Herbivory Amplifies Adverse Effects of Drought on Seedling Recruitment in a Keystone Species of Western North American Rangelands
Abstract
1. Introduction
2. Results
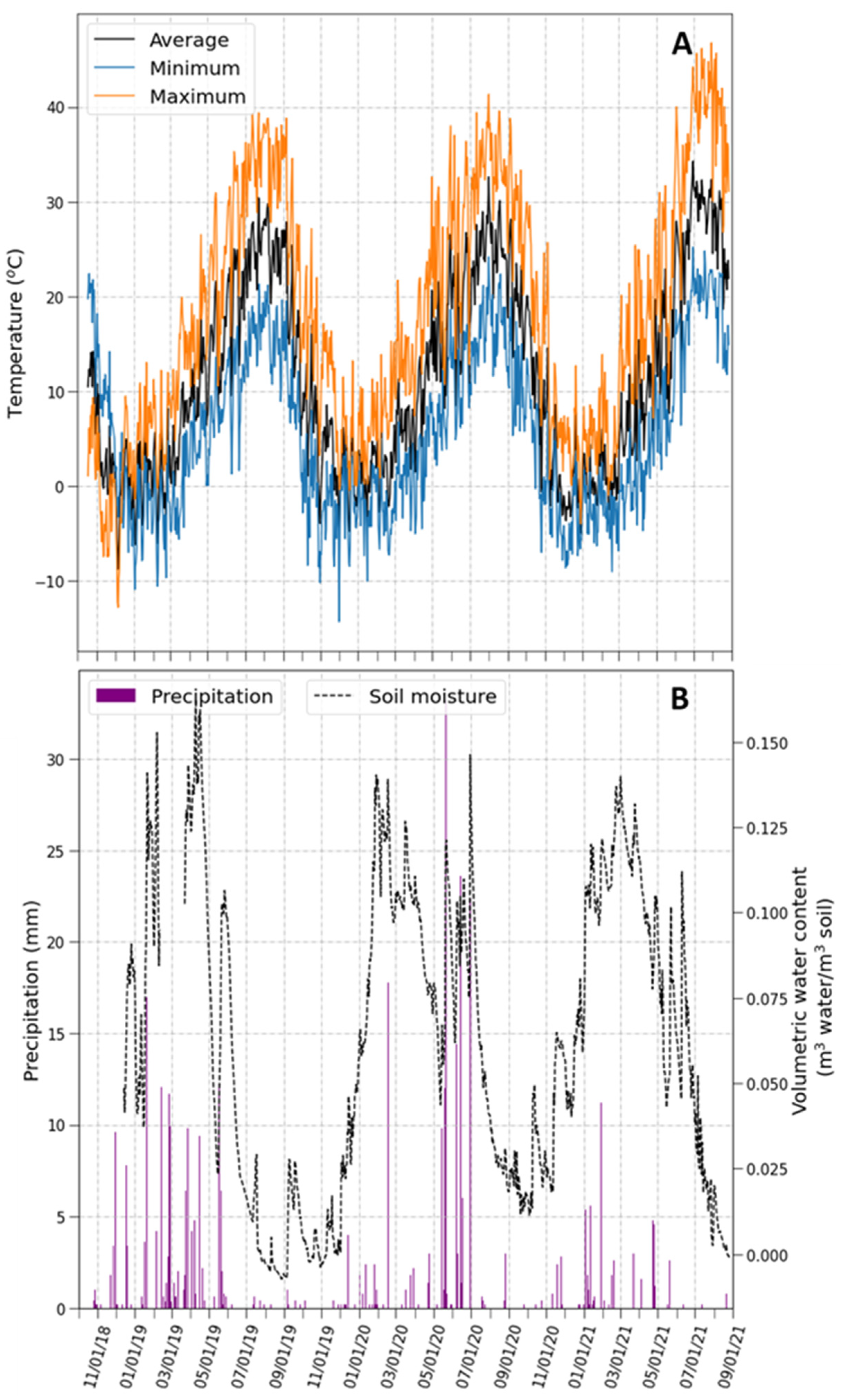
2.1. Climatic Conditions during the Experimental Period
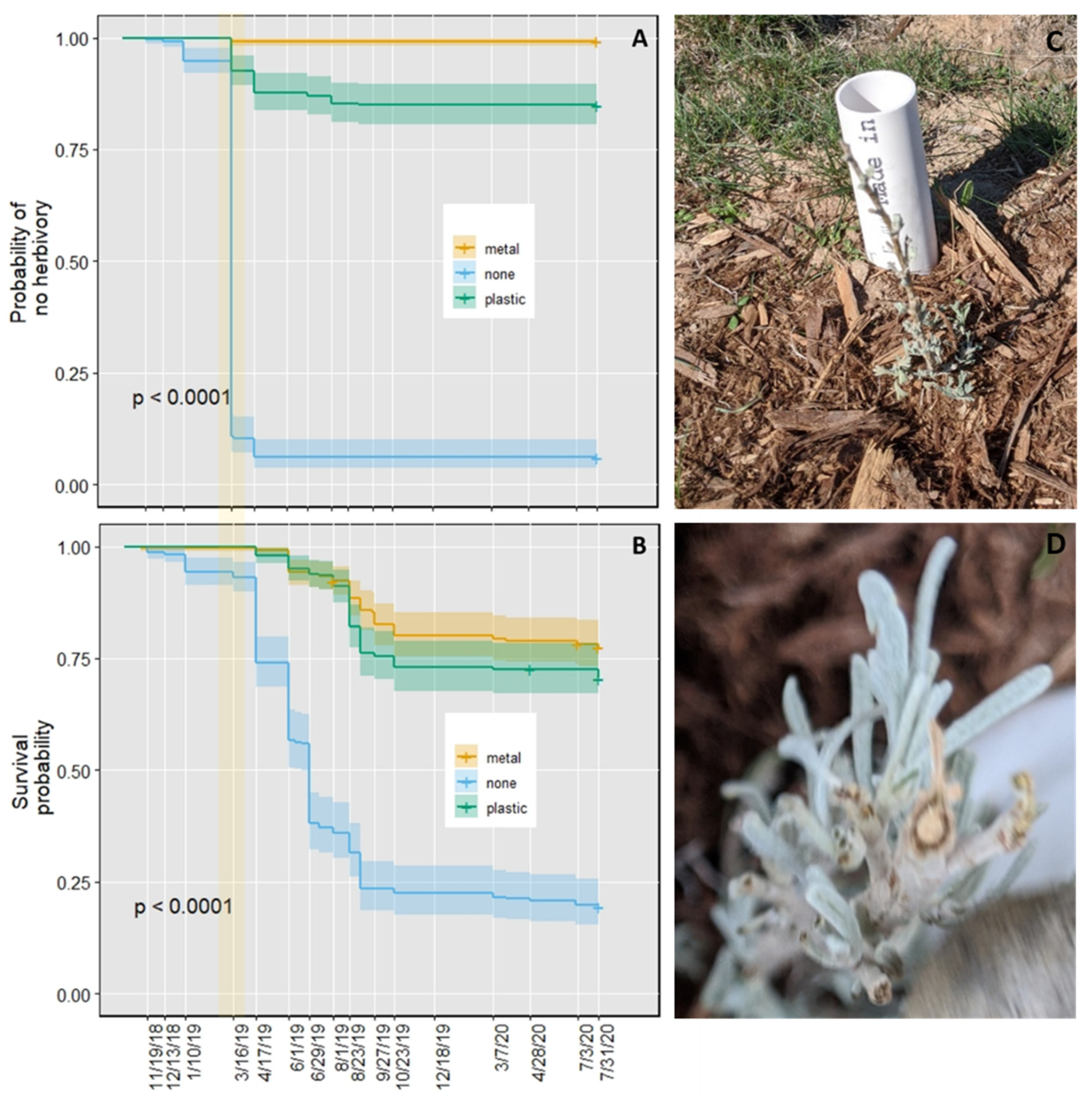
2.2. First Field Experiment
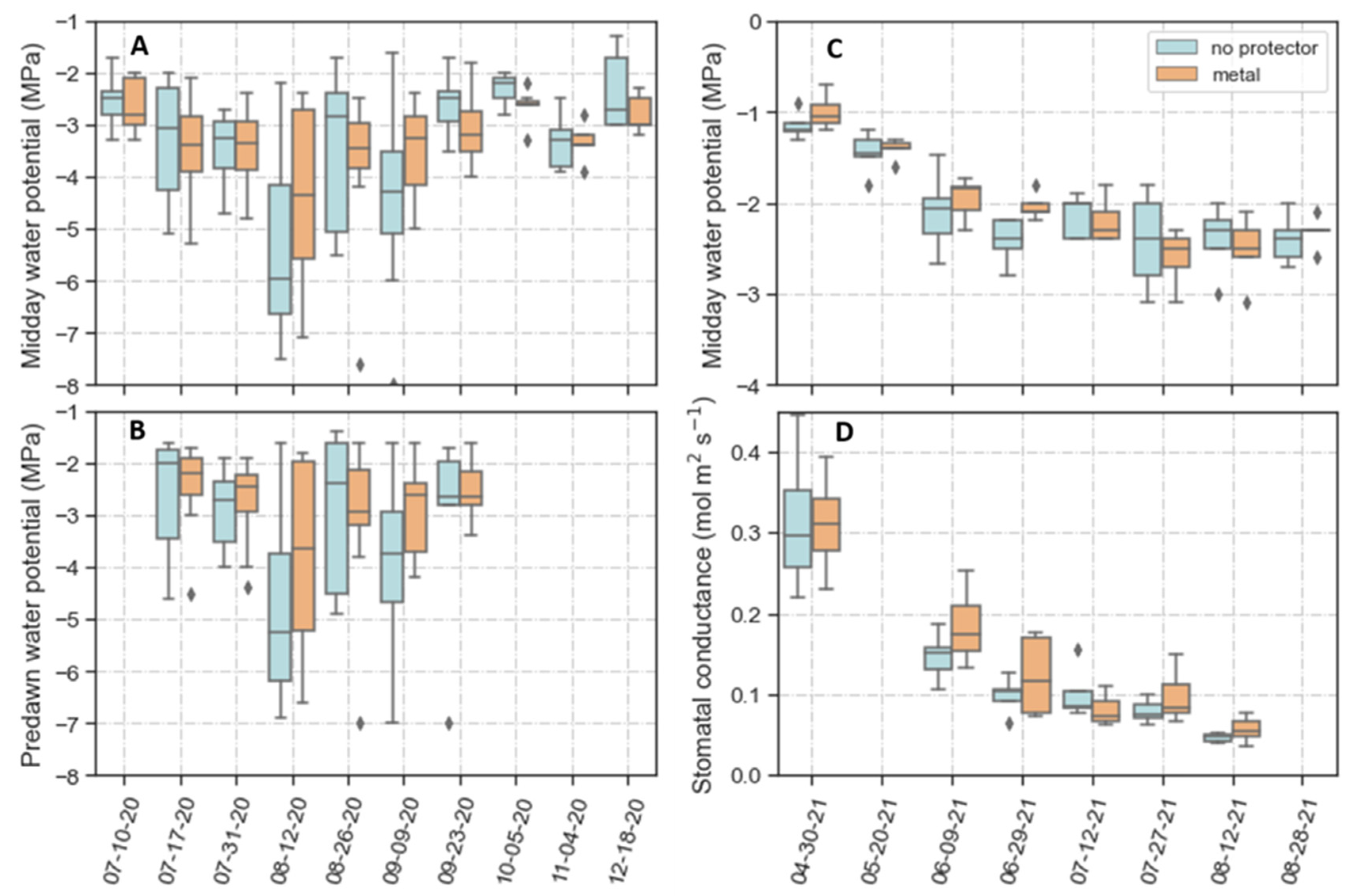
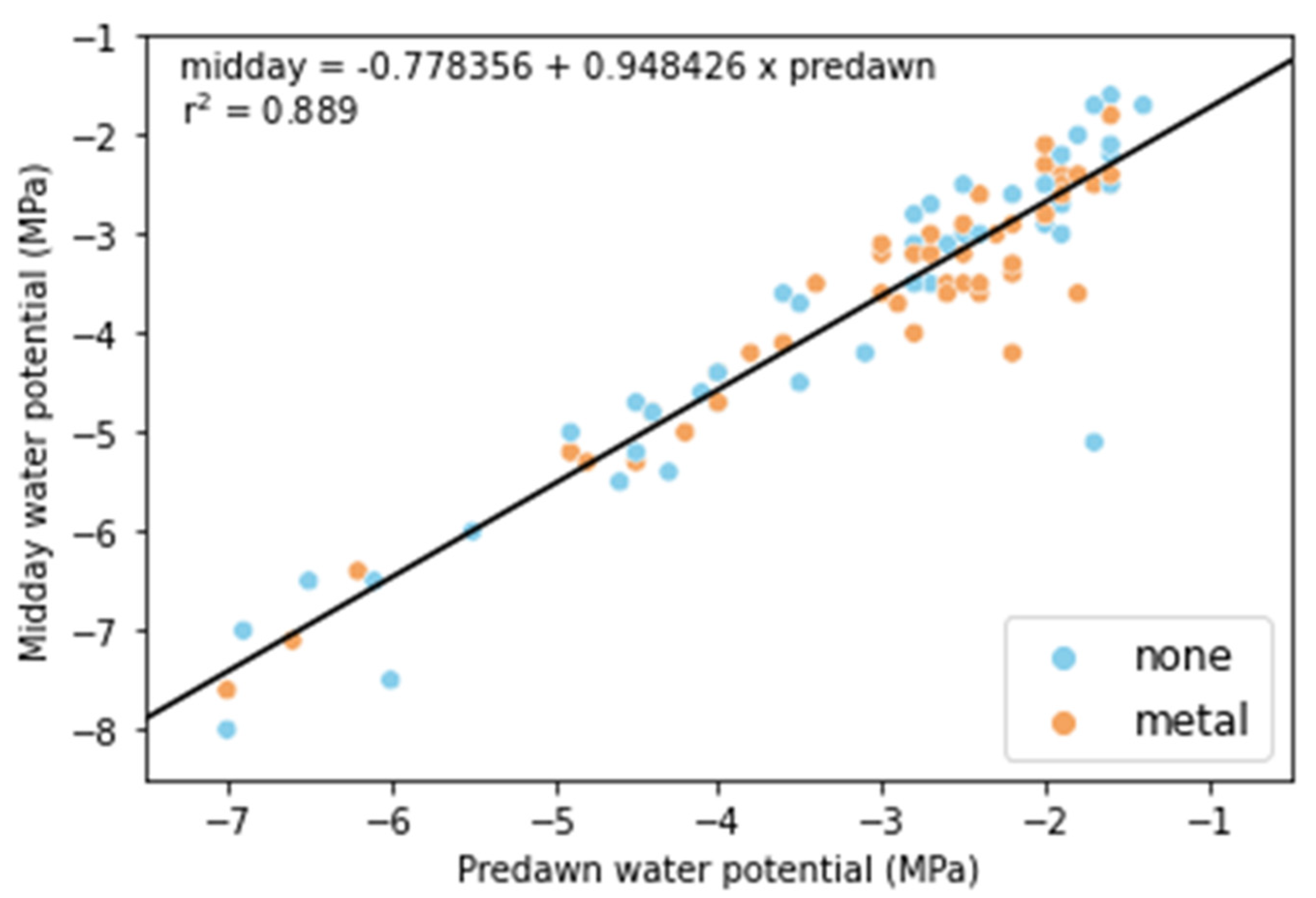
2.3. Second Field Experiment
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Experimental Approach
4.3. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khurana, E.; Singh, J.S. Ecology of seed and seedling growth for conservation and restoration of tropical dry forest: A Review. Environ. Conserv. 2001, 28, 39–52. [Google Scholar] [CrossRef]
- Johnson, D.M.; McCulloh, K.A.; Reinhardt, K. The earliest stages of tree growth: Development, physiology and impacts of microclimate. In Size- and Age-Related Changes in Tree Structure and Function; Meinzer, F.C., Lachenbruch, B., Dawson, T.E., Eds.; Tree Physiology; Springer: Dordrecht, The Netherlands, 2011; pp. 65–87. ISBN 978-94-007-1242-3. [Google Scholar]
- Losso, A.; Bär, A.; Dämon, B.; Dullin, C.; Ganthaler, A.; Petruzzellis, F.; Savi, T.; Tromba, G.; Nardini, A.; Mayr, S.; et al. Insights from in vivo micro-CT analysis: Testing the hydraulic vulnerability segmentation in Acer pseudoplatanus and Fagus sylvatica seedlings. New Phytol. 2019, 221, 1831–1842. [Google Scholar] [CrossRef] [PubMed]
- Vanderwel, M.C.; Lyutsarev, V.S.; Purves, D.W. Climate-related variation in mortality and recruitment determine regional forest-type distributions. Glob. Ecol. Biogeogr. 2013, 22, 1192–1203. [Google Scholar] [CrossRef]
- Cook, B.I.; Mankin, J.S.; Anchukaitis, K.J. Climate change and drought: From past to future. Curr. Clim. Change Rep. 2018, 4, 164–179. [Google Scholar] [CrossRef]
- Barton, K.E.; Hanley, M.E. Seedling–herbivore interactions: Insights into plant defence and regeneration patterns. Ann. Bot. 2013, 112, 643–650. [Google Scholar] [CrossRef]
- Boege, K.; Marquis, R.J. Facing herbivory as you grow up: The ontogeny of resistance in plants. Trends Ecol. Evol. 2005, 20, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Barton, K.E. Low tolerance to simulated herbivory in Hawaiian seedlings despite induced changes in photosynthesis and biomass allocation. Ann. Bot. 2016, 117, 1053–1062. [Google Scholar] [CrossRef]
- Bansal, S. The interactive effects of drought and herbivory on ecophysiology of trees. In Combined Stresses in Plants; Mahalingam, R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 245–259. ISBN 978-3-319-07898-4. [Google Scholar]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L.; Dai, Y.; Wan, X.; Liu, S. Phenology-dependent variation in the non-structural carbohydrates of broadleaf evergreen species plays an important role in determining tolerance to defoliation (or herbivory). Sci. Rep. 2017, 7, 10125. [Google Scholar] [CrossRef]
- Salleo, S.; Trifilò, P.; Gullo, M.A.L.; Salleo, S.; Trifilò, P.; Gullo, M.A.L. Phloem as a possible major determinant of rapid cavitation reversal in stems of Laurus nobilis (laurel). Funct. Plant Biol. 2006, 33, 1063–1074. [Google Scholar] [CrossRef]
- Jacquet, J.-S.; Bosc, A.; O’Grady, A.; Jactel, H. Combined effects of defoliation and water stress on pine growth and non-structural carbohydrates. Tree Physiol. 2014, 34, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, M.; Wang, N.; Liu, S.; Wang, J.; Zhang, W.; Yang, N.; Fan, P.; Wang, R.; Wang, H.; et al. Water use strategies and drought intensity define the relative contributions of hydraulic failure and carbohydrate depletion during seedling mortality. Plant Physiol. Biochem. 2020, 153, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Perkovich, C.; Ward, D. Aboveground herbivory causes belowground changes in twelve oak Quercus species: A phylogenetic analysis of root biomass and non-structural carbohydrate storage. Oikos 2021, 130, 1797–1812. [Google Scholar] [CrossRef]
- Barton, K.E.; Shiels, A.B. Additive and non-additive responses of seedlings to simulated herbivory and drought. Biotropica 2020, 52, 1217–1228. [Google Scholar] [CrossRef]
- James, J.J.; Svejcar, T.J.; Rinella, M.J. Demographic processes limiting seedling recruitment in arid grassland restoration. J. Appl. Ecol. 2011, 48, 961–969. [Google Scholar] [CrossRef]
- Denton, E.M.; Smith, B.S.; Hamerlynck, E.P.; Sheley, R.L. Seedling defoliation and drought stress: Variation in intensity and frequency affect performance and survival. Rangel. Ecol. Manag. 2018, 71, 25–34. [Google Scholar] [CrossRef]
- Clements, C.D.; Harmon, D. Survivability of Wyoming big sagebrush transplants. Rangelands 2019, 41, 88–93. [Google Scholar] [CrossRef]
- Applestein, C.; Caughlin, T.T.; Germino, M.J. Weather affects post-fire recovery of sagebrush-steppe communities and model transferability among sites. Ecosphere 2021, 12, e03446. [Google Scholar] [CrossRef]
- Noss, R.F.; LaRoe, E.T.; Scott, J.M. Endangered Ecosystems of the United States: A Preliminary Assessment of Loss and Degradation; US Department of the Interior, National Biological Service: Washington, DC, USA, 1995; Volume 28. [Google Scholar]
- Anderson, J.E.; Inouye, R.S. Landscape-scale changes in plant species abundance and biodiversity of a sagebrush steppe over 45 years. Ecol. Monogr. 2001, 71, 531–556. [Google Scholar] [CrossRef]
- Brooks, M.L.; D’Antonio, C.M.; Richardson, D.M.; Grace, J.B.; Keeley, J.E.; DiTomaso, J.M.; Hobbs, R.J.; Pellant, M.; Pyke, D. Effects of invasive alien plants on fire regimes. Bioscience 2004, 54, 677–688. [Google Scholar] [CrossRef]
- Aldridge, C.L.; Boyce, M.S. Linking occurrence and fitness to persistence: Habitat-based approach for endangered greater sage-grouse. Ecol. Appl. 2007, 17, 508–526. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.W.; Bates, J.D.; Miller, R.F. The influence of Artemsia tridentata ssp. wyomingensis on microsite and herbaceous vegetation heterogeneity. J. Arid Environ. 2007, 69, 441–457. [Google Scholar] [CrossRef]
- Larrucea, E.S.; Brussard, P.F. Habitat selection and current distribution of the pygmy rabbit in Nevada and California, USA. J. Mammal. 2008, 89, 691–699. [Google Scholar] [CrossRef]
- Baker, W.L. Fire and restoration of sagebrush ecosystems. Wildl. Soc. Bull. 2006, 34, 177–185. [Google Scholar] [CrossRef]
- Barron, R.; Martinez, P.; Serpe, M.; Buerki, S. Development of an in vitro method of propagation for Artemisia tridentata subsp. tridentata to support genome sequencing and genotype-by-environment research. Plants 2020, 9, 1717. [Google Scholar] [CrossRef]
- Brabec, M.M.; Germino, M.J.; Shinneman, D.J.; Pilliod, D.S.; McIlroy, S.K.; Arkle, R.S. Challenges of establishing big sagebrush (Artemisia tridentata) in rangeland restoration: Effects of herbicide, mowing, whole-community seeding, and sagebrush seed sources. Rangel. Ecol. Manag. 2015, 68, 432–435. [Google Scholar] [CrossRef]
- Davidson, B.E.; Novak, S.J.; Serpe, M.D. Consequences of inoculation with native arbuscular mycorrhizal fungi for root colonization and survival of Artemisia tridentata ssp. wyomingensis seedlings after transplanting. Mycorrhiza 2016, 26, 595–608. [Google Scholar] [CrossRef]
- Maier, A.M.; Perryman, B.L.; Olson, R.A.; Hild, A.L. Climatic influences on recruitment of 3 subspecies of Artemisia tridentata. J. Range Manag. 2001, 54, 699–703. [Google Scholar] [CrossRef]
- Schlaepfer, D.R.; Lauenroth, W.K.; Bradford, J.B. Natural regeneration processes in big sagebrush (Artemisia tridentata). Rangel. Ecol. Manag. 2014, 67, 344–357. [Google Scholar] [CrossRef]
- Welch, B.L.; McArthur, E.D. Wintering mule deer preference for 21 accessions of big sagebrush. Great Basin Nat. 1986, 46, 281–286. [Google Scholar]
- Ulappa, A.C.; Kelsey, R.G.; Frye, G.G.; Rachlow, J.L.; Shipley, L.A.; Bond, L.; Pu, X.; Forbey, J.S. Plant protein and secondary metabolites influence diet selection in a mammalian specialist herbivore. J. Mammal. 2014, 95, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Huntly, N. Herbivorous insects reduce growth and reproduction of big sagebrush (Artemisia tridentata). Arthropod-Plant Interact. 2010, 4, 257–266. [Google Scholar] [CrossRef]
- Ishizaki, S.; Shiojiri, K.; Karban, R.; Ohara, M. Seasonal variation of responses to herbivory and volatile communication in sagebrush (Artemisia tridentata) (Asteraceae). J. Plant Res. 2016, 129, 659–666. [Google Scholar] [CrossRef] [PubMed]
- McMunn, M.S. The timing of leaf damage affects future herbivory in mountain sagebrush (Artemisia tridentata). Ecology 2017, 98, 1996–2002. [Google Scholar] [CrossRef]
- Dwinnell, S.P.H.; Sawyer, H.; Randall, J.E.; Beck, J.L.; Forbey, J.S.; Fralick, G.L.; Monteith, K.L. Where to forage when afraid: Does perceived risk impair use of the foodscape? Ecol. Appl. 2019, 29, e01972. [Google Scholar] [CrossRef]
- Manier, D.J.; Hobbs, N.T. Large herbivores in sagebrush steppe ecosystems: Livestock and wild ungulates influence structure and function. Oecologia 2007, 152, 739–750. [Google Scholar] [CrossRef]
- Hanser, S.E.; Huntly, N.J. The biogeography of small mammals of fragmented sagebrush-steppe landscapes. J. Mammal. 2006, 87, 1165–1174. [Google Scholar] [CrossRef]
- Yensen, E. Taxonomy and distribution of the Idaho ground squirrel, Spermophilus brunneus. J. Mammal. 1991, 72, 583–600. [Google Scholar] [CrossRef]
- Lohr, K.; Yensen, E.; Munger, J.C.; Novak, S.J. Relationship between habitat characteristics and densities of southern Idaho ground squirrels. J. Wildl. Manag. 2013, 77, 983–993. [Google Scholar] [CrossRef]
- Karban, R.; Thaler, J.S. Plant phase change and resistance to herbivory. Ecology 1999, 80, 510–517. [Google Scholar] [CrossRef]
- Wise, M.J.; Abrahamson, W.G. Effects of resource availability on tolerance of herbivory: A review and assessment of three opposing models. Am. Nat. 2007, 169, 443–454. [Google Scholar] [CrossRef]
- Levine, M.T.; Paige, K.N. Direct and indirect effects of drought on compensation following herbivory in scarlet gilia. Ecology 2004, 85, 3185–3191. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, J.; Frye, M.J. Effects of resource availability on tolerance of herbivory in the invasive Alternanthera philoxeroides and the native Alternanthera sessilis. Weed Res. 2010, 50, 527–536. [Google Scholar] [CrossRef]
- Shibel, Z.; Heard, S.B. Synergistic and additive effects of drought stress and simulated herbivory on two goldenrods, Solidago altissima and S. gigantea. Botany 2016, 94, 635–642. [Google Scholar] [CrossRef]
- Kolb, K.J.; Sperry, J.S. Differences in drought adaptation between subspecies of sagebrush (Artemisia tridentata). Ecology 1999, 80, 2373–2384. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Hudgeons, J.L.; Knutson, A.E.; Heinz, K.M.; DeLoach, C.J.; Dudley, T.L.; Pattison, R.R.; Kiniry, J.R. Defoliation by introduced Diorhabda elongata leaf beetles (Coleoptera: Chrysomelidae) reduces carbohydrate reserves and regrowth of Tamarix (Tamaricaceae). Biol. Control 2007, 43, 213–221. [Google Scholar] [CrossRef]
- Quentin, A.G.; Beadle, C.L.; O’Grady, A.P.; Pinkard, E.A. Effects of partial defoliation on closed canopy Eucalyptus globulus labilladière: Growth, biomass allocation and carbohydrates. For. Ecol. Manag. 2011, 261, 695–702. [Google Scholar] [CrossRef]
- De Baerdemaeker, N.J.F.; Salomón, R.L.; De Roo, L.; Steppe, K. Sugars from woody tissue photosynthesis reduce xylem vulnerability to cavitation. New Phytol. 2017, 216, 720–727. [Google Scholar] [CrossRef]
- Trifilò, P.; Casolo, V.; Raimondo, F.; Petrussa, E.; Boscutti, F.; Lo Gullo, M.A.; Nardini, A. Effects of prolonged drought on stem non-structural carbohydrates content and post-drought hydraulic recovery in Laurus nobilis L.: The possible link between carbon starvation and hydraulic failure. Plant Physiol. Biochem. 2017, 120, 232–241. [Google Scholar] [CrossRef]
- Sevanto, S. Drought impacts on phloem transport. Curr. Opin. Plant Biol. 2018, 43, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Tomasella, M.; Petrussa, E.; Petruzzellis, F.; Nardini, A.; Casolo, V. The possible role of non-structural carbohydrates in the regulation of tree hydraulics. Int. J. Mol. Sci. 2020, 21, 144. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Leuzinger, S.; Philipson, C.D.; Tay, J.; Hector, A. Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nat. Clim. Change 2014, 4, 710–714. [Google Scholar] [CrossRef]
- Chen, T.; Pei, H.; Zhang, Y.; Qian, Q. Seasonal changes in non-structural carbohydrates and sucrose metabolism enzymes in two Sabina species. Acta Physiol. Plant 2012, 34, 173–180. [Google Scholar] [CrossRef]
- Pommerrenig, B.; Ludewig, F.; Cvetkovic, J.; Trentmann, O.; Klemens, P.A.W.; Neuhaus, H.E. In Concert: Orchestrated changes in carbohydrate homeostasis are critical for plant abiotic stress tolerance. Plant Cell Physiol. 2018, 59, 1290–1299. [Google Scholar] [CrossRef]
- Paige, K.N.; Whitham, T.G. Overcompensation in response to mammalian herbivory: The advantage of being eaten. Am. Nat. 1987, 129, 407–416. [Google Scholar] [CrossRef]
- Brody, A.K.; Irwin, R.E. When Resources Don’t rescue: Flowering phenology and species interactions affect compensation to herbivory in Ipomopsis aggregata. Oikos 2012, 121, 1424–1434. [Google Scholar] [CrossRef]
- Brys, R.; Shefferson, R.P.; Jacquemyn, H. Impact of herbivory on flowering behaviour and life history trade-offs in a polycarpic herb: A 10-year experiment. Oecologia 2011, 166, 293–303. [Google Scholar] [CrossRef]
- Huijser, P.; Schmid, M. The control of developmental phase transitions in plants. Development 2011, 138, 4117–4129. [Google Scholar] [CrossRef]
- Marquis, R.J.; Newell, E.A.; Villegas, A.C. Non-structural carbohydrate accumulation and use in an understorey rain-forest shrub and relevance for the impact of leaf herbivory. Funct. Ecol. 1997, 11, 636–643. [Google Scholar] [CrossRef]
- Dettweiler-Robinson, E.; Bakker, J.D.; Evans, J.R.; Newsome, H.; Davies, G.M.; Wirth, T.A.; Pyke, D.A.; Easterly, R.T.; Salstrom, D.; Dunwiddie, P.W. Outplanting Wyoming big sagebrush following wildfire: Stock performance and economics. Rangel. Ecol. Manag. 2013, 66, 657–666. [Google Scholar] [CrossRef]
- Katjiua, M.L.J.; Ward, D. Resistance and tolerance of Terminalia sericea trees to simulated herbivore damage under different soil nutrient and moisture conditions. J. Chem. Ecol. 2006, 32, 1431–1443. [Google Scholar] [CrossRef] [PubMed]
- Benevenuto, R.F.; Hegland, S.J.; Töpper, J.P.; Rydgren, K.; Moe, S.R.; Rodriguez-Saona, C.; Seldal, T. Multiannual effects of induced plant defenses: Are defended plants good or bad neighbors? Ecol. Evol. 2018, 8, 8940–8950. [Google Scholar] [CrossRef]
- Wang, H.; Ma, C.; Li, Z.; Ma, L.; Wang, H.; Ye, H.; Xu, G.; Liu, B. Effects of exogenous methyl jasmonate on artemisinin biosynthesis and secondary metabolites in Artemisia annua L. Ind. Crops Prod. 2010, 31, 214–218. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Poyatos, R.; Aguadé, D.; Retana, J.; Mencuccini, M. A new look at water transport regulation in plants. New Phytol. 2014, 204, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vilalta, J.; Garcia-Forner, N. Water potential regulation, stomatal behaviour and hydraulic transport under drought: Deconstructing the iso/anisohydric concept. Plant Cell Environ. 2017, 40, 962–976. [Google Scholar] [CrossRef] [PubMed]
- Donovan, L.A.; Richards, J.H.; Linton, M.J. Magnitude and mechanisms of disequilibrium between predawn plant and soil water potentials. Ecology 2003, 84, 463–470. [Google Scholar] [CrossRef]
- Sharma, H.; Reinhardt, K.; Lohse, K.A. Fundamental intra-specific differences in plant–water relations in a widespread desert shrub (Artemisia tridentata). Plant Ecol. 2020, 221, 925–938. [Google Scholar] [CrossRef]
- Franks, P.J.; Drake, P.L.; Froend, R.H. Anisohydric but isohydrodynamic: Seasonally constant plant water potential gradient explained by a stomatal control mechanism incorporating variable plant hydraulic conductance. Plant Cell Environ. 2007, 30, 19–30. [Google Scholar] [CrossRef]
- Melton, A.E.; Beck, J.; Galla, S.J.; Jenkins, J.; Handley, L.; Kim, M.; Grimwood, J.; Schmutz, J.; Richardson, B.A.; Serpe, M.; et al. A draft genome provides hypotheses on drought tolerance in a keystone plant species in western North America threatened by climate change. Ecol. Evol. 2021, 11, 15417–15429. [Google Scholar] [CrossRef]
- Fleege, C.D. Protocols for sagebrush seed processing and seedling production at the Lucky Peak Nursery. In National Proceedings: Forest and Conservation Nursery Associations; US Department of Agriculture: Fort Collins, CO, USA, 2010. [Google Scholar]
- California Soil Resource Lab. SoilWeb: An Online Soil Survey Browser. Available online: https://casoilresource.lawr.ucdavis.edu/gmap/ (accessed on 13 July 2022).
- Rasband, W.S.; ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA. 1997–2018. Available online: https://Imagej.Nih.Gov/Ij/ (accessed on 15 September 2020).
- Kassambara, A.; Kosinski, M.; Biecek, P.; Fabian, S. Survminer: Drawing Survival Curves Using “Ggplot2” 2021. R Package Version 0.4.9. 2021. Available online: https://cran.r-project.org/web/packages/survminer/index.html (accessed on 15 July 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-Project.org/ (accessed on 15 June 2022).
- Waskom, M. Seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Williams, A.P.; Cook, E.R.; Smerdon, J.E.; Cook, B.I.; Abatzoglou, J.T.; Bolles, K.; Baek, S.H.; Badger, A.M.; Livneh, B. Large contribution from anthropogenic warming to an emerging north american megadrought. Science 2020, 368, 314–318. [Google Scholar] [CrossRef] [PubMed]


| Treatment | 2020 | 2021 | 2022 |
|---|---|---|---|
| no-protector | 11 b | 12 b | 66.6 |
| plastic protector | 31 a | 41 a | 76.9 |
| metal protector | 29 a | 30 a | 80.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geisler, M.; Buerki, S.; Serpe, M.D. Herbivory Amplifies Adverse Effects of Drought on Seedling Recruitment in a Keystone Species of Western North American Rangelands. Plants 2022, 11, 2628. https://doi.org/10.3390/plants11192628
Geisler M, Buerki S, Serpe MD. Herbivory Amplifies Adverse Effects of Drought on Seedling Recruitment in a Keystone Species of Western North American Rangelands. Plants. 2022; 11(19):2628. https://doi.org/10.3390/plants11192628
Chicago/Turabian StyleGeisler, Mathew, Sven Buerki, and Marcelo D. Serpe. 2022. "Herbivory Amplifies Adverse Effects of Drought on Seedling Recruitment in a Keystone Species of Western North American Rangelands" Plants 11, no. 19: 2628. https://doi.org/10.3390/plants11192628
APA StyleGeisler, M., Buerki, S., & Serpe, M. D. (2022). Herbivory Amplifies Adverse Effects of Drought on Seedling Recruitment in a Keystone Species of Western North American Rangelands. Plants, 11(19), 2628. https://doi.org/10.3390/plants11192628








