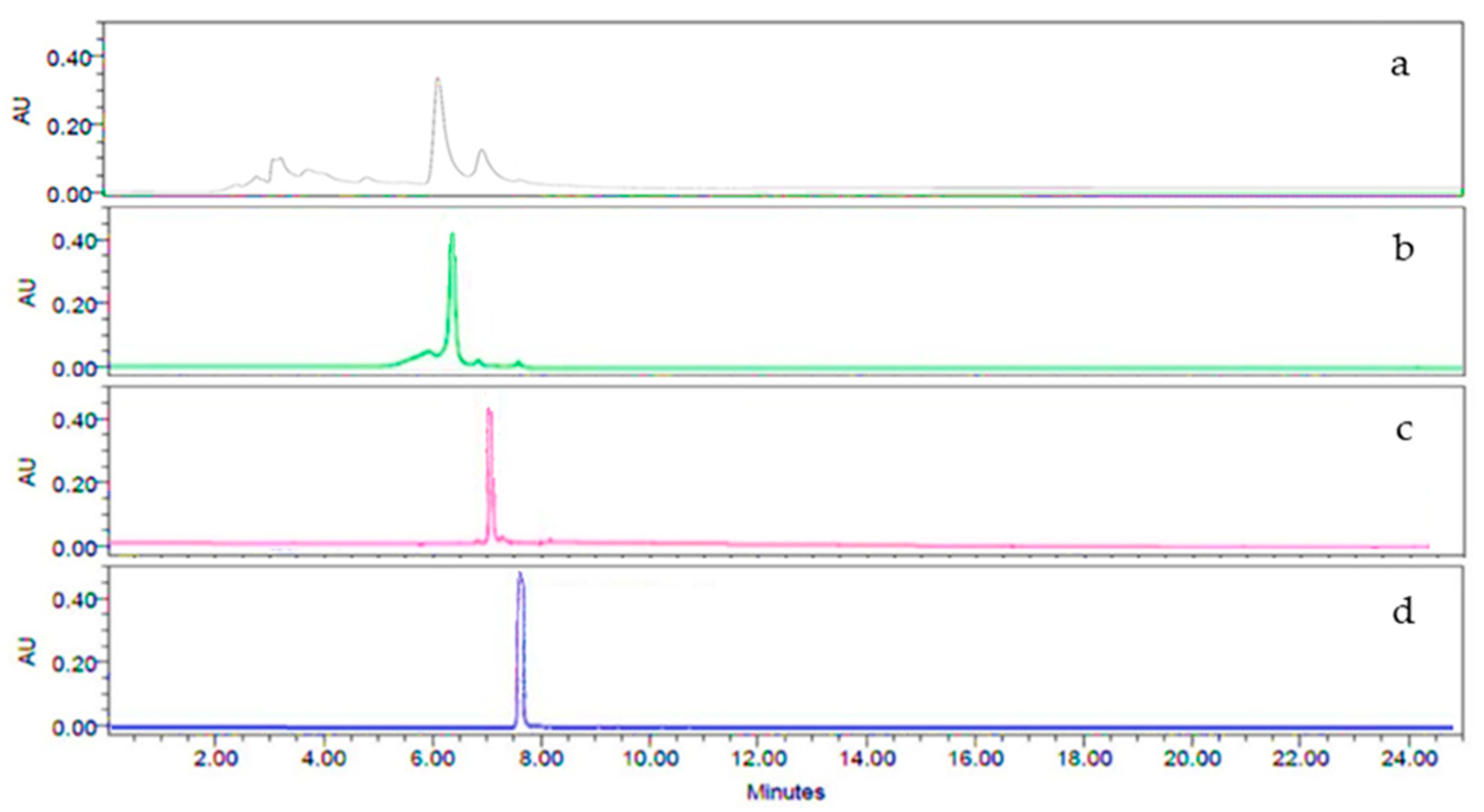
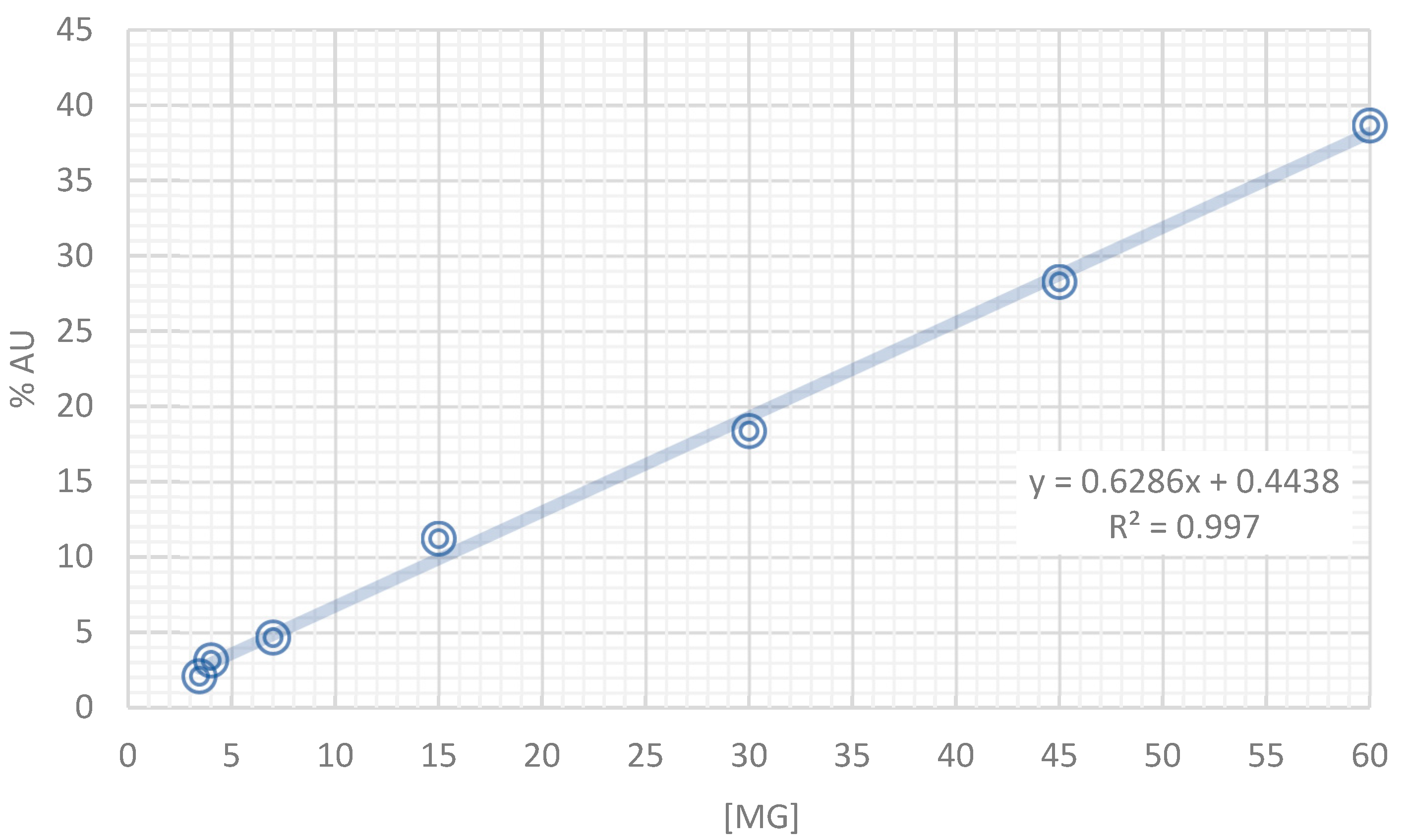
2.2. HPLC-DAD Analysis of the Tea Infusion Extract from Annona cherimola (IELAc)
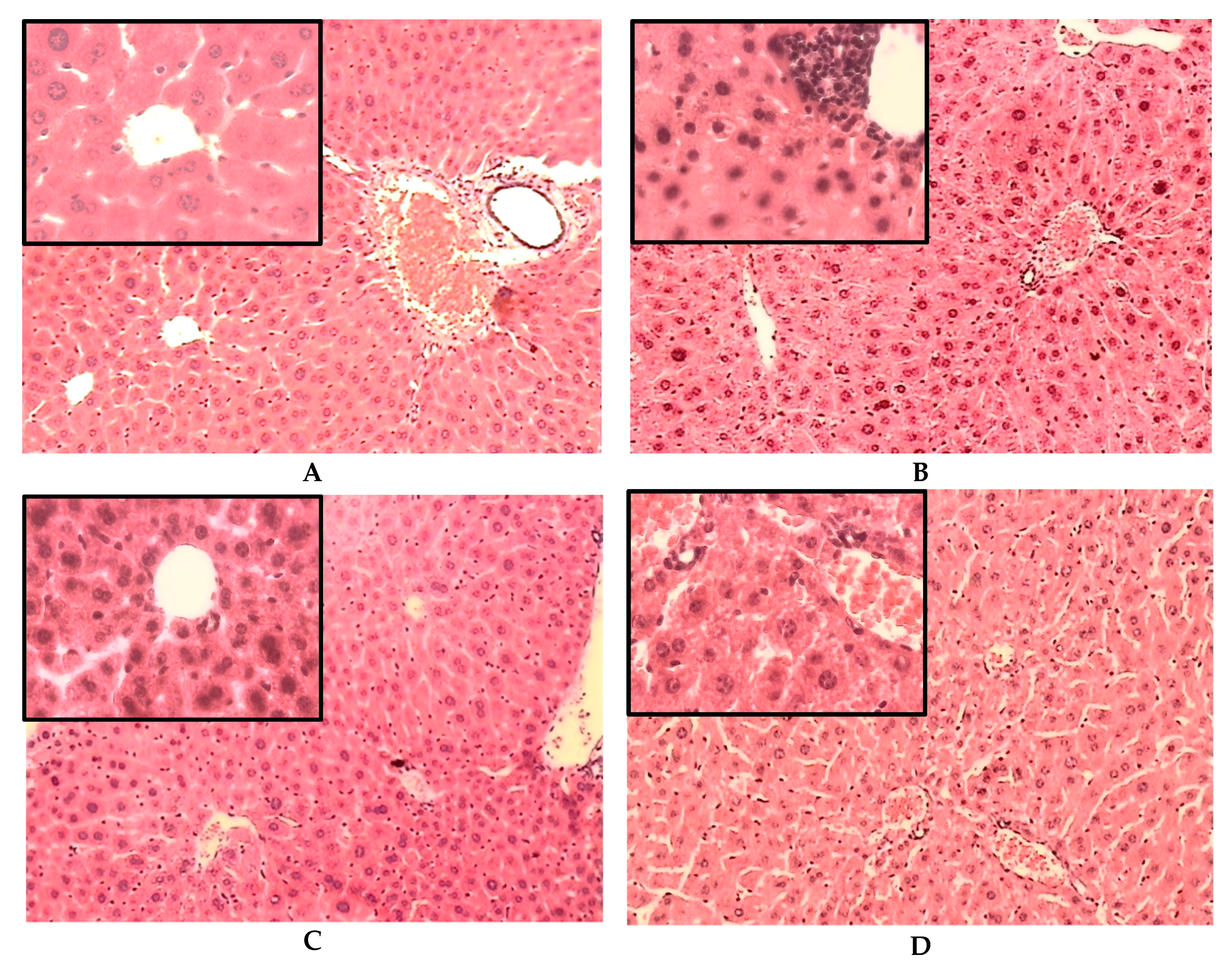
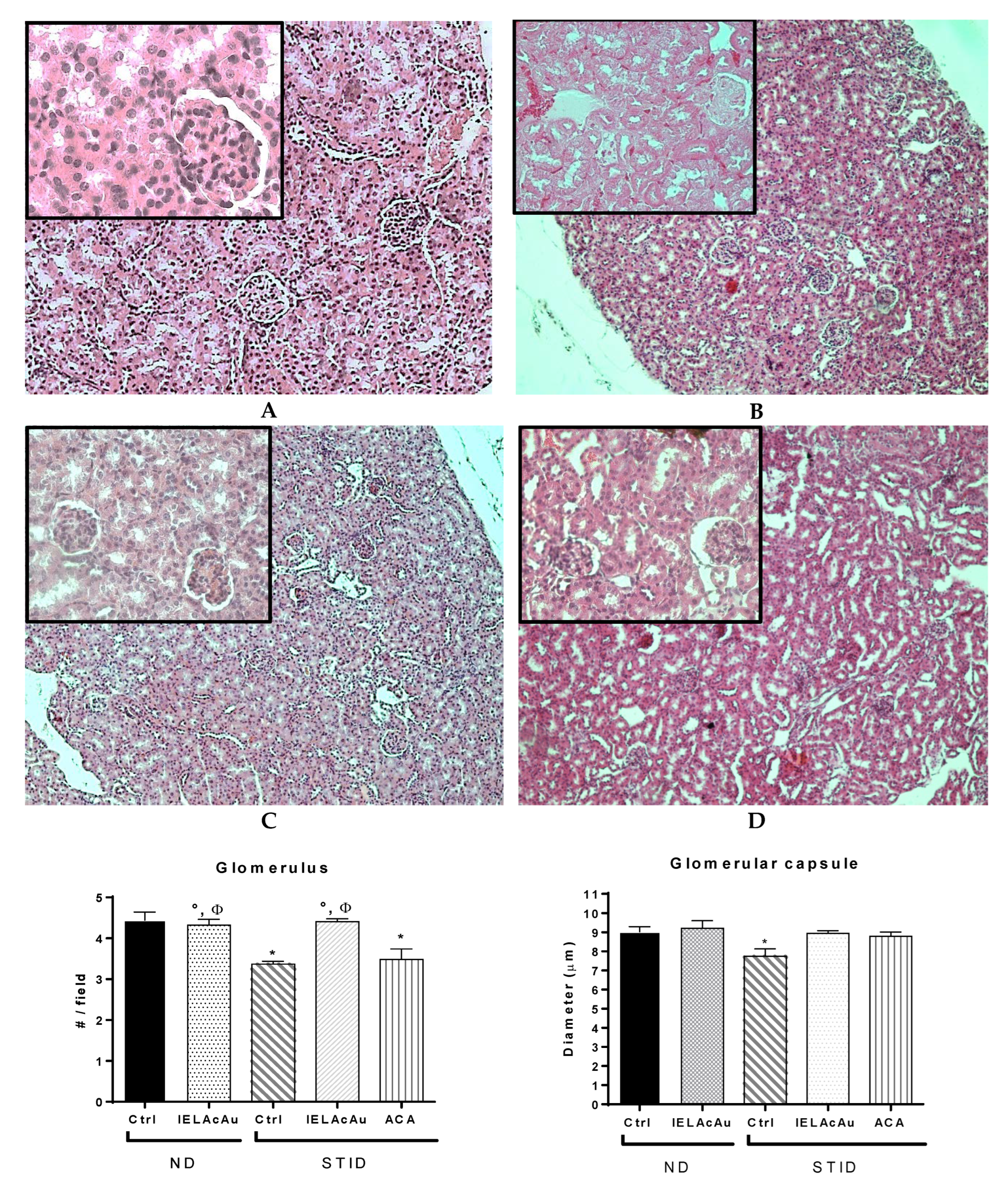
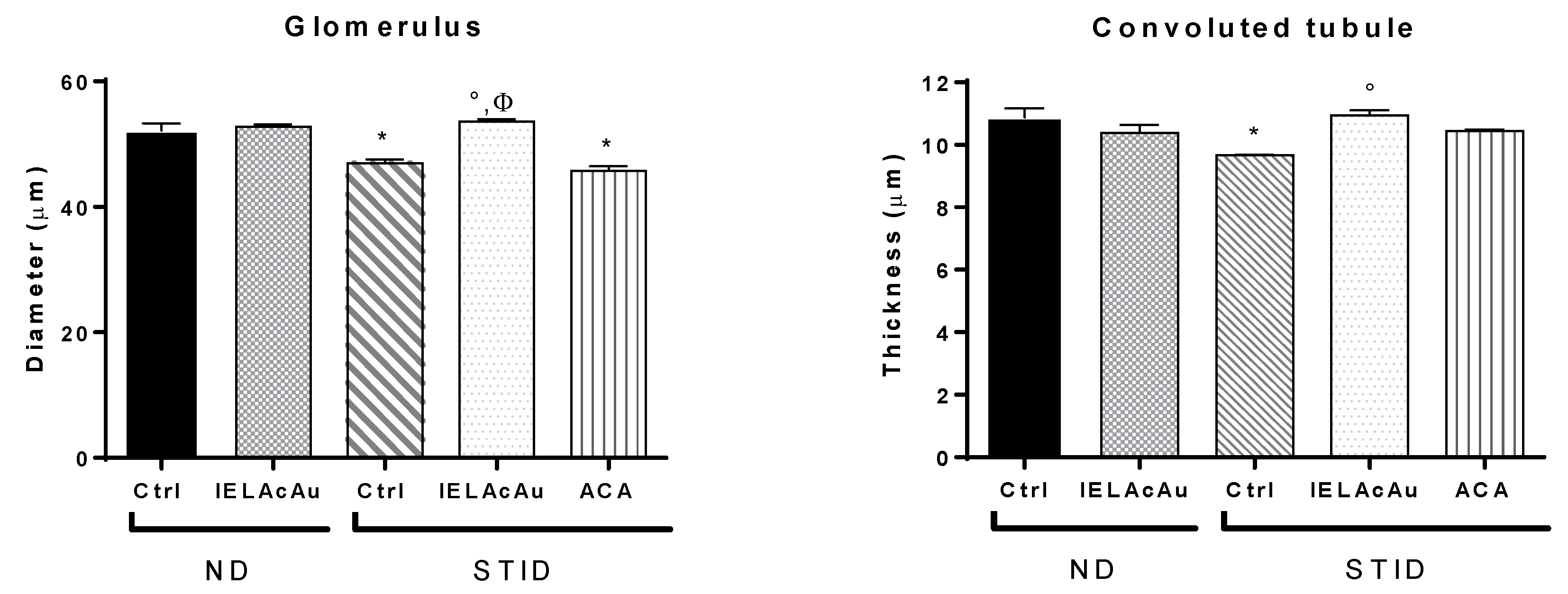
2.3. Histological Results
2.3.1. Histological Changes on the Liver of Non-Diabetic (ND) and STID Mice Treated with the Tea Infusion Extract of Ac
2.3.2. Histological Changes on the Kidneys of Non-Diabetic (ND) and STID Mice Treated with the Tea Infusion Extract of Ac (IELAc)
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Reagents
4.3. Plant Extract Procedure
4.4. Characterization of the Tea Infusion Extract of Leaves from Ac by High-Performance Liquid Chromatography
4.5. Experimental Animals
4.5.1. Grouping
4.5.2. Induction of Experimental Type 2 Diabetes
4.6. Study of Variation of the Antihyperglycemic Effect of the Infusion Extract of Ac
4.7. Evaluation of the Sub-Chronic Effect of the IELAc
4.8. Effect of the Treatment on Internal Organs and Histology
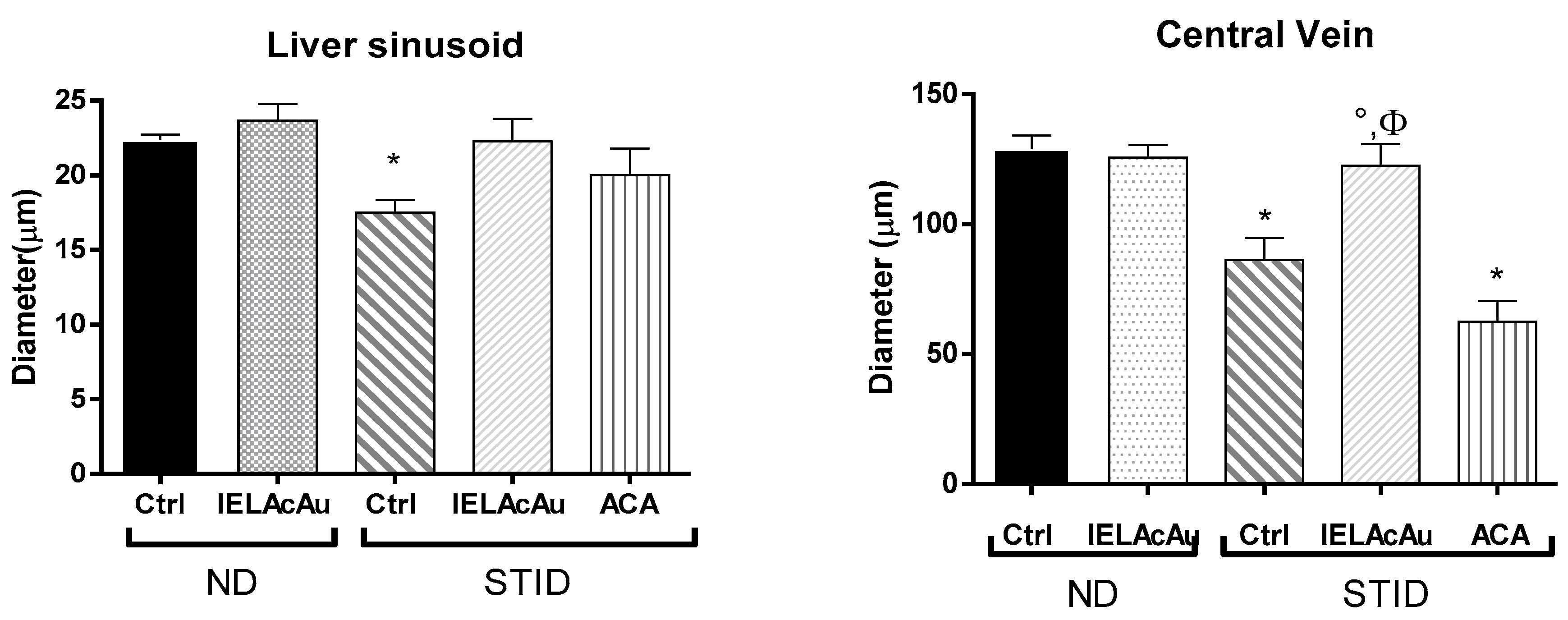
Quantitative Morphometric Measurement
- Diameter of liver sinusoids in the control and the treated groups.
- Diameter of central veins in the liver of the control and the treated groups.
- Number of renal glomeruli per field observed in the control and the treated groups.
- Diameter of the glomerular capsule in the control and the treated groups.
- Diameter of the renal glomerulus in the control and the treated groups.
- Diameter of convoluted tubules in the control and the treated groups.
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Guthrie, R.A.; Guthrie, D.W. Pathophysiology of Diabetes Mellitus. Crit. Care Nurs. Q. 2004, 27, 113–125. [Google Scholar] [CrossRef] [PubMed]
- IDF Diabetes Atlas 10th Edition. 2021. Available online: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf (accessed on 6 May 2022).
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Kapoor, B.; Gulati, M.; Kumar, R.; Ramanunny, A.K.; Awasthi, A.; Dua, K. Treatment strategies against diabetes: Success so far and challenges ahead. Eur. J. Pharmacol. 2019, 862, 172625. [Google Scholar] [CrossRef]
- Atia, T.; Sakr, H.I.; Damanhory, A.A.; Moawad, K.; Alsawy, M. The protective effect of green tea on diabetes-induced hepato-renal pathological changes: A histological and biochemical study. Arch. Physiol. Biochem. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Giri, B.; Dey, S.; Das, T.; Sarkar, M.; Banerjee, J.; Dash, S.K. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed. Pharmacother. 2018, 107, 306–328. [Google Scholar] [CrossRef]
- Oršolić, N.; Bašić, I. Honey bee products and their polyphenolic compounds in the treatment of diabetes. Phytopharm. Ther. Values 2008, 22, 455–471. [Google Scholar]
- Yue, K.K.; Leung, S.-N.; Man, P.-M.; Yeung, W.-F.; Chung, W.-S.; Lee, K.-W.; Leung, A.W.; Cheng, C.H. Alterations in antioxidant enzyme activities in the eyes, aorta and kidneys of diabetic rats relevant to the onset of oxidative stress. Life Sci. 2005, 77, 721–734. [Google Scholar] [CrossRef]
- Obrosova, I.G.; Minchenko, A.G.; Vasupuram, R.; White, L.; Abatan, O.I.; Kumagai, A.K.; Frank, R.N.; Stevens, M.J. Aldose Reductase Inhibitor Fidarestat Prevents Retinal Oxidative Stress and Vascular Endothelial Growth Factor Overexpression in Streptozotocin-Diabetic Rats. Diabetes 2003, 52, 864–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. 1), S111–S124. [Google Scholar] [CrossRef]
- Tan, S.Y.; Wong, J.L.M.; Sim, Y.J.; Wong, S.S.; Elhassan, S.A.M.; Tan, S.H.; Lim, G.P.L.; Tay, N.W.R.; Annan, N.C.; Bhattamisra, S.K.; et al. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 364–372. [Google Scholar] [CrossRef]
- Granados-Pineda, J.; Uribe-Uribe, N.; García-López, P.; Ramos-Godinez, M.D.P.; Rivero-Cruz, J.F.; Pérez-Rojas, J.M. Effect of Pinocembrin Isolated from Mexican Brown Propolis on Diabetic Nephropathy. Molecules 2018, 23, 852. [Google Scholar] [CrossRef] [Green Version]
- Yaseen, G.; Ahmad, M.; Zafar, M.; Sultana, S.; Kayani, S.; Cetto, A.A.; Shaheen, S. Traditional management of diabetes in Pakistan: Ethnobotanical investigation from Traditional Health Practitioners. J. Ethnopharmacol. 2015, 174, 91–117. [Google Scholar] [CrossRef] [PubMed]
- Khalili, M.; Ebrahimzadeh, M.A.; Safdari, Y. Antihaemolytic activity of thirty herbal extracts in mouse red blood cells. Arch. Ind. Hyg. Toxicol. 2014, 65, 399–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirovina, D.; Oršolić, N.; Gregorović, G.; Končić, M.Z. Naringenin ameliorates pathological changes in liver and kidney of diabetic mice: A preliminary study/Naringenin reducira histopatološke promjene u jetri i bubregu miševa s dijabetesom. Arch. Ind. Hyg. Toxicol. 2016, 67, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade-Cetto, A.; Heinrich, M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Quílez, A.; Fernández-Arche, M.; García-Giménez, M.; De la Puerta, R. Potential therapeutic applications of the genus Annona: Local and traditional uses and pharmacology. J. Ethnopharmacol. 2018, 225, 244–270. [Google Scholar] [CrossRef]
- Larranaga, N.; Albertazzi, F.J.; Fontecha, G.; Palmieri, M.; Rainer, H.; van Zonneveld, M.; Hormaza, J.I. A Mesoamerican origin of cherimoya (Annona cherimola Mill.): Implications for the conservation of plant genetic resources. Mol. Ecol. 2017, 26, 4116–4130. [Google Scholar] [CrossRef]
- CABI, Annona cherimola (Cherimoya). Invasive Species Compendium; CAB International: Wallingford, UK, 2014; Available online: https://www.cabi.org/isc/datasheet/5806 (accessed on 8 November 2022).
- Calzada, F.; Correa-Basurto, J.; Barbosa, E.; Mendez-Luna, D.; Yépez-Mulia, L. Antiprotozoal Constituents from Annona cherimola Miller, a Plant Used in Mexican Traditional Medicine for the Treatment of Diarrhea and Dysentery. Pharmacogn. Mag. 2017, 13, 148–152. [Google Scholar] [CrossRef]
- Díaz-De-Cerio, E.; Aguilera-Saez, L.M.; Gómez-Caravaca, A.M.; Verardo, V.; Fernández-Gutiérrez, A.; Fernández, I.; Arráez-Román, D. Characterization of bioactive compounds of Annona cherimola L. leaves using a combined approach based on HPLC-ESI-TOF-MS and NMR. Anal. Bioanal. Chem. 2018, 410, 3607–3619. [Google Scholar] [CrossRef]
- Albuquerque, T.G.; Santos, F.; Sanches-Silva, A.; Oliveira, M.B.; Bento, A.C.; Costa, H.S. Nutritional and phytochemical composition of Annona cherimola Mill. fruits and by-products: Potential health benefits. Food Chem. 2014, 193, 187–195. [Google Scholar] [CrossRef]
- Martínez-Vázquez, M.; Estrada-Reyes, R.; Escalona, A.A.; Velázquez, I.L.; Martínez-Mota, L.; Moreno, J.; Heinze, G. Antidepressant-like effects of an alkaloid extract of the aerial parts of Annona cherimolia in mice. J. Ethnopharmacol. 2012, 139, 164–170. [Google Scholar] [CrossRef]
- Ammoury, C.; Younes, M.; El Khoury, M.; Hodroj, M.H.; Haykal, T.; Nasr, P.; Sily, M.; Taleb, R.I.; Sarkis, R.; Khalife, R.; et al. The pro-apoptotic effect of a Terpene-rich Annona cherimola leaf extract on leukemic cell lines. BMC Complement. Altern. Med. 2019, 19, 365. [Google Scholar] [CrossRef]
- Falé, P.L.; Ferreira, C.; Maruzzella, F.; Florêncio, M.H.; Frazão, F.N.; Serralheiro, M.L. Evaluation of cholesterol absorption and biosynthesis by decoctions of Annona cherimola leaves. J. Ethnopharmacol. 2013, 150, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Calzada, F.; Solares-Pascasio, J.I.; Ordoñez-Razo, R.; Velazquez, C.; Barbosa, E.; García-Hernández, N.; Mendez-Luna, D.; Correa-Basurto, J. Antihyperglycemic activity of the leaves from Annona cherimola miller and rutin on alloxan-induced diabetic rats. Pharmacogn. Res. 2017, 9, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Tantawy, W.H.; Temraz, A. Management of diabetes using herbal extracts: Review. Arch. Physiol. Biochem. 2018, 124, 383–389. [Google Scholar] [CrossRef]
- Sirovina, D.; Oršolić, N.; Končić, M.Z.; Kovačević, G.; Benkovic, V.; Gregorović, G. Quercetin vs chrysin: Effect on liver his-topathology in diabetic mice. Hum. Exp. Toxicol. 2013, 32, 1058–1066. [Google Scholar] [CrossRef]
- Abolfathi, A.A.; Mohajeri, D.; Rezaie, A.; Nazeri, M. Protective Effects of Green Tea Extract against Hepatic Tissue Injury in Streptozotocin-Induced Diabetic Rats. Evid.-Based Complement. Altern. Med. 2012, 2012, 740671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhury, H.; Pandey, M.; Hua, C.K.; Mun, C.S.; Jing, J.K.; Kong, L.; Ern, L.Y.; Ashraf, N.A.; Kit, S.W.; Yee, T.S.; et al. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J. Tradit. Complement. Med. 2018, 8, 361–376. [Google Scholar] [CrossRef]
- Meng, J.-M.; Cao, S.-Y.; Wei, X.-L.; Gan, R.-Y.; Wang, Y.-F.; Cai, S.-X.; Xu, X.-Y.; Zhang, P.-Z.; Li, H.-B. Effects and Mechanisms of Tea for the Prevention and Management of Diabetes Mellitus and Diabetic Complications: An Updated Review. Antioxidants 2019, 8, 170. [Google Scholar] [CrossRef] [Green Version]
- Moradi, B.; Abbaszadeh, S.; Shahsavari, S.; Alizadeh, M.; Beyranvand, F. The most useful medicinal herbs to treat diabetes. Biomed. Res. Ther. 2018, 5, 2538–2551. [Google Scholar] [CrossRef]
- Giovannini, P.; Howes, M.-J.R.; Edwards, S.E. Medicinal plants used in the traditional management of diabetes and its sequelae in Central America: A review. J. Ethnopharmacol. 2016, 184, 58–71. [Google Scholar] [CrossRef] [Green Version]
- Leite, D.O.D.; Nonato, C.D.F.A.; Camilo, C.J.; De Carvalho, N.K.G.; Da Nobrega, M.G.L.A.; Pereira, R.C.; Da Costa, J.G.M. Annona Genus: Traditional Uses, Phytochemistry and Biological Activities. Curr. Pharm. Des. 2020, 26, 4056–4091. [Google Scholar] [CrossRef] [PubMed]
- Florence, N.T.; Benoit, M.Z.; Jonas, K.; Alexandra, T.; Désiré, D.D.P.; Pierre, K.; Théophile, D. Antidiabetic and antioxidant effects of Annona muricata (Annonaceae), aqueous extract on streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2014, 151, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Adewole, S.; Ojewole, J. Protective effects of Annona muricata linn. (Annonaceae) leaf aqueous extract on serum lipid profiles and oxidative stress in hepatocytes of streptozotocin-treated diabetic rats. Afr. J. Tradit. Complement. Altern. Med. 2008, 6, 30–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taderera, T.; Chagonda, L.S.; Gomo, E.; Katerere, D.; Shai, L.J. Annona stenophylla aqueous extract stimulate glucose uptake in established C2Cl2 muscle cell lines. Afr. Health Sci. 2019, 19, 2219–2229. [Google Scholar] [CrossRef] [Green Version]
- Brindis, F.; González-Trujano, M.E.; González-Andrade, M.; Aguirre-Hernández, E.; Villalobos-Molina, R. Aqueous Extract of Annona macroprophyllata: A Potentialα-Glucosidase Inhibitor. BioMed Res. Int. 2013, 2013, 591313. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Solís, J.; Calzada, F.; Barbosa, E.; Valdés, M. Antihyperglycemic and Antilipidemic Properties of a Tea Infusion of the Leaves from Annona cherimola Miller on Streptozocin-Induced Type 2 Diabetic Mice. Molecules 2021, 26, 2408. [Google Scholar] [CrossRef]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Singh, S.; Singh, D.K.; Meena, A.; Dubey, V.; Masood, N.; Luqman, S. Rutin protects t-butyl hydroperoxide-induced oxidative impairment via modulating the Nrf2 and iNOS activity. Phytomedicine 2019, 55, 92–104. [Google Scholar] [CrossRef]
- Kamalakkannan, N.; Prince, P.S.M. Antihyperglycaemic and Antioxidant Effect of Rutin, a Polyphenolic Flavonoid, in Streptozotocin-Induced Diabetic Wistar Rats. Basic Clin. Pharmacol. Toxicol. 2006, 98, 97–103. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of alpha-Glucosidase and alpha-Amylase by Flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Ueda-Wakagi, M.; Nagayasu, H.; Yamashita, Y.; Ashida, H. Green Tea Ameliorates Hyperglycemia by Promoting the Translocation of Glucose Transporter 4 in the Skeletal Muscle of Diabetic Rodents. Int. J. Mol. Sci. 2019, 20, 2436. [Google Scholar] [CrossRef]
- Stepien, M.; Kujawska-Luczak, M.; Szulinska, M.; Kregielska-Narozna, M.; Skrypnik, D.; Suliburska, J.; Skrypnik, K.; Regula, J.; Bogdanski, P. Beneficial dose-independent influence of Camellia sinensis supplementation on lipid profile, glycemia, and insulin resistance in an NaCl-induced hypertensive rat model. J. Physiol. Pharmacol. 2018, 69, 278–282. [Google Scholar] [CrossRef]
- Merlín-Lucas, V.; Ordoñez-Razo, R.M.; Calzada, F.; Solís, A.; García-Hernández, N.; Barbosa, E.; Valdés, M. Antitumor Potential of Annona muricata Linn. An Edible and Medicinal Plant in Mexico: In Vitro, In Vivo, and Toxicological Studies. Molecules 2021, 26, 7675. [Google Scholar] [CrossRef]
- Amador, M.D.C.V.; Rodríguez, F.M.; Rodríguez, Z.M.; Guerra, M.J.M.; Barreiro, M.L. Tamizaje Fitoquímico, Actividad Antiinflamatoria y Toxicidad Aguda de Extractos de Hojas de Annona Squamosa L. Rev. Cuba. Plantas Med. 2006, 11, 1–12. [Google Scholar]
- Auger, J.; Pérez, I.; Esterio, M. Occurrence of Root Rot Disease of Cherimoya (Annona cherimola Mill.) Caused by Dactylonectria macrodidyma in Chile. Plant Dis. 2015, 99, 1282. [Google Scholar] [CrossRef]
- Mwatawala, M.; De Meyer, M.; Makundi, R.; Maerere, A. Host range and distribution of fruit-infesting pestiferous fruit flies (Diptera, Tephritidae) in selected areas of Central Tanzania. Bull. Entomol. Res. 2009, 99, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Saul, W.-C.; Roy, H.E.; Booy, O.; Carnevali, L.; Chen, H.-J.; Genovesi, P.; Harrower, C.A.; Hulme, P.E.; Pagad, S.; Pergl, J.; et al. Assessing patterns in introduction pathways of alien species by linking major invasion data bases. J. Appl. Ecol. 2017, 54, 657–669. [Google Scholar] [CrossRef] [Green Version]
- George, A.; Nissen, R. The effects of temperature, vapour pressure deficit and soil moisture stress on growth, flowering and fruit set of custard apple (Annona cherimola × Annona squamosa) ‘African Pride’. Sci. Hortic. 1988, 34, 183–191. [Google Scholar] [CrossRef]
- Lora, J.; Herrero, M.; Hormaza, J.I. Stigmatic receptivity in a dichogamous early-divergent angiosperm species, Annona cherimola (Annonaceae): Influence of temperature and humidity. Am. J. Bot. 2011, 98, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, R.; Molina-Garcia, A.D.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. High CO2 Atmosphere Modulating the Phenolic Response Associated with Cell Adhesion and Hardening of Annona cherimola Fruit Stored at Chilling Temperature. J. Agric. Food Chem. 2002, 50, 7564–7569. [Google Scholar] [CrossRef] [PubMed]
- González-Agüero, M.; Cifuentes-Esquivel, N.; Ibañez-Carrasco, F.; Gudenschwager, O.; Campos-Vargas, R.; Defilippi, B.G. Identification and Characterization of Genes Differentially Expressed in Cherimoya (Annona cherimola Mill) after Exposure to Chilling Injury Conditions. J. Agric. Food Chem. 2011, 59, 13295–13299. [Google Scholar] [CrossRef] [PubMed]
- Sikder, K.; Kesh, S.B.; Das, N.; Manna, K.; Dey, S. The high antioxidative power of quercetin (aglycone flavonoid) and its glycone (rutin) avert high cholesterol diet induced hepatotoxicity and inflammation in Swiss albino mice. Food Funct. 2014, 5, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Duwaerts, C.C.; Maher, J.J. Macronutrients and the Adipose-Liver Axis in Obesity and Fatty Liver. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 749–761. [Google Scholar] [CrossRef] [Green Version]
- Del Moral, M.L.; Esteban, F.J.; Torres, M.I.; Camacho, M.V.; Hernandez, R.; Jimenez, A.; Aránega, A.; Pedrosa, J.A.; Peinado, M.A. High-Fat Sunflower and Olive Oil Diets Affect Serum Lipid Levels in Steatotic Rat Liver Differently. J. Nutr. Sci. Vitaminol. 1997, 43, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Wanless, I.R.; Belgiorno, J.; Huet, P. Hepatic sinusoidal fibrosis induced by cholesterol and stilbestrol in the rabbit: 1. Morphology and inhibition of fibrogenesis by dipyridamole. Hepatology 1996, 24, 855–864. [Google Scholar] [CrossRef]
- Pessayre, D.; Mansouri, A.; Fromenty, B. Nonalcoholic steatosis, and steatohepatitis. V. Mitochondrial dysfunction in steato-hepatitis. Am. J. Physiol. Gastrointest Liver Physiol. 2002, 282, G193–G199. [Google Scholar] [CrossRef]
- Rivera, L.; Morón, R.; Sánchez, M.; Zarzuelo, A.; Galisteo, M. Quercetin Ameliorates Metabolic Syndrome and Improves the Inflammatory Status in Obese Zucker Rats. Obesity 2008, 16, 2081–2087. [Google Scholar] [CrossRef]
- Li, F.; Chen, Y.; Li, Y.; Huang, M.; Zhao, W. Geniposide alleviates diabetic nephropathy of mice through AMPK/SIRT1/NF-κB pathway. Eur. J. Pharmacol. 2020, 886, 173449. [Google Scholar] [CrossRef]
- Kim, Y.; Park, C.W. Adenosine monophosphate–activated protein kinase in diabetic nephropathy. Kidney Res. Clin. Pract. 2016, 35, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulou-Marketou, N.; Chrousos, G.P.; Kanaka-Gantenbein, C. Diabetic nephropathy in type 1 diabetes: A review of early natural history, pathogenesis, and diagnosis. Diabetes/Metabolism Res. Rev. 2017, 33, e2841. [Google Scholar] [CrossRef]
- Beisswenger, P.J.; Howell, S.K.; Russell, G.B.; Miller, M.E.; Rich, S.S.; Mauer, M. Early Progression of Diabetic Nephropathy Correlates with Methylglyoxal-Derived Advanced Glycation End Products. Diabetes Care 2013, 36, 3234–3239. [Google Scholar] [CrossRef] [Green Version]
- Do, M.H.; Hur, J.; Choi, J.; Kim, M.; Kim, M.J.; Kim, Y.; Ha, S.K. Eucommia ulmoides Ameliorates Glucotoxicity by Suppressing Advanced Glycation End-Products in Diabetic Mice Kidney. Nutrients 2018, 10, 265. [Google Scholar] [CrossRef]
- Busch, M.; Franke, S.; Rüster, C.; Wolf, G. Advanced glycation end-products and the kidney. Eur. J. Clin. Investig. 2010, 40, 742–755. [Google Scholar] [CrossRef]
- Matsuda, H.; Wang, T.; Managi, H.; Yoshikawa, M. Structural requirements of flavonoids for inhibition of protein glycation and radical scavenging activities. Bioorg. Med. Chem. 2003, 11, 5317–5323. [Google Scholar] [CrossRef] [PubMed]
- Kovatchev, B.P. Metrics for glycaemic control—From HbA1c to continuous glucose monitoring. Nat. Rev. Endocrinol. 2017, 13, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Caro-Ordieres, T.; Marín-Royo, G.; Opazo-Ríos, L.; Jiménez-Castilla, L.; Moreno, J.A.; Gómez-Guerrero, C.; Egido, J. The Coming Age of Flavonoids in the Treatment of Diabetic Complications. J. Clin. Med. 2020, 9, 346. [Google Scholar] [CrossRef] [Green Version]
- Larbie, C.; Arthur, F.N.; Woode, E.; Terlabi, E. Evaluation of hepatoprotective effect of aqueous extract of Annona muricata (Linn.) leaf against carbon tetrachloride and acetaminophen-induced liver damage. J. Nat. Pharm. 2012, 3, 25–30. [Google Scholar] [CrossRef]
- Bitar, R.; Fakhoury, R.; Fahmi, R.; Borjac, J. Histopathological Effects of the Annona muricata Aqueous Leaves Extract on the Liver and Kidneys of Albino Mice. Transl. Med. 2017, 7, 2161-1025.100019 . [Google Scholar] [CrossRef]
- Jessica, G.G.; Mario, G.L.; Alejandro, Z.; Cesar, A.P.J.; Ivan, J.V.E.; Ruben, R.R.; Javier, A.-A.F. Chemical Characterization of a Hypoglycemic Extract from Cucurbita Ficifolia Bouche That Induces Liver Glycogen Accumulation in Diabetic Mice. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 218–230. [Google Scholar] [CrossRef] [Green Version]
- Diario Oficial de la Federación. NOM-062-ZOO-1999, 2001. Norma Oficial Mexicana. Especificaciones Técnicas Para La Producción, Cuidado y Uso de Los Animales de Laboratorio; Diario Oficial de La Federación: México City, México, 1999; p. 58. [Google Scholar]
- OECD Test Guidelines for Chemicals—OECD. Available online: http://www.oecd.org/chemicalsafety/testing/oecdguidelinesforthetestingofchemicals.htm (accessed on 6 May 2022).
- Kaleem, M.; Asif, M.; Ahmed, Q.U.; Bano, B. Antidiabetic and antioxidant activity of Annona squamosa extract in strepto-zotocin-induced diabetic rats. Singapore Med. J. 2006, 47, 670–675. [Google Scholar]
- Fernando, C.; Valdes, M.; Garcia, H.N.; Velázquez, C.; Barbosa, E.; Bustos, B.C.; Quijano, L.; Pina, J.E.; Mendieta, W.J.E. Antihyperglycemic activity of the leaves from Annona diversifolia Safford. and farnesol on normal and alloxan-induced diabetic mice. Pharmacogn. Mag. 2019, 15 (Suppl. 1), 5. [Google Scholar] [CrossRef]
- Ngueguim, F.; Théophile, D.; Dzeufiet, P.; Bertin, V.; Etienne, D.; Beauwens, R.; Acha, A.; Louis, Z.; Kamtchouing, P. Anti-diabetic Activities of Methanol-Derived Extract of Dorstenia picta Twigs in Normal and Streptozotocin-Induced Diabetic Rats. Asian J. Tradit. Med. 2007, 2, 140–148. [Google Scholar]
- Luna, L.G. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology, 3rd ed.; McGraw-Hill: New York, NY, USA, 1968. [Google Scholar]
| Group | Blood Glucose Levels (mg/dL) | ||||
|---|---|---|---|---|---|
| 0 h | 1 h | 3 h | 5 h | 7 h | |
| ND Control | 135.3 ± 3.04 b | 140.3 ± 3.18 b | 127.4 ± 5.31 b | 140.6 ± 8.70 b | 122.0 ± 5.94 b |
| AcMa | 335.6 ± 5.95 a | 314.2 ± 2.44 a,b,c | 301.6 ± 5.67 a,b | 308.6 ± 2.95 a,b | 314.3.6 ± 8.38 a,b,c |
| AcJun | 307.5 ± 3.50 a | 253.0 ± 8.12 a,b | 269.1 ± 1.84 a,b,c,d | 295.6 ± 1.14 a,b | 318.3 ± 4.09 a,b,c |
| AcJul | 319.2 ± 6.71 a | 324.3 ± 7.55 a,b | 202.5 ± 9.75 a,b,c,d | 312.1 ± 5.96 a,b | 326.3 ± 10.45 a,b,c |
| AcAu | 325.5 ± 3.90 a | 283.0 ± 8.35 a,b,d | 245.8 ± 6.59 a,b,c,d | 258.8 ± 7.87 a,b | 210.0 ± 6.68 a,b,c |
| ACA | 343.5 ± 1.36 a | 301.0 ± 3.42 a,b | 314.5 ± 9.34 a,b | 295.0 ± 8.37 a,b | 366.0 ± 2.01 a |
| STID control | 343.3 ± 2.50 a | 391.6 ± 1.65 a,c | 379.0 ± 9.33 a,c | 375.5 ± 10.38 a,c | 386.0 ± 2.15 a |
| MONTH | Yield (mg) | (Rutin) (mg/g) | (Narcissin) (mg/g) | (Nicotiflorin) (mg/g) |
|---|---|---|---|---|
| AcMa | 35.67 ± 0.18 c,d | 3.44 ± 0.17 d | 0.58 ± 0.02 c,d | 0.08 ± 0.002 c,d |
| AcJun | 49.24 ± 3.94 d | 4.30 ± 0.21 d | 1.38 ± 0.42 d | 0.31 ± 0.01 d |
| AcJul | 89.81 ± 8.03 a | 4.61 ± 0.43 d | 2.68 ± 0.89 a,d | 0.34 ± 0.07 a,d |
| AcAu | 93.17 ± 17.18 a,b | 7.96 ± 0.89 a,b,c | 4.24 ± 0.68 a,b,c | 0.52 ± 0.02 a,b,c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Solís, J.; Calzada, F.; Barbosa, E.; Gutiérrez-Meza, J.M. Antidiabetic and Toxicological Effects of the Tea Infusion of Summer Collection from Annona cherimola Miller Leaves. Plants 2022, 11, 3224. https://doi.org/10.3390/plants11233224
Martínez-Solís J, Calzada F, Barbosa E, Gutiérrez-Meza JM. Antidiabetic and Toxicological Effects of the Tea Infusion of Summer Collection from Annona cherimola Miller Leaves. Plants. 2022; 11(23):3224. https://doi.org/10.3390/plants11233224
Chicago/Turabian StyleMartínez-Solís, Jesús, Fernando Calzada, Elizabeth Barbosa, and Juan Manuel Gutiérrez-Meza. 2022. "Antidiabetic and Toxicological Effects of the Tea Infusion of Summer Collection from Annona cherimola Miller Leaves" Plants 11, no. 23: 3224. https://doi.org/10.3390/plants11233224
APA StyleMartínez-Solís, J., Calzada, F., Barbosa, E., & Gutiérrez-Meza, J. M. (2022). Antidiabetic and Toxicological Effects of the Tea Infusion of Summer Collection from Annona cherimola Miller Leaves. Plants, 11(23), 3224. https://doi.org/10.3390/plants11233224









