Nutritional and Phyto-Therapeutic Value of the Halophyte Cladium mariscus L. (Pohl.): A Special Focus on Seeds
Abstract
1. Introduction
2. Results and Discussion
2.1. Nutritional Profile
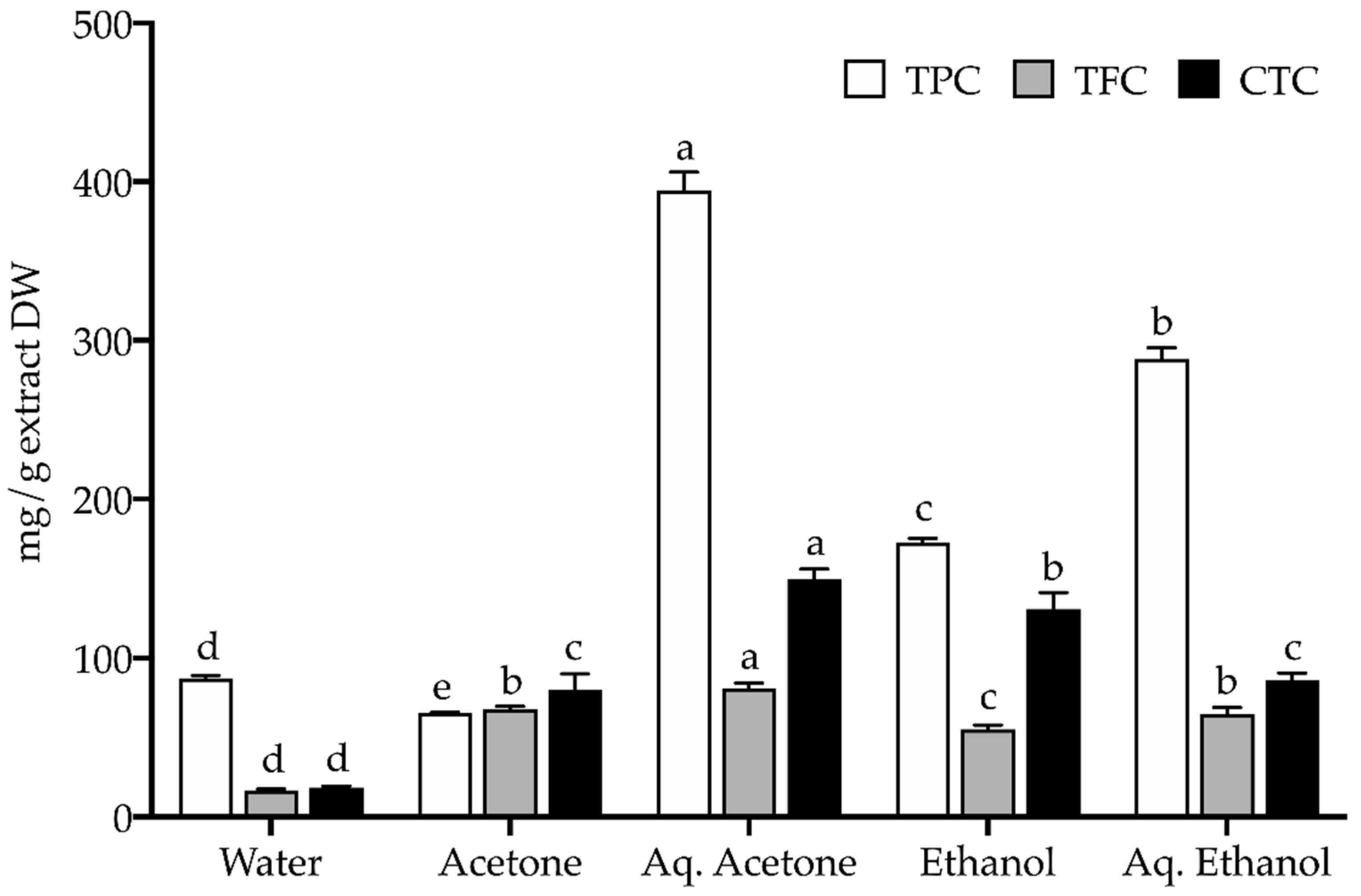
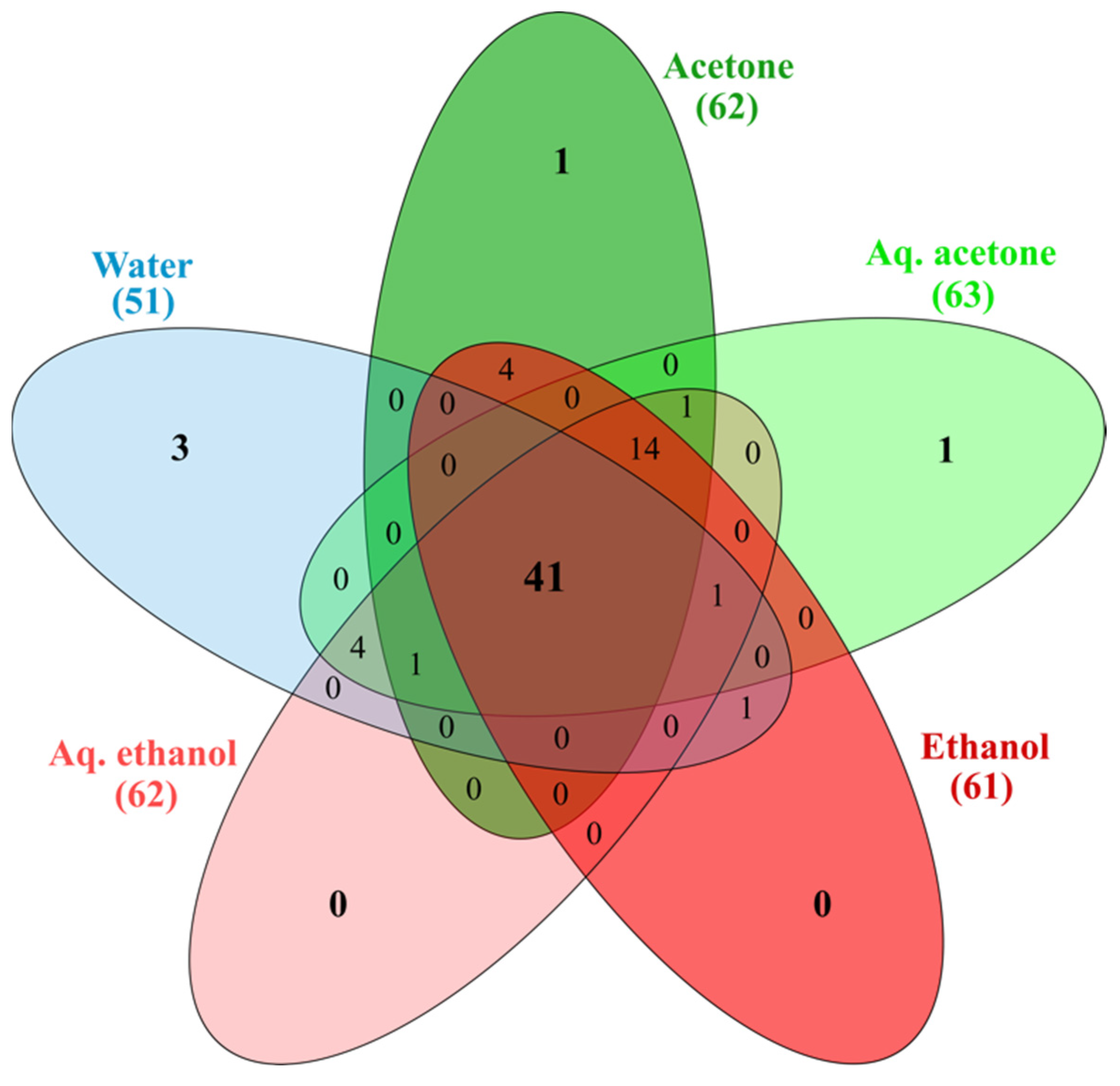
2.2. Chemical Profile
2.3. Bioactivity Profile
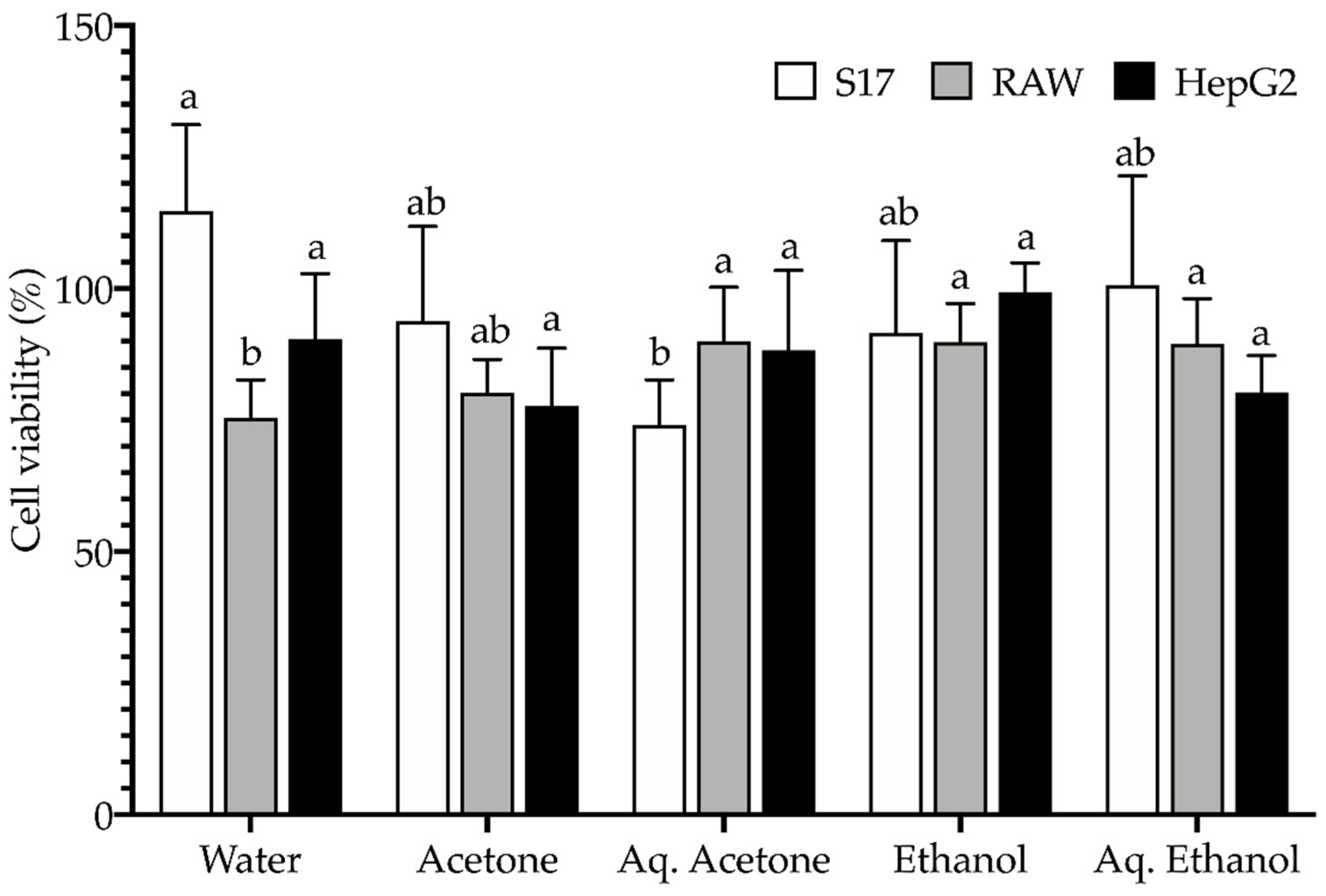
2.4. Toxicological Profile
3. Materials and Methods
3.1. Plant Material and Extraction
3.2. Nutritional Profile
3.3. Chemical Profile
3.3.1. Total Phenolic, Flavonoid, and Condensed Tannin Content
3.3.2. Phytochemical Composition by HPLC-ESI-MS/MS (High Performance Liquid Chromatography Coupled with Electrospray Ionization Mass Spectrometry)
3.4. Bioactivity Profile
3.4.1. In Vitro Antioxidant Activity
3.4.2. In Vitro Enzyme Inhibitory Activity
3.4.3. Cell Culture
3.4.4. In Vitro Anti-Inflammatory Activity
3.5. Toxicological Profile
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edelman, M.; Colt, M. Nutrient value of leaf vs. seed. Front. Chem. 2016, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, M.C.; Ellmore, G.S.; McKeown, N. Seeds—Health benefits, barriers to incorporation, and strategies for practitioners in supporting consumption among consumers. Nutr. Today 2016, 51, 50–59. [Google Scholar] [CrossRef]
- Ros, E.; Hu, F.B. Consumption of plant seeds and cardiovascular health. Circulation 2013, 128, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Oso, A.A.; Ashafa, A.O. Nutritional composition of grain and seed proteins. In Grain and Seed Proteins Functionality; Jimenez-Lopez, J.C., Ed.; IntechOpen: London, UK, 2021; pp. 31–50. [Google Scholar] [CrossRef]
- Kulczyński, B.; Kobus-Cisowska, J.; Taczanowski, M.; Kmiecik, D.; Gramza-Michałowska, A. The chemical composition and nutritional value of chia seeds—Current state of knowledge. Nutrients 2019, 11, 1242. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Sharif, M.K.; Sibt-e-Abbas, M.; Teferra, T.F.; Sultan, M.T.; Anwar, M.J. Nutritional and therapeutic potential of sesame seeds. J. Food Qual. 2022, 2022, 6163753. [Google Scholar] [CrossRef]
- FAO—Food and Agricultural Organization of the United Nations. Climate Change and Food Security: Risks and Responses; FAO: Rome, Italy, 2015; Available online: https://www.fao.org/3/i5188e/I5188E.pdf (accessed on 6 May 2022).
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Cheeseman, J. Food security in the face of salinity, drought, climate change, and population growth. In Halophytes for Food Security in Dry Lands; Khan, M.A., Ozturk, M., Gul, B., Ahmed, M.Z., Eds.; Academic Press: Oxford, UK, 2016; pp. 111–123. [Google Scholar] [CrossRef]
- Peña, R.J.H.; Hernández, D.M.; Ghasemi, M.; Puente, E.O.R. Salt tolerant plants as a valuable resource for sustainable food production in arid and saline coastal zones. Acta Biol. Colomb. 2021, 26, 116–126. [Google Scholar] [CrossRef]
- Angeli, V.; Silva, P.M.; Massuela, D.C.; Khan, M.W.; Hamar, A.; Khajehei, F.; Graeff-Hönninger, S.; Piatti, C. Quinoa (Chenopodium quinoa Willd.): An overview of the potentials of the “Golden Grain” and socio-economic and environmental aspects of its cultivation and marketization. Foods 2020, 9, 216. [Google Scholar] [CrossRef]
- Zarrouk, M.; El Almi, H.; Ben Youssef, N.; Sleimi, N.; Smaoui, A.; Ben Miled, D.; Abdelly, C. Lipid composition of seeds of local halophytes: Cakile maritima, Zygophyllum album and Crithmum maritimum. In Cash Crop Halophytes: Recent Studies. Tasks for Vegetation Science; Lieth, H., Mochtchenko, M., Eds.; Springer: Dordrecht, The Netherlands, 2003; Volume 38, pp. 121–124. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Arampatzis, D.A.; Tsiropoulos, N.G.; Petrović, J.; Soković, M.; Barros, L.; Ferreira, I.C.F.R. Seed oil and seed oil byproducts of common purslane (Portulaca oleracea L.): A new insight to plant-based sources rich in omega-3 fatty acids. LWT 2020, 123, 109099. [Google Scholar] [CrossRef]
- Weber, D.J.; Ansari, R.; Gul, B.; Khan, M.A. Potential of halophytes as source of edible oil. J. Arid Environ. 2007, 68, 315–321. [Google Scholar] [CrossRef]
- Gerdol, R.; Brancaleoni, L.; Lastrucci, L.; Nobili, G.; Pellizzari, M.; Ravaglioli, M.; Viciani, D. Wetland plant diversity in a coastal nature reserve in Italy: Relationships with salinization and eutrophication and implications for nature conservation. Estuaries Coast. 2018, 41, 2079–2091. [Google Scholar] [CrossRef]
- Abouzid, S. An active learning assignment to improve pharmacy students’ knowledge of herbal medicine. J. Appl. Pharm. Sci. 2015, 5, 106–108. [Google Scholar] [CrossRef]
- AbouZid, S.F.; Mohamed, A.A. Survey on medicinal plants and spices used in Beni-Sueif, Upper Egypt. J. Ethnobiol. Ethnomed. 2011, 7, 18. [Google Scholar] [CrossRef]
- Lopes, A.; Rodrigues, M.J.; Pereira, C.; Oliveira, M.; Barreira, L.; Varela, J.; Trampetti, F.; Custódio, L. Natural products from extreme marine environments: Searching for potential industrial uses within extremophile plants. Ind. Crop. Prod. 2016, 94, 299–307. [Google Scholar] [CrossRef]
- Oliveira, M.; Rodrigues, M.J.; Neng, N.R.; Nogueira, J.M.F.; Bessa, R.J.B.; Custódio, L. Seasonal variations of the nutritive value and phytotherapeutic potential of Cladium mariscus L. (Pohl.) targeting ruminant’s production. Plants 2021, 10, 556. [Google Scholar] [CrossRef]
- Trovato, G.M. Behavior, nutrition and lifestyle in a comprehensive health and disease paradigm: Skills and knowledge for a predictive, preventive and personalized medicine. EPMA J. 2012, 3, 8. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Gullón, B.; Pateiro, M.; Tomasevic, I.; Domínguez, R.; Lorenzo, J.M. Natural antioxidants from seeds and their application in meat products. Antioxidants 2020, 9, 815. [Google Scholar] [CrossRef]
- Petropoulos, S.; Fernandes, Â.; Pereira, C.; Tzortzakis, N.; Vaz, J.; Soković, M.; Barros, L.; Ferreira, I.C.F.R. Bioactivities, chemical composition and nutritional value of Cynara cardunculus L. seeds. Food Chem. 2019, 289, 404–412. [Google Scholar] [CrossRef]
- Katunzi-Kilewela, A.; Kaale, L.D.; Kibazohi, O.; Rweyemamu, L.M.P. Nutritional, health benefits and usage of chia seeds (Salvia hispanica): A review. Afr. J. Food Sci. 2021, 15, 48–59. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Zitoun, A. Sesame (Sesamum indicum L.) seeds in food, nutrition, and health. In Nuts and Seeds in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2011; pp. 1029–1036. [Google Scholar] [CrossRef]
- Toqeer, S.; Qasim, M.M. Abideen, Z.; Gul, B.; Rasheed, M.; Khan, M.A. Chemicla composition and antioxidant activity of seeds of various halophytic grasses. J. Am. Oil Chem. Soc. 2018, 95, 1285–1295. [Google Scholar] [CrossRef]
- EFSA—European Food Safety Authority. Dietary reference values for nutrients. Summary Report. EFSA Support. Public. 2017, 14, e15121. [Google Scholar] [CrossRef]
- Otten, J.J.; Hellwig, J.P.; Meyers, L.D. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; The National Academies Press: Washington, DC, USA, 2006; 1329p. [Google Scholar]
- Baxter, I.; Dilkes, B.P. Elemental profiles reflect plant adaptations to the environment. Science 2012, 336, 1661–1663. [Google Scholar] [CrossRef]
- De la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Chapter 12—Phenolic compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing: Duxford, UK, 2019; pp. 253–271. [Google Scholar] [CrossRef]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of polyphenols from aromatic and medicinal plants: An overview of the methods and the effect of extraction parameters. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Academic Press: London, UK, 2019; pp. 243–259. [Google Scholar] [CrossRef]
- Directive 2009/32/EC of the European Parliament and of the Council of 23 April on the approximation of the laws of the Member States on extraction solvents used in the production of foodstuffs and food ingredients. Off. J. Eur. Union 2009, L141, 3–11. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex:32009L0032 (accessed on 14 March 2022).
- Kähkönen, M.P.; Hopia, A.I.; Heikki, J.V.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Soszynski, A.; Martins, A.; Rauter, A.P.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; Barreira, L.; Custódio, L. Unravelling the antioxidant potential and the phenolic composition of different anatomical organs of the marine halophyte Limonium algarvense. Ind. Crop. Prod. 2015, 77, 315–322. [Google Scholar] [CrossRef]
- Hartmann, T. From waste products to ecochemicals: Fifty years research of plant secondary metabolism. Phytochemistry 2007, 68, 2831–2846. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Vidal, S.; Francis, L.; Guyot, S.; Marnet, N.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E. The mouth-feel properties of grape and apple proanthocyanidins in a wine-like medium. J. Sci. Food Agric. 2003, 83, 564–573. [Google Scholar] [CrossRef]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.-M.; Saura-Calixto, F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53, S310–S329. [Google Scholar] [CrossRef]
- Oliveira, M.; Lima, C.S.; Ketavong, S.; Llorent-Martínez, E.J.; Hoste, H.; Custódio, L. Disclosing the bioactives metabolites involved in the in vitro anthelmintic effects of salt-tolerant plants through a combined approach using PVPP and HPLC-ESI-MSn. Sci. Rep. 2021, 11, 24303. [Google Scholar] [CrossRef]
- Ibrahim, N.I.; Fairus, S.; Mohamed, I.N. The effects and potential mechanism of oil palm phenolics in cardiovascular health: A review on current evidence. Nutrients 2020, 12, 2055. [Google Scholar] [CrossRef] [PubMed]
- Del Olmo, A.; Calzada, J.; Nuñez, M. Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: Uses, exposure, and controversy. Crit. Rev. Food 2017, 57, 3084–3103. [Google Scholar] [CrossRef]
- Sakai, S.; Kawamata, H.; Kogure, T.; Mantani, N.; Terasawa, K.; Umatake, M.; Ochiai, H. Inhibitory effect of ferulic acid and isoferulic acid on the production of macrophage inflammatory protein-2 in response to respiratory syncytial virus infection in RAW264.7 Cells. Mediat. Inflamm. 1999, 8, 346214. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; Wang, Q.; Han, Y.-Q.; Jiang, M.; Gao, J.; Miao, Y.; Bai, G. Bioactivity-based UPLC/Q-TOF/MS strategy for screening of anti-inflammatory components from Cimicifugae Rhizoma. Chin. Chem. Lett. 2017, 28, 476–481. [Google Scholar] [CrossRef]
- Serreli, G.; Le Sayec, M.; Thou, E.; Lacour, C.; Diotallevi, C.; Dhunna, M.A.; Deiana, M.; Spencer, J.P.E.; Corona, G. Ferulic acid derivatives and avenanthramides modulate endothelial function through maintenance of nitric oxide balance in HUVEC cells. Nutrients 2021, 13, 2026. [Google Scholar] [CrossRef] [PubMed]
- Karamać, M.; Koleva, L.; Kancheva, V.D.; Amarowicz, R. The Structure–antioxidant activity relationship of ferulates. Molecules 2017, 22, 527. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Chen, D. Evaluation of antioxidant activity of isoferulic acid in vitro. Nat. Prod. Commun. 2011, 6, 1285–1288. [Google Scholar] [CrossRef]
- Dakora, F.D.; Matiru, V.; Kanu, A.S. Rhizosphere ecology of lumichrome and riboflavin, two bacterial signal molecules eliciting developmental changes in plants. Front. Plant Sci. 2015, 6, 700. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.-W.; Hsu, L.-C.; Lai, C.-H.; Wu, C.-C.; Hwang, T.-L.; Lin, Y.-K.; Wu, Y.-C. Evaluation of the bioactivities of extracts of endophytes isolated from Taiwanese herbal plants. World J. Microbiol. Biotechnol. 2009, 25, 1461–1469. [Google Scholar] [CrossRef]
- Chung, B.; Kwon, O.-S.; Shin, J.; Oh, K.-B. Inhibitory effects of Streptomyces sp. MBTH32 metabolites on sortase A and sortase A-mediated cell clumping of Staphylococcus aureus to fibrinogen. J. Microbiol. Biotechnol. 2019, 29, 1603–1606. [Google Scholar] [CrossRef]
- Chantarawong, W.; Kuncharoen, N.; Tanasupawat, S.; Chanvorachote, P. Lumichrome inhibits human lung cancer cell growth and induces apoptosis via a p53-dependent mechanism. Nutr. Cancer 2019, 71, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its main pharmacological activity and potential application in clinical medicine. Oxid. Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef] [PubMed]
- Shirahata, T.; Sunazuka, T.; Yoshida, K.; Yamamoto, D.; Harigaya, Y.; Kuwajima, I.; Nagai, T.; Kiyohara, H.; Yamada, H.; Ōmura, S. Total synthesis, elucidation of absolute stereochemistry, and adjuvant activity of trihydroxy fatty acids. Tetrahedron 2006, 62, 9483–9496. [Google Scholar] [CrossRef]
- Nagai, T.; Shimizu, Y.; Shirahata, T.; Sunazuka, T.; Kiyohara, H.; Omura, S.; Yamada, H. Oral adjuvant activity for nasal influenza vaccines caused by combination of two trihydroxy fatty acid stereoisomers from the tuber of Pinellia ternata. Int. Immunopharmacol. 2010, 10, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Doi, M.; Morita, N.; Sakashita, M.; Okuzawa, T.; Ohgiya, S.; Okamoto, D.; Sato, K.; Ito, Y.; Matsuura, H.; Hashidoko, Y. Pinellic acid isolated from quercetin-rich onions has a peroxisome proliferator-activated receptor-alpha/gamma (PPAR-α/γ) transactivation activity. Planta Med. 2022, 88, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.; Wagner, K.-D. The role of PPARs in disease. Cells 2020, 9, 2367. [Google Scholar] [CrossRef]
- Gramza, A.; Korczak, J.; Amarowicz, R. Tea polyphenols—Their antioxidant properties and biological activity—A review. Pol. J. Food Nutr. Sci. 2005, 55, 219–235. [Google Scholar]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Lozano, R.; Gómez-Serranillos, M.P. The pharmacological activity of Camellia sinensis (L.) Kuntze on metabolic and endocrine disorders: A systematic review. Biomolecules 2020, 10, 603. [Google Scholar] [CrossRef]
- Choi, J.S.; Islam, M.N.; Ali, M.Y.; Kim, Y.M.; Park, H.J.; Sohn, H.S.; Jung, H.A. The effects of C-glycosylation of luteolin on its antioxidant, anti-Alzheimer’s disease, anti-diabetic, and anti-inflammatory activities. Arch. Pharm. Res. 2014, 37, 1354–1363. [Google Scholar] [CrossRef]
- Szulc-Musioł, B.; Sarecka-Hujar, B. The use of micro- and nanocarriers for resveratrol delivery into and across the skin in different skin diseases—A literature review. Pharmaceutics 2021, 13, 451. [Google Scholar] [CrossRef]
- Likhitwitayawuid, K. Oxyresveratrol: Sources, productions, biological activities, pharmacokinetics, and delivery systems. Molecules 2021, 26, 4212. [Google Scholar] [CrossRef] [PubMed]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Krěn, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Qiu, B.; Jia, M.; Liu, W.; Guo, X.-f.; Li, N.; Xu, Z.-x.; Du, F.-l.; Xu, T.; Li, D. Effects of α-linolenic acid intake on blood lipid profiles: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 2894–2910. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Xie, F.; Huang, W.; Hu, M.; Yan, Q.; Chen, Z.; Zheng, Y.; Liu, L. The review of alpha-linolenic acid: Sources, metabolism, and pharmacology. Phytother. Res. 2021, 36, 164–188. [Google Scholar] [CrossRef]
- Valencia-Hernandez, L.J.; Wong-Paz, J.E.; Ascacio-Valdés, J.A.; Chávez-González, M.L.; Contreras-Esquivel, J.C.; Aguilar, C.N. Procyanidins: From agro-industrial waste to food as bioactive molecules. Foods 2021, 10, 3152. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, Ș.C.; Răchișan, A.L.; Negrean, V.; Perné, M.-G.; Donca, V.I.; Alexescu, T.-G.; Para, I.; et al. The effects of flavonoids in cardiovascular diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef] [PubMed]
- Dhanalakshmi, C.; Janakiraman, U.; Manivasagam, T.; Thenmozhi, A.J.; Essa, M.M.; Kalandar, A.; Khan, M.A.S.; Guillemin, G.J. Vanillin attenuated behavioural impairments, neurochemical deficits, oxidative stress and apoptosis against rotenone induced rat model of Parkinson’s disease. Neurochem. Res. 2016, 41, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Choi, D.-K.; Jung, H.J. Neuroprotective effects of vanillyl alcohol in Gastrodia elata Blume through suppression of oxidative stress and anti-apoptotic activity in toxin-induced dopaminergic MN9D cells. Molecules 2011, 16, 5349–5361. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Granado-Casas, M.; Mauricio, D. Oleic acid in the diet and what it does: Implications for diabetes and its complications. In Bioactive Food as Dietary Interventions for Diabetes, 2nd ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2019; pp. 211–229. [Google Scholar] [CrossRef]
- Choque, B.; Catheline, D.; Rioux, V.; Legrand, P. Linoleic acid: Between doubts and certainties. Biochimie 2014, 96, 14–21. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants-an overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Pilluzza, G.; Bullita, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef]
- Castañeda-Loaiza, V.; Placines, C.; Rodrigues, M.J.; Pereira, C.; Zengin, G.; Uysal, A.; Jeko, J.; Cziáky, Z.; Reis, C.P.; Gaspar, M.M.; et al. If you cannot beat them, join them: Exploring the fruits of the invasive species Carpobrotus edulis (L.) N.E. Br as a source of bioactive products. Ind. Crop. Prod. 2020, 144, 112005. [Google Scholar] [CrossRef]
- Meot-Duros, L.; Magné, C. Antioxidant activity and phenol content of Crithmum maritimum L. leaves. Plant Physiol. Biochem. 2009, 47, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Loaiza, V.; Oliveira, M.; Santos, T.; Schüler, L.; Lima, A.R.; Gama, F.; Salazar, M.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; et al. Wild vs cultivated halophytes: Nutritional and functional diferences. Food Chem. 2020, 333, 127536. [Google Scholar] [CrossRef]
- Bernardini, S.; Tiezzi, A.; Masci, V.L.; Ovidi, E. Natural products for human health: An historical overview of the drug discovery approaches. Nat. Prod. Res. 2018, 32, 1926–1950. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.; Romano, A. Inhibitory properties of phenolic compounds against enzymes linked with human diseases. In Phenolic Compounds-Biological Activity; Soto-Hernandez, M., Palma-Tenango, M., Garcia-Mateos, M.D.R., Eds.; InTechOpen: London, UK, 2017; pp. 581–770. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef]
- Zengin, G.; Uysal, A.; Diuzheva, A.; Gunes, E.; Jekő, J.; Cziáky, Z.; Picot-Allain, C.M.N.; Mahomoodally, M.F. Characterization of phytochemical components of Ferula halophila extracts using HPLC-MS/MS and their pharmacological potentials: A multi-functional insight. J. Pharm. Biomed. Anal. 2018, 160, 374–382. [Google Scholar] [CrossRef]
- Pereira, C.G.; Locatelli, M.; Innosa, D.; Cacciagrano, F.; Polesná, L.; Santos, T.F.; Rodrigues, M.J.; Custódio, L. Unravelling the potential of the medicinal halophyte Eryngium maritimum L.: In vitro inhibition of diabetes-related enzymes, antioxidant potential, polyphenolic profile and mineral composition. S. Afr. J. Bot. 2019, 120, 204–212. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Jekő, J.; Cziáky, Z.; Pereira, C.G.; Custódio, L. The medicinal halophyte Frankenia laevis L. (sea heath) has in vitro antioxidant activity, α-glucosidase inhibition, and cytotoxicity towards hepatocarcinoma cells. Plants 2022, 11, 1353. [Google Scholar] [CrossRef]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Wu, G.; Kang, D.; Zhou, Z.; Song, Y.; Liu, X.; Zhan, P. Contemporary medicinal-chemistry strategies for the discovery of selective butyrylcholinesterase inhibitors. Drug Discov. 2019, 24, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Ghalib, R.M.; Sasikala, P.; Ahmed, K.K.M. Cholinesterase inhibitors from botanicals. Pharmacogn. Rev. 2013, 7, 121–130. [Google Scholar] [CrossRef]
- Noufi, P.; Khoury, R.; Jeyakumar, S.; Grossberg, G.T. Use of cholinesterase inhibitors in non-Alzheimer’s dementias. Drugs Aging 2019, 36, 719–731. [Google Scholar] [CrossRef]
- Shafti, S.S.; Khoei, A.A. Evaluation of usefulness of cholinesterase inhibitor in management of schizophrenia: A controlled clinical trial. Arch. Neurol. Neurosci. 2019, 4, 1–8. [Google Scholar] [CrossRef][Green Version]
- Placines, C.; Castañeda-Loaiza, V.; Rodrigues, M.J.; Pereira, C.G.; Stefanucci, A.; Mollica, A.; Zengin, G.; Llorent-Martínez, E.J.; Castilho, P.C.; Custódio, L. Phenolic profile, toxicity, enzyme inhibition, in silico studies, and antioxidant properties of Cakile maritima Scop. (Brassicaceae) from southern Portugal. Plants 2020, 9, 142. [Google Scholar] [CrossRef]
- Mocan, A.; Moldovan, C.; Zengin, G.; Bender, O.; Locatelli, M.; Simirgiotis, M.; Atalay, A.; Vodnar, D.C.; Rohn, S.; Crișan, G. UHPLC-QTOF-MS analysis of bioactive constituents from two Romanian Goji (Lycium barbarum L.) berries cultivars and their antioxidant, enzyme inhibitory and real-time cytotoxicological evaluation. Food Chem. Toxicol. 2018, 115, 414–424. [Google Scholar] [CrossRef]
- Zolgadri, S.; Bahrami, A.; Khan, M.T.H.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enz. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Lobo, J.M.S. Main benefits and applicability of plant extracts in skin care products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Triphati, P.M.; Tripathi, P.; Kashyap, L.; Singh, V. The role of nitric oxide in inflammatory reactions. FEMS Microbiol. Immunol. 2007, 51, 443–452. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Gangadhar, K.N.; Vizzeto-Duarte, C.; Wubshet, S.G.; Nyberg, N.T.; Barreira, L.; Varela, J.; Custódio, L. Maritime halophyte species from Southern Portugal as sources of bioactive molecules. Mar. Drugs 2014, 12, 2228–2244. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.J.; Neves, V.; Martins, A.; Rauter, A.P.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; Barreira, L.; Custódio, L. In vitro antioxidant and anti-inflammatory properties of Limonium algarvense flowers’ infusions and decoctions: A comparison with green tea (Camellia sinensis). Food Chem. 2016, 200, 322–329. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Castañeda-Loaiza, V.; Monteiro, I.; Pinela, J.; Barros, L.; Abreu, R.M.V.; Oliveira, M.C.; Reis, C.; Soares, F.; Pousão-Ferreira, P.; et al. Metabolomic profile and biological properties of sea lavender (Limonium algarvense Erben) plants cultivated with aquaculture wastewaters: Implications for its use in herbal formulations and food additives. Foods 2021, 10, 3104. [Google Scholar] [CrossRef]
- Essoh, A.; Liberal, Â.; Fernandes, Â.; Dias, M.I.; Pereira, C.; Mandim, F.; Moldão-Martins, M.; Cravo, P.; Duarte, M.P.; Moura, M.; et al. Evaluation of the polyphenolic composition and bioactivities of three native Cabo Verde medicinal plants. Pharmaceuticals 2022, 15, 1162. [Google Scholar] [CrossRef]
- De la Fuente, B.; Pinela, J.; Calhelha, R.; Heleno, S.A.; Ferreira, I.C.F.R.; Barba, F.J.; Berrada, H.; Caleja, C.; Barros, L. Sea Bass (Dicentrarchus labrax) and Sea Bream (Sparus aurata) Head Oils Recovered by Microwave-Assisted Extraction: Nutritional Quality and Biological Properties. Food Bioprod. Process. 2022; in press. [Google Scholar] [CrossRef]
- Li, T.; Liu, X.; Liu, J.; Li, D. Synergistic anti-inflammatory effects of quercetin and catechin via inhibiting activation of TLR4-MyD88-mediated NF-κB and MAPK signaling pathways. Phytother. Res. 2019, 33, 756–767. [Google Scholar] [CrossRef]
- Saad, B.; Azaizeh, H.; Abu-Hijleh, G.; Said, O. Safety of traditional Arab herbal medicine. Evid. Based Complement. Altern. Med. 2006, 3, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.G.; Barreira, L.; Bijttebier, S.; Pieters, L.; Marques, C.; Santos, T.F.; Rodrigues, M.J.; Varela, J.; Custódio, L. Health promoting potential of herbal teas and tinctures from Artemisia campestris subsp. maritima: From traditional remedies to prospective products. Sci. Rep. 2018, 8, 4689. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.G.; Barreira, L.; Neng, N.R.; Nogueira, J.M.F.; Marques, C.; Santos, T.F.; Varela, J.; Custódio, L. Searching for new sources of innovative products for the food industry within halophyte aromatic plants: In vitro antioxidant activity and phenolic and mineral contents of infusions and decoctions of Crithmum maritimum L. Food Chem. Toxicol. 2017, 107, 581–589. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Method 942.05. In Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Uslu, L.; Durmaz, Y.; Duyar, H.A.; Bandarra, N.M. Fatty acids, α-tocopherol and proximate composition of four red macroalgae in the Sinop Bay (Turkey). J. Anim. Vet. Adv. 2013, 12, 29–33. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Mozes, A.; Florindo, C.; Polo, C.; Duarte, C.V.; Custódio, L.; Varela, J. Microplate-based high throughput screening procedure for the isolation of lipid-rich marine microalgae. Biotechnol. Biofuels 2011, 4, 61–73. [Google Scholar] [CrossRef] [PubMed]
- FAO—Food and Agricultural Organization of the United Nations. Food energy—Methods of analysis and conversion factors. Report of a Technical Workshop. FAO Food Nutr. Pap. 2003, 77, 66. [Google Scholar]
| Nutritional Profile | Contents |
|---|---|
| Proximate composition | (g/100 g DW) |
| Ash | 3.52 ± 0.50 |
| Crude protein | 6.55 ± 0.46 |
| Total lipids | 0.98 ± 0.07 |
| Carbohydrates | 88.96 ± 0.39 |
| ME (kcal/100 g DW) | 390.81 ± 1.46 |
| Mineral content | (mg/100 g DW) |
| Macro elements | |
| Calcium (Ca) | 138.57 ± 3.51 |
| Potassium (K) | 1164.31 ± 43.8 |
| Magnesium (Mg) | 116.52 ± 3.06 |
| Sodium (Na) | 152.24 ± 6.89 |
| Trace elements | |
| Iron (Fe) | 3.37 ± 0.29 |
| Manganese (Mn) | 2.57 ± 0.12 |
| Zinc (Zn) | 0.86 ± 0.06 |
| Copper (Cu) | 0.35 ± 0.03 |
| Chromium (Cr) | 0.07 ± 0.00 |
| Nickel (Ni) | 0.07 ± 0.00 |
| Cadmium (Cd) | <LOQ 1 |
| Lead (Pb) | <LOQ 2 |
| Nº | Name | Formula | RT | [M + H]+ | [M − H]− | Water | Acetone | Aq. Acetone | Ethanol | Aq. Ethanol |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 1 | Quinic acid | C7H12O6 | 2.00 | 191.05557 | + | + | + | + | + | |
| 2 | Malic acid | C4H6O5 | 2.17 | 133.01370 | + | + | + | + | + | |
| 3 1 | Shikimic acid | C7H10O5 | 2.29 | 173.04500 | + | + | + | + | + | |
| 4 | Nicotinamide | C6H6N2O | 2.44 | 123.05584 | + | + | + | + | + | |
| 5 | Citric acid | C6H8O7 | 2.57 | 191.01918 | + | + | + | + | + | |
| 6 1 | Gallic acid (3,4,5-Trihydroxybenzoic acid) | C7H6O5 | 3.33 | 169.01370 | + | + | + | + | + | |
| 7 | Gallocatechin | C15H14O7 | 5.74 | 305.06613 | - | + | + | + | + | |
| 8 | Procyanidin C isomer 1 | C45H38O18 | 4.11 | 865.19799 | + | - | - | - | - | |
| 9 | Trihydroxybenzoic acid | C7H6O5 | 5.84 | 169.01370 | + | + | + | + | + | |
| 10 | Protocatechuic acid (3,4-Dihydroxybenzoic acid) | C7H6O4 | 5.96 | 153.01879 | + | + | + | + | + | |
| 11 | Vanilloylhexose | C14H18O9 | 7.81 | 329.08726 | + | + | + | + | + | |
| 12 | Hydroxybenzoic acid isomer 1 | C7H6O3 | 9.37 | 137.02387 | + | + | + | + | + | |
| 13 | Hydroxybenzoic acid isomer 2 | C7H6O3 | 10.42 | 137.02387 | + | + | + | + | + | |
| 14 | Procyanidin B isomer 1 | C30H26O12 | 11.19 | 577.13460 | + | + | + | + | + | |
| 15 | Uralenneoside | C12H14O8 | 11.24 | 285.06105 | + | + | + | + | + | |
| 16 | Procyanidin B isomer 2 | C30H26O12 | 12.41 | 577.13460 | + | + | + | + | + | |
| 17 | Hydroxybenzaldehyde | C7H6O2 | 12.96 | 123.04461 | + | + | + | + | + | |
| 18 | Benzoic acid | C7H6O2 | 12.97 | 121.02896 | - | - | + | - | - | |
| 19 | Procyanidin C isomer 2 | C45H38O18 | 13.31 | 865.19799 | + | - | - | - | - | |
| 20 1 | Epigallocatechin | C15H14O7 | 13.52 | 305.06613 | - | + | + | + | + | |
| 21 1 | Catechin | C15H14O6 | 13.57 | 289.07121 | + | + | + | + | + | |
| 22 | Vanillic acid (4-Hydroxy-3-methoxybenzoic acid) | C8H8O4 | 14.06 | 167.03445 | + | + | + | + | + | |
| 23 1 | Chlorogenic acid (3-O-Caffeoylquinic acid) | C16H18O9 | 14.38 | 355.10291 | + | + | + | + | + | |
| 24 | Caffeic acid | C9H8O4 | 14.63 | 179.03444 | + | + | + | + | + | |
| 25 | Procyanidin B isomer 3 | C30H26O12 | 15.21 | 577.13460 | + | - | + | - | + | |
| 26 1 | Vanillin (4-Hydroxy-3-methoxybenzaldehyde) | C8H8O3 | 15.91 | 153.05517 | + | + | + | + | + | |
| 27 | Procyanidin B isomer 4 | C30H26O12 | 16.92 | 577.13460 | + | - | + | - | + | |
| 28 | Unidentified glucoside | C14H24O10 | 17.06 | 351.12912 | + | - | + | - | + | |
| 29 | Syringaldehyde (3,5-Dimethoxy-4-hydroxybenzaldehyde) | C9H10O4 | 17.45 | 183.06574 | + | + | + | + | + | |
| 30 | 1-Benzofuranecarbaldehyde | C9H6O2 | 17.90 | 147.04461 | + | + | + | + | + | |
| 31 1 | 4-Coumaric acid | C9H8O3 | 17.94 | 163.03952 | + | + | + | + | + | |
| 32 | Caffeoylshikimic acid | C16H16O8 | 17.97 | 335.07670 | + | - | - | - | - | |
| 33 1 | Taxifolin (Dihydroquercetin) | C15H12O7 | 19.25 | 303.05048 | + | + | + | + | + | |
| 34 | Scytalone or isomer | C10H10O4 | 19.32 | 195.06574 | + | + | + | + | + | |
| 35 1 | Ferulic acid | C10H10O4 | 19.34 | 193.05009 | + | + | + | + | + | |
| 36 | Isoorientin (Luteolin-6-C-glucoside) | C21H20O11 | 20.36 | 449.10839 | - | + | + | + | + | |
| 37 | Cudranin (Oxyresveratrol) | C14H12O4 | 20.38 | 245.08139 | - | + | + | + | + | |
| 38 | Sinapyl aldehyde (3,5-Dimethoxy-4-hydroxycinnamaldehyde) | C11H12O4 | 20.42 | 209.08139 | - | + | - | + | - | |
| 39 | Isoferulic acid | C10H10O4 | 20.51 | 193.05009 | + | - | - | + | - | |
| 40 | Isovitexin (Apigenin-6-C-glucoside) | C21H20O10 | 21.98 | 433.11348 | + | + | + | + | + | |
| 41 | Luteolin-O-hexoside | C21H20O11 | 22.06 | 447.09274 | - | + | + | + | + | |
| 42 1 | Isoquercitrin (Quercetin-3-O-glucoside) | C21H20O12 | 22.56 | 463.08765 | - | + | + | + | + | |
| 43 1 | Resveratrol | C14H12O3 | 22.83 | 229.08647 | - | + | + | + | + | |
| 44 | Luteolin-C-pentoside | C20H18O10 | 23.13 | 419.09783 | - | + | - | - | - | |
| 45 | Lumichrome | C12H10N4O2 | 23.80 | 243.08821 | + | - | + | - | + | |
| 46 | Methoxy-trihydroxy(iso)flavone isomer 1 | C16H12O6 | 24.34 | 299.05556 | + | + | + | + | + | |
| 47 | N-trans-Feruloyltyramine | C18H19NO4 | 24.53 | 314.13924 | + | + | + | + | + | |
| 48 | Azelaic acid (Nonanedioic acid) | C9H16O4 | 24.63 | 187.09704 | + | + | + | + | + | |
| 49 1 | Eriodictyol (3′,4′,5,7-Tetrahydroxyflavanone) | C15H12O6 | 24.89 | 287.05556 | + | + | + | + | + | |
| 50 | Pentahydroxy(iso)flavone | C15H10O7 | 25.40 | 303.05048 | - | + | + | + | + | |
| 51 | Methoxy-pentahydroxy(iso)flavone | C16H12O8 | 25.56 | 331.04540 | - | + | + | + | + | |
| 52 | Tetrahydroxyxanthone | C13H8O6 | 25.84 | 259.02427 | - | + | + | - | + | |
| 53 | Methoxy-trihydroxy(iso)flavone isomer 2 | C16H12O6 | 26.01 | 299.05556 | + | + | + | + | + | |
| 54 | Methoxy-trihydroxy(iso)flavone isomer 3 | C16H12O6 | 26.38 | 299.05556 | - | + | + | + | + | |
| 55 1 | Quercetin (3,3′,4′,5,7-Pentahydroxyflavone) | C15H10O7 | 26.72 | 301.03483 | + | + | + | - | + | |
| 56 1 | Luteolin (3′,4′,5,7-Tetrahydroxyflavone) | C15H10O6 | 27.55 | 285.03991 | + | + | + | + | + | |
| 57 | Methoxy-trihydroxy(iso)flavone isomer 4 | C16H12O6 | 27.70 | 299.05556 | - | + | + | + | + | |
| 58 | Sebacic acid (Decanedioic acid) | C10H18O4 | 28.11 | 201.11268 | + | + | + | + | + | |
| 59 1 | Apigenin (4′,5,7-Trihydroxyflavone) | C15H10O5 | 29.44 | 269.04500 | + | + | + | + | + | |
| 60 | Dimethoxy-tetrahydroxy(iso)flavone | C17H14O8 | 29.51 | 345.06105 | - | + | + | + | + | |
| 61 | Dimethoxy-trihydroxy(iso)flavone isomer 1 | C17H14O7 | 29.57 | 329.06613 | - | + | - | + | - | |
| 62 | Chrysoeriol (3′-Methoxy-4′,5,7-trihydroxyflavone) | C16H12O6 | 29.63 | 299.05556 | + | + | + | + | + | |
| 63 | Dimethoxy-trihydroxy(iso)flavone isomer 2 | C17H14O7 | 30.45 | 329.06613 | - | + | - | + | - | |
| 64 | Undecanedioic acid | C11H20O4 | 31.02 | 215.12834 | + | + | + | + | + | |
| 65 | Dimethoxy-trihydroxy(iso)flavone isomer 3 | C17H14O7 | 31.36 | 329.06613 | - | + | - | + | - | |
| 66 | Hydroxydodecenoic acid | C12H22O3 | 32.47 | 213.14907 | + | + | + | + | + | |
| 67 | Pinellic acid | C18H34O5 | 33.61 | 329.23280 | + | - | + | + | + | |
| 68 | Hydroxyoctadecadienoic acid | C18H32O3 | 41.09 | 295.22732 | + | + | + | + | + | |
| 69 1 | α-Linolenic acid | C18H30O2 | 44.82 | 277.21676 | - | + | + | + | + | |
| 70 | 2-Hydroxyhexadecanoic acid | C16H32O3 | 45.11 | 271.22732 | - | + | + | + | + | |
| 71 1 | Linoleic acid | C18H32O2 | 45.81 | 279.23241 | + | + | + | + | + | |
| 72 1 | Oleic acid | C18H34O2 | 46.89 | 281.24806 | + | + | + | + | + |
| Sample | DPPH• | ABTS•+ | NO | FRAP | CCA | ICA |
|---|---|---|---|---|---|---|
| Water | 5.10 ± 0.27 e | 1.38 ± 0.16 c | 8.19 ± 0.89 b | 2.23 ± 0.07 d | 2.10 ± 0.06 d | 2.64 ± 0.05 b |
| Acetone | 1.50 ± 0.07 d | 1.85 ± 0.20 d | >10 | 0.97 ± 0.02 c | 2.09 ± 0.02 d | >10 |
| Aq. Acetone | 0.21 ± 0.01 a | 0.13 ± 0.01 a | >10 | 0.13 ± 0.00 a | 0.83 ± 0.01 b | >10 |
| Ethanol | 0.93 ± 0.12 c | 0.68 ± 0.02 b | >10 | 0.37 ± 0.01 b | 2.93 ± 0.06 e | >10 |
| Aq. Ethanol | 0.38 ±0.02 ab | 0.22 ± 0.01 a | >10 | 0.36 ± 0.01 b | 1.14 ± 0.04 c | >10 |
| BHA * | 0.60 ± 0.03 bc | 0.33 ± 0.02 a | 0.16 ± 0.01 a | |||
| EDTA * | 0.16 ± 0.00 a | 0.03 ± 0.01 a | ||||
| Ascorbic acid * | 1.71 ± 0.02 a |
| Sample | AChE (mg GALAE/g) | BChE (mg GALAE/g) | Tyrosinase (mg KAE/g) | α-Glucosidase (mmol ACAE/g) | α-Amylase (mmol ACAE/g) |
|---|---|---|---|---|---|
| Water | 3.73 ± 0.13 b | 5.13 ± 0.78 ab | 61.81 ± 0.50 c | 1.05 ± 0.01 b | 1.12 ± 0.02 a |
| Acetone | 3.89 ± 0.17 ab | 5.05 ± 0.31 ab | 55.04 ± 0.69 d | 1.05 ± 0.01 b | 0.81 ± 0.01 c |
| Aq. Acetone | 3.92 ± 0.05 ab | 3.47 ± 0.48 b | 70.26 ± 1.59 ab | 1.10 ± 0.02 a | 0.82 ± 0.02 c |
| Ethanol | 4.21 ± 0.19 a | n.a. | 68.64 ± 0.30 b | 1.06 ± 0.01 b | 0.95 ± 0.02 b |
| Aq. Ethanol | 3.83 ± 0.17 ab | 6.02 ± 1.39 a | 70.99 ± 0.57 a | 1.07 ± 0.01 b | 0.92 ± 0.03 b |
| Sample | NO Decrease (%) |
|---|---|
| Water | 36.45 ± 7.37 cd |
| Acetone | 26.98 ± 3.80 d |
| Aq. Acetone | n.a. |
| Ethanol | 38.05 ± 3.02 c |
| Aq. Ethanol | 60.09 ± 4.11 b |
| L-NAME * | 73.40 ± 4.28 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, M.J.; Custódio, L.; Mecha, D.; Zengin, G.; Cziáky, Z.; Sotkó, G.; Pereira, C.G. Nutritional and Phyto-Therapeutic Value of the Halophyte Cladium mariscus L. (Pohl.): A Special Focus on Seeds. Plants 2022, 11, 2910. https://doi.org/10.3390/plants11212910
Rodrigues MJ, Custódio L, Mecha D, Zengin G, Cziáky Z, Sotkó G, Pereira CG. Nutritional and Phyto-Therapeutic Value of the Halophyte Cladium mariscus L. (Pohl.): A Special Focus on Seeds. Plants. 2022; 11(21):2910. https://doi.org/10.3390/plants11212910
Chicago/Turabian StyleRodrigues, Maria João, Luísa Custódio, Débora Mecha, Gokhan Zengin, Zoltán Cziáky, Gyula Sotkó, and Catarina Guerreiro Pereira. 2022. "Nutritional and Phyto-Therapeutic Value of the Halophyte Cladium mariscus L. (Pohl.): A Special Focus on Seeds" Plants 11, no. 21: 2910. https://doi.org/10.3390/plants11212910
APA StyleRodrigues, M. J., Custódio, L., Mecha, D., Zengin, G., Cziáky, Z., Sotkó, G., & Pereira, C. G. (2022). Nutritional and Phyto-Therapeutic Value of the Halophyte Cladium mariscus L. (Pohl.): A Special Focus on Seeds. Plants, 11(21), 2910. https://doi.org/10.3390/plants11212910








