Immunomodulatory Effects of Plant Extracts from Salvia deserta Schang. and Salvia sclarea L.
Abstract
1. Introduction
2. Results
2.1. Testing the Quality of Raw Materials
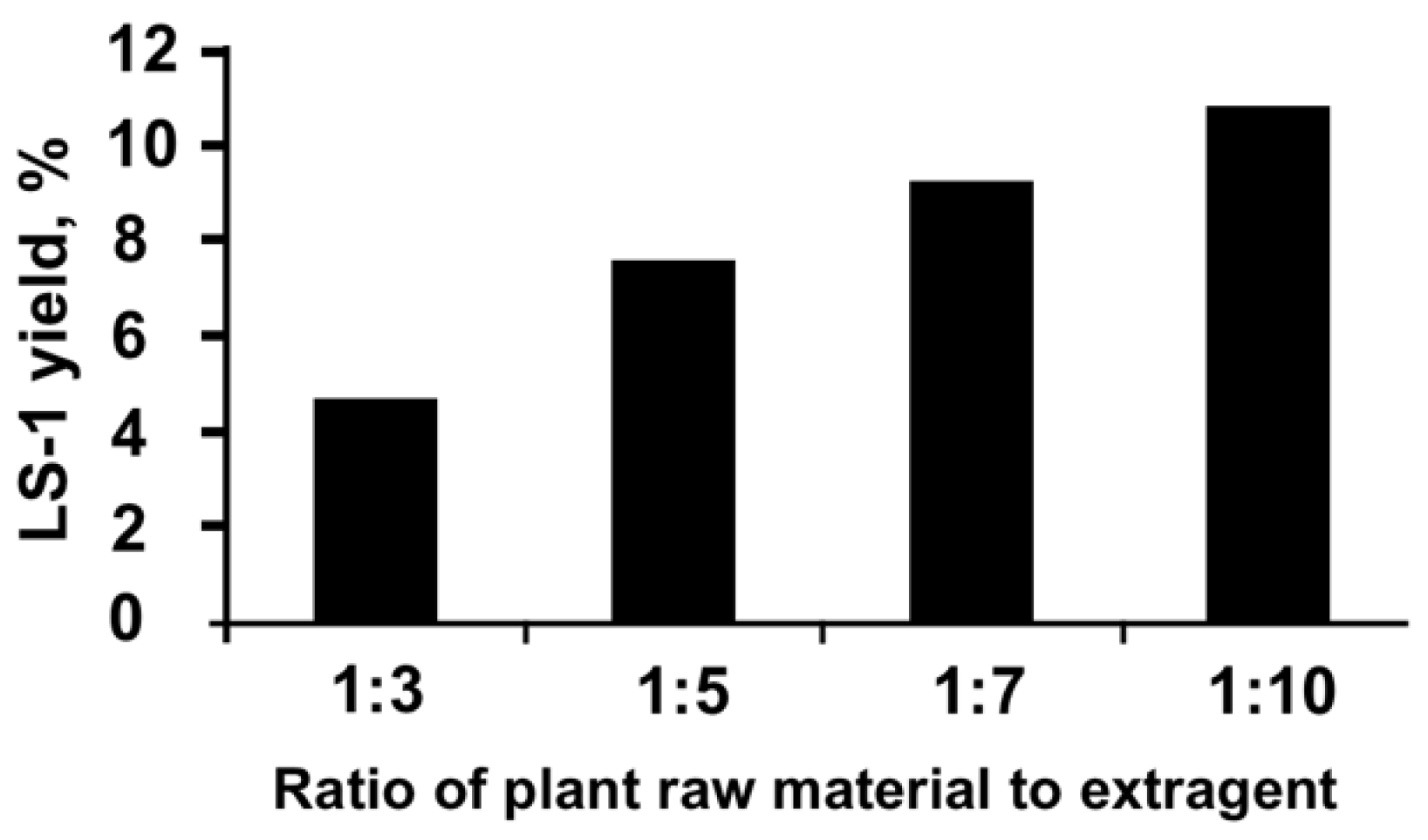
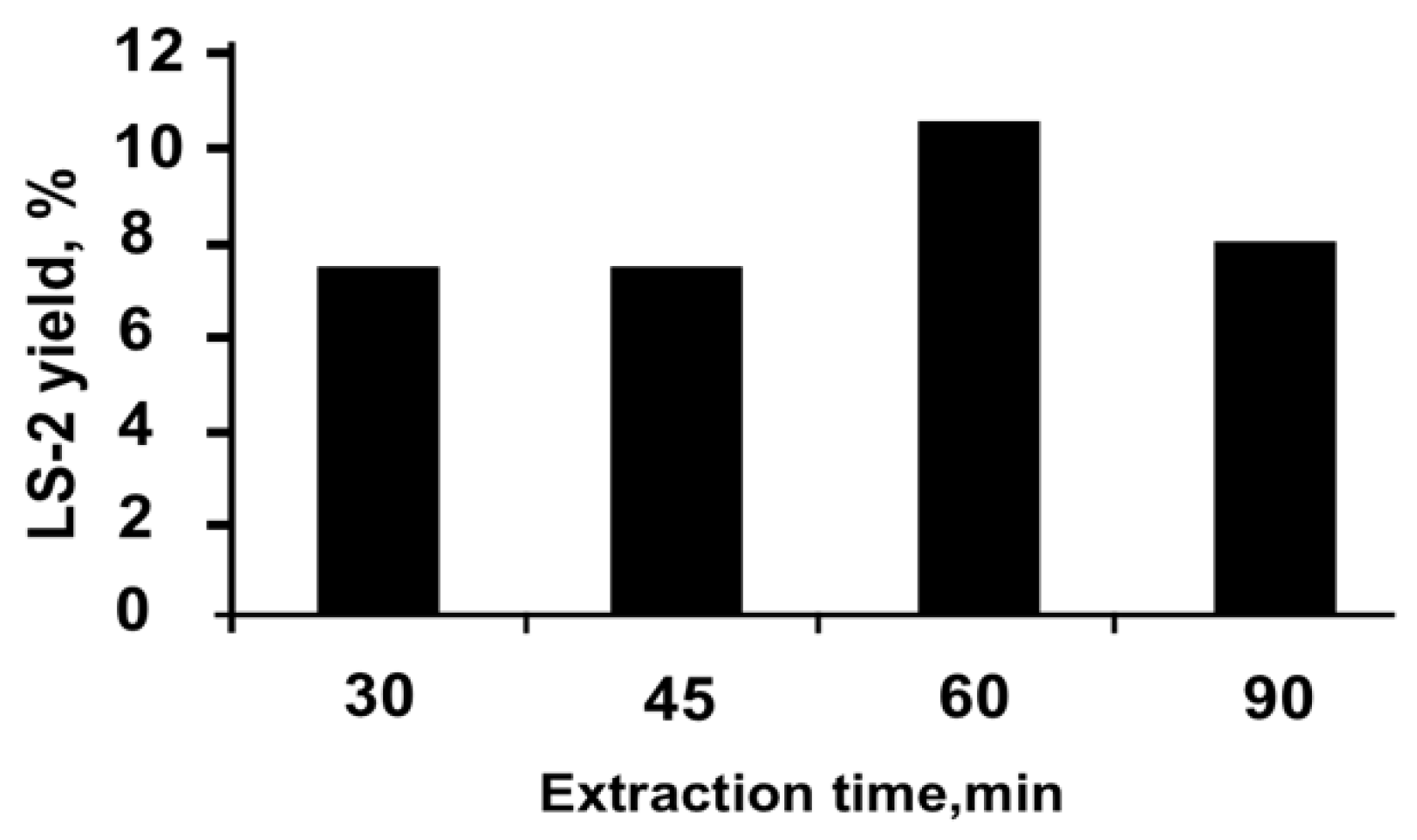
2.2. Preparation of the Extracts
2.3. Chemical Profiling of LS-2 and LS-4 Extracts Using LC-DAD-QTOF-MS
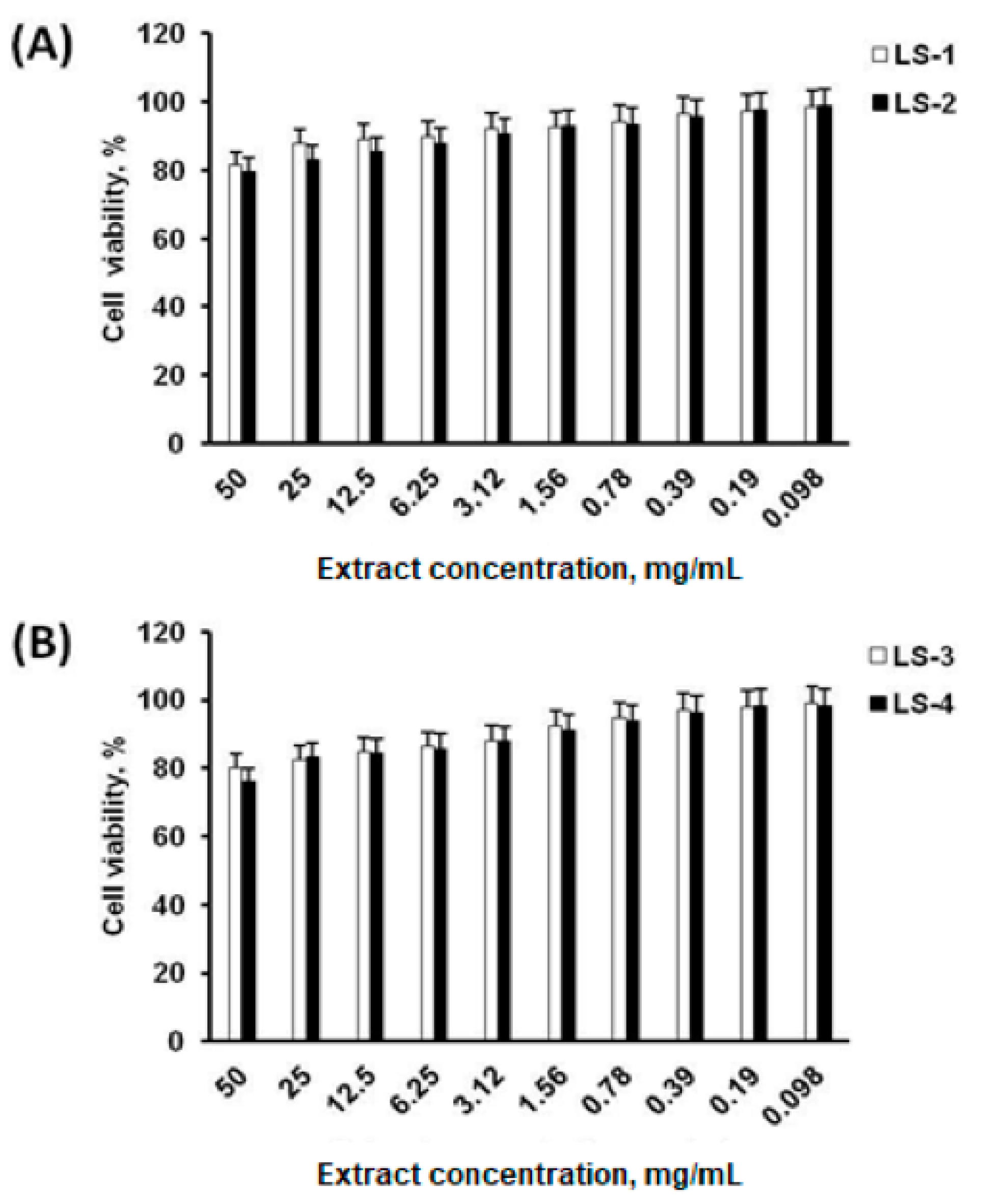
2.4. Cell Viability
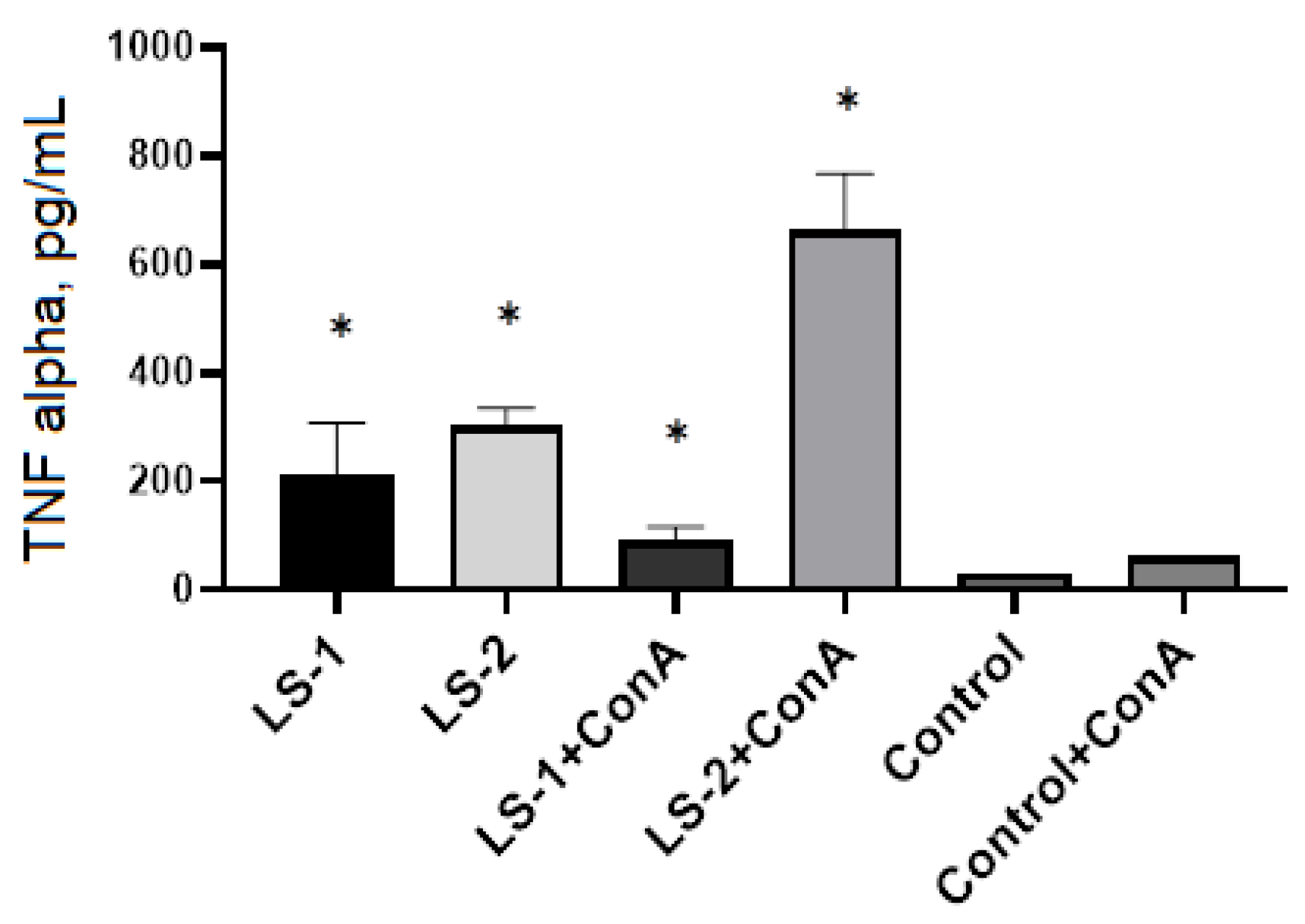
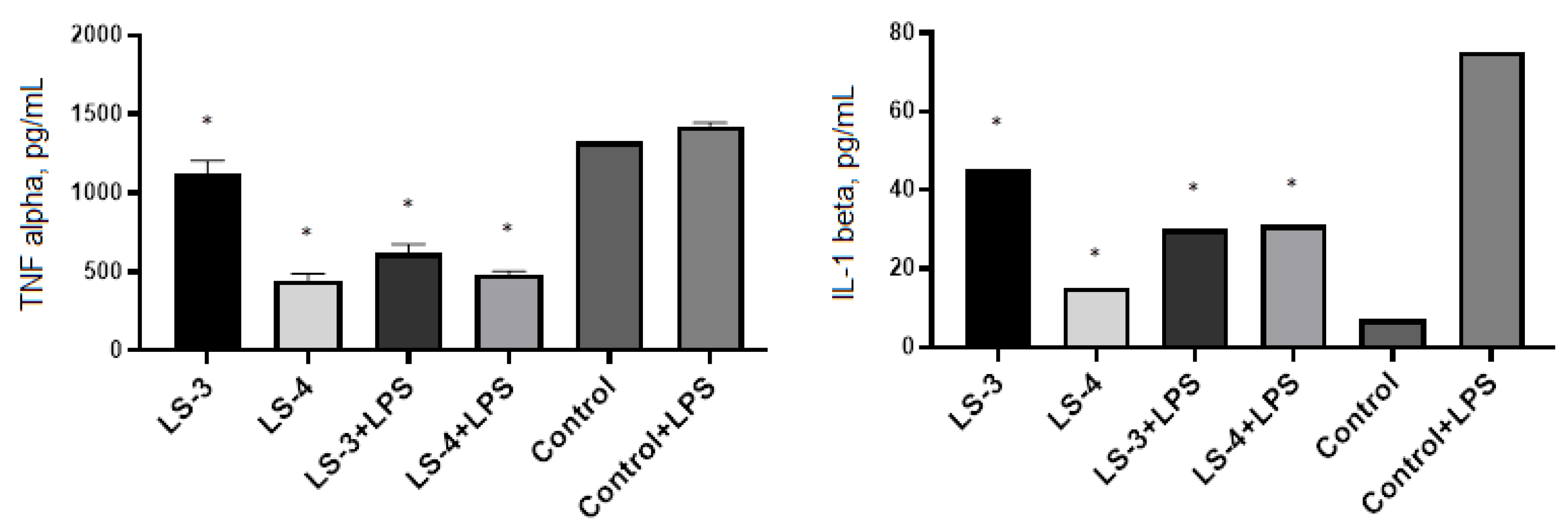
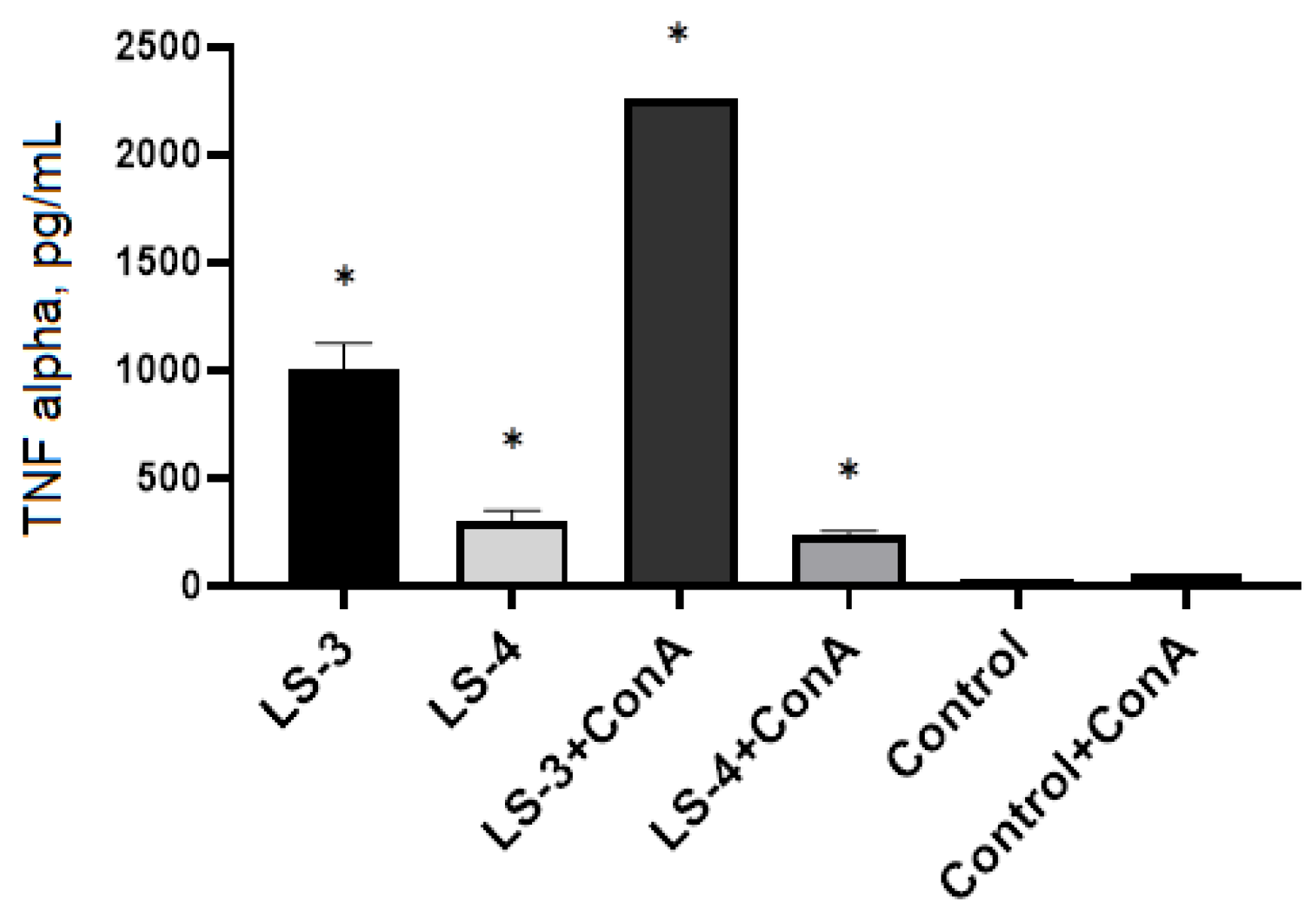
2.5. Immunomodulatory Effects of Sage Extracts
3. Discussion
4. Materials and Methods
4.1. Checking the Quality of Plant Materials and Obtaining the Extracts
4.2. Preparation of Extracts
4.3. Chemical Profiling of LS-2 and LS-4 Extracts Using LC-DAD-QTOF-MS
4.4. Animals
4.5. Cell Line
4.6. Isolation of Peritoneal Macrophages
4.7. Isolation of Spleen Lymphocytes
4.8. Cytotoxicity Test
4.9. Cytokine Detection in Cell Supernatants
4.10. Phagocytosis Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| # | RT (min) | Compound Name | Mol. Formula | [M+H]+ | Fragment Ions | [M-H]− | Fragment Ions | Identities | |
|---|---|---|---|---|---|---|---|---|---|
| S. deserta Schang. | S sclarea L. | ||||||||
| 1 | 1.58 | Valine | C5H11NO2 | 118.0865 (118.0863) | 72.0812 | - | - | + | + |
| 2 | 1.6 | Proline | C5H9NO2 | 116.0709 (116.0706) | 70.0657 | - | - | ND | + |
| 3 | 1.8 | Luecine/ Isoleucine | C6H13NO2 | 132.1015 (132.1019) | - | - | - | + | + |
| 4 | 1.94 | ||||||||
| 5 | 1.85 | Tyrosine | C9H11NO3 | 182.0810 (182.0812) | 70.0655 | - | - | + | + |
| 6 | 1.99 | Succinic acid | C4H6O4 | - | - | 117.0195 (117.0193) | - | + | + |
| 7 | 2.4 | Phenylalanine | C9H11NO2 | 166.0862 (166.0863) | 120.0786, 103.0524 | - | - | + | + |
| 8 | 2.57 | Danshensu (Danshensuan A, 3,4-dihydroxy- phenyllactic acid) | C9H10O5 | 199.0603 (199.0601) | 135.0461 | 197.0455 (197.0455) | 179.0349 [M-H-H2O]− 135.0454 [M-H-H2O-CO2]− | + | + |
| 9 | 2.68 | Vanillic acid | C8H8O4 | 169.0493 (169.0495) | - | 167.0349 (167.0350) | - | + | + |
| 10 | 3.5 | Tryptophan | C11H12N2O2 | 205.0974 (205.0972) | 188.0707, 115.0543, 91.0545 | - | - | + | + |
| 11 | 3.8 | dihydrocaffeic acid or DHCA | C9H10O4 | - | - | 181.0501 (181.0506) | 161.0239; 135.0445 | + | + |
| 12 | 4.24 | Protocatechualdehyde/Monohydroxy benzoic acid (HBA) | C7H6O3 | 139.0391 (139.039) | 111.0441 [M+H-CO]+ | 137.0239 (137.0244) | 109.0297 [M+H-CO]− | + | + |
| 13 | 5.1 | Caffeic acid | C9H8O4 | 181.0493 (181.0495) | 163.0391 [M+H-H2O]+ | 179.0350 (179.035) | 135.0454 [M-H-CO2]− | + | + |
| 14 | 6.64 | Quercetin glycoside (Flavonol glycoside) | C21H20O12 | 465.1025 (465.1028) | 303.0501 | 463.0885 (463.0882) | 300.0181 | + | ND |
| 15 | 7.2 | Salviaflaside | C24H26O13 | - | - | 521.1298 (521.1301) | 359.0715 | ND | + |
| 16 | 7.42 | Luteolin 7-O-β-rutinoside | C27H30O15 | 595.1660 (595.1657) | 287.0552 [M+H-162-146]+ | 593.1508 (593.1512) | 285.0404 [M+H-162-146]+ | + | ND |
| 17 | 7.65 | Ferulic acid | C10H10O4 | 195.0653 (195.0652) | - | 193.0507 (193.0506) | 149.0609 [M-H-CO2]− 134.0370 [M-H-CO2-CH3]− | + | ND |
| 18 | 7.83 | Isoquercitrin (Quercetin 3-O-β-D-glucopyranoside) | C21H20O12 | 465.1029 (465.1028) | 303.0490 [M+H-162]+ | 463.0881 (463.0882) | 300.0188 | + | ND |
| 19 | 7.84 | Luteolin 7-O-glucoside | C21H20O11 | 449.1079 (449.1078) | 287.0545 [M+H-Glc]+ | 447.0931 (447.0933) | 285.0399 | + | + |
| 20 | 7.89 | Luteolin 7-O-glucuronide/Kaempferol-glucoronide | C21H18O12 | 463.0875 (463.0871) | 287.0537 [M+H-176]+ | 461.0723 (461.0725) | 285.0405 | + | + |
| 21 | 9.75 | ND | + | ||||||
| 22 | 8.61 | Apigenin-rutinoside isomer | C27H30O14 | 579.1706 (579.1708) | 271.0591 [M+H-162-146]+ | 577.1561 (577.1563) | 269.0455 | + | ND |
| 23 | 9.11 | Apigenin-7-O-glucoside (Flavone Glycosides) | C21H20O10 | 433.1131 (433.1129) | 271.0580 [M+H-162]+ | 431.0987 (431.0984) | 269.0441 | + | + |
| 24 | 9.23 | Apigenin 7-O-glucuronide | C21H18O11 | 447.0919 (447.0922) | 271.0595 [M+H-176]+ | 445.0775 (445.0776) | 269.0435 | + | + |
| 25 | 9.41 | Salvianolic acid K | C27H24O13 | 555.1147 (555.1144) | 359.0738, 161.0231, 135.0439 | + | + | ||
| 26 | 9.5 | Chrysoeriol 7-glucuronide | C22H20O12 | 477.1031 (477.1028) | 301.0711 | 475.0885 (487.0882) | 299.0565 | + | + |
| 27 | 9.6 | Rosmarinic acid | C18H16O8 | 361.0920 (361.0918) | 163.0390 [M+H-C9H10O5]+ or [caffeic acid-H-H2O]+; 135.0441 [caffeic acid-H- H2O-CO]+ | 359.0774 (359.0772) | 197.0456 [M-H-162]−; 179.0352 [M-H-180]−; 161.0247 [M-H- C9H10O5]− | + | + |
| 28 | 10.3 | Lithospermic acid B | C27H22O12 | 539.1190 (539.1188) | - | 537.1041 (537.1038) | 493.1137 [M-H-CO2]; 313.0718 [M-H-CO2-180]− 295.0610 [M-H-CO2-198]− 135.0455 | + | + |
| 29 | 11.7 | Tetramethoxyflavone | C19H18O8 | 375.1071 (375.1074) | 177.0550 | 373.0929 (373.0929) | 197.0457, 175.0402, 135.0455 | + | + |
| 30 | 12.2 | Horminone | C20H28O4 | - | - | 331.1916 (331.1915) | - | + | ND |
| 31 | 12.3 | Luteolin | C15H10O6 | 287.0555 (287.055) | 153.0176 (C7H5O4) 135.0421 (RDA fragments) | 285.0408 (285.0405) | - | + | + |
| 32 | 13.3 | Methoxy- coumarin | C10H8O3 | 177.0547 (177.0546) | 163.0391, 145.0286, 117.0335, 89.0390 | - | - | + | + |
| 33 | 13.6 | Salvianolic acid B | C36H30O16 | 719.1602 (719.1607) | 717.1462 (717.1461) | + | ND | ||
| 34 | 13.91 | Apigenin (Flavone) | C15H10O5 | 271.0603 (271.0601) | 269.0451 (269.0455) | + | + | ||
| 35 | 14.3 | Hispidulin (Flavone) | C16H12O6 | 301.0698 (301.0707) | 286.0499 | 299.0565 (299.0561) | 284.0311 [M-H-GluA-CH3]− | ND | + |
| 36 | 14.41 | 3′,4′,5-Trihydroxy-7-methoxyflavone | C16H12O6 | 301.0710 (301.0707) | 286.0471 | ND | + | ||
| 37 | 14.54 | Jaceosidin (trihydroxyflavone) | C17H14O7 | 331.0815 (331.0812) | 315.0517 | 329.0657 (329.0667) | 314.0328 | + | + |
| 38 | 14.6 | Viscosine | C17H14O7 | 331.0801 (331.0812) | 315.0498 | 329.0617 (329.0667) | - | + | + |
| 39 | 15.2 | Rosmanol | C20H26O5 | - | - | 345.1708 (345.1707) | 301.1808 | + | ND |
| 40 | 15.9 | Dihydroxy-dimethoxyflavone | C17H14O6 | 315.0860 (315.0863) | 254.0569 | - | - | ND | + |
| 41 | 16.54 | ND | + | ||||||
| 42 | 15.83 | Hydroxycarnosic acid | C20H28O5 | - | - | 347.1859 (347.1864) | - | + | ND |
| 43 | 16.07 | Cirsimaritin | C17H14O6 | 315.0861 (315.0863) | - | 313.0715 (313.0718) | 298.0481 [M-H-CH3]; 283.0235 [M-H-CH3-CH3]− | + | + |
| 44 | 16.3 | Eupatorin (3′,5-Dihydroxy-4′,6,7-tri methoxyflavone) | C18H16O7 | 345.0971 (345.0969) | 330.0655 [M+H-15]+, 312.0599 [M+H-15-18]+, 269.0411 [M+H-15-18-28-15]+ | - | - | + | + |
| 45 | 17.36 | Genkwanin (Flavone) | C16H12O5 | 285.0760 (285.0757) | 242.0569 | 283.0613 (283.0612) | 268.0384 | + | + |
| 46 | 17.7 | Pectolinarigenin (Dihydroxy-dimethoxyflavone) | C17H14O6 | 315.0869 (315.0863) | 300.0624 [M+H-15]+; 282.0522 [M+H-15-18]+ | 313.0718 (313.0718) | 298.0470 283.0233 255.0300 | t | + |
| 47 | 18.3 | Carnosic acid | C20H28O4 | 333.2061 (333.2060) | 315.1958 [M+H-H2O]+ 287.2008 | 331.1917 (331.1915) | - | + | ND |
| 48 | 18.5 | labdadien-olides | C25H38O5 | 441.2613 (441.2611) [M+Na]+ | 401.2689 [M+H-H2O]+ | - | - | + | ND |
| 49 | 18.9 | Tormentic acid | C30H48O5 | 487.3435 (487.3429) | 469.3301 [M-H-18]− | + | ND | ||
| 50 | 19.2 | Salvigenin (5-hydroxy-4′, 6, 7-trimethoxy flavone) | C18H16O6 | 329.1021 (329.1020) | 296.0671 [M+H-15-18]+; 268.0735 [M+H-15-18-28]+ | 327.0875 (327.0874) | - | + | + |
| 51 | 20.43 | 1,11,20-Trihydroxy-3-lupanone (1β,11α)-form | C30H50O4 | - | - | 475.3789 (475.3782) | - | + | ND |
| 52 | 21.5 | Maslinic acid | C30H48O4 | - | - | 471.3477 (471.3480) | 417.3000 | + | + |
| 53 | 21.73 | Hydroxyursolic acid | C30H48O4 | 473.3625 (473.3625) | 455.3516 [M+H-H2O]+ 437.3402; 409.3484 | 471.3477 (471.348) | - | + | + |
| 54 | 21.81 | 2α-Hydroxyursolic acid | C30H48O4 | 473.3625 (473.3625) | 455.3516 [M+H-H2O]+ | 471.3481 (471.3480) | - | + | + |
| 55 | 24.1 | Ursolic acid/Oleanolic acid/3-Epiursolic acid | C30H48O3 | 457.3680 (457.3676) | 439.3573 [M+H-H2O]+ | 455.3530 (455.3531) | - | + | ND |
| 56 | 24.6 | + | + | ||||||
| 57 | 24.7 | + | + | ||||||
References
- Muyumba, N.W.; Mutombo, S.C.; Sheridan, H.; Nachtergael, A.; Duez, P. Quality control of herbal drugs and preparations: The methods of analysis, their relevance and applications. Talanta Open 2021, 4, 100070. [Google Scholar] [CrossRef]
- Sarsenbayev, K.N. Chapter 10. Medicinally important plants of Kazakhstan. In Vegetation of Central Asia and Environs; Egamberdieva, D., Öztürk, M., Eds.; Springer: Cham, Switzerland, 2018; p. 264. [Google Scholar]
- Zhusupova, G.E.; Litvinenko, Y.A.; Zhussupova, A.I. Methodology for Processing Medicinal Plant Materials; Qazaq University Press: Almaty, Kazakhstan, 2018; 150p. [Google Scholar]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Aleksovski, A.; Sovova, H. Supercritical CO2 extraction of Salvia officinalis L. J. Supercrit. Fluids 2007, 40, 239–245. [Google Scholar] [CrossRef]
- Sa, C.; Ramos, A.; Azevedo, M.; Lima, C.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Sage tea drinking improves lipid profile and antioxidant defences in humans. Int. J. Mol. Sci. 2009, 10, 3937–3950. [Google Scholar] [CrossRef]
- Lu, Y.; Yeap Foo, L. Salvianolic acid L, a potent phenolic antioxidant from Salvia officinalis. Tetrahedron Lett. 2001, 42, 8223–8225. [Google Scholar] [CrossRef]
- Hamidpour, M.; Hamidpour, R.; Hamidpour, S.; Shahlari, M. Chemistry, pharmacology, and medicinal property of sage (Salvia) to prevent and cure illnesses such as obesity, diabetes, depression, dementia, lupus, autism, heart disease, and cancer. J. Tradit. Complement. Med. 2014, 4, 82–88. [Google Scholar] [CrossRef]
- Ayoub, I.M.; George, M.Y.; Menze, E.T.; Mahmoud, M.; Botros, M.; Essam, M.; Ashmawy, I.; Shendi, P.; Hany, A.; Galal, M.; et al. Insights into the neuroprotective effects of Salvia officinalis L. and Salvia microphylla Kunth in the memory impairment rat model. Food Funct. 2022, 13, 2253–2268. [Google Scholar] [CrossRef]
- Christensen, K.B.; Jorgenson, M.; Kotowska, D.; Peterson, R.K.; Kristiansen, K.; Christensen, L.P. Activation of the nuclear receptor PPARγ by metabolites isolated from sage (Salvia officinalis L.). J. Ethnopharmacol. 2010, 132, 127–133. [Google Scholar] [CrossRef]
- Javid, H.; Moein, S.; Moein, M. An investigation of the inhibitory effects of dichloromethane and methanol extracts of Salvia macilenta, Salvia officinalis, Salvia santolinifola and Salvia mirzayanii on diabetes marker enzymes, an approach for the treatment diabetes. Clin. Phytosci. 2022, 8, 7. [Google Scholar] [CrossRef]
- el Hadri, A.; Gomez del Rio, M.A.; Sanz, J.; Coloma, A.G.; Idaomar, M.; Ribas Ozanas, B.; Benedí González, J.; Sánchez Reus, M.I. Cytotoxic activity of α-humulene and transcaryo-phyllene from Salvia officinalis in animal and human tumor cells. An. R. Acad. Nac. Farm. 2010, 76, 343–356. [Google Scholar]
- Privitera, G.; Luca, T.; Castorina, S.; Passanisi, R.; Ruberto, G.; Napoli, E. Anticancer activity of Salvia officinalis essential oil and its principal constituents against hormone-dependent tumour cells. Asian Pac. J. Trop. Biomed. 2019, 9, 24–28. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Menichini, F.; Saab, A.M.; Statti, G.A.; Menichini, F. Cytotoxic activity of essential oils from Labiatae and Lauraceae families against in vitro human tumor models. Anticancer Res. 2007, 27, 3293–3299. [Google Scholar]
- Capek, P.; Hríbalová, V. Water-soluble polysaccharides from Salvia officinalis L. possessing immunomodulatory activity. Phytochemistry 2004, 65, 1983–1992. [Google Scholar] [CrossRef]
- Bonesi, M.; Loizzo, M.R.; Acquaviva, R.; Malfa, G.A.; Aiello, F.; Tundis, R. Anti-inflammatory and antioxidant agents from Salvia genus (Lamiaceae): An assessment of the current state of knowledge. Med. Chem. 2017, 16, 70–86. [Google Scholar] [CrossRef]
- Bekut, M.; Brkic, S.; Kladar, N.; Dragovic, G.; Gavaric, N.; Bozin, B. Potential of selected Lamiaceae plants in anti (retro) viral therapy. Pharmacol. Res. 2018, 133, 301–314. [Google Scholar] [CrossRef]
- Kylyshbaeva, G.B.; Bozshataeva, G.T.; Ospanova, G.S. Study of biologically active compounds in species of the genus Salvia (Salvia L., Lamiaceae) in the conditions of the South Kazakhstan region. Int. J. Appl. Fund. Res. 2013, 10, 76–77. [Google Scholar]
- Cui, N.; Chen, T.; Liao, B.; Xu, J.; Li, X. The biology of medicinal resource substitution in Salvia. Chin. Med. 2021, 16, 141. [Google Scholar] [CrossRef]
- Wu, Y.-B.; Ni, Z.-Y.; Shi, Q.-W.; Dong, M.; Kiyota, H.; Gu, Y.-C.; Cong, B. Constituents from Salvia species and their biological activities. Chem. Rev. 2012, 112, 5967–6026. [Google Scholar] [CrossRef]
- Vosoughi, N.; Gomarian, M.; Ghasemi Pirbalouti, A.; Khaghani, S.; Malekpoor, F. Essential oil composition and total phenolic, flavonoid contents, and antioxidant activity of sage (Salvia officinalis L.) extract under chitosan application and irrigation frequencies. Ind. Crops Prod. 2018, 117, 366–374. [Google Scholar] [CrossRef]
- Pourhosseini, M.; Asgarpanah, J. Essential and fixed oil chemical profiles of Salvia aegyptiaca L. flowers and seeds. J. Chil. Chem. Soc. 2015, 60, 2747–2748. [Google Scholar] [CrossRef][Green Version]
- Kasimu, R.; Wang, X.; Wang, X.; Hu, J.; Wang, X.; Mu, Y. Antithrombotic effects and related mechanisms of Salvia deserta Schang root EtOAc extracts. Sci. Rep. 2018, 8, 17753. [Google Scholar] [CrossRef]
- Li, M.; Li, Q.; Zhang, C. An ethnopharmacological investigation of medicinal Salvia plants (Lamiaceae) in China. Acta Pharm. Sin. B 2013, 3, 273–280. [Google Scholar] [CrossRef]
- Zheng, X.; Kadir, A.; Zheng, G.; Jin, P.; Qin, D.; Maiwulanjiang, M.; Aisa, H.A.; Yao, G. Antiproliferative abietane quinone diterpenoids from the roots of Salvia deserta. Bioorg. Chem. 2020, 104, 104261. [Google Scholar] [CrossRef]
- Llurba-Montesino, N.; Schmidt, T.J. Salvia species as sources of natural products with antiprotozoal activity. Int. J. Mol. Sci. 2018, 19, 264. [Google Scholar] [CrossRef]
- Aćimović, M.; Kiprovski, B.; Rat, M.; Sikora, V.; Popović, V.; Koren, A.; Brdar-Jokanović, M. Salvia sclarea: Chemical composition and biological activity. J. Agron. Technol. Eng. Manag. 2018, 1, 18–28. [Google Scholar]
- Yalcin, H.; Ozturk, I.; Tulukcu, E.; Sagdic, O. Effect of γ-irradiation on bioactivity, fatty acid compositions and volatile compounds of clary sage seed (Salvia sclarea L.). J. Food Sci. 2011, 76, C1056–C1061. [Google Scholar] [CrossRef]
- Wong, J.; Chiang, Y.F.; Shih, Y.H.; Chiu, C.-H.; Chen, H.-Y.; Shieh, T.-M.; Wang, K.-L.; Huang, T.-C.; Hong, Y.-H.; Hsia, S.-M. Salvia sclarea L. essential oil extract and its antioxidative phytochemical sclareol inhibit oxytocin-induced uterine hypercontraction dysmenorrhea model by inhibiting the Ca2+-MLCK-MLC20 signaling cascade: An ex vivo and in vivo study. Antioxidants 2020, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M. Clary sage essential oil and its biological activities. Adv. Tradit. Med. 2020, 20, 517–528. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Zhou, H.; Zhao, C.; Lin, L. Antimicrobial activity and mechanisms of Salvia sclarea essential oil. Bot. Stud. 2015, 56, 16. [Google Scholar] [CrossRef] [PubMed]
- Jasicka-Misiak, I.; Poliwoda, A.; Petecka, M.; Buslovych, O.; Shlyapnikov, V.; Wieczorek, P.P. Antioxidant phenolic compounds in Salvia officinalis L. and Salvia sclarea L. Ecol. Chem. Eng. S 2018, 25, 133–142. [Google Scholar] [CrossRef]
- State Pharmacopeia of the Republic of Kazakhstan; Zhibek Zholy: Almaty, Kazakhstan, 2008; Volume 1, p. 592.
- State Pharmacopeia of the Republic of Kazakhstan; Zhibek Zholy: Almaty, Kazakhstan, 2009; Volume 2, p. 804.
- State Pharmacopeia of the Republic of Kazakhstan; Zhibek Zholy: Almaty, Kazakhstan, 2014; Volume 3, p. 872.
- Pagare, S.; Bhatia, M.; Tripathi, N.; Pagare, S.; Bansal, Y.K. Secondary metabolites of plants and their role: Overview. Curr. Trends Biotechnol. Pharm. 2015, 9, 293–304. [Google Scholar]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Jantan, I.; Ahmad, W.; Bukhari, S.N.A. Plant-derived immunomodulators: An insight on their preclinical evaluation and clinical trials. Front. Plant Sci. 2015, 6, 655. [Google Scholar] [CrossRef]
- Samec, M.; Liskova, A.; Koklesova, L.; Mathews, S.; Radovan, S.; Pavol, M. The role of plant—Derived natural substances as immunomodulatory agents in carcinogenesis. J. Cancer Res. Clin. Oncol. 2020, 146, 3137–3154. [Google Scholar] [CrossRef]
- Wong, C.K.; Bao, Y.X.; Wong, E.L.; Leung, P.C.; Fung, K.P.; Lam, C.W. Immunomodulatory activities of Yunzhi and Danshen in post-treatment breast cancer patients. Am. J. Chin. Med. 2005, 33, 381–395. [Google Scholar] [CrossRef]
- Wong, C.K.; Tse, P.S.; Wong, E.L.; Leung, P.C.; Fung, K.P.; Lam, C.W. Immunomodulatory effects of yun zhi and danshen capsules in health subjects—A randomized, double-blind, placebo-controlled, crossover study. Int. Immunopharmacol. 2004, 4, 201–211. [Google Scholar] [CrossRef]
- Moon, S.; Shin, S.; Kim, S.; Oh, H.E.; Han, S.; Lee, S.; Kim, K. Role of Salvia miltiorrhiza for modulation of Th2-derived cytokines in the resolution of inflammation. Immune Netw. 2011, 11, 288–298. [Google Scholar] [CrossRef]
- Kang, B.Y.; Chung, S.W.; Kim, S.H.; Ryu, S.Y.; Kim, T.S. Inhibition of interleukin-12 and interferon-gamma production in immune cells by tanshinones from Salvia miltiorrhiza. Immunopharmacology 2000, 49, 355–361. [Google Scholar] [CrossRef]
- Shin, J.; Kim, O.K.; Kim, S.; Bae, D.; Lee, J.; Park, J.; Jun, W. Immunomodulatory effect of a Salvia plebeian R. aqueous extract in forced swimming exercise-induced mice. Nutrients 2020, 12, 2260. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, Y.; Li, H. Advances in research on immunoregulation of macrophages by plant polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef]
- Im, S.A.; Lee, Y.R.; Lee, Y.H.; Oh, S.T.; Gerelchuluun, T.; Kim, B.H.; Kim, Y.; Yun, Y.P.; Song, S.; Lee, C.K. Synergistic activation of monocytes by polysaccharides isolated from Salicornia herbacea and interferon-gamma. J. Ethnopharmacol. 2007, 111, 365–370. [Google Scholar] [CrossRef]
- Han, C.; Yang, J.; Song, P.; Wang, X.; Shi, W. Effects of Salvia miltiorrhiza polysaccharides on lipopolysaccharide-induced inflammatory factor release in RAW264.7 Cells. J. Interferon Cytokine Res. 2018, 38, 29–37. [Google Scholar] [CrossRef]
- Wang, H.; Ma, C.; Sun-Waterhouse, D.; Geoffrey, W.; Waterhouse, J.I.N.; Kang, W. Imunoregulatory polysaccharides from Apocynum venetum L. flowers stimulate phagocytosis and cytokine expression via activating the NF-κB/MAPK signaling pathways in RAW264.7 cells. Food Sci. Hum. Wellness 2022, 11, 806–814. [Google Scholar] [CrossRef]
- Cui, L.; Chen, L.; Yang, G.; Li, Y.; Qiao, Z.; Liu, Y.; Meng, Y.; Zhou, Y.; Sun, L. Structural characterization and immunomodulatory activity of a heterogalactan from Panax ginseng flowers. Food Res. Int. 2021, 140, 109859. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Li, H.; Yue, X.L.; Xie, J.Y.; Zhang, Y.Y.; Di, H.Y.; Chen, D.F. Macrophage immunomodulatory activity of the polysaccharides from the roots of Bupleurum smithii var. parvifolium. J. Ethnopharmacol. 2010, 130, 363–368. [Google Scholar] [CrossRef]
- Huston, D.P. The biology of the immune system. JAMA 1997, 278, 1804–1814. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Li, M.; Niu, S.; Wang, J.; Shao, H.; Li, T.; Wang, H. Salvia miltiorrhiza polysaccharide activates T Lymphocytes of cancer patients through activation of TLRs mediated-MAPK and -NF-κB signaling pathways. J. Ethnopharmacol. 2017, 200, 165–173. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Shekar, H.S.; Rajamma, A.J.; Sateesha, S.B. Application of ultrasound to pharmaceutical industry: An overview. J. Pharm. Drug Deliv. Res. 2017, 6, 1–5. [Google Scholar]
- Hemingway, R.W.; Karchesy, J.J. (Eds.) Chemistry and Significance of Condensed Tannins; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; 553p. [Google Scholar]
- Avula, B.; Bae, J.Y.; Chittiboyina, A.G.; Wang, Y.H.; Wang, M.; Srivedavyasasri, R.; Ali, Z.; Li, J.; Wu, C.; Khan, I.A. Comparative analysis of five Salvia species using LC-DAD-QToF. J. Pharm. Biomed. Anal. 2022, 209, 114520. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Guide for the Care and Use of Laboratory Animals; The National Academies Press: Washington, DC, USA, 1996; 140p. [Google Scholar]
- Zhang, X.; Goncalves, R.; Mosser, D.M. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 2008, 83, 14.1.1–14.1.14. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.F.; Berger, H.; Su, I.H. Isolation and activation of murine lymphocytes. J. Vis. Exp. 2016, 116, 54596. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, L.J.; Sato, V.L. “Panning” for lymphocytes: A method for cell selection. Proc. Natl. Acad. Sci. USA 1978, 75, 2844–2848. [Google Scholar] [CrossRef]






| Numerical Indicators, % | S. deserta Schang. | S. sclarea L. |
|---|---|---|
| Moisture | 8.14 | 8.82 |
| Total ash | 9.67 | 10.05 |
| Ash insoluble in 10% HCl | 1.19 | 1.33 |
| Sulphated ash | 9.98 | 10.89 |
| Main Groups, % | S. deserta Schang. | S. sclarea L. |
|---|---|---|
| Polysaccharides | 1.76 | 2.63 |
| Flavonoids | 8.72 | 5.56 |
| Organic acids | 0.111 | 0.112 |
| Tannins | 16.42 | 9.98 |
| Main Groups, % | LS-1 | LS-2 |
|---|---|---|
| Polysaccharides | 1.81 | 1.83 |
| Flavonoids | 9.79 | 10.14 |
| Organic acids | 2.78 | 2.67 |
| Tannins | 23.35 | 24.06 |
| Main Groups, % | LS-3 | LS-4 |
|---|---|---|
| Polysaccharides | 2.67 | 2.68 |
| Flavonoids | 8.61 | 7.71 |
| Organic acids | 2.13 | 2.24 |
| Tannins | 9.98 | 10.12 |
| Species | Collection Area | GPS Coordinates | Phenophase | Collected Part | Phytomass, kg |
|---|---|---|---|---|---|
| S. sclarea L. | Zhambyl region, Korday region, ridge Zhetyzhol, env. pos. Ulken Sulutor | H = 1326 m above the sea level N = 43°13′368″ E = 075°10′971″ | budding, beginning of flowering. sp-sol, blooming | aerial part (fresh) | 26.2 |
| S. deserta Schang. | Zhambyl region, Korday region, ridge Zhetyzhol, env. pos. Ulken Sulutor | H = 1339 m above the sea level N = 43°12′655″ E = 075°11′226″ | budding, blooming, cop 3 | aerial part (fresh) | 20.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhussupova, A.; Zhumaliyeva, G.; Ogay, V.; Issabekova, A.; Ross, S.A.; Zhusupova, G.E. Immunomodulatory Effects of Plant Extracts from Salvia deserta Schang. and Salvia sclarea L. Plants 2022, 11, 2690. https://doi.org/10.3390/plants11202690
Zhussupova A, Zhumaliyeva G, Ogay V, Issabekova A, Ross SA, Zhusupova GE. Immunomodulatory Effects of Plant Extracts from Salvia deserta Schang. and Salvia sclarea L. Plants. 2022; 11(20):2690. https://doi.org/10.3390/plants11202690
Chicago/Turabian StyleZhussupova, Aizhan, Gaziza Zhumaliyeva, Vyacheslav Ogay, Assel Issabekova, Samir A. Ross, and Galiya E. Zhusupova. 2022. "Immunomodulatory Effects of Plant Extracts from Salvia deserta Schang. and Salvia sclarea L." Plants 11, no. 20: 2690. https://doi.org/10.3390/plants11202690
APA StyleZhussupova, A., Zhumaliyeva, G., Ogay, V., Issabekova, A., Ross, S. A., & Zhusupova, G. E. (2022). Immunomodulatory Effects of Plant Extracts from Salvia deserta Schang. and Salvia sclarea L. Plants, 11(20), 2690. https://doi.org/10.3390/plants11202690







