Aluminum Stress Induces Irreversible Proteomic Changes in the Roots of the Sensitive but Not the Tolerant Genotype of Triticale Seedlings
Abstract
1. Introduction
2. Results
2.1. Biometric and Biochemical Evaluation of Tested Materials
2.2. Two-Dimensional Electrophoresis (2-DE)
2.3. Identification of Differential Proteins
3. Discussion
3.1. Evaluation of the Al Stress Response of Tolerant (L198) and Sensitive (L17, L444) Triticale Lines
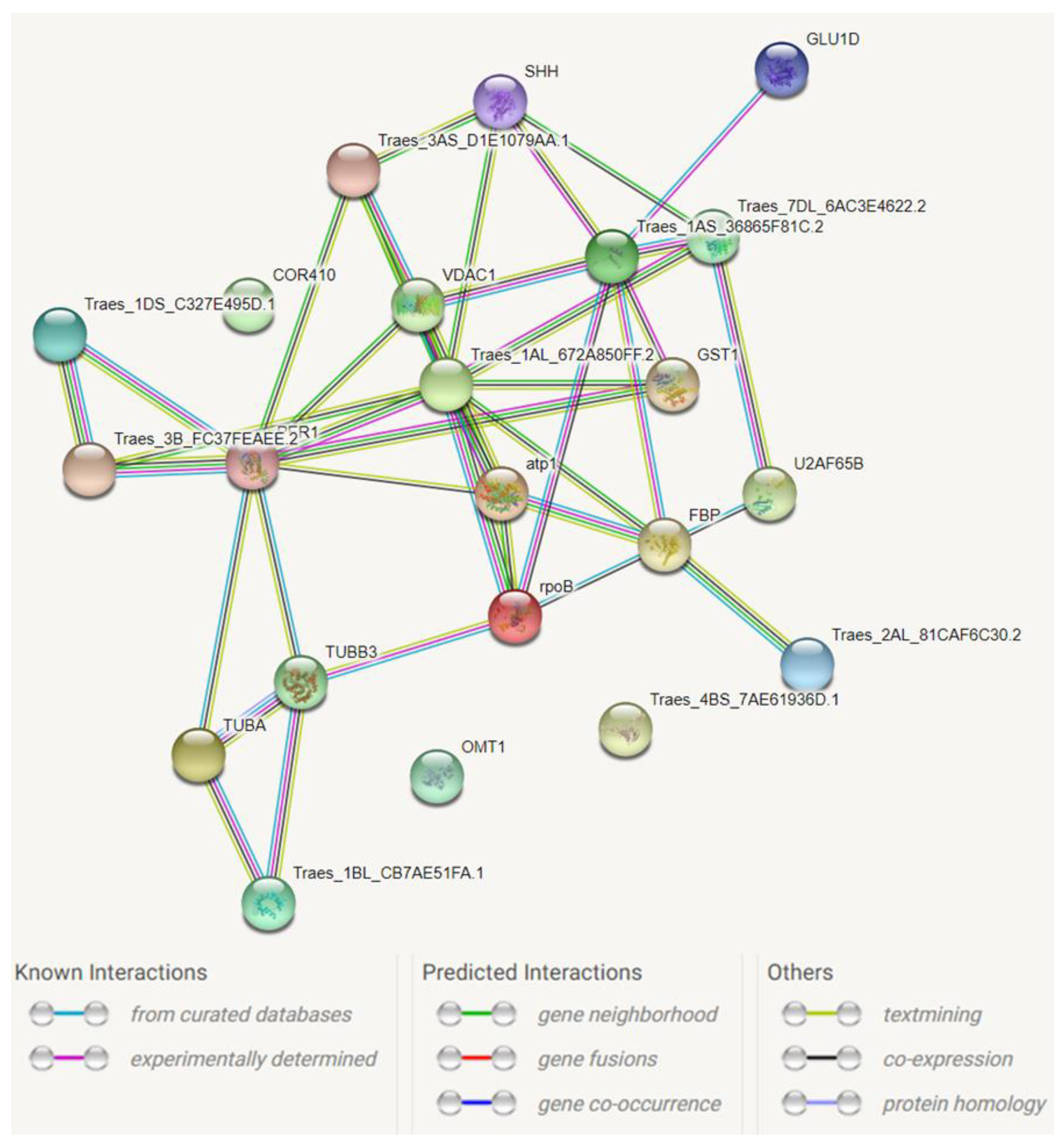
3.2. Annotation of Protein Spots
4. Materials and Methods
4.1. Plant Materials
4.2. Antioxidant Potential Determination
4.3. Proteomic Studies
Phenol-SDS Buffer Extraction with Sonication (PSWS)
4.4. Two-Dimensional Electrophoresis (2-DE)
4.5. Analysis of 2D PAGE Gel Images
4.6. Protein Identification by Mass Spectrometry and Database Search
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.S.; Schmidt, M.; Sturchio, N.; Nagy, K.; Fenter, P. Effect of pH on the Formation of Gibbsite-Layer Films at the Muscovite (001)−Water Interface. J. Phys. Chem. C 2019, 123, 6560–6571. [Google Scholar] [CrossRef]
- Kochian, L.V.; Piñeros, M.A.; Hoekenga, O.A. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. In Root Physiology: From Gene to Function; Lambers, H., Colmer, T.D., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 175–195. [Google Scholar]
- Barceló, J.; Poschenrieder, C. Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: A review. Environ. Exp. Bot. 2002, 48, 75–92. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Q.L.; Geng, M.J.; Guo, Z.H.; Zhao, Z. Rhizosphere pH difference regulated by plasma membrane H+-ATPase is related to differential Al tolerance of two wheat cultivars. Plant Soil Envion. 2011, 57, 201–206. [Google Scholar] [CrossRef]
- Blamey, F.P.C. The Role of the Root Cell Wall in Aluminum Toxicity. In Plant Nutrient Acquisition; Ae, N., Arihara, J., Okada, K., Srinivasan, A., Eds.; Springer: Tokyo, Japan, 2001; pp. 201–226. [Google Scholar]
- Matsumoto, H. Cell biology of aluminium toxicity and tolerancein higher plants. Int. Rev. Cytol. 2000, 200, 1–46. [Google Scholar] [CrossRef]
- Kochian, L.V. Cellular mechanisms of aluminium toxicity and tolerance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995, 46, 237–260. [Google Scholar] [CrossRef]
- Fontecha, G.; Silva-Navas, J.; Benito, C.; Mestres, M.A.; Espino, F.J.; Hernández-Riquer, M.V.; Gallego, F.J. Candidate gene identification of an aluminum-activated organic acid transporter gene at the Alt4 locus for aluminum tolerance in rye (Secale cereale L.). Theor. Appl. Genet. 2007, 114, 249–260. [Google Scholar] [CrossRef]
- Silva-Navas, J.; Benito, C.; Téllez-Robledo, B.; Abd El-Moneim, D.; Gallego, F.J. The ScAACT1 gene at the Qalt5locus as a candidate for increased aluminum tolerance in rye (Secale cereale L.). Mol. Breed. 2012, 30, 845–856. [Google Scholar] [CrossRef]
- Ryan, P.R.; Raman, H.; Gupta, S.; Horst, W.J.; Delhaize, E. A Second Mechanism for Aluminum Resistance in Wheat Relies on the Constitutive Efflux of Citrate from Roots. Plant Physiol. 2009, 149, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Oliveira, A.L.; Benito, C.; Prieto, P.; de Andrade Menezes, R.; Rodrigues-Pousada, C.; Guedes-Pinto, H.; Martins-Lopes, P. Molecular characterization of TaSTOP1 homoeologues and their response to aluminium and proton (H+) toxicity in bread wheat (Triticum aestivum L.). BMC Plant Biol. 2013, 13, 134. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Dong, D.; Jia, X.; McCouch, S.; Kochian, L. Natural variation underlies alterations in Nramp aluminum transporter (NRAT1) expression and function that play a key role in rice aluminum tolerance. Proc. Natl. Acad. Sci. USA 2014, 111, 6503–6508. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, W.; Yumnam, J.S.; Sen, D.; Rai, M. Root transcriptome reveals efficient cell signaling and energy conservation key to aluminum toxicity tolerance in acidic soil adapted rice genotype. Sci. Rep. 2020, 10, 4580. [Google Scholar] [CrossRef]
- Niedziela, A.; Bednarek, P.T.; Labudda, M.; Mańkowski, D.R.; Anioł, A. Genetic mapping of a 7R Al tolerance QTL in triticale (× Triticosecale Wittmack). J. Appl. Genet. 2014, 55, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Niedziela, A.; Bednarek, P.T.; Cichy, H.; Budzianowski, G.; Kilian, A.; Anioł, A. Aluminum tolerance association mapping in triticale. BMC Genom. 2012, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Budzianowski, G.; Woś, H. The effect of single D-genome chromosomes on aluminum tolerance of triticale. Euphytica 2004, 137, 165–172. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Urban, M.O.; Klíma, M.; Roy, A.; Tom Prášil, I. Biological networks underlying abiotic stress tolerance in temperate crops-a proteomic perspective. Int. J. Mol. Sci. 2015, 16, 20913–20942. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Prášil, I.T. Proteomics of stress responses in wheat and barley—Search for potential protein markers of stress tolerance. Front. Plant Sci. 2014, 5, 711. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Li, Y.Y.; Zhang, Y.J.; Zhang, S.S.; Wu, Y.R.; Wu, P.; Zheng, S.J. Cell Wall Polysaccharides Are Specifically Involved in the Exclusion of Aluminum from the Rice Root Apex. Plant Physiol. 2008, 146, 602–611. [Google Scholar] [CrossRef]
- Wang, C.; Shen, R.F.; Wang, W. Root protein profile changes induced by Al exposure in two rice cultivars differing in Al tolerance. J. Proteom. 2012, 78, 281–293. [Google Scholar] [CrossRef]
- Oh, M.W.; Roy, S.K.; Kamal, A.H.M.; Cho, K.; Cho, S.-W.; Park, C.-S.; Choi, J.-S.; Komatsu, S.; Woo, S.-H. Proteome analysis of roots of wheat seedlings under aluminum stress. Mol. Biol. Rep. 2014, 41, 671–681. [Google Scholar] [CrossRef]
- Dai, J.; Bai, G.; Zhang, D.; Hong, D. Validation of quantitative trait loci for aluminum tolerance in Chinese wheat landrace FSW. Euphytica 2013, 192, 171–179. [Google Scholar] [CrossRef]
- Duressa, D.; Soliman, K.; Taylor, R.; Senwo, Z. Proteomic Analysis of Soybean Roots under Aluminum Stress. Int. J. Plant Genom. 2011, 2011, 282531. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Lan, P.; Shen, R.F.; Li, W.F. Proteomics of aluminum tolerance in plants. Proteimics 2014, 14, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Saito, A.; Wasaki, J.; Shinano, T.; Osaki, M. Metabolic alterations proposed by proteome in rice roots grown under low P and high Al concentration under low pH. Plant Sci. 2007, 172, 1157–1165. [Google Scholar] [CrossRef]
- Zhou, S.; Sauvé, R.; Thannhauser, T.W. Proteome changes induced by aluminium stress in tomato roots. J. Exp. Bot. 2009, 60, 1849–1857. [Google Scholar] [CrossRef]
- Ezaki, B.; Katsuhara, M.; Kawamura, M.; Matsumoto, H. Different Mechanisms of Four Aluminum (Al)-Resistant Transgenes for Al Toxicity in Arabidopsis. Plant Physiol. 2001, 127, 918–927. [Google Scholar] [CrossRef]
- Wei, Y.; Jiang, C.; Han, R.; Xie, Y.; Liu, L.; Yu, Y. Plasma membrane proteomic analysis by TMT-PRM provides insight into mechanisms of aluminum resistance in tamba black soybean roots tips. PeerJ 2020, 8, e9312. [Google Scholar] [CrossRef] [PubMed]
- Grębosz, J.; Badowiec, A.; Weidner, S. Changes in the root proteome of Triticosecale grains germinating under osmotic stress. Acta Physiol. Plant. 2014, 36, 825–835. [Google Scholar] [CrossRef]
- Meriño-Gergichevich, C.; Ondrasek, G.; Zovko, M.; Šamec, D.; Alberdi, M.; Reyes-Díaz, M. Comparative study of methodologies to determine the antioxidant capacity of Al-toxified blueberry amended with calcium sulfate. J. Soil Sci. Plant Nutr. 2015, 15, 965–978. [Google Scholar] [CrossRef]
- Arnao, M.B. Some methodological problems in the determination of antioxidant activity using chromogen radicals: A practical case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef]
- Matsumoto, H.; Senoo, Y.; Kasai, M.; Maeshima, M. Response of the plant root to aluminum stress: Analysis of the inhibition of the root elongation and changes in membrane function. J. Plant Res. 1996, 109, 99–105. [Google Scholar] [CrossRef]
- Vartapetian, B.B.; Andeeva, I.N.; Generozova, I.P.; Polyakova, L.I.; Maslova, I.P.; Dogikh, Y.I.; Stepanova, A.Y. Functional Electron Microscopy in Studies of Plant response and adaptation to Anaerobic Stress. Ann. Bot. 2003, 91, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Szewińska, J.; Różańska, E.; Papierowska, E.; Labudda, M. Proteolytic and Structural Changes in Rye and Triticale Roots under Aluminum Stress. Cells 2021, 10, 3046. [Google Scholar] [CrossRef] [PubMed]
- Aniol, A.; Gustafson, J. Chromosome location of genes controlling aluminium tolerance in wheat, rye, and triticale. Genome 1984, 26, 701–705. [Google Scholar] [CrossRef]
- Aniol, A. Induction of Aluminum Tolerance in Wheat Seedlings by Low Doses of Aluminum in the Nutrient Solution. Plant Physiol. 1984, 76, 551–555. [Google Scholar] [CrossRef]
- Kim, B.Y.; Baier, A.C.; Somers, D.J.; Gustafson, J.P. Aluminum tolerance in triticale, wheat and rye. Euphytica 2001, 120, 329–337. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, Y.; Cao, Y.; Liu, J. Aluminum-activated root malate and citrate exudation is independent of NIP1;2-facilitated root-cell-wall aluminum removal in Arabidopsis. Plant Signal. Behav. 2018, 13, e1422469. [Google Scholar] [CrossRef]
- Li, M.; Pu, Y.; Yoo, C.G.; Ragauskas, A. The occurrence of tricin and its derivatives in plants. Green Chem. 2016, 18, 1439–1454. [Google Scholar] [CrossRef]
- Chandran, D.; Sharopova, N.; Ivashuta, S.; Gantt, J.S.; VandenBosch, K.A.; Samac, D.A. Transcriptome profiling identified novel genes associated with aluminum toxicity, resistance and tolerance in Medicago truncatula. Planta 2008, 228, 151–166. [Google Scholar] [CrossRef]
- Jung, J.; Hong, M.; Kim, D.; Kim, J.; Heo, H.; Kim, T.; Jang, C.; Seo, Y.W. Structural and expressional divergence of genes encoding O-methyltransferase in wheat. Genome 2008, 51, 856–869. [Google Scholar] [CrossRef]
- Zhou, J.-M.; Seo, Y.W.; Ibrahim, R.K. Biochemical characterization of a putative wheat caffeic acid O-methyltransferase. Plant Physiol. Biochem. 2009, 47, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Ge, C.; Xu, C.; Wang, X.; Wang, S.; Wang, Q. Expression Analysis of Oxalate Metabolic Pathway Genes Reveals Oxalate Regulation Patterns in Spinach. Molecules 2018, 23, 1286. [Google Scholar] [CrossRef]
- Tamás, L.; Budíková, S.; Huttová, J.; Mistrík, I.; Simonovicová, M.; Siroká, B. Aluminum-induced cell death of barley-root border cells is correlated with peroxidase- and oxalate oxidase-mediated hydrogen peroxide production. Plant Cell Rep. 2005, 24, 189–194. [Google Scholar] [CrossRef]
- Delisle, G.; Champoux, M.; Houde, M. Characterization of Oxalate Oxidase and Cell Death in Al-Sensitive and Tolerant Wheat Roots. Plant Cell Physiol. 2001, 42, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Arisi, A.; Jouanin, L.; Kunert, K.; Rennenberg, H.; Foyer, C. Review article. Glutathione: Biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J. Exp. Bot. 1998, 49, 623–647. [Google Scholar] [CrossRef]
- Dmitriev, A.A.; Krasnov, G.S.; Rozhmina, T.A.; Kishlyan, N.V.; Zyablitsin, A.V.; Sadritdinova, A.F.; Snezhkina, A.V.; Fedorova, M.S.; Yurkevich, O.Y.; Muravenko, O.V.; et al. Glutathione S-transferases and UDP-glycosyltransferases Are Involved in Response to Aluminum Stress in Flax. Front. Plant Sci. 2016, 7, 1920. [Google Scholar] [CrossRef]
- Cancado, G.M.A.; De Rosa, V.E.; Fernandez, J.H.; Maron, L.G.; Jorge, R.A.; Menossi, M. Glutathione S-transferase and aluminum toxicity in maize. Funct. Plant Biol. 2005, 32, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Matsumoto, H. Changes in antioxidant gene expression and induction of oxidative stress in pea (Pisum sativum L.) under Al stress. Biometals 2010, 23, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Hirakawa, Y.; Sawa, S. Peptide signaling in vascular development. Curr. Opin. Plant Biol. 2007, 10, 477–482. [Google Scholar] [CrossRef]
- Kim, B.-G.; Sung, S.H.; Chong, Y.; Lim, Y.; Ahn, J.-H. Plant Flavonoid O-Methyltransferases: Substrate Specificity and Application. J. Plant Biol. 2010, 53, 321–329. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Tolrà, R.; Barceló, J. A role for cyclic hydroxamates in aluminum resistance in maize? J. Inorg. Biochem. 2005, 99, 1830–1836. [Google Scholar] [CrossRef]
- Neal, A.L.; Ahmad, S.; Gordon-Weeks, R.; Ton, J. Benzoxazinoids in Root Exudates of Maize Attract Pseudomonas putida to the Rhizosphere. PLoS ONE 2012, 7, e35498. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Pytela, J.; Hotta, T.; Kato, T.; Hamada, T.; Akamatsu, R.; Ishida, Y.; Kutsuna, N.; Hasezawa, S.; Nomura, Y.; et al. An Atypical Tubulin Kinase Mediates Stress-Induced Microtubule Depolymerization in Arabidopsis. Curr. Biol. 2013, 23, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Sivaguru, M.; Baluška, F.; Volkmann, D.; Felle, H.H.; Horst, W.J. Impacts of Aluminum on the Cytoskeleton of the Maize Root Apex. Short-Term Effects on the Distal Part of the Transition Zone. Plant Physiol. 1999, 119, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Ren, J.; Goss, D.J. Wheat Germ Translation Initiation Factor eIF4B Affects eIF4A and eIFiso4F Helicase Activity by Increasing the ATP Binding Affinity of eIF4A. Biochemistry 2000, 39, 5758–5765. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, N.; Vashisht, A.; Tuteja, R. Translation initiation factor 4A: A prototype member of dead-box protein family. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2008, 14, 101–107. [Google Scholar] [CrossRef]
- Pham, X.H.; Reddy, M.K.; Ehtesham, N.Z.; Matta, B.; Tuteja, N. A DNA helicase from Pisum sativum is homologous to translation initiation factor and stimulates topoisomerase I activity. Plant J. 2000, 24, 219–229. [Google Scholar] [CrossRef]
- Vashisht, A.A.; Pradhan, A.; Tuteja, R.; Tuteja, N. Cold- and salinity stress-induced bipolar pea DNA helicase 47 is involved in protein synthesis and stimulated by phosphorylation with protein kinase C. Plant J. 2005, 44, 76–87. [Google Scholar] [CrossRef]
- Santosh, R.B.R.T.; Vijaya Naresh, J.; Sudhakar, R.P.; Reddy, M.K.; Mallikarjuna, G. Expression of Pennisetum glaucum Eukaryotic Translational Initiation Factor 4A (PgeIF4A) Confers Improved Drought, Salinity, and Oxidative Stress Tolerance in Groundnut. Front. Plant Sci. 2017, 8, 453. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Li, R.; Yuan, L.; Dai, Y.; Wang, X. Identification and Functional Analysis of a Protein Disulfide Isomerase (AtPDI1) in Arabidopsis thaliana. Front. Plant Sci. 2018, 9, 913. [Google Scholar] [CrossRef]
- Khan, R.; Siddiqui, M.; Salahuddin, P. Protein Disulfide Isomerase: Structure, Mechanism of Oxidative Protein Folding and Multiple Functional Roles. J. Biochem. Mol. Biol. Res. 2016, 2, 173–179. [Google Scholar] [CrossRef]
- Zhu, C.; Luo, N.; He, M.; Chen, G.; Zhu, J.; Yin, G.; Li, X.; Hu, Y.; Li, J.; Yan, Y. Molecular Characterization and Expression Profiling of the Protein Disulfide Isomerase Gene Family in Brachypodium distachyon L. PLoS ONE 2014, 9, e94704. [Google Scholar] [CrossRef]
- Kayum, M.A.; Park, J.-I.; Nath, U.K.; Saha, G.; Biswas, M.K.; Kim, H.-T.; Nou, I.-S. Genome-wide characterization and expression profiling of PDI family gene reveals function as abiotic and biotic stress tolerance in Chinese cabbage (Brassica rapa ssp. pekinensis). BMC Genom. 2017, 18, 885. [Google Scholar] [CrossRef]
- Lyzenga, W.J.; Stone, S.L. Abiotic stress tolerance mediated by protein ubiquitination. J. Exp. Bot. 2011, 63, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.-Y.; Guo, X.-G.; Xie, L.-P.; Xie, C.-G.; Zhang, X.-H.; Yang, Y.; Xiao, L.; Tang, Y.-Y.; Pan, X.-L.; Guo, A.-G.; et al. Molecular Characterization, Gene Evolution, and Expression Analysis of the Fructose-1, 6-bisphosphate Aldolase (FBA) Gene Family in Wheat (Triticum aestivum L.). Front. Plant Sci. 2017, 8, 1030. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.W. Malonyl-CoA: The regulator of fatty acid synthesis and oxidation. J. Clin. Investig. 2012, 122, 1958–1959. [Google Scholar] [CrossRef]
- Wagatsuma, T. The membrane lipid bilayer as a regulated barrier to cope with detrimental ionic conditions: Making new tolerant plant lines with altered membrane lipid bilayer. Soil Sci. Plant Nutr. 2017, 63, 507–516. [Google Scholar] [CrossRef]
- Lindberg, S.; Griffiths, G. Aluminium Effects on ATPase Activity and Lipid Composition of Plasma Membranes in Sugar Beet Roots. J. Exp. Bot. 1993, 44, 1543–1550. [Google Scholar] [CrossRef]
- Zhang, G.; Slaski, J.J.; Archambault, D.J.; Taylor, G.J. Alternation of plasma membrane lipids in aluminum-resistant and aluminum-sensitive wheat genotypes in response to aluminum stress. Physiol. Plant. 1997, 99, 302–308. [Google Scholar] [CrossRef]
- Maejima, E.; Watanabe, T. Proportion of phospholipids in the plasma membrane is an important factor in Al tolerance. Plant Signal. Behav. 2014, 9, e29277. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, L.; Singh, A.; Wang, H.; Du, L.; Poovaiah, B.W. Involvement of calmodulin and calmodulin-like proteins in plant responses to abiotic stresses. Front. Plant Sci. 2015, 6, 600. [Google Scholar] [CrossRef] [PubMed]
- Hanin, M.; Brini, F.; Ebel, C.; Toda, Y.; Takeda, S.; Masmoudi, K. Plant dehydrins and stress tolerance. Plant Signal. Behav. 2011, 6, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Niedziela, A. The influence of Al3+ on DNA methylation and sequence changes in the triticale (× Triticosecale Wittmack) genome. J. Appl. Genet. 2018, 59, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Hurkman, W.J.; Tanaka, C.K. Solubilization of Plant Membrane Proteins for Analysis by Two-Dimensional Gel Electrophoresis. Plant Physiol. 1986, 81, 802–806. [Google Scholar] [CrossRef]
- Laemmli, U. Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Nykiel, M.; Lisik, P.; Dębski, J.; Florea, B.I.; Rybka, K. Chl a fluorescence and proteomics reveal the protection of photosynthetic apparatus in tolerant but not susceptible to dehydration wheat cultivar. Biol. Plant. 2019, 63, 287–297. [Google Scholar] [CrossRef]
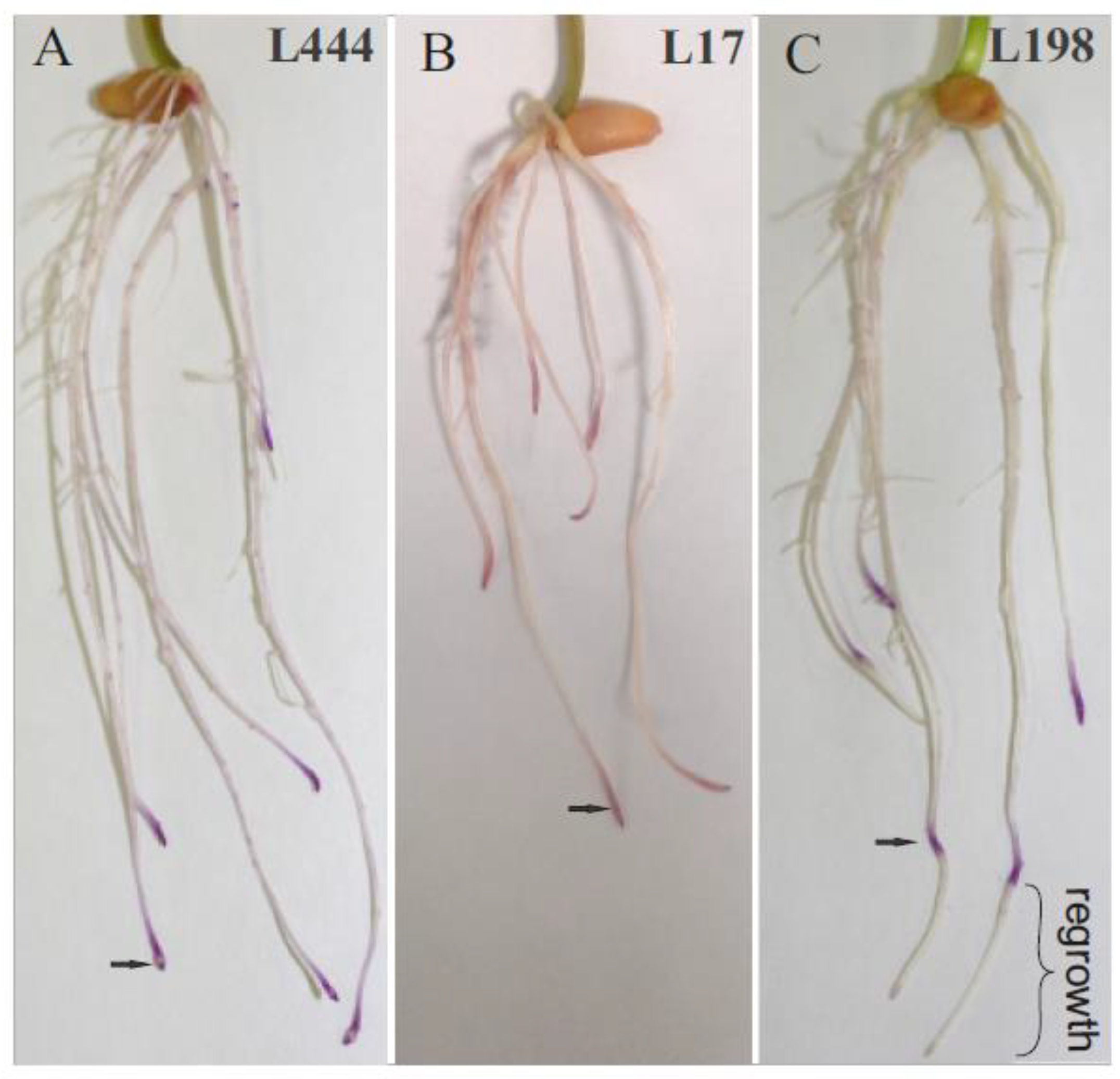
| Line | Control/Stress | Root Regrowth (cm) | DPPH*− (μmol TEAC/mg) | ABTS*+ (μmol TEAC/mg) |
|---|---|---|---|---|
| L198 | control | - | 15.264 ± 1.17 a | 8.706 ± 0.39 ab |
| L198 | stress (16ppm Al) | 0.3–2.5 | 15.786 ± 1.29 a | 9.170 ± 0.39 a |
| L444 | control | - | 13.854 ± 1.00 a | 8.072 ± 0.11 b |
| L444 | stress (16ppm Al) | no regrowth | 16.643 ± 3.34 a | 9.124 ± 0.25 a |
| L17 | control | - | 12.544 ± 2.05 a | 8.086 ± 0.58 b |
| L17 | stress (16ppm Al) | no regrowth | 12.602 ± 0.9 a | 8.870 ± 0.10 ab |
| Line | Tolerant | Sensitive | L17-L444 | |
|---|---|---|---|---|
| Spots Characteristic | L198 | L17 | L444 | Common Spots (with p ≤ 0.01) |
| 579 | 584 | 602 | |
| 0 | 23 (14 ≥ 2×) | 23 (18 ≥ 2×) | 13 |
| 0 | 21 (8 ≥ 2×) | 8 (0 ≥ 2×) | 2 |
| 0 | 12 (3 ≥ 0.2) | 9 (1 ≥ 0.2) | 3 |
| 0 | 15 (9 ≥ 0.2) | 3 (0 ≥ 0.2) | 2 |
| Spot No. | Pathway/Protein Name | UniProt ID | Mascot Score | Mass | pI | 1 MP | Fold Changed | |
|---|---|---|---|---|---|---|---|---|
| L17 | L444 | |||||||
| Cell signaling | ||||||||
| 1 | Calmodulin | P04464 | 25 | 16,893 | 4.9 | 1 | +2.52 | * n.s. |
| Metabolic pathway | ||||||||
| 2 | ATP synthase subunit alpha, mitochondrial | P12862 | 201/96 | 55,515 | 6.6 | 4 | +3.54 | +2.73 |
| 3 | Adenosylhomocysteinase | P32112 | 34 | 54,086 | 7.85 | 1 | −2.00 | −0.83 |
| 4 | Phosphoglycerate kinase, cytosolic | P12783 | 372 | 42,153 | 5.6 | 7 | +3.13 | +5.00 |
| 5 | Fructose-1,6-bisphosphatase | P09195 | 24 | 44,703 | 7.1 | 1 | +2.03 | * n.s. |
| Metabolic pathway/Flavonoid metabolism | ||||||||
| 6 | Flavone O-methyltransferase 1 | Q84N28 | 1053 | 39,177 | 5.7 | 22 | +7.61 | see Table 3 |
| Methyl cycle | ||||||||
| 7 | S-adenosylmethionine synthase | B0LXM0 | 448 | 43,609 | 5.51 | 7 | +2.91 | n.s. |
| Protease inhibitor | ||||||||
| 8 | Serpin-Z1C | Q9ST58 | 185 | 42,969 | 5.45 | 4 | +2.03 | +1.72 |
| Protein degradation/cell signaling | ||||||||
| 9 | Ubiquitin | P69326 | 42 | 8648 | 7.2 | 1 | +2.01 | +3.6 |
| 10 | Ubiquitin | P69326 | 38 | 8648 | 6.79 | 1 | +2.05 | n.s. |
| 11 | Ubiquitin | P69326 | 55 | 8648 | 7.25 | 1 | +2.43 | n.s. |
| Protein synthesis | ||||||||
| 12 | Protein disulfide-isomerase | P52589 | 113 | 56,726 | 4.9 | 4 | +2.51 | +3.61 |
| 13 | Eukaryotic initiation factor 4A | P41378 | 114 | 47,183 | 5.25 | 2 | +4.12 | +1.92 |
| 14 | Protein disulfide-isomerase | P52589 | 81 | 56,726 | 5.11 | 3 | +2.65 | +1.78 |
| Stress related | ||||||||
| 15 | Dehydrin COR410 | P46524 | 50 | 28,166 | 6.9 | 1 | +2.50 | +1.34 |
| 16 | Oxalate oxidase | P26759 | 341/394 | 23,711 | 6.35 | 5 | +2.75 | +5.00 |
| 17 | Glutathione S-transferase | O04437 | 218/474 | 24,022 | 6,2 | 11 | +2.85 | +4.52 |
| 18 | 1-Cys peroxiredoxin | Q6W8Q2 | 298 | 24178 | 6 | 5 | +2.87 | +3.51 |
| Transcription control | ||||||||
| 19 | Splicing factor U2af large subunit B | Q2QKB4 | 334 | 60,720 | 5.2 | 4 | +2.01 | +1.51 |
| 20 | DNA-directed RNA polymerase subunit beta | Q9XPS9 | 14 | 170,794 | 6.25 | 1 | +2.05 | n.s. |
| Transport | ||||||||
| 21 | Mitochondrial outer membrane porin | P46274 | 16 | 28,944 | 6.5 | 1 | +2.33 | +1.62 |
| Lignin synthesis | ||||||||
| 22 | 2 DIMBOA1b, chloroplastic | Q1XH05 | 56 | 64,898 | 5.25 | 2 | n.s. | +2.87 |
| 23 | 2 DIMBOA 1c, chloroplastic | Q1XH04 | 80 | 64,980 | 5.4 | 2 | n.s. | +2.16 |
| Unassigned peptides | ||||||||
| 24 | Unassigned peptide | - | - | - | - | - | −5.2 | n.s. |
| 25 | Unassigned peptide | - | - | - | - | - | +2.65 | n.s. |
| Spot No. | Pathway/Protein Name | UniProt/String (mloc) ID | Mascot Score | Mass | pI | 1 MP | 2 Spot Intensity | |
|---|---|---|---|---|---|---|---|---|
| L17 | L444 | |||||||
| Cell division/Cytoskeleton | ||||||||
| 26 | Tubulin beta-3 chain | Q9ZRB0 | 791 | 50,555 | 4.9 | 18 | −0.21 | * n.s. |
| 27 | Tubulin alpha chain | Q9ZRB7 | 2061 | 50,396 | 4.95 | 26 | −0.20 | n.s. |
| Lignin synthesis | ||||||||
| 28 | 3 DIMBOA 1b, chloroplastic | Q1XH05 | 328 | 64,898 | 5.55 | 7 | +0.21 | n.s. |
| 29 | 3 DIMBOA 1b, chloroplastic | Q1XH05 | 215 | 64,898 | 5.45 | 4 | +0.23 | n.s. |
| Metabolic pathway | ||||||||
| 30 | Phosphomannomutase | Q1W374 | 505 | 28,405 | 6 | 11 | n.s. | −0.20 |
| Metabolic pathway/Flavonoid metabolism | ||||||||
| 31 | Flavone O-methyltransferase 1 | Q84N28 | 1053 | 39,177 | 5.7 | 22 | see Table 2 | +0.26 |
| Methyl cycle | ||||||||
| 32 | Adenosylhomocysteinase | P32112 | 137 | 54,086 | 6.8 | 3 | −0.20 | n.s. |
| Protease inhibitor | ||||||||
| 33 | Ubiquitin | P69326 | 67 | 8648 | 7.6 | 1 | +0.21 | n.s. |
| 34 | Ubiquitin | P69326 | 70 | 8648 | 6.45 | 1 | +0.22 | n.s. |
| 35 | Ubiquitin | P69326 | 40 | 8648 | 7.25 | 1 | +0.28 | +0.26 |
| Unassigned peptides | ||||||||
| 36 | Unassigned peptide | - | - | - | - | n.s. | −0.24 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niedziela, A.; Domżalska, L.; Dynkowska, W.M.; Pernisová, M.; Rybka, K. Aluminum Stress Induces Irreversible Proteomic Changes in the Roots of the Sensitive but Not the Tolerant Genotype of Triticale Seedlings. Plants 2022, 11, 165. https://doi.org/10.3390/plants11020165
Niedziela A, Domżalska L, Dynkowska WM, Pernisová M, Rybka K. Aluminum Stress Induces Irreversible Proteomic Changes in the Roots of the Sensitive but Not the Tolerant Genotype of Triticale Seedlings. Plants. 2022; 11(2):165. https://doi.org/10.3390/plants11020165
Chicago/Turabian StyleNiedziela, Agnieszka, Lucyna Domżalska, Wioletta M. Dynkowska, Markéta Pernisová, and Krystyna Rybka. 2022. "Aluminum Stress Induces Irreversible Proteomic Changes in the Roots of the Sensitive but Not the Tolerant Genotype of Triticale Seedlings" Plants 11, no. 2: 165. https://doi.org/10.3390/plants11020165
APA StyleNiedziela, A., Domżalska, L., Dynkowska, W. M., Pernisová, M., & Rybka, K. (2022). Aluminum Stress Induces Irreversible Proteomic Changes in the Roots of the Sensitive but Not the Tolerant Genotype of Triticale Seedlings. Plants, 11(2), 165. https://doi.org/10.3390/plants11020165






