1. Introduction
Deforestation continues and the net annual change in global forest cover remains negative, despite growing reforestation efforts and widespread recognition of the importance of forests for human wellbeing, biodiversity, and overall biosphere health [
1,
2]. Forests provide a multitude of ecosystem functions and services, including biodiversity support (wildlife habitat), carbon storage, climate regulation, and provisioning of air, water, and food [
1,
2,
3]. However, resources and human support for the protection of intact forest habitats and restoration of lost or degraded habitats are finite, so it has been recommended that we prioritize forests that most effectively mitigate climate change and provide important local socio-ecological functions, e.g., providing food in areas with high food insecurity or wildlife habitat in areas with high biodiversity or conservation value [
3].
In the Lower Rio Grande Valley (RGV) of southern Texas and in northeastern Mexico, less than 2% of the historic Tamaulipan (or Mezquital) thornscrub forests (or thornforests) remain [
4]. These forests exhibit high biodiversity, with hundreds of plant species supporting an array of migratory and resident birds, insects (especially bees and butterflies), mammals, and reptiles, including many species endemic to the region and threatened at the state or national level [
5,
6], such as the federally endangered ocelot (
Leopardus pardalis) [
7,
8,
9,
10]. Tamaulipan thornforests also support a critical ecotourism industry valued at 59–300 million USD per year in a region with high poverty and food insecurity [
11]. Nevertheless, thornforests are threatened by rapid urbanization and are already heavily fragmented due to over a century of agricultural modification, which has reduced biodiversity and forest cover across the region [
5,
12,
13]. Given its high biodiversity and ecological importance paired with its high risk from human impacts, the Tamaulipan ecoregion has been identified as a conservation hotspot [
14]. As a result, restoration of Tamaulipan thornforests are a high priority for various governmental, conservation, and commercial organizations who operate in the region and collaborate to produce and plant native thornforest species on both public and private lands [
4,
14].
Native plant seedling availability is currently the greatest limiting factor for Tamaulipan thornforest restoration [
15]. Current best practices for forest restoration in the Rio Grande Valley (RGV) of south Texas require the planting of seedlings, rather than seeds, and for all seedlings to be grown from locally collected seed to increase genetic diversity in these habitats while promoting locally adapted genotypes [
16]. However, native seed availability is also a limiting factor. Native seed is only commercially available in small quantities and for a few of the 75+ species regularly planted for restoration, and wild collection and processing of native seed is both labor-intensive and requires significant expertise and local knowledge. Similarly, propagation of these 75+ native species from seed is also labor- and knowledge-intensive, and germination and/or seedling survival are often low or inconsistent, partly because many knowledge gaps remain about best practices for nursery propagation [
15]. Consequently, nursery production of thornforest seedlings is high risk and difficult for commercial growers, which limits the number of qualified growers producing seedlings and creates a major bottleneck in reforestation capacity. Greater understanding of the horticultural techniques required to break seed dormancy, enhance germination, and maximize post-germination and post-transplant survival for Tamaulipan thornforest species is urgently needed [
15].
Multiple mechanisms underlie seed dormancy and the requirements to break dormancy can vary widely among species within the same region [
17]. Baskin and Baskin [
18] identified five classes of seed dormancy: physical, physiological, morphological, morphophysiological, and combinational. Generally, seeds experience a combination of natural processes that can act to break dormancy, including daily and seasonal temperature fluctuations, weathering by wind and water, gut passage, and desiccation [
17,
19]. In the RGV, the climate is semiarid and borderline tropical-subtropical [
5], which promotes considerable variation among thornforest plant species in both the timing of flowering, fruiting, and germination, and in the nature of phenological cues, with rainfall pulses and seasonal shifts in temperature or day length hypothesized to be most common, but the exact nature of phenological and germination triggers are poorly understood for most species [
20,
21]. Our understanding of the role temperature plays in the seed and seedling behavior of Tamaulipan thornforest species is underdeveloped [
21,
22,
23]. Long-established horticulture practices, such as cold stratification, mimic the seasonal temperature conditions that govern dormancy in many species [
24], for example, by exposing seeds to an identified number of cold hours at 4–10 °C via refrigeration [
17]. Alternatively, ovens or driers can be used to affect other environmental triggers, such as after ripening, seed desiccation, and exposure to high temperatures [
25,
26]. Although these methods are not new, their applications to thornscrub species are largely untested, despite demonstrated utility for some species [
23]. Understanding thermal triggers of germination will help us better understand thornscrub ecology and community dynamics and would be of significant practical use in breaking seed dormancy for propagation.
In our previous study, Luera et al. [
15] performed a series of scarification and phytohormone trials to investigate the factors governing seed dormancy in three focal thornforest species:
Ebenopsis ebano (Berl.) Barneby & Grimes (Texas ebony),
Cordia boissieri A. DC. (Mexican olive), and
Zanthoxylum fagara (L.) Sarg. (colima). Seed scarification with sulfuric acid increased
E. ebano germination, suggesting physical dormancy, whereas seed treatment with the phytohormone gibberellic acid increased
C. boissieri germination, suggesting physiological dormancy [
15]. However, scarification and phytohormone treatments were tested in isolation, so we could not rule out combinational (physical plus physiological) dormancy, and, although we investigated the effects of temperature and soil type on seedling growth and root morphology, we did not study their effects on germination. This study builds on our prior work in three important ways. (1) We included seed treatments that combined scarification and different phytohormones. (2) We investigated the effects of temperature and soil type—in conjunction with scarification and phytohormones—on germination. (3) We tested germination outdoors in soil, which better reflects the realities of thornforest seedling production.
This study focused on the same three focal Tamaulipan thornforest species as Luera et al. [
15] and employed two factorial experiments for each species. The first experiment quantified the effects of the optimal dosages of sulfuric, gibberellic, and indole-3-butyric acids identified by Luera et al. [
15] on germination when applied to seeds alone and in all possible combinations. The second experiment quantified the effects of a subset of five chemical seed treatments, six soil mixture types, and three stratification treatments on the germination of our three focal species and on the seedling performance of
E. ebano, using a full factorial design. Many knowledge gaps remain regarding the propagation of Tamaulipan thornforest plant species, which, when filled, should reduce risk and increase profitability for commercial growers seeking to produce native thornforest species [
15]. In turn, commercial viability of thornforest seedling production should promote increased availability of thornforest seedlings, which currently limits restoration in the region. This study increased our understanding of thornforest seed dormancy and further elucidated best practices for enhancing germination and seedling performance in a nursery setting.
3. Discussion
Large-scale reforestation requires large-scale seedling production in many regions, including the Tamaulipan biotic province, where seedling availability is the principal limiting factor [
15,
27]. Enhanced production of native plant seedlings in south Texas could both increase the acreage of thornforests restored each year and promote regional economic growth if the best practices for successful nursery propagation are identified and made readily accessible to current and future growers. Luera et al. [
15] previously studied the effects of single-chemical seed treatments on the germination and early growth of Tamaulipan thornforest tree species. They tested different dosages of GA, IBA, and SA but did not test combinational treatments, which were a key focus of the current study and may be required for species that exhibit combinational dormancy. We used the optimal dosages for single-chemical treatments identified by Luera at al. [
15] as the basis for our combinational treatments and included the same single-chemical treatments in the current study to permit direct comparisons. Our results both corroborate prior results and provide new findings that enable additional refinement of current best practices for Tamaulipan thornforest species propagation.
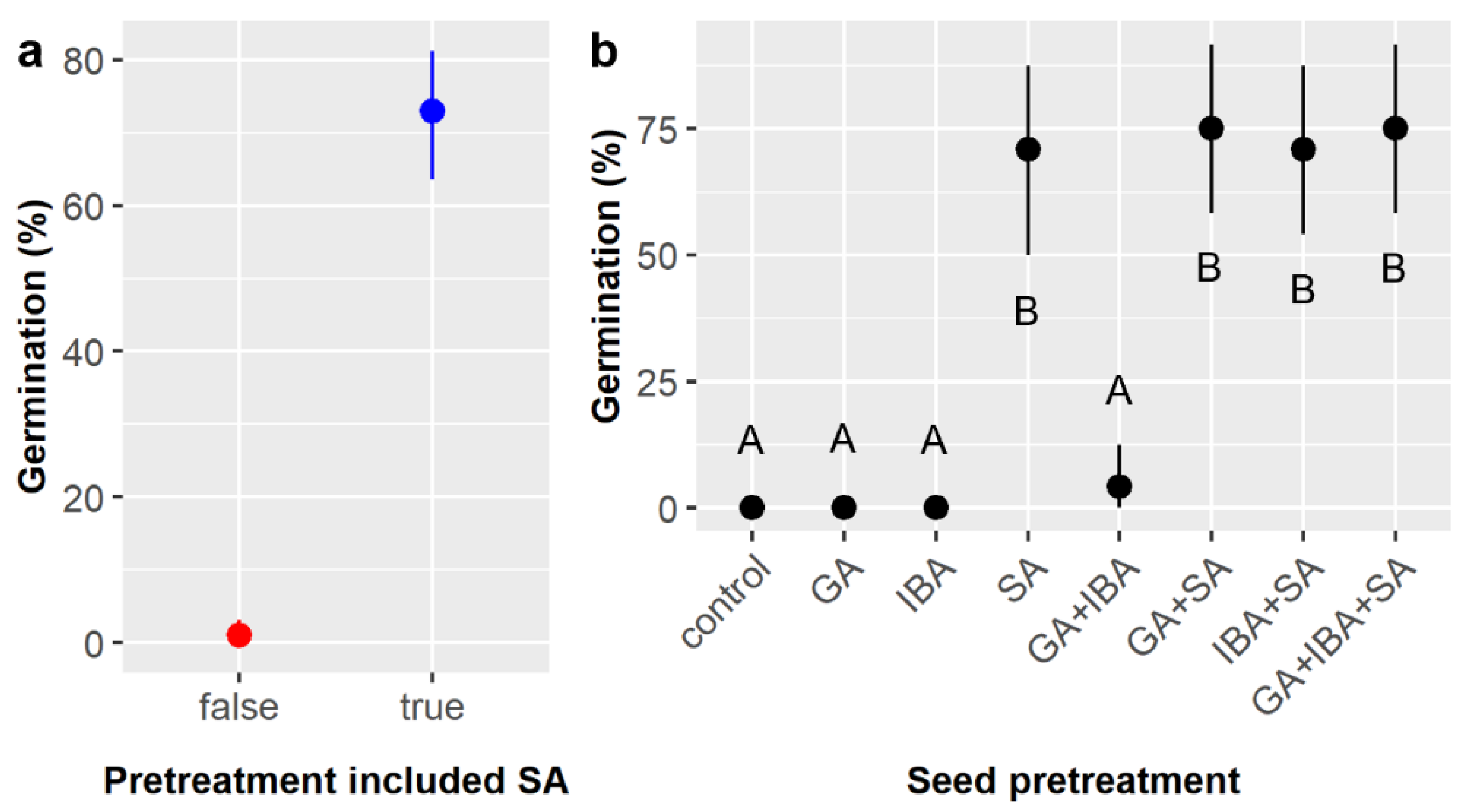
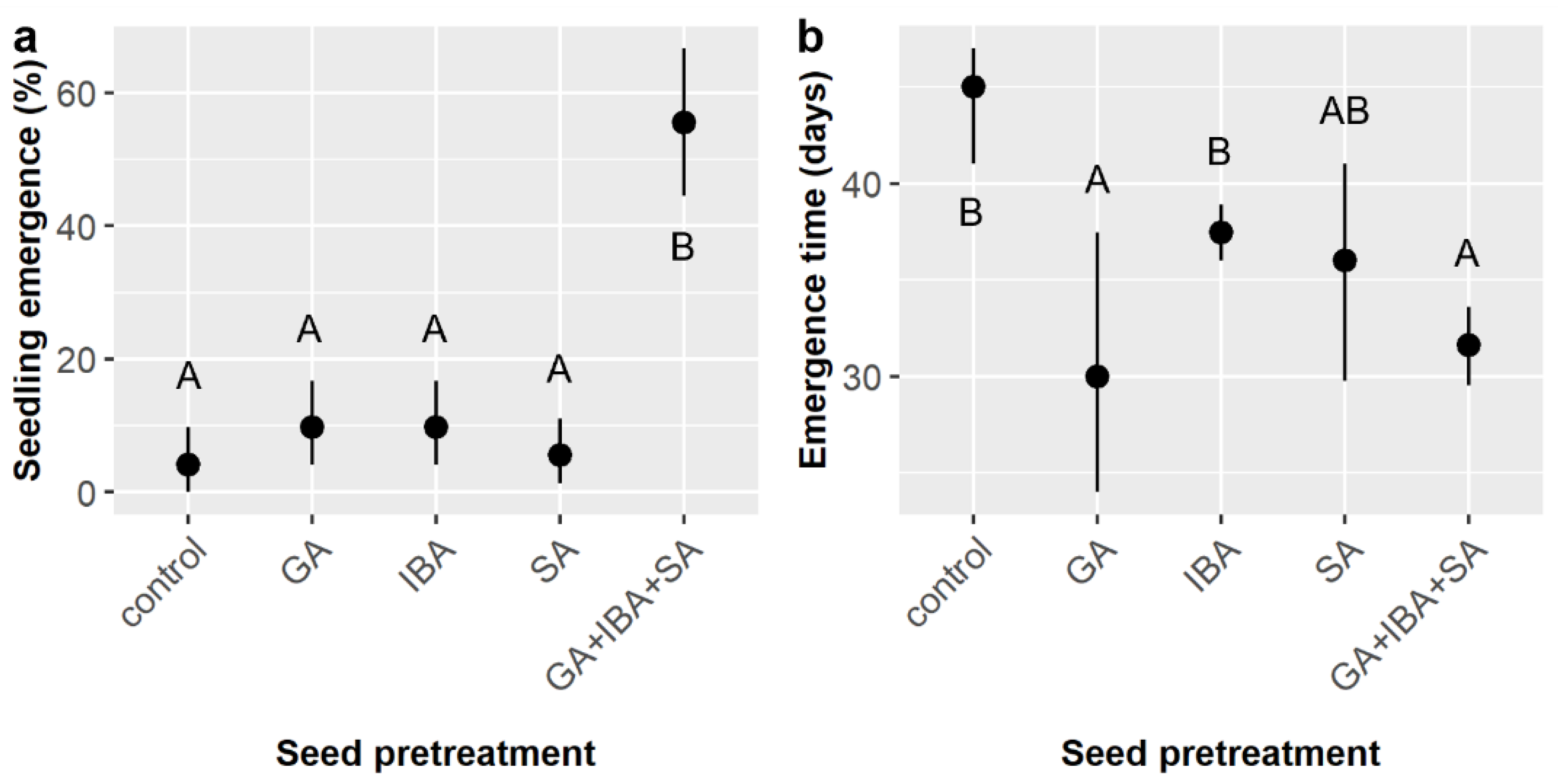
First, this study confirmed that treatment of
E. ebano seeds with SA for 50 min effectively triggered germination and demonstrated for the first time that neither preliminary stratification treatments nor subsequent treatment of SA-treated seeds with GA and/or IBA further increased germination likelihood beyond the effect of SA (
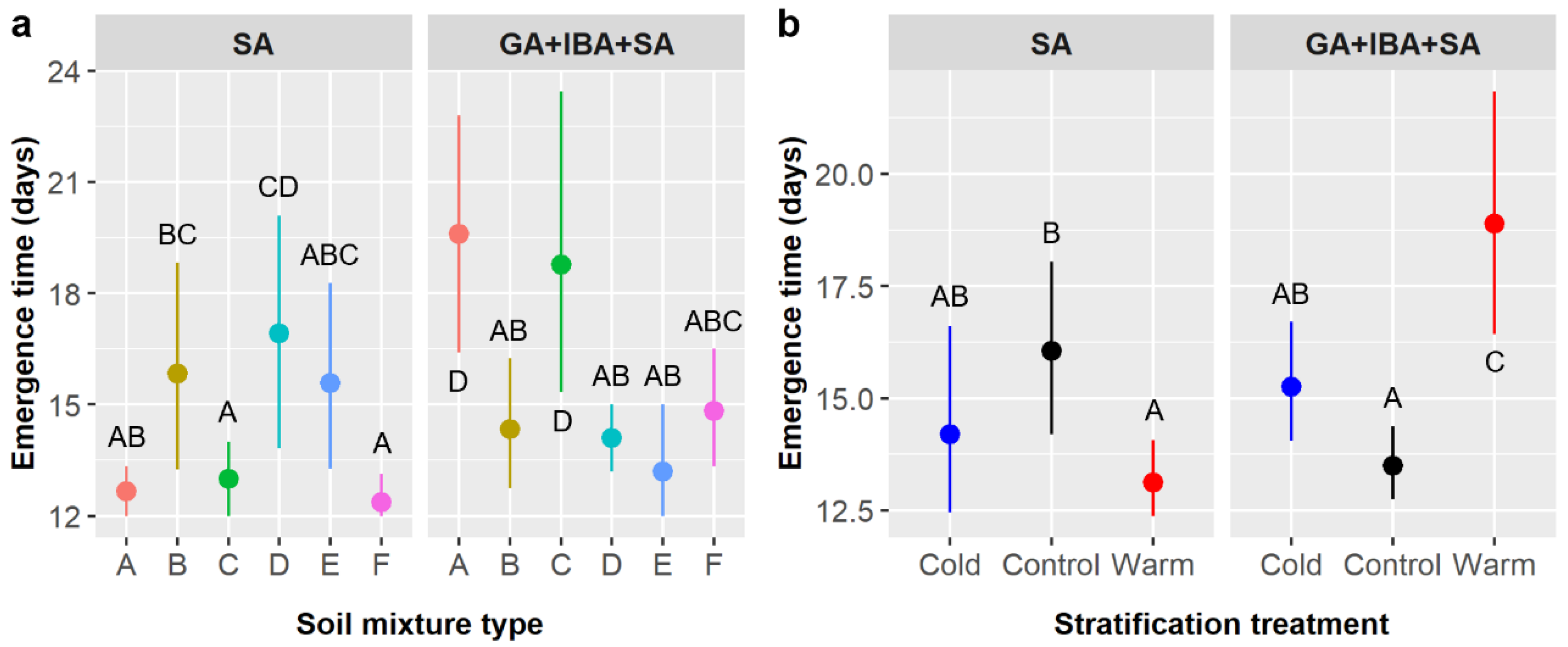
Figure 1). However, subsequent GA and/or IBA treatment did reduce time to germination by ca. 2–3 days (
Figure 2), and germination likelihood was ca. 4% higher (not statistically significant) (
Figure 1 and
Figure 3) with subsequent GA treatment in both our small and large
E. ebano experiments. These impacts are probably too minor to justify the use (and cost) of GA in practice, and the use of IBA in powder form is even less justified, but this modest increase suggests that
E. ebano may also exhibit some weak physiological dormancy mechanisms that are relatively easily overcome. Though not directly tested previously, some level of physiological dormancy is suggested by prior thornforest studies that found environmental impacts on
E. ebano germination [
21,
22,
23]. Although combinational chemical treatments did not significantly enhance germination beyond the previously identified optimal methods [
15], there is value in ruling out unnecessary treatments and materials. Fortunately, the best practice identified herein of treating
E. ebano seeds with SA only is both cost- and labor-efficient at large scales, especially compared to more labor-intensive scarification methods like nicking [
28]. Treatment with GA remains worth considering for hard-to-germinate
E. ebano seeds, and the weak effects of IBA may be related to our use of its powder form, which could be inherently less effective when treating seeds; both merit further evaluation.
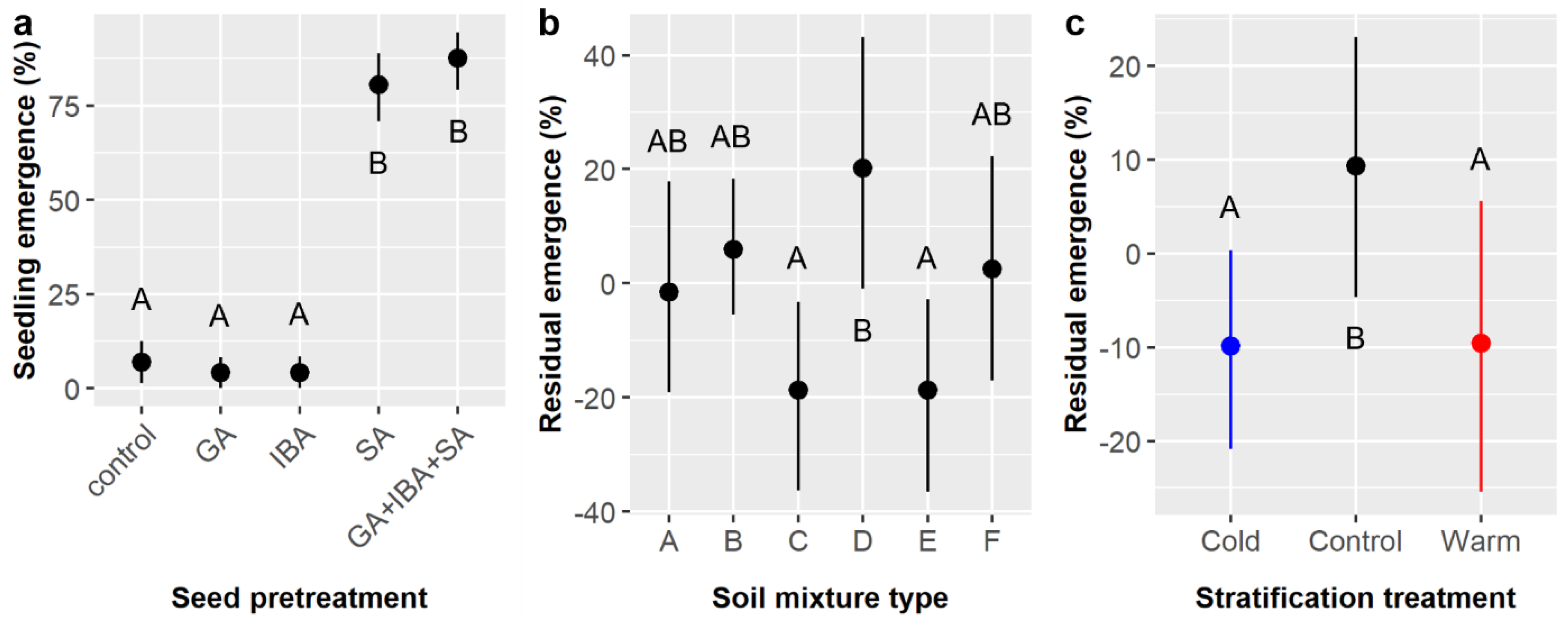
Prior studies showed that soil mixture composition had weak but significant effects on
E. ebano seedling survival and somewhat stronger effects on seedling growth, especially belowground [
15]. In our large factorial experiments, we tested germination (i.e., emergence) when seeds were planted outdoors in soil, which is much closer to normal nursery conditions than prior germination tests using incubated petri dishes, and we found that soil impacted germination likelihood (
Table 3,
Figure 3b) more strongly than it influenced seedling survival or performance (
Table 5 and
Table 7,
Figure 5). Other studies involving
E. ebano and other thornforest species recognize the importance of edaphic properties to germination and early seedling growth and survival but did not manipulate soil types or soil properties as was done in this study [
22,
23].
Ebenopsis ebano seeds were 15% more likely to germinate and emerge in soil type D than in soils C or E, and residuals analysis suggests even greater variation in emergence (ca. 40%) between these groups that is attributable explicitly to soil type (
Figure 3b). We observed only a marginal effect of soil on survival (
p = 0.076), but the differences between soil types was greater (83–100%) than in Luera et al. [
15] (92–100% in unheated treatments). In both studies, the same soil types were used and soil types A, B, and E tended to have lower survival overall than soils C, D, and F. All soil mixture types tested had a low bulk density (0.48–0.89 g/cm
3 dry weight) [
15], which appears unrelated to survival in this study, partly because C and F have the highest and lowest bulk densities, respectively, yet both exhibited 100% seedling survival. This is somewhat surprising because bulk density impacts plant performance and distributions, but all soil types tested had densities below levels associated with suppression of root growth [
29]. Survival likelihoods appear to correlate more with water-holding capacity, which we estimated to be highest in soils D and F and lowest in B. This finding agrees with the many studies that have shown drought stress and soil water holding capacity are critical to plant recruitment and regeneration in thornforests and other dryland ecosystems [
23,
30,
31].
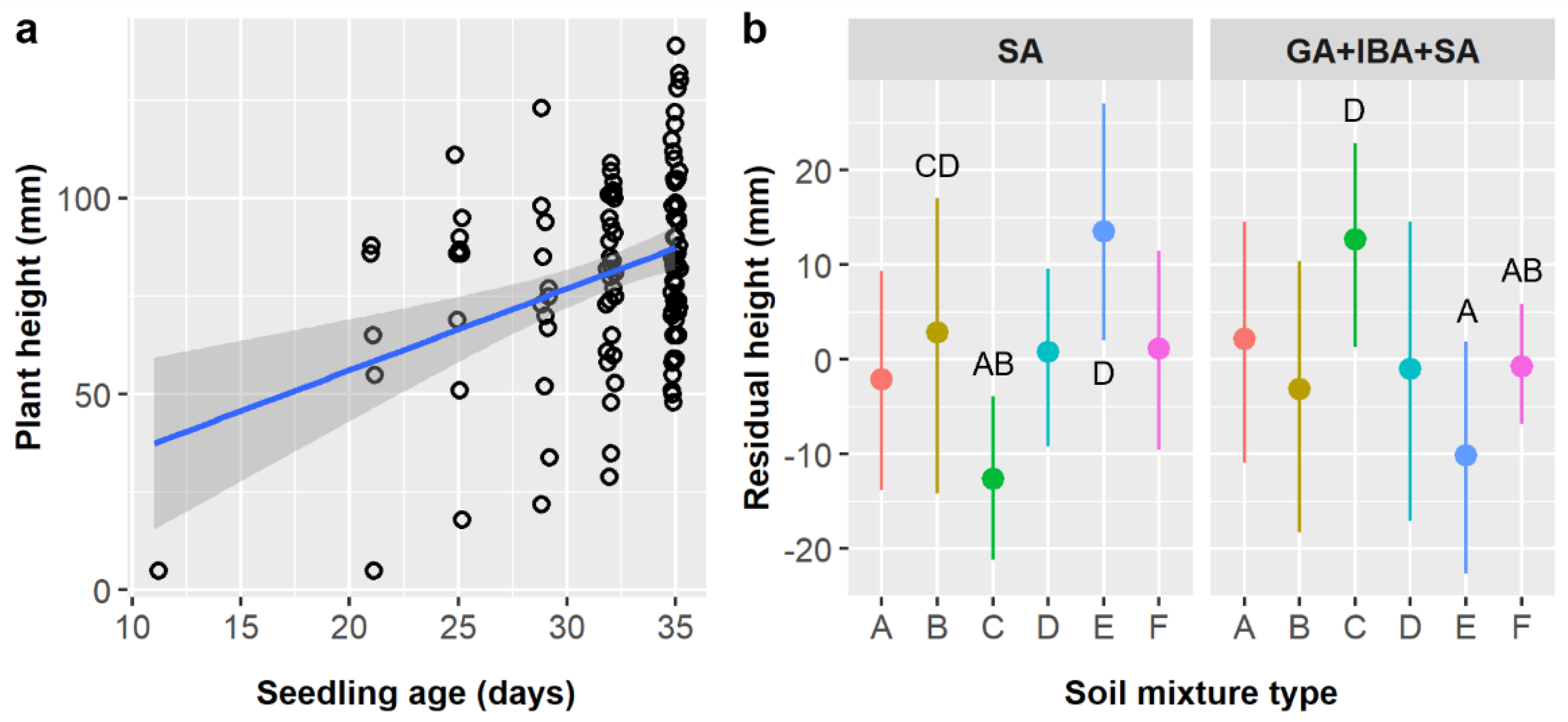
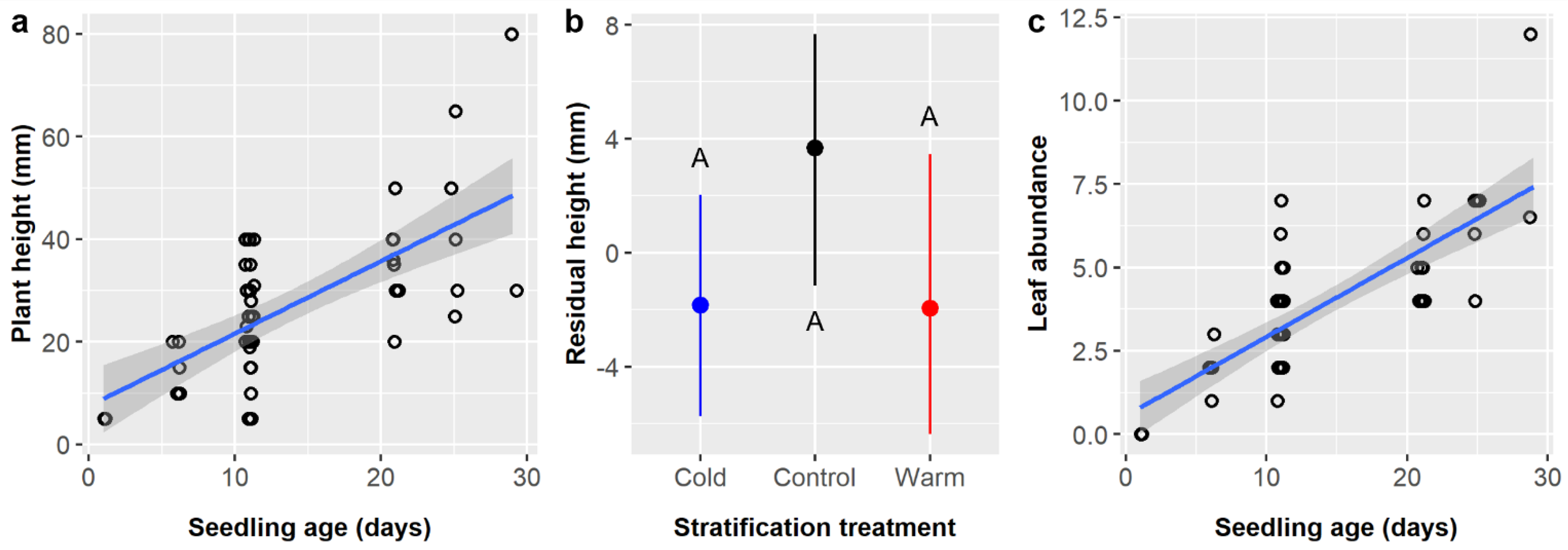
Soil had only weak and marginally significant main effects on seedling height in both this study and its predecessor (
Table 6), yet, in both, soil type F produced the shortest seedlings while soils B, C, and D produced the tallest [
15]. This is somewhat surprising, given the importance of water availability for all plant growth and that the soil mixtures tested varied in their water holding capacity, but these studies were performed in a nursery context with watering regimes designed to keep plants well-watered. Notably, the effect of treating
E. ebano seeds with GA and IBA after SA treatment depended on soil type (pretreatment × soil interaction,
p = 0.0172). There was no effect for most soil types, but, in soils C and E, the residual height difference was ca. 2 cm, which is considerable given the average seedling was only 8.0 cm (
Figure 5b). The mechanism underlying this interactive effect is unclear, but this pattern may suggest that the powder form of IBA had an impact, but its ability to persist and take effect is governed by soil properties.
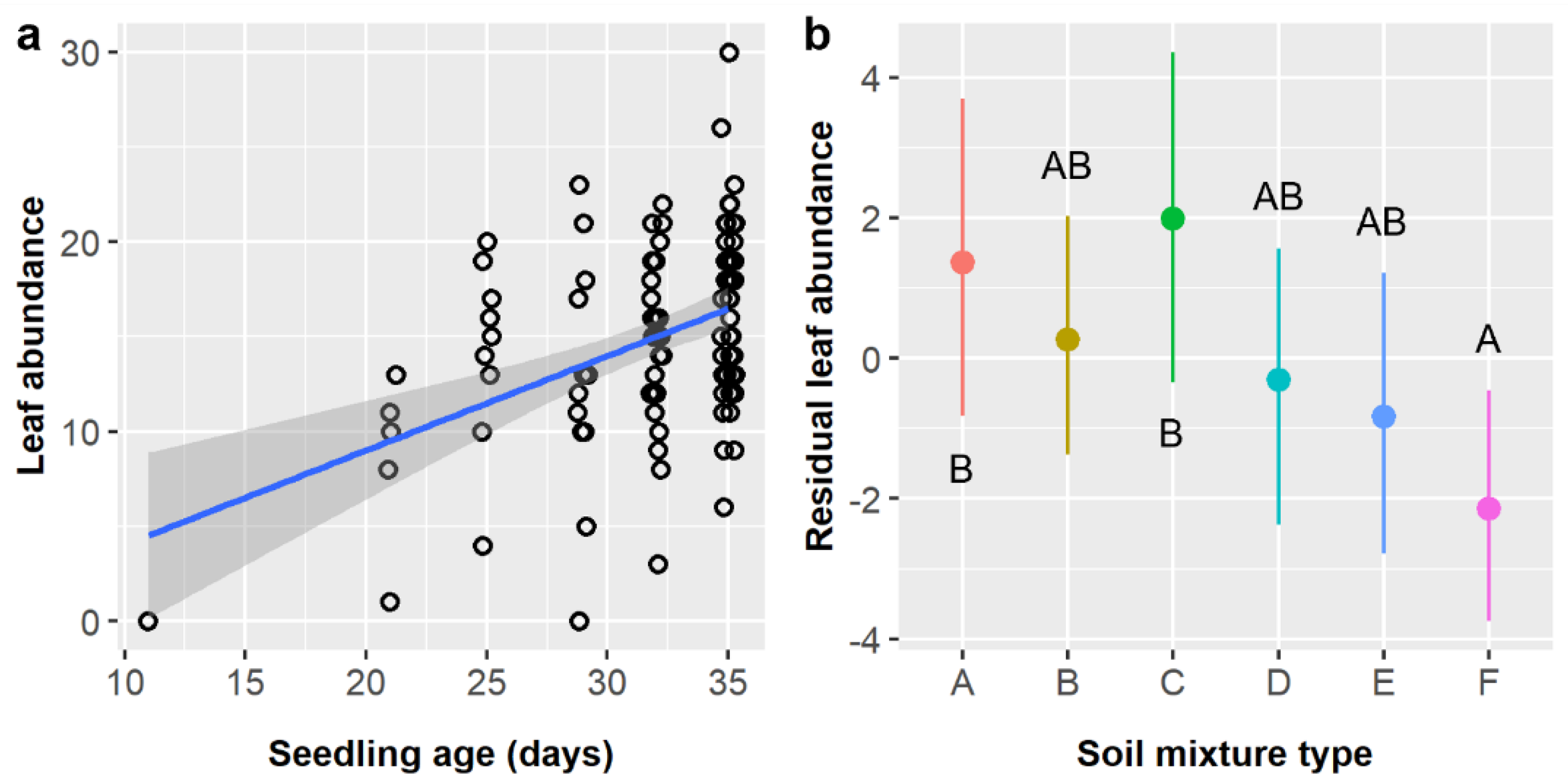
Leaf abundance was significantly influenced by soil type in this study and its precursor (
Table 6), but which soil types had the most leaves differed between studies [
15]. Previously, in unheated controls, soils E and F had the most leaves and soils A and C had the fewest, whereas the opposite was true in this study (
Figure 6b). Given that watering and shade protocols were the same and climatic conditions were comparable in this experiment and in Luera et al. [
15], these differences in leaf abundance most likely reflect age-related differences in plant growth (and growth strategies) and the fact that seedlings of different ages were more evenly distributed among soil treatments in the prior study. Previously, seedlings were assigned to soil treatments upon germination in petri dishes, but here, seeds were planted and allowed to germinate in assigned soil treatments, which differed in their germination timing in interaction with other factors (
Table 4). This resulted in differences in seedling ages between treatment groups, and age was the strongest predictor of leaf abundance in both studies. The current results are more applicable in a practical nursery context, where differences in germination times could overshadow differences in growth rates between soils (depending on the magnitudes of differences and the duration of the growth period).
Prior examination of
E. ebano performance within these soil types suggested that soil microbial communities may play an important role in seedling growth. This is broadly true across plant communities and may be particularly important in stressful environments [
32,
33,
34]. Soil types D (50% peat moss, 25% perlite, and 25% vermiculite) and E (50% peat moss, 25% perlite, 25% sand) contained no live topsoil, and D is a standard substrate utilized in many commercial nurseries, yet the presence of topsoil was not associated with any clear trends in seedling survival or performance.
Ebenopsis ebano, like other members of the Fabaceae, are capable of fixing nitrogen through soil microbial interactions, so the use of fertilizer in this study may have masked some microbial impacts on
E. ebano growth that are more important outside of a nursery context (i.e., post-transplantation). Future studies of microbial impacts on seedling performance and post-transplant establishment are merited across thornforest species, in part because inclusion of topsoil or soil inoculants may have strong effects for some species [
34].
Stratification was not tested in the prior study [
15], but here it had a marginal main effect on
E. ebano emergence likelihood, and it had interactive effects on emergence likelihood (
Table 3) and timing (
Table 4) and marginally on seedling height (
Table 6). However, there was no evidence to warrant cold or warm stratification treatment when propagating
E. ebano from seed; rather, stratification generally had subtle negative effects that varied in intensity among soil and chemical treatments. These findings agree with prior studies that found little effect of temperature on
E. ebano germination and early growth [
22]. However, these findings have larger implications for seed storage, which is an important practical consideration. The lack of significant negative effects of cold stratification on germination likelihood validates the use of cold storage, which can buffer against seed shortages in years with low seed production. However, our cold stratification treatment was only 30 days and not as cold as most cold storage facilities, so due caution is merited when storing
E. ebano seeds, but our results agree with years of observations by United States Fish and Wildlife Service (USFWS) personnel, who have been using cold storage for
E. ebano for years and observed no obvious negative effects of the practice [
35]. Seed longevity (viability over time) for different thornforest species merits future evaluation, as it has scarcely been quantified, as do tests of whether different storage regimes (e.g., cold vs. dry) can improve longevity.
Our lab tests (small factorial experiment) with
C. boissieri confirmed prior findings by Luera et al. [
15] that the main effects of GA treatment had a strong positive effect and SA had no effect on germination likelihood (
Table 8,
Figure 6). However, the prior study found IBA had no effect, but we found that the main effect of IBA significantly decreased germination likelihood. The positive effects of GA on germination are well-established for many species [
36,
37,
38], whereas evidence for germination benefits from IBA treatment are much more limited [
39], but a negative effect of IBA was unexpected. Unlike
E. ebano, interactions among chemical treatments had clear and relatively strong effects on germination likelihood. The positive effect of GA appears to be mediated by IBA (
Figure 6a), and the negative effect of IBA appears to be mediated by SA (
Figure 6b). There was not a significant GA × SA interaction, but germination was significantly (13%) lower with GA + SA treatment than GA alone, suggesting that sulfuric acid may have damaged the embryo, or that residual SA in the pericarp reduced the efficacy of gibberellic acid.
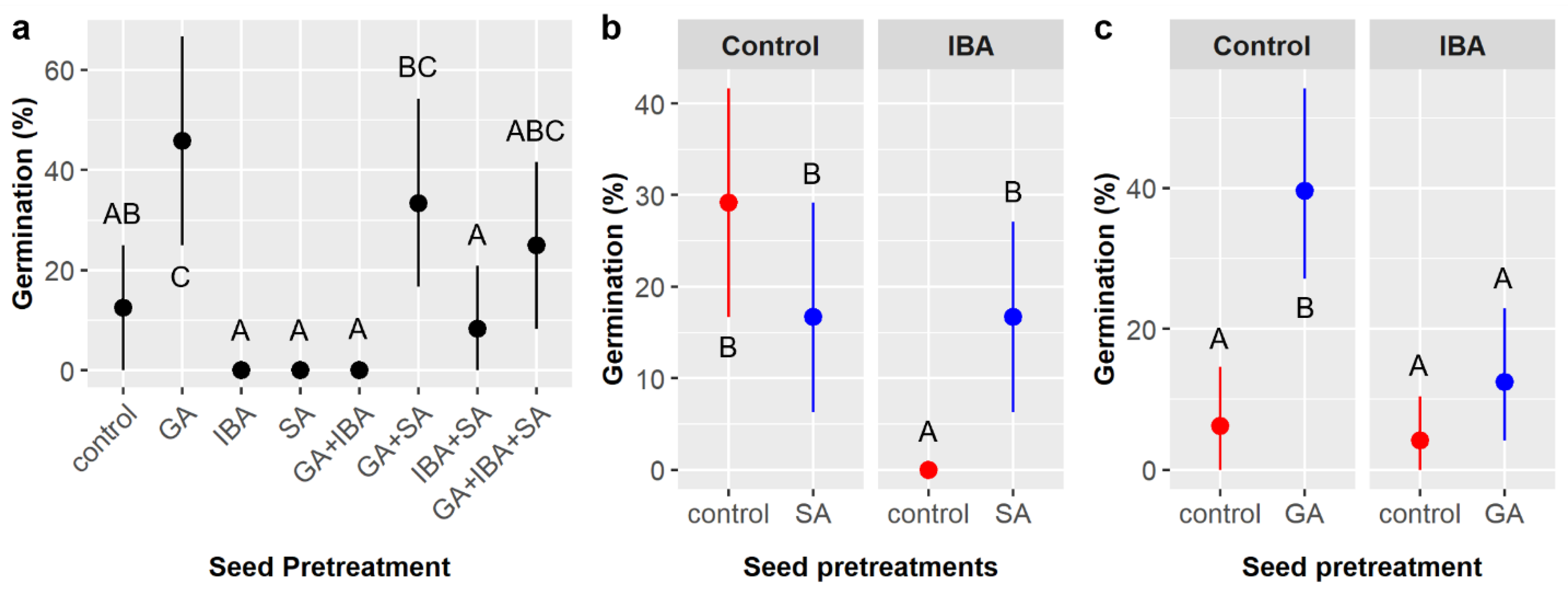
Importantly, our outdoor tests (large factorial experiment) disagreed with the current and prior lab tests [
15] in a critical way: under realistic nursery conditions, treatment with GA alone did not significantly increase germination likelihood relative to other single chemical treatments or controls (
Table 9,
Figure 7a). However, the combined GA + IBA + SA treatment increased germination likelihood over five-fold relative to single-chemical seed treatments and the control. The exact mechanisms underlying these differing results are uncertain, but we hypothesize that both endocarp permeability and the leaching of phytohormones out of containers are important factors. In lab tests, seeds were in closed petri dishes and only ever rinsed according to surface sterilization protocols to minimize molding, as described in Luera et al. [
15], whereas, in nursery tests, seeds were in well-drained containers that were watered regularly and exposed to rain. Thus, any residual GA, IBA, or SA on or in the porous endocarp of
C. boissieri likely leached away much faster in nursery tests compared to lab tests, thereby reducing the total amount of aqueous GA able to penetrate the endocarp and diffuse into embryonic tissue. Meanwhile, the scarifying effect of SA treatment should render the endocarp more permeable to GA, more porous overall (which would increase the endocarp surface area and allow it to absorb and hold more aqueous GA), or both, thereby potentially offsetting the reduction in GA reaching the
C. boissieri embryo due to increased leaching. Therefore, in nursery conditions, GA would only be effective if seeds were first treated with SA.
There is evidence to support this hypothesized mechanism, yet additional tests to explain the discrepancy in the effects of GA on
C. boissieri germination between nursery and lab experiments are merited. First, although SA had no effect on
C. boissieri germination in this lab experiment or prior studies, Luera et al. [
15] showed that physically cracking
C. boissieri seeds increased germination likelihood from 9% to 40%. Together, these findings suggest that the permeability of the
C. boissieri endocarp influences germination, but that SA does not effectively render the endocarp permeable to water. Rather, heating and drying of
C. boissieri seeds resulted in the fissuring of the endocarp, which is far more likely to occur outdoors where temperatures and soil moisture levels fluctuate much more frequently and strongly than in an incubator. This would also explain why seeds treated with GA after being treated with SA were not any more likely to germinate than seeds treated with only GA in the current laboratory experiment (
Figure 6a), but they were more likely to germinate in the outdoor experiment (
Figure 7a). SA treatment likely both increases the porosity of
C. boissieri endocarps and facilitates thermal/desiccative cracking. A prior study found that warm stratification increased
C. boissieri germination [
40], which concurs with this notion of heating to induce fissuring of the endocarp, but more directly disagrees with our finding that warm stratification had a negative effect on
C. boissieri germination.
The same leaching effect may also explain why IBA significantly reduced
C. boissieri germination likelihood in our indoor experiment (
Table 8,
Figure 6) but had no effect in our outdoor experiment (
Table 9,
Figure 7). IBA did not reduce germination in a previous indoor experiment with
C. boissieri, but its germination was so low overall that such an effect was likely undetectable, and the same study found that IBA increased the rate of seed molding, likely due to the anti-caking agent present in the powder form of IBA utilized [
15]. If IBA presence was deleterious, then the higher leaching rates in the outdoor experiment could have reduced IBA concentrations and its overall effect. Alternatively, if IBA reduced germination by promoting molding, the regular cycle of soils drying out between watering in the outdoor experiment may have mitigated the negative effects of mold proliferation.
The mechanism behind the mediating effect of SA on the negative effect of IBA (
Figure 6b) is unknown. It is conceivable that residual SA left in the endocarp after scarification created a high-pH barrier that acted to degrade IBA diffusing toward the embryo, impeded mold penetration, or both. If residual SA degraded IBA entering the seed, it could have had a similar effect on GA, and this may explain why germination was lower in the GA + SA treatment compared to the GA only treatment in the lab experiment with less leaching (
Figure 6a) but higher in the GA + IBA + SA treatment compared to GA only in the outdoor experiment with more leaching (
Figure 7a).
Future studies could investigate the hypothesized mechanisms for our current observations or investigate additional approaches to enhancing propagation of these thornforest species. For example, ethylene is another natural occurring hormone produced during rapid cell division and fruit ripening [
41]. Ethylene is the only major plant hormone that occurs as a gas and is a very small molecule [
42], so it may more easily penetrate the endocarp and reach the seed embryo in
C. boissieri.
Cordia boissieri fruits are relatively large fleshy drupes that often fall from the parent tree shortly after ripening. The ripening process releases ethylene, which can induce seed maturation and fruit ripening in nearby immature fruits [
42]. Collection of fruits from trees prior to full maturation may explain the low germination rates frequently observed for
C. boissieri, but this could potentially be countered with ethylene treatment, or even just storage practices that promote ethylene accumulation around harvested seeds or fruits.
Fewer comparisons with prior studies are possible for
C. boissieri because soil mixture types and the effects of chemical seed treatments on seedling survival and performance have not previously been tested for
C. boissieri. Unlike
E. ebano, soil mixture treatments had only an interactive effect on
C. boissieri emergence likelihood (pretreatment × soil, discussed above) (
Table 9), and no effect on time to emergence or on seedling survival, height, or leaf abundance (
Table 10,
Table 11,
Table 12 and
Table 13). Nevertheless, soil B appears to have exhibited the highest emergence (23.3%) but the lowest seedling growth (16.5 mm height, 2.5 leaves), whereas soils C and F had the highest seedling growth (33.8 and 31.4 mm height, 4.8 and 5.8 leaves, respectively) but moderate emergence (16.7% and 15.0%), and soil C had the lowest seedling survival (80%). The effects of different soil types on
C. boissieri germination and seedling performance merit additional study and, given the large proportion of
C. boissieri seeds that experience mammalian gut passage, so do the nature and impacts of certain soil microbial associations.
Stratification also had fewer effects on germination and performance for
C. boissieri than it did for
E. ebano. However, like
E. ebano, the effects stratification had were weakly negative, and there is no justification for either including stratification treatments in propagation protocols or avoiding cold storage for the sake of seed banking. As aforementioned, this finding contradicts a prior study on
C. boissieri germination by Schuch et al. [
40], who found warm stratification significantly increased germination. Schuch et al. [
40] also found that
C. boissieri seed longevity was relatively limited and called for further study of different storage methods, which we agree merit investigation.
Zanthoxylum fagara propagation remains a major challenge and an unresolved mystery.
Zanthoxylum fagara germination was effectively zero, just as in a prior study using similar methods [
15]. The likely causes for this paucity of germination were discussed at length previously [
15], and the same factors likely applied in this study. Most importantly, we suspect that the low germination rates in this study were due to near-zero viability of the seeds tested. Unfortunately, this means we cannot even conclude that the current treatments were ineffective or unimportant, as they could have had strong effects on seed with higher viability. Use of fresh seed is now standard procedure for
Z. fagara propagation at the regional USFWS nursery, but
Z. fagara germination remains low and highly variable [
35]. The current suite of factors tested remain worth investigating in the future, but perhaps more urgent are tests of
Z. fagara seed longevity and evaluations of different seed harvesting and processing methods.
Ebenopsis ebano, C. boissieri, and Z. fagara are only three of the 75+ plant species that regularly make up Tamaulipan thornscrub forest communities, and many common horticulture techniques that could increase their propagation remain untested. Filling these knowledge gaps and developing a quantitative foundation to provide better, evidence-based propagation guidelines for thornforest species can directly and immediately increase not only seedling production, but also restored acreage and, by extension, regional biodiversity and other ecosystem services. Urbanization and human land use change continue to increase in the Lower Rio Grande Valley as its human population continues to grow rapidly. Now, more than ever, major advances in thornforest habitat restoration are needed if we are to return Tamaulipan thornforests to a regular part of the regional landscape, rather than a few scattered jewels with enemies at their gates.