QTL Mapping Low-Temperature Germination Ability in the Maize IBM Syn10 DH Population
Abstract
1. Introduction
2. Results
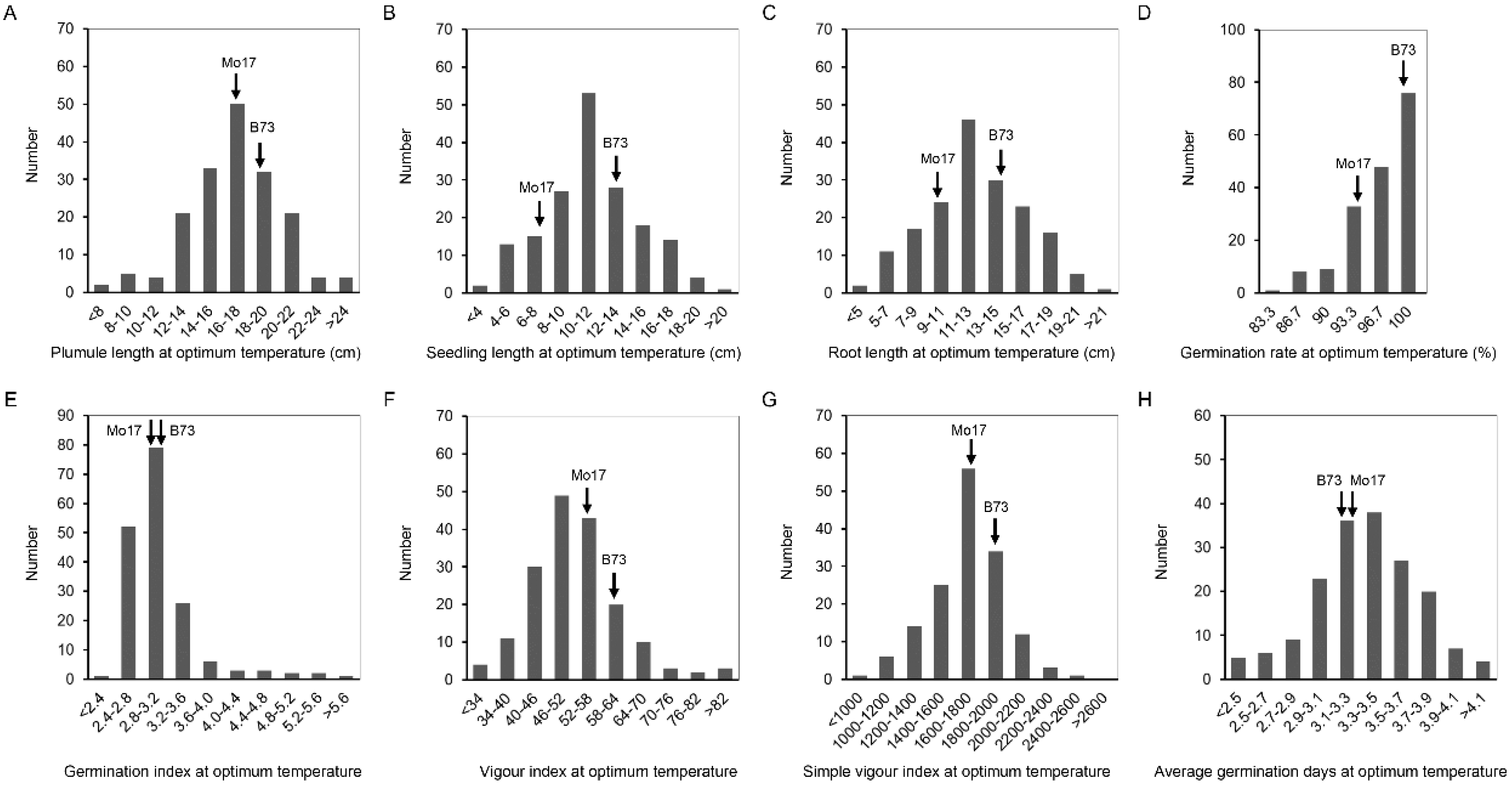
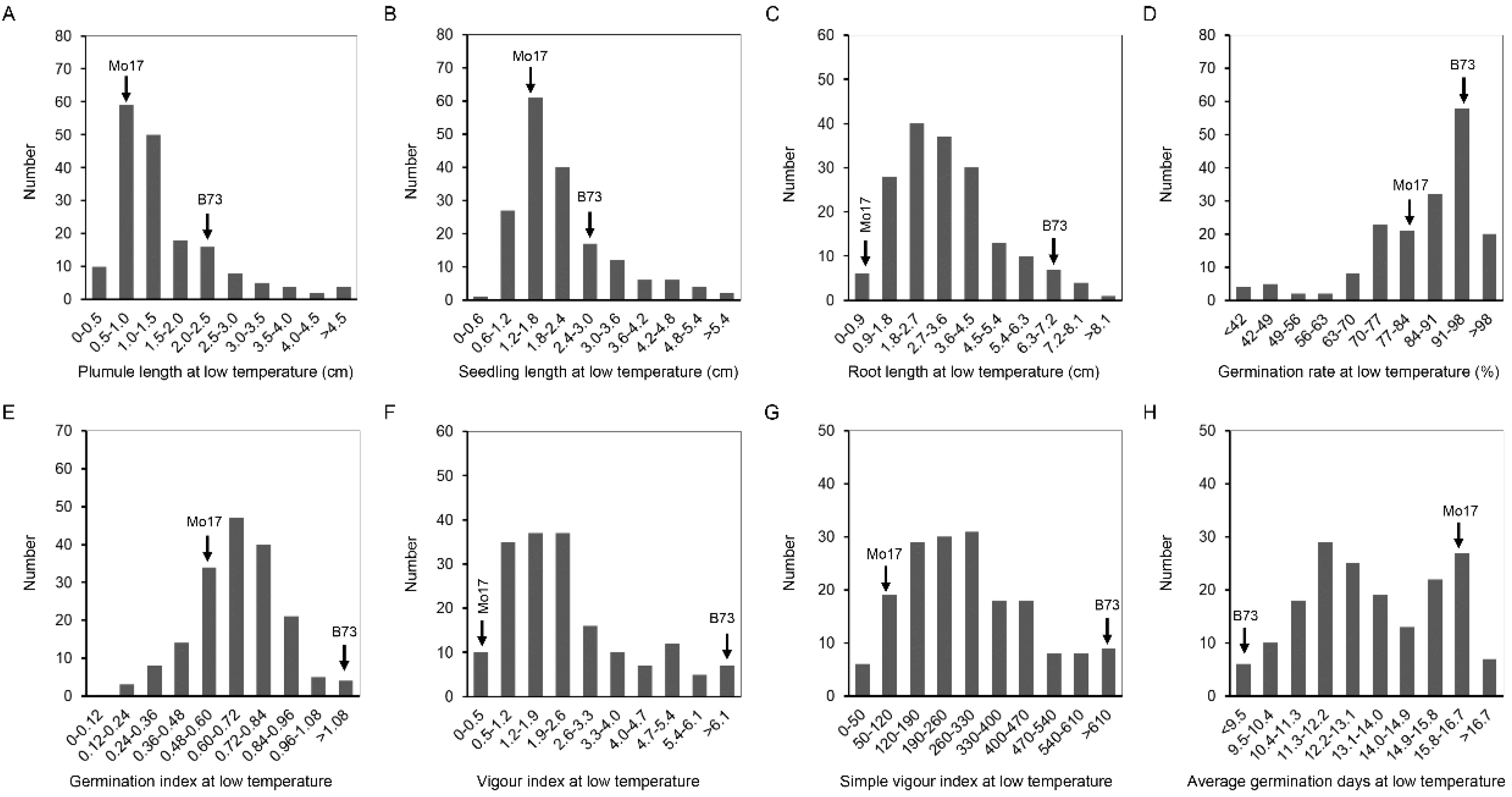
2.1. Phenotypic Analysis of Germination-Related Traits under Optimum-Temperature Condition and Low-Temperature Condition
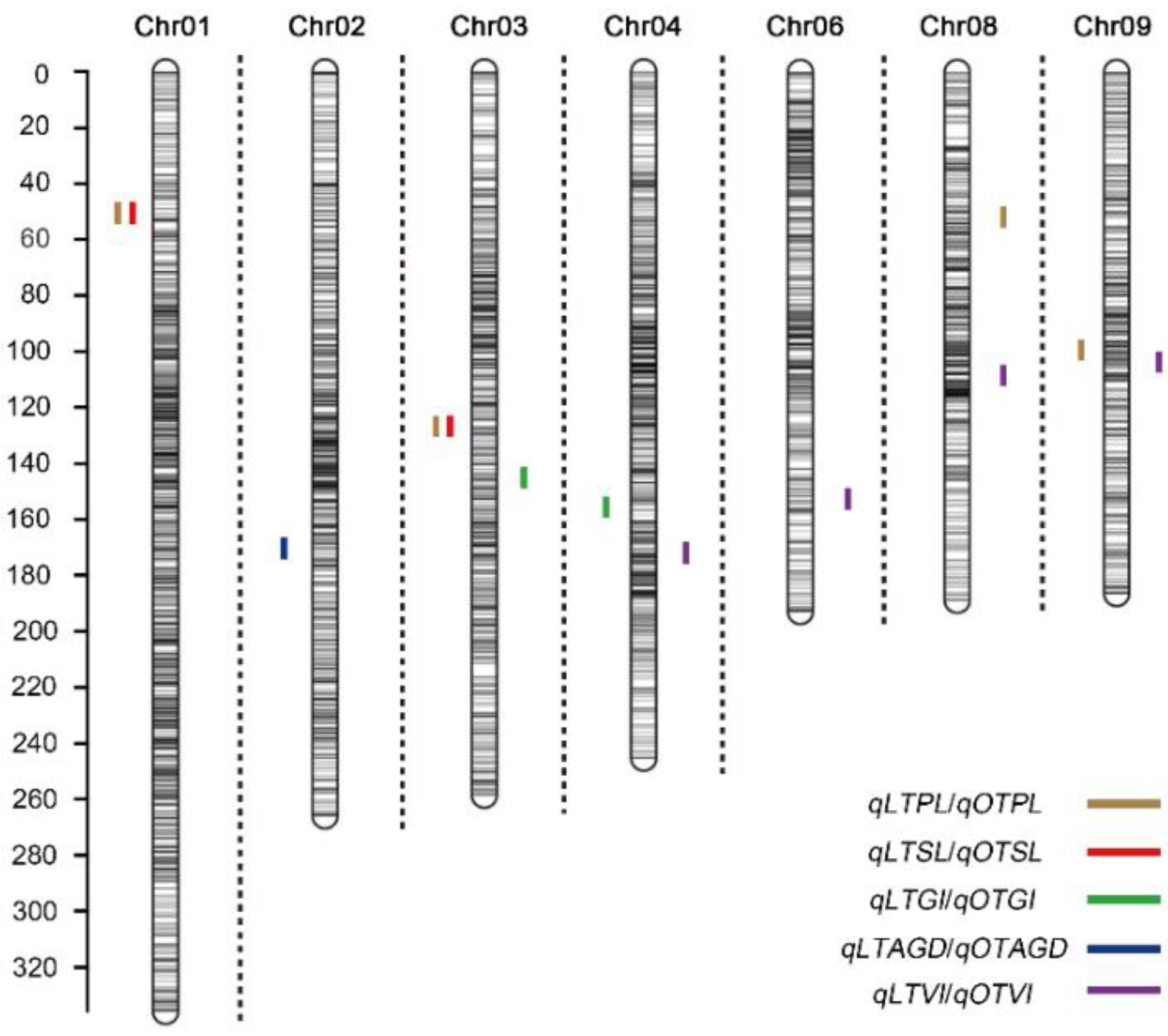
2.2. Construction of a Molecular Linkage Map and QTL Analysis for Germination-Related Traits
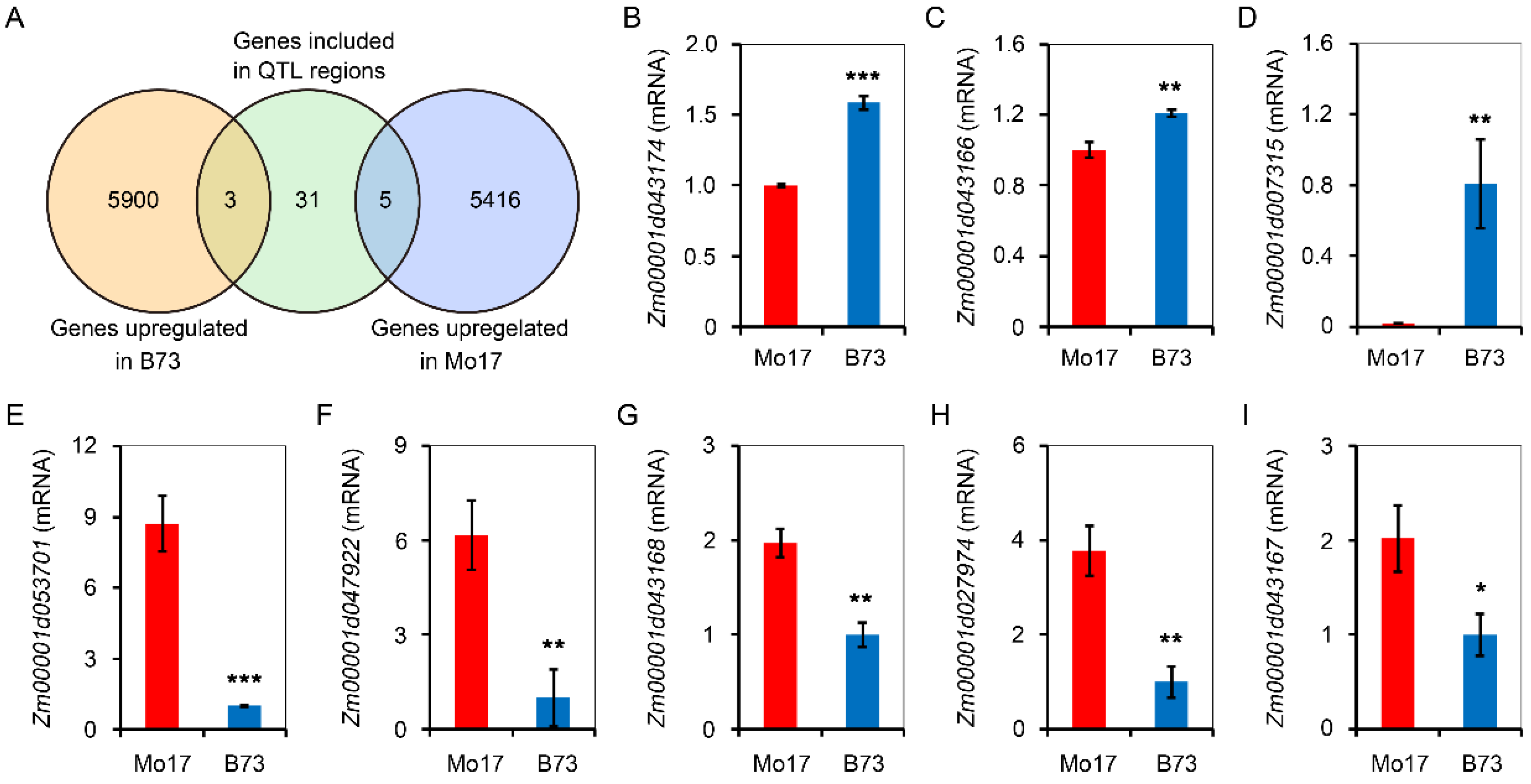
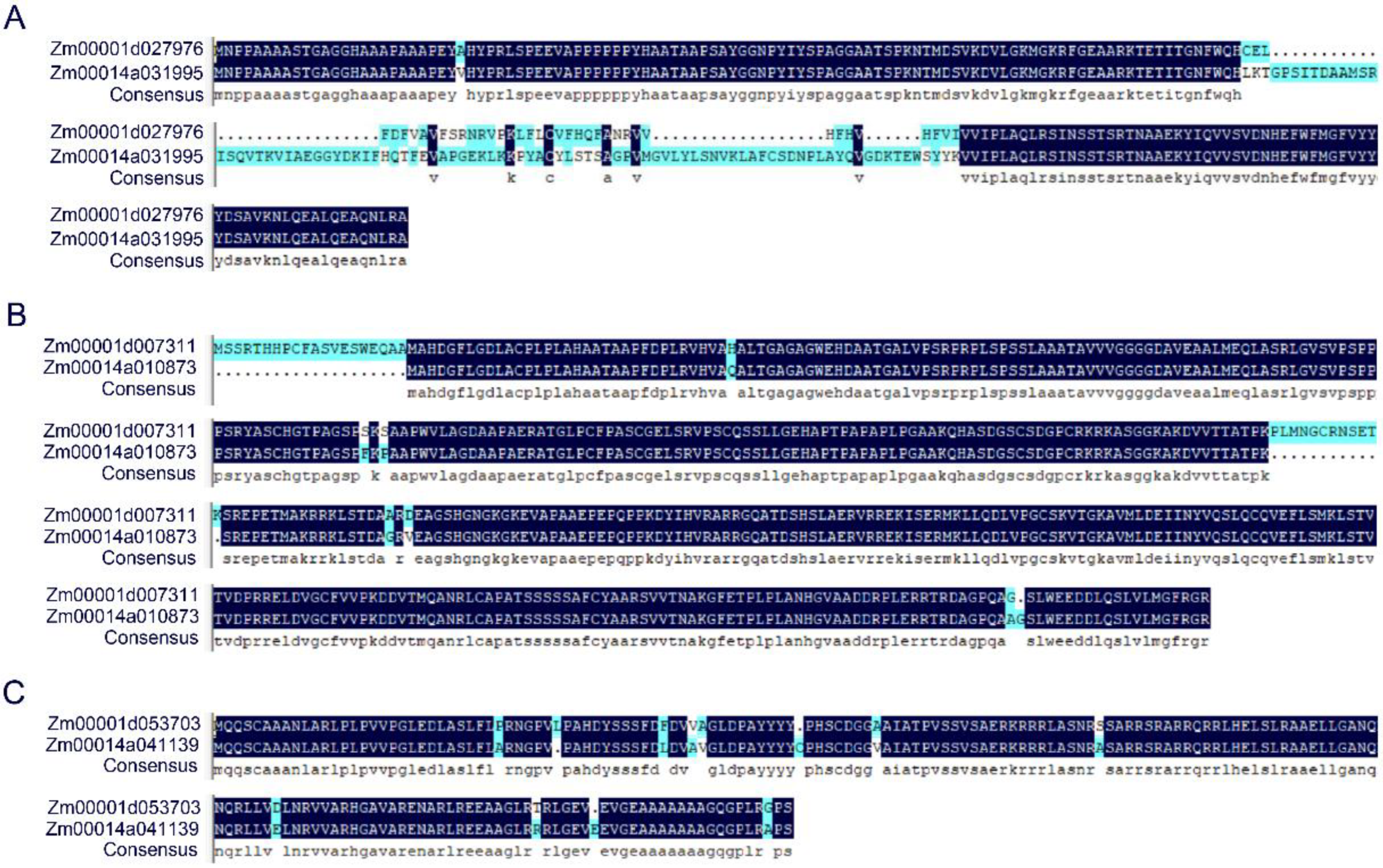
2.3. Candidate Gene Screening
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Germination Conditions and Measurement Indexes
4.3. QTL Analysis and Candidate Gene Mining
4.4. RNA-Seq Analysis and Candidate Gene Selection
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Liu, P.; Wang, C.; Zhang, N.; Shen, Y. Genome-wide association study uncovers new genetic loci and candidate genes underlying seed chilling-germination in maize. Peer J. 2021, 9, e11707. [Google Scholar] [CrossRef]
- Rodríguez, V.M.; Butrón, A.; Malvar, R.A.; Ordás, A.; Revilla, P. Quantitative Trait Loci for Cold Tolerance in the Maize IBM Population. Int. J. Plant Sci. 2008, 169, 551–556. [Google Scholar] [CrossRef]
- Fujino, K. Mapping of quantitative trait loci controlling low-temperature germinability in rice (Oryza sativa L.). Theor. Appl. Genet. 2004, 108, 118. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, X.; Xie, K.; Wang, Y.; Liu, F.; Lin, Q.; Wang, W.; Yang, C.; Lu, B.; Liu, S. qLTG-9, a stable quantitative trait locus for low-temperature germination in rice (Oryza sativa L.). Theor. Appl. Genet. 2013, 126, 2313–2322. [Google Scholar] [CrossRef]
- Fujino, K.; Sekiguchi, H.; Matsuda, Y.; Sugimoto, K.; Ono, K.; Yano, M. Molecular identification of a major quantitative trait locus, qLTG3-1, controlling low-temperature germinability in rice. Proc. Natl. Acad. Sci. USA 2008, 105, 12623–12628. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zou, B.; Shao, Q.; Cui, Y.; Lu, S.; Zhang, Y.; Huang, Q.; Huang, J.; Hua, J. Natural variation reveals that OsSAP16 controls low-temperature germination in rice. J. Exp. Bot. 2018, 11, 413–421. [Google Scholar] [CrossRef]
- Hodges, D.M.; Andrews, C.J.; Johnson, D.A.; Hamilton, R.I. Sensitivity of Maize Hybrids to Chilling and Their Combining Abilities at Two Developmental Stages. Crop. Sci. 1997, 37, 850–856. [Google Scholar] [CrossRef]
- Fracheboud, Y.; Jompuk, C.; Ribaut, J.M.; Stamp, P.; Leipner, J. Genetic analysis of cold-tolerance of photosynthesis in maize. Plant. Mol. Biol. 2004, 56, 241–253. [Google Scholar] [CrossRef]
- Hund, A.; Fracheboud, Y.; Soldati, A.; Frascaroli, E.; Salvi, S.; Stamp, P. QTL controlling root and shoot traits of maize seedlings under cold stress. Theor. Appl. Genet. 2004, 109, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Malvar, R.A.; Alvarez-Iglesias, L.; Ordas, B.; Revilla, P. Dissecting the genetics of cold tolerance in a multiparental maize population. Theor. Appl. Genet. 2020, 133, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Revilla, P.; Rodríguez, V.M.; Ordás, A.; Rincent, R.; Charcosset, A.; Giauffret, C.; Melchinger, A.E.; Schön, C.-C.; Bauer, E.; Altmann, T.; et al. Association mapping for cold tolerance in two large maize inbred panels. BMC Plant. Biol. 2016, 16, 127. [Google Scholar] [CrossRef]
- Allam, M.; Revilla, P.; Djemel, A.; Tracy, W.F.; Ordás, B. Identification of QTLs involved in cold tolerance in sweet x field corn. Euphytica Int. J. Plant Breed. 2016, 208, 353–365. [Google Scholar] [CrossRef]
- Yi, Q.; Alvarez-Iglesias, L.; Malvar, R.A.; Romay, M.C.; Revilla, P. A worldwide maize panel revealed new genetic variation for cold tolerance. Theor. Appl. Genet. 2021, 134, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Zhu, J.; Zhu, J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Lübberstedt, T.; Zhao, G.; Lee, M. QTL mapping of low-temperature germination ability in the maize IBM Syn4 RIL population. PLoS ONE 2016, 11, e0152795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Xu, Q.; Wang, D.; Zhou, Y. Identification of candidate tolerance genes to low-temperature during maize germination by GWAS and RNA-seqapproaches. BMC Plant Biol. 2020, 20, 333. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Gao, C.; Guo, G.; Liu, J.; Gao, Y.; Pan, R.; Guan, Y.; Hu, J. Maize annexin genes ZmANN33 and ZmANN35 encode proteins that function in cell membrane recovery during seed germination. J. Exp. Bot. 2019, 70, 1183–1195. [Google Scholar] [CrossRef]
- Collard, B.; Jahufer, M.; Brouwer, J.B.; Pang, E.K. An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: The basic concepts. Euphytica 2005, 142, 169–196. [Google Scholar] [CrossRef]
- Liu, H.; Niu, Y.; Gonzalez-Portilla, P.J.; Zhou, H.; Wang, L.; Zuo, T.; Qin, C.; Tai, S.; Jansen, C.; Shen, Y.; et al. An ultra-high-density map as a community resource for discerning the genetic basis of quantitative traits in maize. BMC Genom. 2015, 16, 1078. [Google Scholar] [CrossRef]
- Frascaroli, E.; Landi, P. Registration of Maize Inbred Line Bo23 with High Cold Tolerance and Agronomic Performance for Early Sowing. J. Plant Regist. 2017, 11, 172–177. [Google Scholar] [CrossRef][Green Version]
- Gao, Z.; Feng, H.Y.; Liang, X.G.; Lin, S.; Zhao, X.; Shen, S.; Du, X.; Cui, Y.H.; Zhou, S.L. Adjusting the sowing date of spring maize did not mitigate against heat stress in the North China Plain. Agric. For. Meteorol. 2021, 298, 7. [Google Scholar] [CrossRef]
- Frascaroli, E.; Landi, P. Divergent selection in a maize population for germination at low temperature in controlled environment: Study of the direct response, of the trait inheritance and of correlated responses in the field. Theor. Appl. Genet. 2013, 126, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Hubbard, K.G.; Lin, X.M.; Yang, X.G. Negative effects of climate warming on maize yield are reversed by the changing of sowing date and cultivar selection in Northeast China. Glob. Chang. Biol. 2013, 19, 3481–3492. [Google Scholar] [CrossRef]
- Yang, L.M.; Liu, H.L.; Lei, L.; Zhao, H.W.; Wang, J.G.; Li, N.; Sun, J.; Zheng, H.L.; Zou, D.T. Identification of QTLs controlling low-temperature germinability and cold tolerance at the seedling stage in rice (Oryza Sativa L.). Euphytica 2018, 214, 13. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Hagihara, S.; Hirai, D. Field assessment of a major QTL associated with tolerance to cold-induced seed coat discoloration in soybean. Breed. Sci. 2019, 69, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Knoll, J.; Gunaratna, N.; Ejeta, G. QTL analysis of early-season cold tolerance in sorghum. Theor. Appl. Genet. 2008, 116, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Yagcioglu, M.; Jiang, B.; Wang, P.; Wang, Y.; Ellialtioglu, S.S.; Weng, Y. QTL mapping of low temperature germination ability in cucumber. Euphytica 2019, 215, 84. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Pan, Y.; Li, J.; Zhou, L.; Shi, H.; Zeng, Y.; Guo, H.; Yang, S.; Zheng, W. Natural variation in CTB4a enhances rice adaptation to cold habitats. Nat. Commun. 2017, 8, 14788. [Google Scholar] [CrossRef]
- Rodríguez, V.; Butrón, A.; Rady, M.; Soengas, P.; Revilla, P. Identification of quantitative trait loci involved in the response to cold stress in maize (Zea mays L.). Mol. Breed. 2014, 33, 363–371. [Google Scholar] [CrossRef]
- Jompuk, C. Mapping of quantitative trait loci associated with chilling tolerance in maize (Zea mays L.) seedlings grown under field conditions. J. Exp. Bot. 2005, 56, 1153–1163. [Google Scholar] [CrossRef]
- Han, Q.H.; Shen, Y.; Lv, L.; Lee, M.; Lubberstedt, T.; Zhao, G.W. QTL analysis of deep-sowing tolerance during seed germination in the maize IBM Syn4 RIL population. Plant Breed. 2020, 139, 1125–1134. [Google Scholar] [CrossRef]
- Liu, H.; Lin, Z.; Wang, J.; Li, C.; Xing, Z.; Xie, S.; Zhang, Y.; Liu, S.; Hu, S.; Wang, J. Quantitative Trait Locus Analysis for Deep-Sowing Germination Ability in the Maize IBM Syn10 DH Population. Front. Plant Sci. 2017, 8, 813. [Google Scholar] [CrossRef]
- Thapa, R.; Tabien, R.E.; Thomson, M.J.; Septiningsih, E.M. Genome-Wide Association Mapping to Identify Genetic Loci for Cold Tolerance and Cold Recovery During Germination in Rice. Front. Genet. 2020, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.H.; Sami, A.; Xu, Q.Q.; Wu, L.L.; Zheng, W.Y.; Chen, Z.P.; Jin, X.Z.; Zhang, H.; Li, Y.; Yu, Y.; et al. Effects of seed priming treatments on the germination and development of two rapeseed (Brassica napus L.) varieties under the co-influence of low temperature and drought. PLoS ONE 2021, 16, e0257236. [Google Scholar]
- Li, X.H.; Wang, G.H.; Fu, J.J.; Li, L.; Jia, G.Y.; Ren, L.S.; Lubberstedt, T.; Wang, G.Y.; Wang, J.H.; Gu, R.L. QTL Mapping in Three Connected Populations Reveals a Set of Consensus Genomic Regions for Low Temperature Germination Ability in Zea mays L. Front. Plant Sci. 2018, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, Y.J.; Zhang, F.J.; Zhang, G.Z.; Jiang, X.Y.; Yu, H.M.; Hou, B.K. The Arabidopsis UDP-glycosyltransferases UGT79B2 and UGT79B3, contribute to cold, salt and drought stress tolerance via modulating anthocyanin accumulation. Plant J. 2017, 89, 85–103. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, S.; Tian, H.; He, Y.; Xiong, W.; Guo, L.; Wu, Y. CPK3-phosphorylated RhoGDI1 is essential in the development of Arabidopsis seedlings and leaf epidermal cells. J. Exp. Bot. 2013, 64, 3327–3338. [Google Scholar] [CrossRef]
- Kamimoto, Y.; Terasaka, K.; Hamamoto, M.; Takanashi, K.; Fukuda, S.; Shitan, N.; Sugiyama, A.; Suzuki, H.; Shibata, D.; Wang, B.; et al. Arabidopsis ABCB21 is a Facultative Auxin Importer/Exporter Regulated by Cytoplasmic Auxin Concentration. Plant Cell Physiol. 2012, 53, 2090–2100. [Google Scholar] [CrossRef]
- Wang, X.; Bi, S.; Wang, L.; Li, H.; Wang, C. GLABRA2 Regulates Actin Bundling Protein VILLIN1 in Root Hair Growth in Response to Osmotic Stress. Plant Physiol. 2020, 184, 176–193. [Google Scholar] [CrossRef]
- Huang, X.S.; Wang, W.; Zhang, Q.; Liu, J.H. A Basic Helix-Loop-Helix Transcription Factor PtrbHLH of Poncirus trifoliata Confers Cold Tolerance and Modulates POD-mediated Scavenging of H2O2. Plant Physiol. 2013, 162, 1178–1194. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.B.; Li, S.; Zhang, R.F.; Zhao, J.; Chen, Y.C.; Zhao, Q.; Yao, Y.-X.; You, C.-X.; Zhang, X.-S.; Hao, Y.-J. The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples. Plant Cell Environ. 2012, 35, 1884–1897. [Google Scholar] [CrossRef]
- Yu, C.M.; Yan, M.; Dong, H.Z.; Luo, J.; Ke, Y.C.; Guo, A.F.; Chen, Y.H.; Zhang, J.; Huang, X.S. Maize bHLH55 functions positively in salt tolerance through modulation of AsA biosynthesis by directly regulating GDP-mannose pathway genes. Plant Sci. 2021, 302, 110676. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.J.; Gan, S.F.; Jiao, J.Q.; He, Y.H.; Liu, H.; Yin, X.R.; Zhu, Q.G.; Rao, J.P. Genome-wide analysis of the bZIP gene family and the role of AchnABF1 from postharvest kiwifruit (Actinidia chinensis cv. Hongyang) in osmotic and freezing stress adaptations. Plant Sci. 2021, 308, 110927. [Google Scholar] [CrossRef]
- Cai, W.; Yang, Y.; Wang, W.; Guo, G.; Liu, W.; Bi, C. Overexpression of a wheat (Triticum aestivum L.) bZIP transcription factor gene, TabZIP6, decreased the freezing tolerance of transgenic Arabidopsis seedlings by down-regulating the expression of CBFs. Plant Physiol. Biochem. 2018, 124, 786. [Google Scholar] [CrossRef]
- Zhu, M.; Yu, X.; Zhao, G.; Wang, L. Effects of harvest time on seed vigor, enzyme activity and gene expression of conventional japonica rice. Arch. Agron. Soil Sci. 2020, 16, 5836. [Google Scholar] [CrossRef]
- Mccouch, S.; Cho, Y.; Yano, M.; Paul, E.; Blinstrub, M.; Morishima, H.; Mccouch, S.; Cho, Y.; Paul, E.; Morishima, H. Report on QTL nomenclature. Rice Genet. Newsl. 1997, 14, 113. [Google Scholar]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 7. [Google Scholar] [CrossRef] [PubMed]
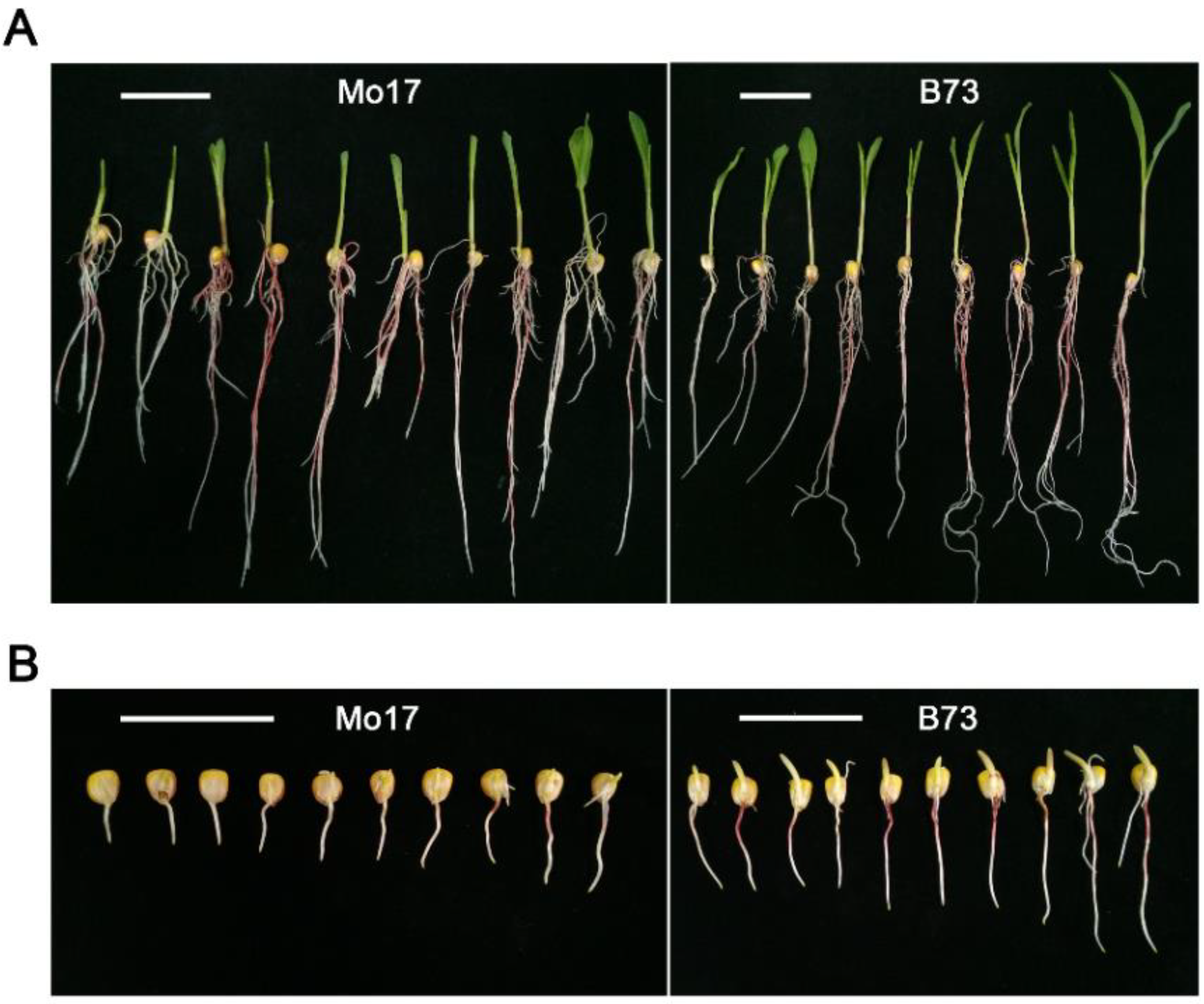
| Traits a | Parent b | Syn10 Population | ||||
|---|---|---|---|---|---|---|
| Mo17 | B73 | Range | Mean | S c | CV d | |
| OTPL * | 16.9 | 18.5 | 7.9–28.0 | 16.9 | 3.4 | 20 |
| OTSL ** | 8 | 13.6 | 3.3–21.8 | 11.2 | 3.5 | 31.5 |
| OTRL * | 9.2 | 15 | 4.0–23.0 | 12.6 | 3.6 | 28.7 |
| OTGR | 93.3 | 100 | 85.0–100.0 | 96.6 | 3.8 | 4 |
| OTGI | 3 | 3.1 | 2.3–5.9 | 3.1 | 0.6 | 18.4 |
| OTVI * | 54.5 | 59.4 | 24.8–85.4 | 51.8 | 10.1 | 19.5 |
| OTSVI * | 1695.4 | 1899.7 | 981.7–2600.0 | 1668.5 | 261.6 | 15.7 |
| OTAGD | 3.2 | 3.3 | 2.1–4.5 | 3.3 | 0.4 | 12.2 |
| LTPL *** | 0.8 | 2.2 | 0.3–5.5 | 1.5 | 1.0 | 67.8 |
| LTSL ** | 1.3 | 2.7 | 0.5–6.3 | 2.1 | 1.1 | 51.7 |
| LTRL *** | 0.6 | 6.8 | 0.1–9.1 | 3.3 | 1.7 | 52.7 |
| LTGR * | 83.3 | 96.7 | 20.0–100.0 | 84.8 | 15.6 | 18.4 |
| LTGI ** | 0.5 | 2.6 | 0.1–2.3 | 0.7 | 0.3 | 38.3 |
| LTVI *** | 0.4 | 18.1 | 0.1–21.8 | 2.6 | 2.4 | 94.2 |
| LTSVI ** | 57.7 | 661.7 | 19.0–853.7 | 294.6 | 170.0 | 57.7 |
| LTAGD ** | 16 | 7.4 | 8.0–19.9 | 13.4 | 2.3 | 17.1 |
| Traits | OTPL (cm) | OTSL (cm) | OTRL (cm) | OTGR (%) | OTGI | OTVI | OTSVI | OTAGD (d) | LTPL (cm) | LTSL (cm) | LTRL (cm) | GR (%) | LTGI | LTVI | LTSVI | LTAGD (d) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OTPL (cm) | 1.000 | |||||||||||||||
| OTSL (cm) | 0.627 ** | 1.000 | ||||||||||||||
| OTRL (cm) | 0.626 ** | 0.997 ** | 1.000 | |||||||||||||
| OTGR (%) | 0.177 * | 0.165 * | 0.172 * | 1.000 | ||||||||||||
| OTGI | 0.101 | 0.184 * | 0.187 * | 0.591 ** | 1.000 | |||||||||||
| OTVI | 0.553 ** | 0.452 ** | 0.448 ** | 0.522 ** | 0.782 ** | 1.000 | ||||||||||
| OTSVI | 0.672 ** | 0.468 ** | 0.466 ** | 0.693 ** | 0.424 ** | 0.811 ** | 1.000 | |||||||||
| OTAGD (d) | −0.001 | −0.098 | −0.097 | 0.065 | −0.716 ** | −0.510 ** | 0.025 | 1.000 | ||||||||
| LTPL (cm) | 0.053 | 0.078 | 0.079 | −0.063 | 0.227 ** | 0.139 | −0.049 | −0.310 ** | 1.000 | |||||||
| LTSL (cm) | 0.064 | 0.076 | 0.077 | −0.056 | 0.238 ** | 0.152 * | −0.036 | −0.326 ** | 0.982 ** | 1.000 | ||||||
| LTRL (cm) | 0.255 ** | 0.347 ** | 0.354 ** | 0.003 | 0.132 | 0.169 * | 0.102 | −0.161 * | 0.670 ** | 0.675 ** | 1.000 | |||||
| LTGR (%) | 0.170 * | 0.264 ** | 0.269 ** | 0.115 | 0.181 * | 0.219 ** | 0.176 * | −0.176 * | 0.204 ** | 0.254 ** | 0.375 ** | 1.000 | ||||
| LTGI | 0.043 | 0.128 | 0.133 | 0.177 * | 0.265 ** | 0.166 * | 0.072 | −0.198 ** | 0.380 ** | 0.405 ** | 0.574 ** | 0.537 ** | 1.000 | |||
| LTVI | 0.123 | 0.211 ** | 0.216 ** | 0.080 | 0.151 * | 0.114 | 0.055 | −0.125 | 0.498 ** | 0.500 ** | 0.817 ** | 0.346 ** | 0.875 ** | 1.000 | ||
| LTSVI | 0.265 ** | 0.367 ** | 0.374 ** | 0.013 | 0.132 | 0.183 * | 0.123 | −0.163 * | 0.646 ** | 0.658 ** | 0.971 ** | 0.547 ** | 0.634 ** | 0.823 ** | 1.000 | |
| LTAGD (d) | −0.037 | −0.094 | −0.095 | −0.007 | −0.295 ** | −0.219 ** | −0.019 | 0.368 ** | −0.605 ** | −0.642 ** | −0.503 ** | −0.200 ** | −0.692 ** | −0.597 ** | −0.486 ** | 1.000 |
| QTL Symbol a | Chr | Position (cm) | Left Marker | Right Marker | LOD b | Additive Effect c | PVE d (%) | 2-LOD Confidence Interval | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GC e Start (cM) | GC End (cM) | PC f Start (Mb) | PC End (Mb) | ||||||||
| qOTPL8-1 | 8 | 53 | chr08.243.5 | chr08.244.5 | 2.68 | −0.97 | 7.06 | 52.5 | 53.5 | 24.275 | 24.4 |
| qOTGI3-1 | 3 | 143 | chr03.2044.5 | chr03.2045.5 | 2.79 | 0.14 | 6.24 | 142.5 | 143.5 | 204.4 | 204.5 |
| qOTVI4-1 | 4 | 168 | chr04.2370.5 | chr04.2371.5 | 3.06 | −4.08 | 7.09 | 167.5 | 169.5 | 237 | 237.1 |
| qOTVI6-1 | 6 | 132 | chr06.1642.5 | chr06.1643.5 | 4.35 | −5.17 | 8.32 | 131.5 | 132.5 | 164.2 | 164.3 |
| qOTVI8-1 | 8 | 103 | chr08.1655.5 | chr08.1656.5 | 2.51 | 3.68 | 5.51 | 102.5 | 103.5 | 165.5 | 165.6 |
| qOTVI9-1 | 9 | 101 | chr09.1427.5 | chr09.1428.5 | 2.53 | 3.34 | 4.81 | 100.5 | 101.5 | 142.7 | 142.8 |
| qLTPL1-1 | 1 | 50 | chr01.187.5 | chr01.190 | 7.02 | 0.37 | 11.81 | 49.5 | 50.5 | 18.575 | 18.875 |
| qLTPL3-1 | 3 | 126 | chr03.1873.5 | chr03.1876 | 3.67 | 0.25 | 5.56 | 125.5 | 126.5 | 187.475 | 187.725 |
| qLTPL9-1 | 9 | 101 | chr09.1427.5 | chr09.1428.5 | 4.26 | 0.28 | 6.82 | 100.5 | 101.5 | 142.7 | 142.8 |
| qLTSL1-1 | 1 | 50 | chr01.187.5 | chr01.190 | 8.17 | 0.47 | 11.28 | 49.5 | 50.5 | 18.575 | 18.875 |
| qLTSL3-1 | 3 | 126 | chr03.1873.5 | chr03.1876 | 3.02 | 0.27 | 3.73 | 125.5 | 126.5 | 187.475 | 187.725 |
| qLTGI4-1 | 4 | 156 | chr04.2343.5 | chr04.2344.5 | 2.92 | −0.07 | 6.82 | 155.5 | 156.5 | 234.3 | 234.4 |
| qLTAGD2-1 | 2 | 172 | chr02.2206.5 | chr02.2207.5 | 3.85 | −0.64 | 6.99 | 171.5 | 172.5 | 220.475 | 221 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Q.; Zhu, Q.; Shen, Y.; Lee, M.; Lübberstedt, T.; Zhao, G. QTL Mapping Low-Temperature Germination Ability in the Maize IBM Syn10 DH Population. Plants 2022, 11, 214. https://doi.org/10.3390/plants11020214
Han Q, Zhu Q, Shen Y, Lee M, Lübberstedt T, Zhao G. QTL Mapping Low-Temperature Germination Ability in the Maize IBM Syn10 DH Population. Plants. 2022; 11(2):214. https://doi.org/10.3390/plants11020214
Chicago/Turabian StyleHan, Qinghui, Qingxiang Zhu, Yao Shen, Michael Lee, Thomas Lübberstedt, and Guangwu Zhao. 2022. "QTL Mapping Low-Temperature Germination Ability in the Maize IBM Syn10 DH Population" Plants 11, no. 2: 214. https://doi.org/10.3390/plants11020214
APA StyleHan, Q., Zhu, Q., Shen, Y., Lee, M., Lübberstedt, T., & Zhao, G. (2022). QTL Mapping Low-Temperature Germination Ability in the Maize IBM Syn10 DH Population. Plants, 11(2), 214. https://doi.org/10.3390/plants11020214






