Biostimulants in Viticulture: A Sustainable Approach against Biotic and Abiotic Stresses
Abstract
1. Introduction
2. History of Biostimulants
- (i)
- nutrient use efficiency
- (ii)
- tolerance to abiotic stress
- (iii)
- quality traits
- (iv)
- availability of confined nutrients in the soil or rhizosphere.”
3. Mechanisms of Action
4. Categories of Biostimulants
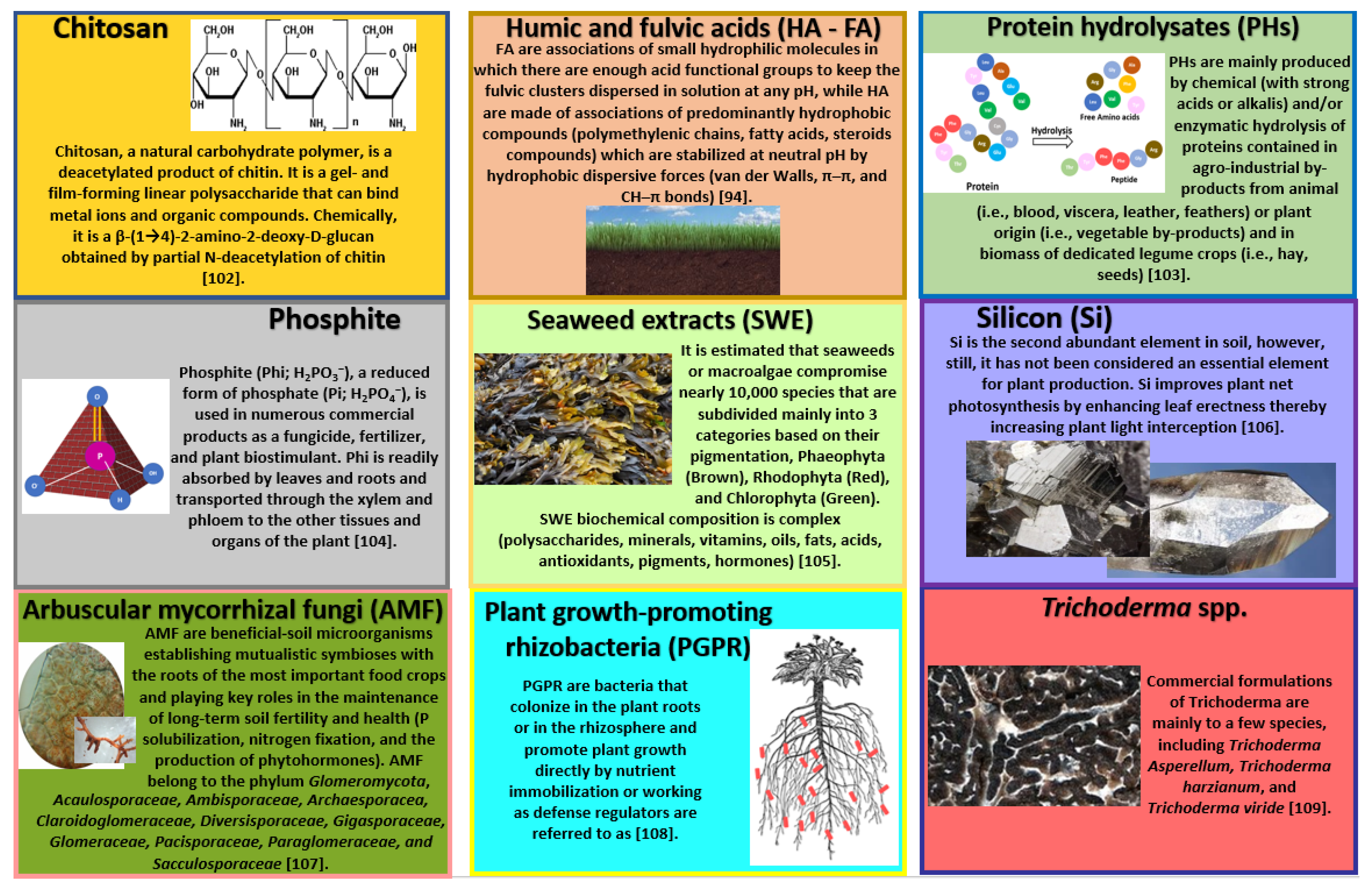
4.1. Seaweed Extracts (SWEs)
4.2. Protein Hydrolysates (PHs)
4.3. Humic Acid (HA) and Fulvic Acid (FA)
4.4. Chitosan
4.5. Trichoderma spp.
4.6. Plant-Growth-Promoting Rhizobacteria (PGPR)
4.7. Arbuscular Mycorrhizal Fungi (AMF)
4.8. Silicon (Si)
4.9. Phosphite
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Neset, T.S.; Wiréhn, L.; Klein, N.; Käyhkö, J.; Juhola, S. Maladaptation in Nordic agriculture. Clim. Risk Manag. 2019, 23, 78–87. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Verdugo-Vásquez, N.; Díaz-Gálvez, I. Influence of type of management and climatic conditions on productive behavior, oenological potential, and soil characteristics of a ‘Cabernet Sauvignon’ vineyard. Agronomy 2019, 9, 64. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Zheng, W.; Martínez de Toda, F. Strategies in vineyard establishment to face global warming in viticulture: A mini review. J. Sci. Food Agric. 2021, 101, 1261–1269. [Google Scholar] [CrossRef]
- Perrino, E.V.; Ladisa, G.; Calabrese, G. Flora and plant genetic resources of ancient olive groves of Apulia (southern Italy). Genet. Resour. Crop Evol. 2014, 61, 23–53. [Google Scholar] [CrossRef]
- Perrino, E.V.; Calabrese, G. Vascular flora of vineyards in the DOC area “Gioia del Colle” (Apulia, Southern Italy): Preliminary data. Nat. Croat. 2018, 27, 41–55. [Google Scholar] [CrossRef]
- Della Chiesa, S.; Genova, G.; la Cecilia, D.; Niedrist, G. Phytoavailable phosphorus (P2O5) and potassium (K2O) in topsoil for apple orchards and vineyards, South Tyrol, Italy. J. Maps 2019, 15, 555–562. [Google Scholar] [CrossRef]
- Parris, K. Impact of agriculture on water pollution in OECD countries: Recent trends and future prospects. Int. J. Water Resour. Dev. 2011, 27, 33–52. [Google Scholar] [CrossRef]
- Howarth, R.W. Coastal nitrogen pollution: A review of sources and trends globally and regionally. Harmful Algae 2008, 8, 14–20. [Google Scholar] [CrossRef]
- Ngatia, L.; Grace, J.M., III; Moriasi, D.; Taylor, R. Nitrogen and phosphorus eutrophication in marine ecosystems. Monit. Mar. Pollut. 2019, 5, 1–17. [Google Scholar]
- Ramos, M.C.; Martínez-Casasnovas, J.A. Nutrient losses by runoff in vineyards of the Mediterranean Alt Penedès region (NE Spain). Agric. Ecosyst. Environ. 2006, 113, 356–363. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Moreno-Simunovic, Y. Seaweeds in viticulture: A review focused on grape quality. Ciênc Técnic Vitiviníc 2021, 36, 9–21. [Google Scholar] [CrossRef]
- Komárek, M.; Čadková, E.; Chrastný, V.; Bordas, F.; Bollinger, J.C. Contamination of vineyard soils with fungicides: A review of environmental and toxicological aspects. Environ. Int. 2010, 36, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Miguéns, T.; Leirós, M.C.; Gil-Sotres, F.; Trasar-Cepeda, C. Biochemical properties of vineyard soils in Galicia, Spain. Sci. Total Environ. 2007, 378, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Nóvoa-Muñoz, J.C.; Queijeiro, J.M.G.; Blanco-Ward, D.; Álvarez-Olleros, C.; Martínez-Cortizas, A.; García-Rodeja, E. Total copper content and its distribution in acid vineyards soils developed from granitic rocks. Sci. Total Environ. 2007, 378, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Calviño, D.; Rodríguez-Suárez, J.A.; López-Periago, E.; Arias-Estévez, M.; Simal-Gándara, J. Copper content of soils and river sediments in a winegrowing area, and its distribution among soil or sediment components. Geoderma 2008, 145, 91–97. [Google Scholar] [CrossRef]
- Munishi, L.K.; Ndakidemi, P.A.; Blake, W.; Comber, S.; Hutchinson, T.H. Toxic metals in East African agroecosystems: Key risks for sustainable food production. J. Environ. 2021, 294, 112973. [Google Scholar]
- Helling, B.; Reinecke, S.A.; Reinecke, A.J. Effects of the fungicide copper oxychloride on the growth and reproduction of Eisenia fetida (Oligochaeta). Ecotoxicol. Environ. Saf. 2000, 46, 108–116. [Google Scholar] [CrossRef]
- Jacobson, A.R.; Dousset, S.; Guichard, N.; Baveye, P.; Andreux, F. Diuron mobility through vineyard soils contaminated with copper. Environ. Pollut. 2005, 138, 250–259. [Google Scholar] [CrossRef]
- Gachene, C.K.; Nyawade, S.O.; Karanja, N.N. Soil and water conservation: An overview. Zero Hunger 2020, 810–823. [Google Scholar] [CrossRef]
- Hummes, A.P.; Bortoluzzi, E.C.; Tonini, V.; da Silva, L.P.; Petry, C. Transfer of copper and zinc from soil to grapevine-derived products in young and centenarian vineyards. Water Air Soil Pollut. 2019, 230, 1–11. [Google Scholar] [CrossRef]
- European Commission. EC (European Commission) Regulation 473/2002 Amending Annexes I, II and VI to Council Regulation (EEC) No 2092/91 on Organic Production of Agricultural Products and Indications Referring Thereto on Agricultural Products and Foodstuffs and Laying Down Detailed Rules as Regards the Transmission of Information on the Use of Copper Compounds; European Commission: Brussels, Belgium, 2002. [Google Scholar]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Zang, F.; Wang, S.; Nan, Z.; Ma, J.; Zhang, Q.; Chen, Y.; Li, Y. Accumulation, spatio-temporal distribution, and risk assessment of heavy metals in the soil-corn system around a polymetallic mining area from the Loess Plateau, northwest China. Geoderma 2017, 305, 188–196. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Song, J.; Xiao, R.; Luo, L.; Yang, Z.; Chai, L. Stabilization of Cd-, Pb-, Cu-and Zn-contaminated calcareous agricultural soil using red mud: A field experiment. Environ. Geochem. Health 2018, 40, 2143–2153. [Google Scholar] [CrossRef]
- Li, P.; Lin, C.; Cheng, H.; Duan, X.; Lei, K. Contamination and health risks of soil heavy metals around a lead/zinc smelter in southwestern China. Ecotoxicol. Environ. Saf. 2015, 113, 391–399. [Google Scholar] [CrossRef]
- Milićević, T.; Urošević, M.A.; Relić, D.; Vuković, G.; Škrivanj, S.; Popović, A. Bioavailability of potentially toxic elements in soil–grapevine (leaf, skin, pulp and seed) system and environmental and health risk assessment. Sci. Total Environ. 2018, 626, 528–545. [Google Scholar] [CrossRef]
- Milićević, T.; Urošević, M.A.; Relić, D.; Jovanović, G.; Nikolić, D.; Vergel, K.; Popović, A. Environmental pollution influence to soil-plant–air system in organic vineyard: Bioavailability, environmental, and health risk assessment. Environ. Sci. Pollut. Res. 2021, 28, 3361–3374. [Google Scholar] [CrossRef]
- Yang, L.; Ren, Q.; Zheng, K.; Jiao, Z.; Ruan, X.; Wang, Y. Migration of heavy metals in the soil-grape system and potential health risk assessment. Sci. Total Environ. 2022, 806, 150646. [Google Scholar] [CrossRef] [PubMed]
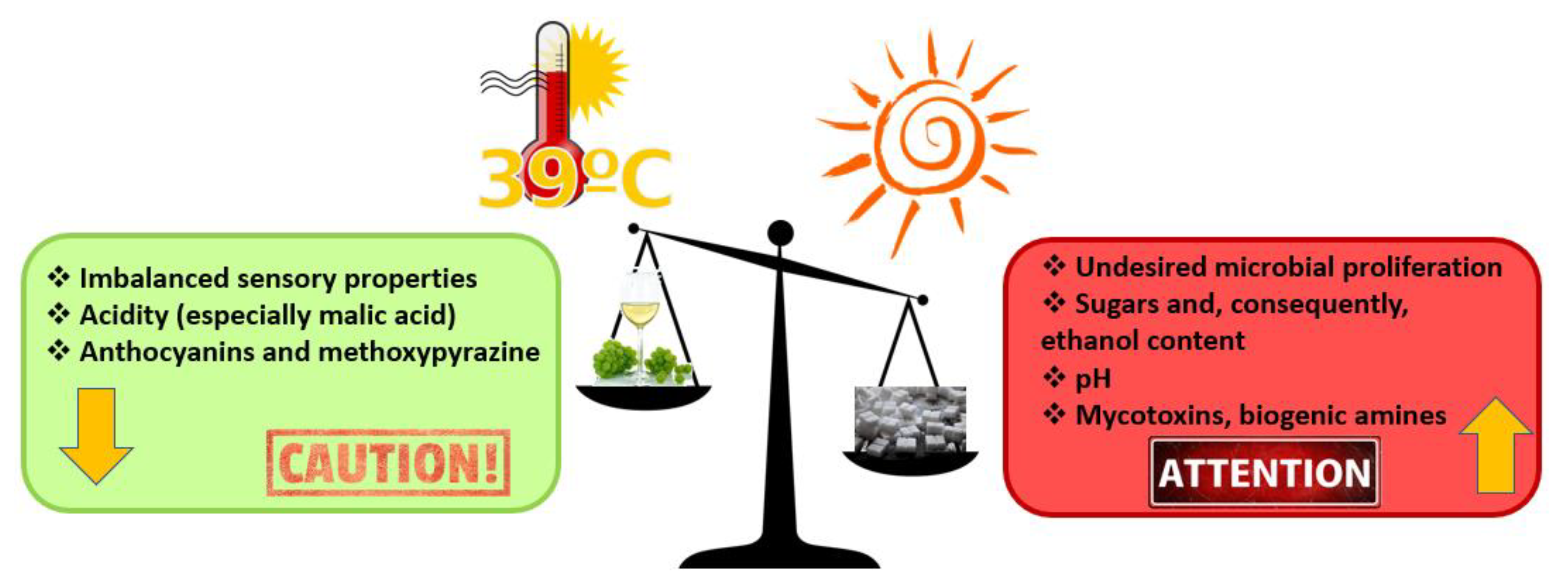
- Venios, X.; Korkas, E.; Nisiotou, A.; Banilas, G. Grapevine responses to heat stress and global warming. Plants 2020, 9, 1754. [Google Scholar] [CrossRef]
- Skirycz, A.; Inzé, D. More from less: Plant growth under limited water. Curr. Opin. Biotechnol. 2010, 21, 197–203. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef]
- Sharma, H.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Schmidt, R.; Schippers, J.H. ROS-mediated redox signaling during cell differentiation in plants. Biochim. Biophys. Acta (BBA) Gen. Subj. 2015, 1850, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Liu, J.H.; Ma, X.; Luo, D.X.; Gong, Z.H.; Lu, M.H. The plant heat stress transcription factors (HSFs): Structure, regulation, and function in response to abiotic stresses. Front. Plant Sci. 2016, 7, 114. [Google Scholar] [CrossRef]
- Tester, M.; Bacic, A. Abiotic stress tolerance in grasses. From model plants to crop plants. Plant Physiol. 2005, 137, 791–793. [Google Scholar] [CrossRef]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Spayd, S.E.; Tarara, J.M.; Mee, D.L.; Ferguson, J.C. Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot berries. Am. J. Enol. Vitic. 2002, 53, 171–182. [Google Scholar]
- Bernardo, S.; Dinis, L.T.; Machado, N.; Moutinho-Pereira, J. Grapevine abiotic stress assessment and search for sustainable adaptation strategies in Mediterranean-like climates. A review. Agron. Sustain. Dev. 2018, 38, 1–20. [Google Scholar] [CrossRef]
- De Orduna, R.M. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Ferrandino, A.; Pagliarani, C.; Carlomagno, A.; Novello, V.; Schubert, A.; Agati, G. Improved fluorescence-based evaluation of flavonoid in red and white winegrape cultivars. Aust. J. Grape Wine Res. 2017, 23, 207–214. [Google Scholar] [CrossRef]
- Keller, M. Managing grapevines to optimise fruit development in a challenging environment: A climate change primer for viticulturists. Aust. J. Grape Wine Res. 2010, 16, 56–69. [Google Scholar] [CrossRef]
- Berbegal, C.; Fragasso, M.; Russo, P.; Bimbo, F.; Grieco, F.; Spano, G.; Capozzi, V. Climate changes and food quality: The potential of microbial activities as mitigating strategies in the wine sector. Fermentation 2019, 5, 85. [Google Scholar] [CrossRef]
- Mozell, M.R.; Thach, L. The impact of climate change on the global wine industry: Challenges & solutions. Wine Econ. Policy 2014, 3, 81–89. [Google Scholar]
- Millar, A.A. Thermal regime of grapevines. Am. J. Enol. Vitic. 1972, 23, 173–176. [Google Scholar]
- Smart, R.E.; Sinclair, T.R. Solar heating of grape berries and other spherical fruits. Agric. Meteorol. 1976, 17, 241–259. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Sbraci, S.; Storchi, P.; Mattii, G.B. Sustainable viticulture: Effects of soil management in Vitis vinifera. Agronomy 2020, 10, 1949. [Google Scholar] [CrossRef]
- Filatov, V.P. Tissue Therapy in Ophthalmology. Am. Rev. Sov. Med. 1944, 2, 53–66. [Google Scholar]
- Gordon, D.M. The treatment of retinitis pigmentosa with special reference to the Filatov method. Am. J. Ophthalmol. 1974, 30, 565–580. [Google Scholar] [CrossRef]
- Filatov, V.P. Tissue treatment. (Doctrine on biogenic stimulators). II. Hypothesis of tissue therapy, or the doctrine on biogenic stimulators. Priroda 1951, 12, 20–28. [Google Scholar]
- Blagoveshchensky, A.V. Biogenic stimulants in agriculture. Priroda 1955, 7, 43–47. [Google Scholar]
- Blagoveshchensky, A.V. Biogenic stimulants and biochemical nature of their action. Bull. Main Bot. Gard. 1956, 25, 79–86. [Google Scholar]
- Berlyn, G.P.; Russo, R.O. The use of organic biostimulants to promote root growth. Belowground Ecol. 1990, 2, 12–13. [Google Scholar]
- Schmidt, R.E. Biostimulants. Grounds Maint. 1992, 27, 38–56. [Google Scholar]
- Goatley, J.M.; Schmidt, R.E. Biostimulator enhancement of Kentucky bluegrass sod. HortScience 1991, 26, 254–255. [Google Scholar] [CrossRef]
- Naumov, G.F.; Bozhkov, A.I.; Leontovich, V.P.; Sklyar, A.I.; Belous, A.M. Polyfunctionality of allelopathic substance allelostim. Dokl. Akad. Nauk Ukr. 1993, 11, 166–169. [Google Scholar]
- Herve, J.J. Biostimulants, a new concept for the future; prospects offered by the chemistry of synthesis and biotechnology. C. R. Acad. Agric. Fr. 1994, 80, 91–102. [Google Scholar]
- Elliott, M.L.; Prevatte, M. Response of ‘Tifdwarf’ Bermudagrass to Seaweed-derived Biostimulants. HortTechnology 1996, 6, 261–263. [Google Scholar] [CrossRef]
- Zhang, X.; Schmidt, R. Biostimulating turfgrasses. Grounds Maint. 1999, 34, 14–15. [Google Scholar]
- Schmidt, R.E.; Ervin, E.H.; Zhang, X. Questions and answers about biostimulants. Golf Course Manag. 2003, 71, 91–94. [Google Scholar]
- Doak, S.O.; Schmidt, R.E.; Ervin, E.H. Metabolic enhancer impact on creeping bentgrass leaf sodium and physiology under salinity. Int. Turfgrass Soc. Res. J. 2005, 10, 845–849. [Google Scholar]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Ciavatta, C.; Cavani, L. Problematiche per l’inserimento dei biostimolanti nella legislazione dei fertilizzanti. Fertil. Agrorum 2006, 1, 11–15. [Google Scholar]
- Kauffman, G.L.; Kneivel, D.P.; Watschke, T.L. Effects of a biostimulant on the heat tolerance associated with photosynthetic capacity, membrane thermostability, and polyphenol production of perennial ryegrass. Crop Sci. 2007, 47, 261–267. [Google Scholar] [CrossRef]
- Crouch, I.J.; Smith, M.T.; Van Staden, J.; Lewis, M.J.; Hoad, G.V. Identification of auxins in a commercial seaweed concentrate. J. Plant Physiol. 1992, 139, 590–594. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H. Cytokinin-containing seaweed and humic acid extracts associated with creeping bentgrass leaf cytokinins and drought resistance. Crop Sci. 2004, 44, 1737–1745. [Google Scholar] [CrossRef]
- Apone, F.; Arciello, S.; Colucci, G.; Filippini, L.; Portoso, D. Alle radici della biostimolazione: Indagini scientifiche a supporto. Fertil. Agrorum 2006, 1, 55–63. [Google Scholar]
- Kumar, D.; Shivay, Y.S. Definitional Glossary of Agricultural Terms; I.K. International Publishing House Pvt Ltd.: New Delhi, India, 2008; Volume I. [Google Scholar]
- Parrado, J.; Bautista, J.; Romero, E.J.; García-Martínez, A.M.; Friaza, V.; Tejada, M. Production of a carob enzymatic extract: Potential use as a biofertilizer. Bioresour. Technol. 2008, 99, 2312–2318. [Google Scholar] [CrossRef] [PubMed]
- Basak, A. Biostimulators–Definitions, Classification and Legislation. Biostimulators in Modern Agriculture: General Aspects; Editorial House Wie Jutra: Warsaw, Poland, 2008; pp. 7–17. [Google Scholar]
- Toscano, S.; Romano, D.; Massa, D.; Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulant applications in low input horticultural cultivation systems. Italus Hortus 2018, 25, 27–36. [Google Scholar] [CrossRef]
- Du Jardin, P. The Science of Plant Biostimulants—A Bibliographic Analysis; Ad hoc Study Report; European Commission: Brussels, Belgium, 2012; Available online: http://hdl.handle.net/2268/169257 (accessed on 29 November 2021).
- Drobek, M.; Frąc, M.; Cybulska, J. Plant biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—A review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- EU Regulation. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union 2019, 62, 1–114. [Google Scholar]
- MacCarthy, P. The principles of humic substances. Soil Sci. 2001, 166, 738–751. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolism. Sci. Agric. 2016, 73, 18–23. [Google Scholar] [CrossRef]
- Popko, M.; Michalak, I.; Wilk, R.; Gramza, M.; Chojnacka, K.; Górecki, H. Effect of the new plant growth biostimulants based on amino acids on yield and grain quality of winter wheat. Molecules 2018, 23, 470. [Google Scholar] [CrossRef] [PubMed]
- Mphande, W.; Kettlewell, P.S.; Grove, I.G.; Farrell, A.D. The potential of antitranspirants in drought management of arable crops: A review. Agric. Water Manag. 2020, 236, 106143. [Google Scholar] [CrossRef]
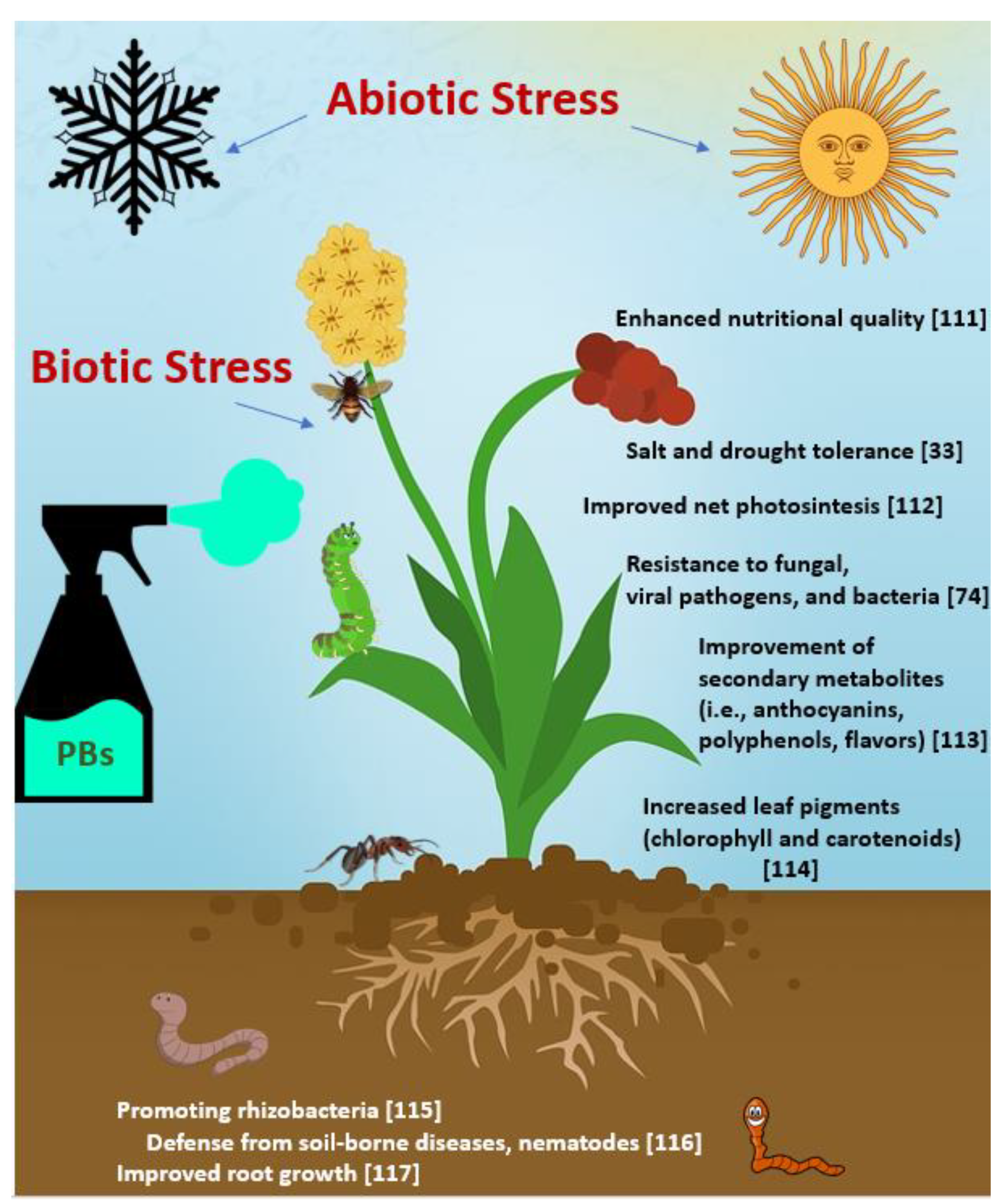
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Aeron, A.; Dubey, R.C.; Maheshwari, D.K. Next-Generation biofertilizers and novel biostimulants: Documentation and validation of mechanism of endophytic plant growth-promoting rhizobacteria in tomato. Arch. Microbiol. 2021, 203, 3715–3726. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Tzortzakis, N.; Petropoulos, S.A. Sustainable Agriculture Systems in Vegetable Production Using Chitin and Chitosan as Plant Biostimulants. Biomolecules 2021, 11, 819. [Google Scholar] [CrossRef]
- Tian, S.; Lu, L.; Xie, R.; Zhang, M.; Jernstedt, J.; Hou, D.; Ramsier, C.; Brown, P. Supplemental macronutrients and microbial fermentation products improve the uptake and transport of foliar applied zinc in sunflower (Helianthus annuus L.) plants. Studies utilizing micro X-ray florescence. Front. Plant Sci. 2015, 5, 808. [Google Scholar] [CrossRef] [PubMed]
- Aremu, A.O.; Stirk, W.A.; Kulkarni, M.G.; Tarkowská, D.; Turečková, V.; Gruz, J.; Šubrtová, M.; Pěnčík, A.; Novák, O.; Doležal, K.; et al. Evidence of phytohormones and phenolic acids variability in garden-waste-derived vermicompost leachate, a well-known plant growth stimulant. Plant Growth Regul. 2015, 75, 483–492. [Google Scholar] [CrossRef]
- Jannin, L.; Arkoun, M.; Etienne, P.; Laîné, P.; Goux, D.; Garnica, M.; Fuentes, M.; San Francisco, S.; Baigorri, R.; Cruz, F.; et al. Brassica napus growth is promoted by Ascophyllum nodosum (L.) Le Jol. seaweed extract: Microarray analysis and physiological characterization of N, C, and S metabolisms. J. Plant Growth Regul. 2013, 32, 31–52. [Google Scholar] [CrossRef]
- Santaniello, A.; Giorgi, F.M.; Di Tommaso, D.; Di Tommaso, G.; Piaggesi, A.; Perata, P. Genomic approaches to unveil the physiological pathways activated in Arabidopsis treated with plant-derived raw extracts. Acta Hortic. 2013, 1009, 161–174. [Google Scholar] [CrossRef]
- Goñi, O.; Fort, A.; Quille, P.; Mckeown, P.C.; Spillane, C.; O’Connell, S. Comparative transcriptome analysis of two Ascophyllum nodosum extract biostimulants: Same seaweed but different. J. Agric. Food Chem. 2016, 64, 2980–2989. [Google Scholar] [CrossRef]
- Martínez-Esteso, M.J.; Vilella-Antón, M.T.; Sellés-Marchart, S.; Martínez-Márquez, A.; Botta-Català, A.; Piñol-Dastis, R.; Bru-Martínez, R. A DIGE proteomic analysis of wheat flag leaf treated with TERRA-SORB® foliar, a free amino acid high content biostimulant. J. Integr. Omics 2016, 6, 9–17. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant Sci. 2014, 5, 375. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y. Biostimulants in horticulture. Sci. Hortic. 2015, 196, 1–134. [Google Scholar] [CrossRef]
- Pichyangkura, R.; Chadchawan, S. Biostimulant activity of chitosan in horticulture. Sci. Hortic. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Gómez-Merino, F.C.; Trejo-Téllez, L.I. Biostimulant activity of phosphite in horticulture. Sci. Hortic. 2015, 196, 82–90. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Savvas, D.; Ntatsi, G. Biostimulant activity of silicon in horticulture. Sci. Hortic. 2015, 196, 66–81. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Ruzzi, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Islam, N.; Dmour, I.; Taha, M.O. Degradability of chitosan micro/nanoparticles for pulmonary drug delivery. Heliyon 2019, 5, e01684. [Google Scholar] [CrossRef]
- Maini, P. The experience of the first biostimulant, based on amino acids and peptides: A short retrospective review on the laboratory researches and the practical results. Fertil. Agrorum 2006, 1, 29–43. [Google Scholar]
- Wu, L.; Gao, X.; Xia, F.; Joshi, J.; Borza, T.; Wang-Pruski, G. Biostimulant and fungicidal effects of phosphite assessed by GC-TOF-MS analysis of potato leaf metabolome. Physiol. Mol. Plant Pathol. 2019, 106, 49–56. [Google Scholar] [CrossRef]
- EL Boukhari, M.E.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.O.K.; Park, B.S.; Adnan, M.; Germ, M.; Kreft, I.; Woo, S.H.; Park, C.H. Silicon biostimulant enhances the growth characteristics and fortifies the bioactive compounds in common and Tartary buckwheat plant. J. Crop Sci. Biotechnol. 2021, 24, 51–59. [Google Scholar] [CrossRef]
- Giovannini, L.; Palla, M.; Agnolucci, M.; Avio, L.; Sbrana, C.; Turrini, A.; Giovannetti, M. Arbuscular mycorrhizal fungi and associated microbiota as plant biostimulants: Research strategies for the selection of the best performing inocula. Agronomy 2020, 10, 106. [Google Scholar] [CrossRef]
- Kumari, B.; Mallick, M.A.; Solanki, M.K.; Solanki, A.C.; Hora, A.; Guo, W. Plant growth promoting rhizobacteria (PGPR): Modern prospects for sustainable agriculture. In Plant Health under Biotic Stress; Springer: Cham, Switzerland, 2019; pp. 109–127. [Google Scholar]
- Fernando, D.; Milagrosa, S.; Francisco, C.; Francisco, M. Biostimulant activity of Trichoderma saturnisporum in melon (Cucumis melo). HortScience 2018, 53, 810–815. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 1–12. [Google Scholar] [CrossRef]
- Desoky, E.S.M.; ElSayed, A.I.; Merwad, A.R.M.; Rady, M.M. Stimulating antioxidant defenses, antioxidant gene expression, and salt tolerance in Pisum sativum seedling by pretreatment using licorice root extract (LRE) as an organic biostimulant. Plant Physiol. Biochem. 2019, 142, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gamboa, G.; Romanazzi, G.; Garde-Cerdán, T.; Pérez-Álvarez, E.P. A review of the use of biostimulants in the vineyard for improved grape and wine quality: Effects on prevention of grapevine diseases. J. Sci. Food Agric. 2019, 99, 1001–1009. [Google Scholar] [CrossRef]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- D’Addabbo, T.; Laquale, S.; Perniola, M.; Candido, V. Biostimulants for plant growth promotion and sustainable management of phytoparasitic nematodes in vegetable crops. Agronomy 2019, 9, 616. [Google Scholar] [CrossRef]
- Vernieri, P.; Borghesi, E.; Ferrante, A.; Magnani, G. Application of biostimulants in floating system for improving rocket quality. J. Food Agric. Environ. 2005, 3, 86. [Google Scholar]
- Ganugi, P.; Martinelli, E.; Lucini, L. Microbial biostimulants as a sustainable approach to improve the functional quality in plant-based foods: A review. Curr. Opin. Food Sci. 2021, 41, 217–223. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Jabaji, S. Metabolomics—A robust bioanalytical approach for the discovery of the modes-of-action of pesticides: A review. Pestic. Biochem. Physiol. 2011, 100, 105–117. [Google Scholar] [CrossRef]
- Halmann, M. Synthetic plant growth regulators. Adv. Agron. 1990, 43, 47–105. [Google Scholar]
- Rathore, S.S.; Chaudhary, D.R.; Boricha, G.N.; Ghosh, A.; Bhatt, B.P.; Zodape, S.T.; Patolia, J.S. Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. S. Afr. J. Bot. 2009, 75, 351–355. [Google Scholar] [CrossRef]
- Rafiee, H.; Naghdi Badi, H.; Mehrafarin, A.; Qaderi, A.; Zarinpanjeh, N.; Sękara, A.; Zand, E. Application of plant biostimulants as new approach to improve the biological responses of medicinal plants-A critical review. Med. Plant Res. 2016, 15, 6. [Google Scholar]
- Schiavon, M.; Ertani, A.; Nardi, S. Effects of an alfalfa protein hydrolysate on the gene expression and activity of enzymes of the tricarboxylic acid (TCA) cycle and nitrogen metabolism in Zea mays L. J. Agric. Food Chem. 2008, 56, 11800–11808. [Google Scholar] [CrossRef]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. J. Plant. Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Mahfouz, S.A.; Sharaf-Eldin, M.A. Effect of mineral vs. biofertilizer on growth, yield, and essential oil content of fennel [Foeniculum vulgare Mill.]. Int. Agrophys. 2007, 21, 361–366. [Google Scholar]
- Machado, V.P.D.O.; Pacheco, A.C.; Carvalho, M.E.A. Effect of biostimulant application on production and flavonoid content of marigold (Calendula officinalis L.). Rev. Ceres 2014, 61, 983–988. [Google Scholar] [CrossRef]
- Van Overbeek, J. Plant Hormones and Regulators: Gibberellins, cytokinins, and auxins may regulate plant growth via nucleic acid and enzyme synthesis. Science 1966, 152, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Mady, M.A. Effect of foliar application with yeast extract and zinc on fruit setting and yield of faba bean (Vicia faba L.). J. Biol. Chem. Environ. Sci. 2009, 4, 109–127. [Google Scholar]
- Colla, G.; Svecova, E.; Rouphael, Y.; Cardarelli, M.; Reynaud, H.; Canaguier, R.; Planques, B. Effectiveness of a plant-derived protein Hydrolysate to improve crop performances under different growing conditions. Acta Hortic. 2013, 1009, 175–180. [Google Scholar] [CrossRef]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Moreno, M.B.M.; Reynaud, H.; Canaguier, R.; Trtílek, M. A combined phenotypic and metabolomic approach for elucidating the biostimulant action of a plant-derived protein hydrolysate on tomato grown under limited water availability. Front. Plant Sci. 2019, 10, 493. [Google Scholar] [CrossRef]
- Ceccarelli, A.V.; Miras-Moreno, B.; Buffagni, V.; Senizza, B.; Pii, Y.; Cardarelli, M.; Rouphael, Y.; Colla, G.; Lucini, L. Foliar application of different vegetal-derived protein hydrolysates distinctively modulates tomato root development and metabolism. Plants 2021, 10, 326. [Google Scholar] [CrossRef]
- Calabrese, V.; Giordano, J.; Ruggieri, M.; Berritta, D.; Trovato, A.; Ontario, M.L.; Bianchini, R.; Calabrese, E.J. Hormesis, cellular stress response, and redox homeostasis in autism spectrum disorders. J. Neurosci. Res. 2016, 94, 1488–1498. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Altissimo, A.; Franceschi, C.; Nardi, S. Phenol-Containing organic substances stimulate phenylpropanoid metabolism in Zea mays. J. Plant. Nutr. Soil Sci. 2011, 174, 496–503. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Yun, C.S.; Matsuda, F.; Sasaki, T.; Saito, K.; Tozawa, Y. Expression of bacterial tyrosine ammonia-lyase creates a novel p-coumaric acid pathway in the biosynthesis of phenylpropanoids in Arabidopsis. Planta 2010, 232, 209–218. [Google Scholar] [CrossRef]
- Xu, L.; Trinh, H.K.; Geelen, D. Biostimulant mode of action: Impact of PBs on molecular level. In The Chemical Biology of Plant Biostimulants; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 245–259. [Google Scholar]
- Ertani, A.; Sambo, P.; Nicoletto, C.; Santagata, S.; Schiavon, M.; Nardi, S. The use of organic biostimulants in hot pepper plants to help low input sustainable agriculture. Chem. Biol. Technol. Agric. 2015, 2, 1–10. [Google Scholar] [CrossRef]
- Ali, A.; Mohamed, M.T.M.; Siddiqui, Y. Control of anthracnose by chitosan through stimulation of defence-related enzymes in Eksotika II papaya (Carica papaya L.) fruit. J. Biol. Life Sci. 2012, 3, 114–126. [Google Scholar] [CrossRef]
- Kim, H.J.; Chen, F.; Wang, X.; Rajapakse, N.C. Effect of chitosan on the biological properties of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 2005, 53, 3696–3701. [Google Scholar] [CrossRef]
- Pretali, L.; Bernardo, L.; Butterfield, T.S.; Trevisan, M.; Lucini, L. Botanical and biological pesticides elicit a similar induced systemic response in tomato (Solanum lycopersicum) secondary metabolism. Phytochemistry 2016, 130, 56–63. [Google Scholar] [CrossRef]
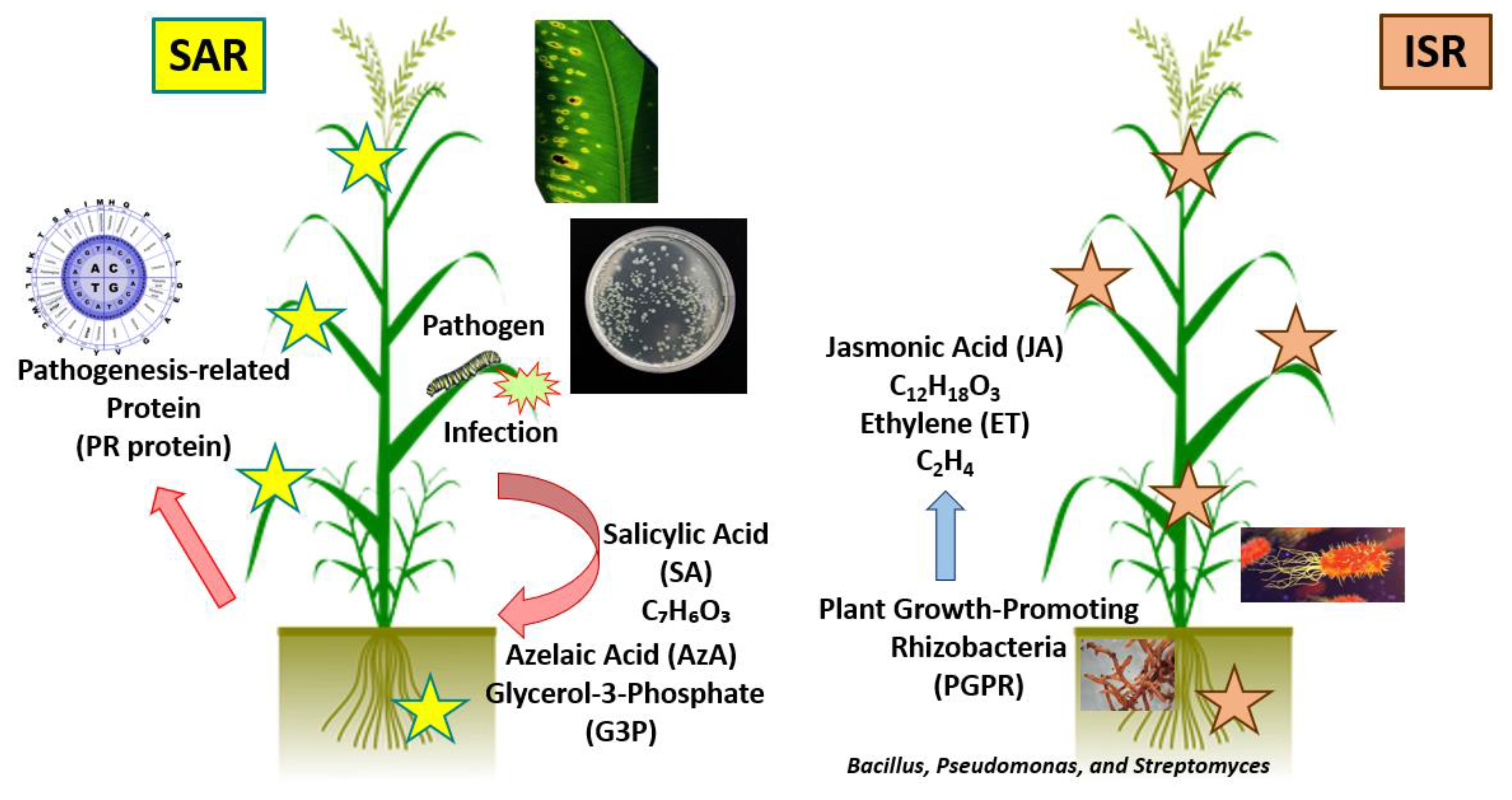
- Pieterse, C.M.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.H.; Shine, M.B.; de Lorenzo, L.; Yu, K.; Cui, W.; Navarre, D.; Hunt, A.G.; Lee, J.Y.; Kachroo, A.; Kachroo, P. Plasmodesmata localizing proteins regulate transport and signaling during systemic acquired immunity in plants. Cell Host Microbe 2016, 19, 541–549. [Google Scholar] [CrossRef]
- Luo, J.; Xia, W.; Cao, P.; Xiao, Z.A.; Zhang, Y.; Liu, M.; Zhan, C.; Wang, N. Integrated transcriptome analysis reveals plant hormones jasmonic acid and salicylic acid coordinate growth and defense responses upon fungal infection in poplar. Biomolecules 2019, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Kamle, M.; Borah, R.; Bora, H.; Jaiswal, A.K.; Singh, R.K.; Kumar, P. Systemic Acquired Resistance (SAR) and Induced Systemic Resistance (ISR): Role and mechanism of action against phytopathogens. In Fungal Biotechnology and Bioengineering; Springer: Cham, Switzerland, 2020; pp. 457–470. [Google Scholar]
- Vargas-Hernandez, M.; Macias-Bobadilla, I.; Guevara-Gonzalez, R.G.; Romero-Gomez, S.D.J.; Rico-Garcia, E.; Ocampo-Velazquez, R.V.; Alvarez-Arquieta, L.L.; Torres-Pacheco, I. Plant hormesis management with biostimulants of biotic origin in agriculture. Front. Plant Sci. 2017, 8, 1762. [Google Scholar] [CrossRef]
- Han, X.; Xi, Y.; Zhang, Z.; Mohammadi, M.A.; Joshi, J.; Borza, T.; Wang-Pruski, G. Effects of phosphite as a plant biostimulant on metabolism and stress response for better plant performance in Solanum tuberosum. Ecotoxicol. Environ. Saf. 2021, 210, 111873. [Google Scholar] [CrossRef]
- Kolomazník, K.; Pecha, J.; Friebrová, V.; Janáčová, D.; Vašek, V. Diffusion of biostimulators into plant tissues. Heat Mass Transf. 2012, 48, 1505–1512. [Google Scholar] [CrossRef]
- Pecha, J.; Fürst, T.; Kolomazník, K.; Friebrová, V.; Svoboda, P. Protein biostimulant foliar uptake modeling: The impact of climatic conditions. AIChE J. 2012, 58, 2010–2019. [Google Scholar] [CrossRef]
- Rindi, F.; Soler-Vila, A.; Guiry, M.D. Taxonomy of marine macroalgae used as sources of bioactive compounds. In Marine Bioactive Compounds; Springer: Cham, Switzerland, 2012; pp. 1–53. [Google Scholar]
- Arioli, T.; Mattner, S.W.; Winberg, P.C. Applications of seaweed extracts in Australian agriculture: Past, present and future. J. Appl. Phycol. 2015, 27, 2007–2015. [Google Scholar] [CrossRef]
- Winberg, P.C.; Fitton, H.J.; Stringer, D.; Karpiniec, S.S.; Gardiner, V.A. Controlling seaweed biology, physiology and metabolic traits in production for commercially relevant bioactives in glycobiology. Adv. Bot. Res. 2014, 71, 221–252. [Google Scholar]
- Cornish, M.L.; Monagail, M.M.; Critchley, A.T. The Animal Kingdom, Agriculture and Seaweeds. J. Mar. Sci. Eng. 2020, 8, 574. [Google Scholar] [CrossRef]
- Okolie, C.L.; Mason, B.; Critchley, A.T. Seaweeds as a source of proteins for use in pharmaceuticals and high-value applications. In Novel Proteins for Food, Pharmaceuticals, and Agriculture: Sources, Applications and Advances; John Wiley & Sons: Hoboken, NJ, USA, 2018; p. 217. [Google Scholar]
- Offei, F.; Mensah, M.; Thygesen, A.; Kemausuor, F. Seaweed bioethanol production: A process selection review on hydrolysis and fermentation. Fermentation 2018, 4, 99. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Bajpai, S.; Shukla, P.S.; Asiedu, S.; Pruski, K.; Prithiviraj, B. A biostimulant preparation of brown seaweed Ascophyllum nodosum suppresses powdery mildew of strawberry. Plant Pathol. J. 2019, 35, 406. [Google Scholar] [CrossRef] [PubMed]
- De Saeger, J.; Van Praet, S.; Vereecke, D.; Park, J.; Jacques, S.; Han, T.; Depuydt, S. Toward the molecular understanding of the action mechanism of Ascophyllum nodosum extracts on plants. J. Appl. Phycol. 2020, 32, 573–597. [Google Scholar] [CrossRef]
- Cai, Z.; Kastell, A.; Mewis, I.; Knorr, D.; Smetanska, I. Polysaccharide elicitors enhance anthocyanin and phenolic acid accumulation in cell suspension cultures of Vitis vinifera. Plant Cell Tissue Organ Cult. (PCTOC) 2012, 108, 401–409. [Google Scholar] [CrossRef]
- Aziz, A.; Poinssot, B.; Daire, X.; Adrian, M.; Bézier, A.; Lambert, B.; Joubert, J.M.; Pugin, A. Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol. Plant Microbe Interact. 2003, 16, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Taskos, D.; Stamatiadis, S.; Yvin, J.C.; Jamois, F. Effects of an Ascophyllum nodosum (L.) Le Jol. extract on grapevine yield and berry composition of a Merlot vineyard. Sci. Hortic. 2019, 250, 27–32. [Google Scholar] [CrossRef]
- Popescu, G.C.; Popescu, M. Effect of the brown alga Ascophyllum nodosum as biofertilizer on vegetative growth in grapevine (Vitis vinifera L.). Curr. Trends Nat. Sci. 2014, 3, 61–67. [Google Scholar]
- Arioli, T.; Mattner, S.W.; Hepworth, G.; McClintock, D.; McClinock, R. Effect of seaweed extract application on wine grape yield in Australia. J. Appl. Phycol. 2021, 33, 1883–1891. [Google Scholar] [CrossRef]
- Salvi, L.; Brunetti, C.; Cataldo, E.; Niccolai, A.; Centritto, M.; Ferrini, F.; Mattii, G.B. Effects of Ascophyllum nodosum extract on Vitis vinifera: Consequences on plant physiology, grape quality and secondary metabolism. Plant Physiol. Biochem. 2019, 139, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Frioni, T.; Sabbatini, P.; Tombesi, S.; Norrie, J.; Poni, S.; Gatti, M.; Palliotti, A. Effects of a biostimulant derived from the brown seaweed Ascophyllum nodosum on ripening dynamics and fruit quality of grapevines. Sci. Hortic. 2018, 232, 97–106. [Google Scholar] [CrossRef]
- Petoumenou, D.G.; Patris, V.E. Effects of Several Preharvest Canopy Applications on Yield and Quality of Table Grapes (Vitis vinifera L.) Cv. Crimson Seedless. Plants 2021, 10, 906. [Google Scholar] [CrossRef] [PubMed]
- Salvi, L.; Brunetti, C.; Cataldo, E.; Storchi, P.; Mattii, G.B. Eco-physiological traits and phenylpropanoid profiling on potted Vitis vinifera L. cv Pinot noir subjected to Ascophyllum nodosum treatments under post-veraison low water availability. Appl. Sci. 2020, 10, 4473. [Google Scholar] [CrossRef]
- Abbas, M.; Anwar, J.; Zafar-ul-Hye, M.; Iqbal Khan, R.; Saleem, M.; Rahi, A.A.; Danish, S.; Datta, R. Effect of seaweed extract on productivity and quality attributes of four onion cultivars. Horticulturae 2020, 6, 28. [Google Scholar] [CrossRef]
- Frioni, T.; VanderWeide, J.; Palliotti, A.; Tombesi, S.; Poni, S.; Sabbatini, P. Foliar vs. soil application of Ascophyllum nodosum extracts to improve grapevine water stress tolerance. Sci. Hortic. 2021, 277, 109807. [Google Scholar] [CrossRef]
- Tombesi, S.; Frioni, T.; Sabbatini, P.; Poni, S.; Palliotti, A. Ascophyllum nodosum extract improves leaf thermoregulation by reducing stomatal sensitivity to VPD in Vitis vinifera L. J. Appl. Phycol. 2021, 33, 1293–1304. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Garde-Cerdán, T.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Seaweed foliar applications at two dosages to Tempranillo blanco (Vitis vinifera L.) grapevines in two seasons: Effects on grape and wine volatile composition. Food Res. Int. 2020, 130, 108918. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Garde-Cerdán, T.; Martínez-Lapuente, L.; Costa, B.S.D.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Phenolic composition of Tempranillo Blanco (Vitis vinifera L.) grapes and wines after biostimulation via a foliar seaweed application. J. Sci. Food Agric. 2020, 100, 825–835. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Garde-Cerdán, T.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Effects on must and wine volatile composition after biostimulation with a brown alga to Tempranillo grapevines in two seasons. J. Sci. Food Agric. 2021, 101, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Hernández, J.M.; Benítez-García, I.; Mazorra-Manzano, M.A.; Ramírez-Suárez, J.C.; Sánchez, E. Strategies for production, characterization and application of protein-based biostimulants in agriculture: A review. Chil. J. Agric. Res. 2020, 80, 274–289. [Google Scholar] [CrossRef]
- Parrado, J.; Escudero-Gilete, M.L.; Friaza, V.; García-Martínez, A.; González-Miret, M.L.; Bautista, J.D.; Heredia, F.J. Enzymatic vegetable extract with bio-active components: Influence of fertiliser on the colour and anthocyanins of red grapes. J. Sci. Food Agric. 2007, 87, 2310–2318. [Google Scholar] [CrossRef]
- Boselli, M.; Bahouaoui, M.A.; Lachhab, N.; Sanzani, S.M.; Ferrara, G.; Ippolito, A. Protein hydrolysates effects on grapevine (Vitis vinifera L., cv. Corvina) performance and water stress tolerance. Sci. Hortic. 2019, 258, 108784. [Google Scholar] [CrossRef]
- Meggio, F.; Trevisan, S.; Manoli, A.; Ruperti, B.; Quaggiotti, S. Systematic Investigation of the Effects of a Novel Protein Hydrolysate on the Growth, Physiological Parameters, Fruit Development and Yield of Grapevine (Vitis vinifera L., cv Sauvignon Blanc) under Water Stress Conditions. Agronomy 2020, 10, 1785. [Google Scholar] [CrossRef]
- Bavaresco, L.; Lucini, L.; Squeri, C.; Zamboni, M.; Frioni, T. Protein hydrolysates modulate leaf proteome and metabolome in water-stressed grapevines. Sci. Hortic. 2020, 270, 109413. [Google Scholar] [CrossRef]
- Lachhab, N.; Sanzani, S.M.; Adrian, M.; Chiltz, A.; Balacey, S.; Boselli, M.; Ippolito, A.; Poinssot, B. Soybean and casein hydrolysates induce grapevine immune responses and resistance against Plasmopara viticola. Front. Plant Sci. 2014, 5, 716. [Google Scholar] [CrossRef]
- Nebbioso, A.; Piccolo, A. Advances in humeomics: Enhanced structural identification of humic molecules after size fractionation of a soil humic acid. Anal. Chim. Acta 2012, 720, 77–90. [Google Scholar] [CrossRef]
- Derrien, M.; Lee, Y.K.; Park, J.E.; Li, P.; Chen, M.; Lee, S.H.; Lee, S.H.; Lee, J.B.; Hur, J. Spectroscopic and molecular characterization of humic substances (HS) from soils and sediments in a watershed: Comparative study of HS chemical fractions and the origins. Environ. Sci. Pollut. Res. 2017, 24, 16933–16945. [Google Scholar] [CrossRef]
- Islam, M.A.; Morton, D.W.; Johnson, B.B.; Angove, M.J. Adsorption of humic and fulvic acids onto a range of adsorbents in aqueous systems, and their effect on the adsorption of other species: A review. Sep. Purif. Technol. 2020, 247, 116949. [Google Scholar] [CrossRef]
- Popescu, G.C.; Popescu, M. Yield, berry quality and physiological response of grapevine to foliar humic acid application. Bragantia 2018, 77, 273–282. [Google Scholar] [CrossRef]
- Aljabary, A.M.O.; Al-Baytie, M.R.S.; Ahmed, Z.S. Effect of number eyes left after pruning, fertilization with humic acid and spraying with gibberellic acid in some mineral content of vineyards thompson cv. vitis viniferal. Plant Arch. 2018, 18, 2061–2067. [Google Scholar]
- Imam, N.M.A.A.; Al-Obaidi, H.S.F. Effect of adding the chemical fertilizer NPK and humic acid on the growth and mineral percentage for seedlings of three grape cultivars (Vitis vinifera L.). Euphrates J. Agric. Sci. 2020, 2, 473–486. [Google Scholar]
- Al-Atrushy, S.M.; Mustafa, S.A. Foliar Application of Humic Acid, Iron and Sprays Number on Chemical Quality of Grape (Vitis vinifera L.) cv. TAIFI. ICNS 2016, 49–57. Available online: https://www.researchgate.net/profile/Muhamad-Hamad/publication/334730080_Simulation_of_Cu_Electrodeposition_from_Aqueous_Solution_for_Solar_Absorption_Using_COMSOL_Model/links/5d3d68a3299bf1995b509561/Simulation-of-Cu-Electrodeposition-from-Aqueous-Solution-for-Solar-Absorption-Using-COMSOL-Model.pdf#page=53 (accessed on 19 December 2021).
- EL Ghayaty, S.H.; Abdrabboh, G.A.; Hamdy, A.E.; Ahmed, A.F. Effect of soil applications anti-salinity agent on growth, yield and fruit quality of superior seedless grapevines (Vitis vinifera L.). Al-Azhar J. Agric. Res. 2019, 44, 24–34. [Google Scholar] [CrossRef]
- Asgharzade, A.; Babaeian, M. Investigating the effects of humic acid and acetic acid foliar application on yield and leaves nutrient content of grape (Vitis vinifera). Afr. J. Microbiol. Res. 2012, 6, 6049–6054. [Google Scholar] [CrossRef]
- Sabir, A.; Sagdıç, K.; Sabır, F.K. Vermicompost, humic acid and urea pulverizations as sustainable practices on the face of climatic extremities to increase grape yield and quality. Int. J. Agric. Nat. Sci. 2021, 14, 114–123. [Google Scholar]
- Li, W.; Yao, H.; Chen, K.; Ju, Y.; Min, Z.; Sun, X.; Cheng, Z.; Liao, Z.; Zhang, K.; Fang, Y. Effect of foliar application of fulvic acid antitranspirant on sugar accumulation, phenolic profiles and aroma qualities of Cabernet Sauvignon and Riesling grapes and wines. Food Chem. 2021, 351, 129308. [Google Scholar] [CrossRef]
- El-Boray, M.S.; Mostafa, M.F.; Shaltout, A.D.; Hassan, K.H. Influence of fulvic acid plus some microelements and microorganisms on yield and quality characteristics of superior seedless grapevines. J. Plant Prod. 2015, 6, 287–305. [Google Scholar] [CrossRef]
- Mostafa, M.F.M.; EL-Boray, M.S.; El-Baz, E.L.; Omar, A.S. Effect of Fulvic Acid and Some Nutrient Elements on King Ruby Grapevines Growth, Yield and Chemical Properties of Berries. J. Plant Prod. 2017, 8, 321–328. [Google Scholar] [CrossRef]
- Xu, D.; Deng, Y.; Xi, P.; Yu, G.; Wang, Q.; Zeng, Q.; Jiang, Z.; Gao, L. Fulvic acid-induced disease resistance to Botrytis cinerea in table grapes may be mediated by regulating phenylpropanoid metabolism. Food Chem. 2019, 286, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Tassoni, A.; Franceschetti, M.; Righetti, L.; Naldrett, M.J.; Bagni, N. Chitosan treatment induces changes of protein expression profile and stilbene distribution in Vitis vinifera cell suspensions. Proteomics 2009, 9, 610–624. [Google Scholar] [CrossRef]
- Elshaer, E.E.; Elwakil, B.H.; Eskandrani, A.; Elshewemi, S.S.; Olama, Z.A. Novel Clotrimazole and Vitis vinifera loaded chitosan nanoparticles: Antifungal and Wound Healing Efficiencies. Saudi J. Biol. Sci. 2021. [Google Scholar] [CrossRef]
- Godana, E.A.; Yang, Q.; Wang, K.; Zhang, H.; Zhang, X.; Zhao, L.; Abdelhai, M.H.; Legrand, N.N.G. Bio-Control activity of Pichia anomala supplemented with chitosan against Penicillium expansum in postharvest grapes and its possible inhibition mechanism. LWT 2020, 124, 109188. [Google Scholar] [CrossRef]
- Lucini, L.; Baccolo, G.; Rouphael, Y.; Colla, G.; Bavaresco, L.; Trevisan, M. Chitosan treatment elicited defence mechanisms, pentacyclic triterpenoids and stilbene accumulation in grape (Vitis vinifera L.) bunches. Phytochemistry 2018, 156, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Soares, B.; Goufo, P.; Castro, I.; Cosme, F.; Pinto-Sintra, A.L.; Ines, A.; Oliveira, A.A.; Falco, V. Chitosan upregulates the genes of the ROS pathway and enhances the antioxidant potential of grape (Vitis vinifera L. ‘Touriga Franca’ and ’Tinto Cão’) tissues. Antioxidants 2019, 8, 525. [Google Scholar] [CrossRef]
- Nia, A.E.; Taghipour, S.; Siahmansour, S. Pre-harvest application of chitosan and postharvest Aloe vera gel coating enhances quality of table grape (Vitis vinifera L. cv. ‘Yaghouti’) during postharvest period. Food Chem. 2021, 347, 129012. [Google Scholar]
- Singh, R.K.; Martins, V.; Soares, B.; Castro, I.; Falco, V. Chitosan application in vineyards (Vitis vinifera L. cv. Tinto Cão) induces accumulation of anthocyanins and other phenolics in berries, mediated by modifications in the transcription of secondary metabolism genes. Int. J. Mol. Sci. 2020, 21, 306. [Google Scholar] [CrossRef] [PubMed]
- Miliordos, D.E.; Tsiknia, M.; Kontoudakis, N.; Dimopoulou, M.; Bouyioukos, C.; Kotseridis, Y. Impact of Application of Abscisic Acid, Benzothiadiazole and Chitosan on Berry Quality Characteristics and Plant Associated Microbial Communities of Vitis vinifera L. var. Mouhtaro Plants. Sustainability 2021, 13, 5802. [Google Scholar] [CrossRef]
- Vitalini, S.; Ruggiero, A.; Rapparini, F.; Neri, L.; Tonni, M.; Iriti, M. The application of chitosan and benzothiadiazole in vineyard (Vitis vinifera L. cv Groppello Gentile) changes the aromatic profile and sensory attributes of wine. Food Chem. 2014, 162, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gamboa, G.; Pérez-Álvarez, E.P.; Rubio-Bretón, P.; Garde-Cerdán, T. Changes on grape volatile composition through elicitation with methyl jasmonate, chitosan, and a yeast extract in Tempranillo (Vitis vinifera L.) grapevines. Sci. Hortic. 2019, 244, 257–262. [Google Scholar] [CrossRef]
- Esparza-Reynoso, S.; Pelagio-Flores, R.; López-Bucio, J. Mechanism of plant immunity triggered by Trichoderma. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 57–73. [Google Scholar] [CrossRef]
- Carro-Huerga, G.; Compant, S.; Gorfer, M.; Cardoza, R.E.; Schmoll, M.; Gutiérrez, S.; Casquero, P.A. Colonization of Vitis vinifera L. by the endophyte Trichoderma sp. strain T154: Biocontrol activity against Phaeoacremonium minimum. Front. Plant Sci. 2020, 11, 1170. [Google Scholar] [CrossRef]
- Carro-Huerga, G.; Mayo-Prieto, S.; Rodríguez-González, Á.; González-López, Ó.; Gutiérrez, S.; Casquero, P.A. Influence of Fungicide Application and Vine Age on Trichoderma Diversity as Source of Biological Control Agents. Agronomy 2021, 11, 446. [Google Scholar] [CrossRef]
- Kamble, M.V.; Joshi, S.M.; Hadimani, S.; Jogaiah, S. Biopriming with rhizosphere Trichoderma harzianum elicit protection against grapevine downy mildew disease by triggering histopathological and biochemical defense responses. Rhizosphere 2021, 19, 100398. [Google Scholar] [CrossRef]
- Sawant, I.S.; Wadkar, P.N.; Ghule, S.B.; Salunkhe, V.P.; Chavan, V.; Sawant, S.D. Induction of systemic resistance in grapevines against powdery mildew by Trichoderma asperelloides strains. Australas. Plant Pathol. 2020, 49, 107–117. [Google Scholar] [CrossRef]
- Bigot, G.; Sivilotti, P.; Stecchina, M.; Lujan, C.; Freccero, A.; Mosetti, D. Long-term effects of Trichoderma asperellum and Trichoderma gamsii on the prevention of esca in different vineyards of Northeastern Italy. Crop Prot. 2020, 137, 105264. [Google Scholar] [CrossRef]
- Lazazzara, V.; Vicelli, B.; Bueschl, C.; Parich, A.; Pertot, I.; Schuhmacher, R.; Perazzolli, M. Trichoderma spp. volatile organic compounds protect grapevine plants by activating defense-related processes against downy mildew. Physiol. Plant. 2021, 172, 1950–1965. [Google Scholar] [CrossRef]
- Poveda, J. Trichoderma as biocontrol agent against pests: New uses for a mycoparasite. Biol. Control 2021, 159, 104634. [Google Scholar] [CrossRef]
- Rodríguez-González, Á.; Carro-Huerga, G.; Mayo-Prieto, S.; Lorenzana, A.; Gutiérrez, S.; Peláez, H.J.; Casquero, P.A. Investigations of Trichoderma spp. and Beauveria bassiana as biological control agent for Xylotrechus arvicola, a major insect pest in Spanish vineyards. J. Econ. Entomol. 2018, 111, 2585–2591. [Google Scholar] [CrossRef] [PubMed]
- Azizoglu, U.; Yilmaz, N.; Simsek, O.; Ibal, J.C.; Tagele, S.B.; Shin, J.H. The fate of plant growth-promoting rhizobacteria in soilless agriculture: Future perspectives. 3 Biotech 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sathya, A.; Vijayabharathi, R.; Gopalakrishnan, S. Plant growth-promoting actinobacteria: A new strategy for enhancing sustainable production and protection of grain legumes. 3 Biotech 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Rani, K.; Wati, L. The Rhizosphere Actinobacteria and Biological Control: A Review. Environ. Ecol. 2020, 38, 765–770. [Google Scholar]
- Azizoglu, U. Bacillus thuringiensis as a biofertilizer and biostimulator: A mini-review of the little-known plant growth-promoting properties of Bt. Curr. Microbiol. 2019, 76, 1379–1385. [Google Scholar] [CrossRef]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clement, C.; Mugnai, L.; Fontaine, F. Grapevine trunk diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef]
- Laassami, A.; Yekkour, A.; Meklat, A.; Djemouai, N.; Zitouni, A.; Mokrane, S.; Lecomte, P.; Rey, P.; Berraf-Tebbal, A. Actinobacteria Associated with Vineyard Soils of Algeria: Classification, Antifungal Potential Against Grapevine Trunk Pathogens and Plant Growth-Promoting Features. Curr. Microbiol. 2020, 77, 2831–2840. [Google Scholar] [CrossRef]
- Wu, H.; Spagnolo, A.; Marivingt-Mounir, C.; Clément, C.; Fontaine, F.; Chollet, J.F. Evaluating the combined effect of a systemic phenylpyrrole fungicide and the plant growth-promoting rhizobacteria Paraburkholderia phytofirmans (strain PsJN::gfp2x) against the grapevine trunk pathogen Neofusicoccum parvum. Pest Manag. Sci. 2020, 76, 3838–3848. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, A.; Vega-Celedón, P.; Fiaschi, G.; Agnolucci, M.; Avio, L.; Giovannetti, M.; D’Onofrio, C.; Seeger, M. Responses of Vitis vinifera cv. Cabernet Sauvignon roots to the arbuscular mycorrhizal fungus Funneliformis mosseae and the plant growth-promoting rhizobacterium Ensifer meliloti include changes in volatile organic compounds. Mycorrhiza 2020, 30, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Andreolli, M.; Zapparoli, G.; Lampis, S.; Santi, C.; Angelini, E.; Bertazzon, N. In Vivo Endophytic, Rhizospheric and Epiphytic Colonization of Vitis vinifera by the Plant-Growth Promoting and Antifungal Strain Pseudomonas protegens MP12. Microorganisms 2021, 9, 234. [Google Scholar] [CrossRef]
- Torres, N.; Yu, R.; Kurtural, S.K. Inoculation with Mycorrhizal Fungi and Irrigation Management Shape the Bacterial and Fungal Communities and Networks in Vineyard Soils. Microorganisms 2021, 9, 1273. [Google Scholar] [CrossRef]
- Moukarzel, R.; Ridgway, H.J.; Guerin-Laguette, A.; Jones, E.E. Grapevine rootstocks drive the community structure of arbuscular mycorrhizal fungi in New Zealand vineyards. J. Appl. Microbiol. 2021, 131, 2941–2956. [Google Scholar] [CrossRef]
- Velásquez, A.; Valenzuela, M.; Carvajal, M.; Fiaschi, G.; Avio, L.; Giovannetti, M.; D’Onofrio, C.; Seeger, M. The arbuscular mycorrhizal fungus Funneliformis mosseae induces changes and increases the concentration of volatile organic compounds in Vitis vinifera cv. Sangiovese leaf tissue. Plant Physiol. Biochem. 2020, 155, 437–443. [Google Scholar] [CrossRef]
- Agudelo, M.B.; Meyer, E.; Lovato, P.E. Growth, heavy metal uptake, and photosynthesis in ‘Paulsen 1103’ (Vitis berlandieri x rupestris) grapevine rootstocks inoculated with arbuscular mycorrhizal fungi from vineyard soils with high copper contents. Vitis J. Grapevine Res. 2020, 59, 169–180. [Google Scholar]
- Agudelo, M.B.; Meyer, E.; Lovato, P.E. Arbuscular mycorrhizal fungus richness in the soil and root colonization in vineyards of different ages. Rhizosphere 2021, 17, 100307. [Google Scholar] [CrossRef]
- Nogales, A.; Rottier, E.; Campos, C.; Victorino, G.; Costa, J.M.; Coito, J.L.; Pereira, H.S.; Viegas, W.; Lopes, C. The effects of field inoculation of arbuscular mycorrhizal fungi through rye donor plants on grapevine performance and soil properties. Agric. Ecosyst. Environ. 2021, 313, 107369. [Google Scholar] [CrossRef]
- Massa, N.; Bona, E.; Novello, G.; Todeschini, V.; Boatti, L.; Mignone, F.; Gamalero, E.; Lingua, G.; Berta, G.; Cesaro, P. AMF communities associated to Vitis vinifera in an Italian vineyard subjected to integrated pest management at two different phenological stages. Sci. Rep. 2020, 10, 1–12. [Google Scholar]
- Landi, L.; Foglia, R.; Murolo, S.; Romanazzi, G. The Mycorrizal Status in Vineyards Affected by Esca. J. Fungi 2021, 7, 869. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H. Can interaction between silicon and plant growth promoting rhizobacteria benefit in alleviating abiotic and biotic stresses in crop plants? Agric. Ecosyst. Environ. 2018, 253, 98–112. [Google Scholar] [CrossRef]
- Bakhat, H.F.; Bibi, N.; Zia, Z.; Abbas, S.; Hammad, H.M.; Fahad, S.; Ashraf, M.R.; Shah, G.M.; Rabbani, F.; Saeed, S. Silicon mitigates biotic stresses in crop plants: A review. Crop Prot. 2018, 104, 21–34. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Feizi, M.; Kumari, A.; Khan, M.; Mandzhieva, S.; Sushkova, S.; El-Ramady, H.; Verma, K.K.; Singh, A.; et al. Effects of silicon and silicon-based nanoparticles on rhizosphere microbiome, plant stress and growth. Biology 2021, 10, 791. [Google Scholar] [CrossRef] [PubMed]
- Schabl, P.; Gabler, C.; Kührer, E.; Wenzel, W. Effects of silicon amendments on grapevine, soil and wine. Plant Soil Environ. 2020, 66, 403–414. [Google Scholar] [CrossRef]
- Amato, D.; Montanaro, G.; Summerer, S.; Briglia, N.; Attia, F.; Challet, E.; Nuzzo, V. The effects of calcite silicon-mediated particle film application on leaf temperature and grape composition of Merlot (Vitis vinifera L.) vines under different irrigation conditions: This article is published in cooperation with the XIIIth International Terroir Congress November 17–18 2020, Adelaide, Australia. Guest editors: Cassandra Collins and Roberta De Bei. OENO One 2020, 54, 1007–1020. [Google Scholar]
- Farouk, S.; Belal, B.E.A.; El-Sharkawy, H.H.A. The role of some elicitors on the management of Roumy Ahmar grapevines downy mildew disease and it’s related to inducing growth and yield characters. Sci. Hortic. 2017, 225, 646–658. [Google Scholar] [CrossRef]
- Farouk, S.; El-Metwally, I.M. Synergistic responses of drip-irrigated wheat crop to chitosan and/or silicon under different irrigation regimes. Agric. Water Manag. 2019, 226, 105807. [Google Scholar] [CrossRef]
- Habibi, G. Effects of soil-and foliar-applied silicon on the resistance of grapevine plants to freezing stress. Acta Biol. Szeged. 2015, 59, 109–117. [Google Scholar]
- Qin, L.; Kang, W.H.; Qi, Y.L.; Zhang, Z.W.; Wang, N. The influence of silicon application on growth and photosynthesis response of salt stressed grapevines (Vitis vinifera L.). Acta Physiol. Plant. 2016, 38, 68. [Google Scholar] [CrossRef]
- Aguín, O.; Mansilla, J.P.; Sainz, M.J. In vitro selection of an effective fungicide against Armillaria mellea and control of white root rot of grapevine in the field. Pest Manag. Sci. Former. Pestic. Sci. 2006, 62, 223–228. [Google Scholar] [CrossRef]
- Pereira, V.F.; Resende, M.L.V.D.; Ribeiro Junior, P.M.; Regina, M.D.A.; Mota, R.V.D.; Vitorino, L.R.R. Potassium phosphite on the control of downy mildew of grapevine and physicochemical characteristics of Merlot grapes. Pesqui. Agropecuária Bras. 2012, 47, 1581–1588. [Google Scholar] [CrossRef]
- Pinto, K.M.S.; do Nascimento, L.C.; de Souza Gomes, E.C.; da Silva, H.F.; dos Reis Miranda, J. Efficiency of resistance elicitors in the management of grapevine downy mildew Plasmopara viticola: Epidemiological, biochemical and economic aspects. Eur. J. Plant Pathol. 2012, 134, 745–754. [Google Scholar] [CrossRef]
- Buffara, C.R.S.; Angelotti, F.; Tessmann, D.J.; de Souza, C.D.; Vida, J.B. Potassium phosphite pre-and post-infection activities against Phakopsora euvitis in grapevine leaves. Semin. Ciências Agrárias 2013, 34 (Suppl. S1), 3333–3340. [Google Scholar] [CrossRef]
- Da Silva, H.F.; Pinto, K.M.S.; do Nascimento, L.C.; da Silva, E.C.; de Souza, W.C.O. Evaluation of the use of biotic and abiotic resistance elicitors against anthracnose in grapevine (Vitis labrusca L.). Summa Phytopathol. 2019, 45, 70–75. [Google Scholar]
- Burdziej, A.; Bellée, A.; Bodin, E.; Valls Fonayet, J.; Magnin, N.; Szakiel, A.; Richard, T.; Cluzet, S.; Corio-Costet, M.F. Three types of elicitors induce grapevine resistance against downy mildew via common and specific immune responses. J. Agric. Food Chem. 2021, 69, 1781–1795. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cataldo, E.; Fucile, M.; Mattii, G.B. Biostimulants in Viticulture: A Sustainable Approach against Biotic and Abiotic Stresses. Plants 2022, 11, 162. https://doi.org/10.3390/plants11020162
Cataldo E, Fucile M, Mattii GB. Biostimulants in Viticulture: A Sustainable Approach against Biotic and Abiotic Stresses. Plants. 2022; 11(2):162. https://doi.org/10.3390/plants11020162
Chicago/Turabian StyleCataldo, Eleonora, Maddalena Fucile, and Giovan Battista Mattii. 2022. "Biostimulants in Viticulture: A Sustainable Approach against Biotic and Abiotic Stresses" Plants 11, no. 2: 162. https://doi.org/10.3390/plants11020162
APA StyleCataldo, E., Fucile, M., & Mattii, G. B. (2022). Biostimulants in Viticulture: A Sustainable Approach against Biotic and Abiotic Stresses. Plants, 11(2), 162. https://doi.org/10.3390/plants11020162