Genetic Evaluation of In Vitro Micropropagated and Regenerated Plants of Cannabis sativa L. Using SSR Molecular Markers
Abstract
1. Introduction
2. Results
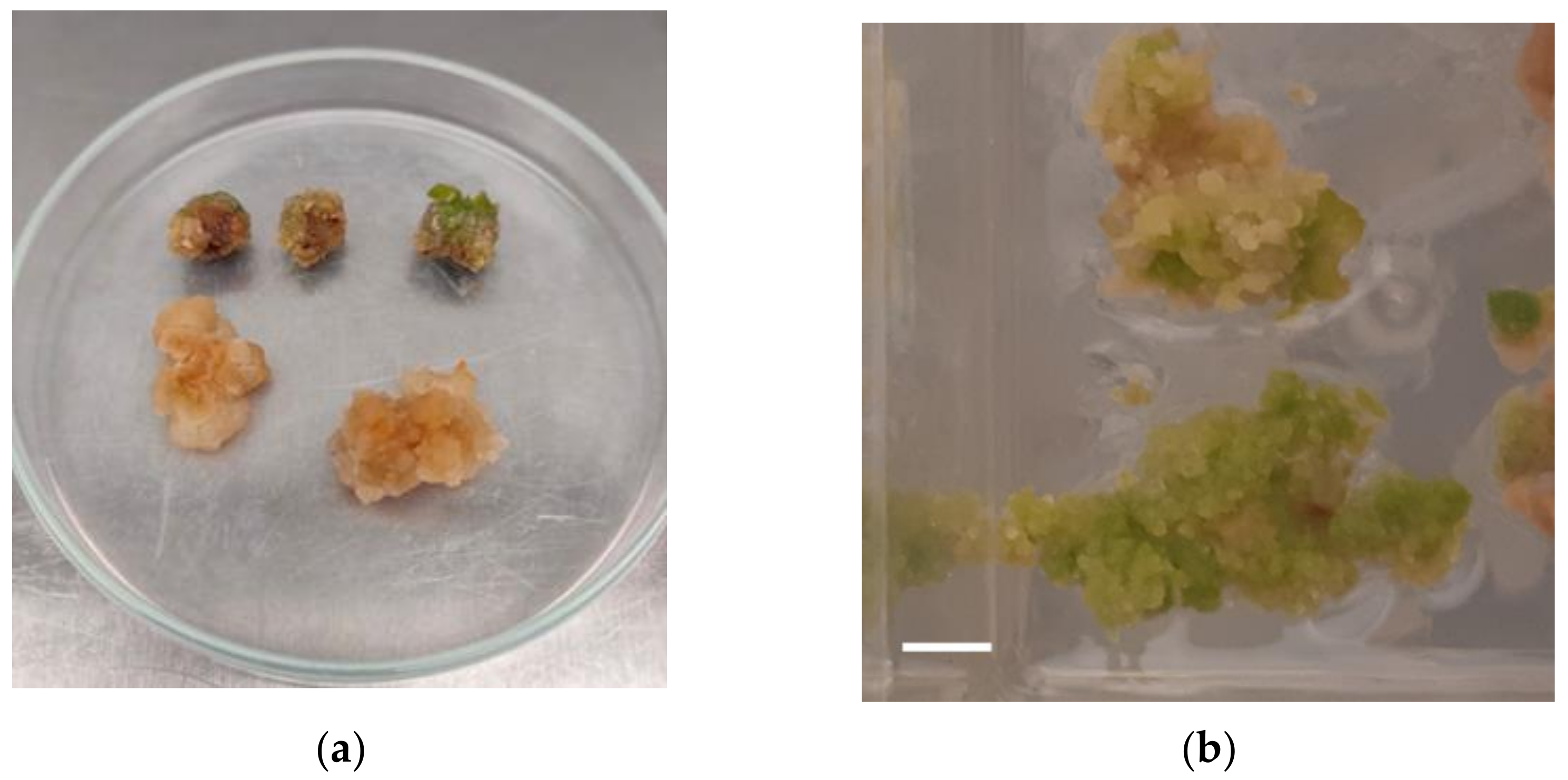
2.1. Plant Regeneration through Callus Formation
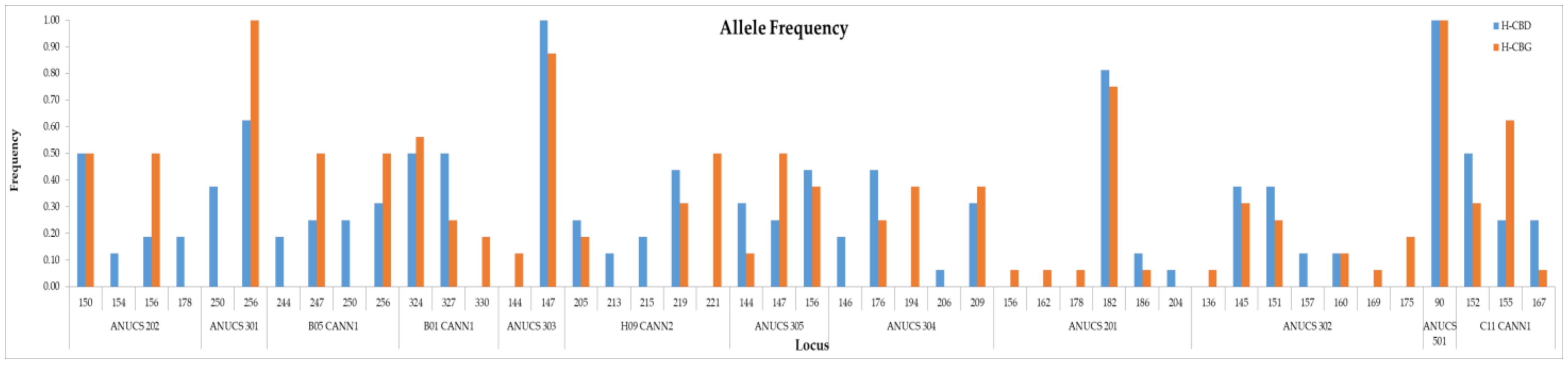
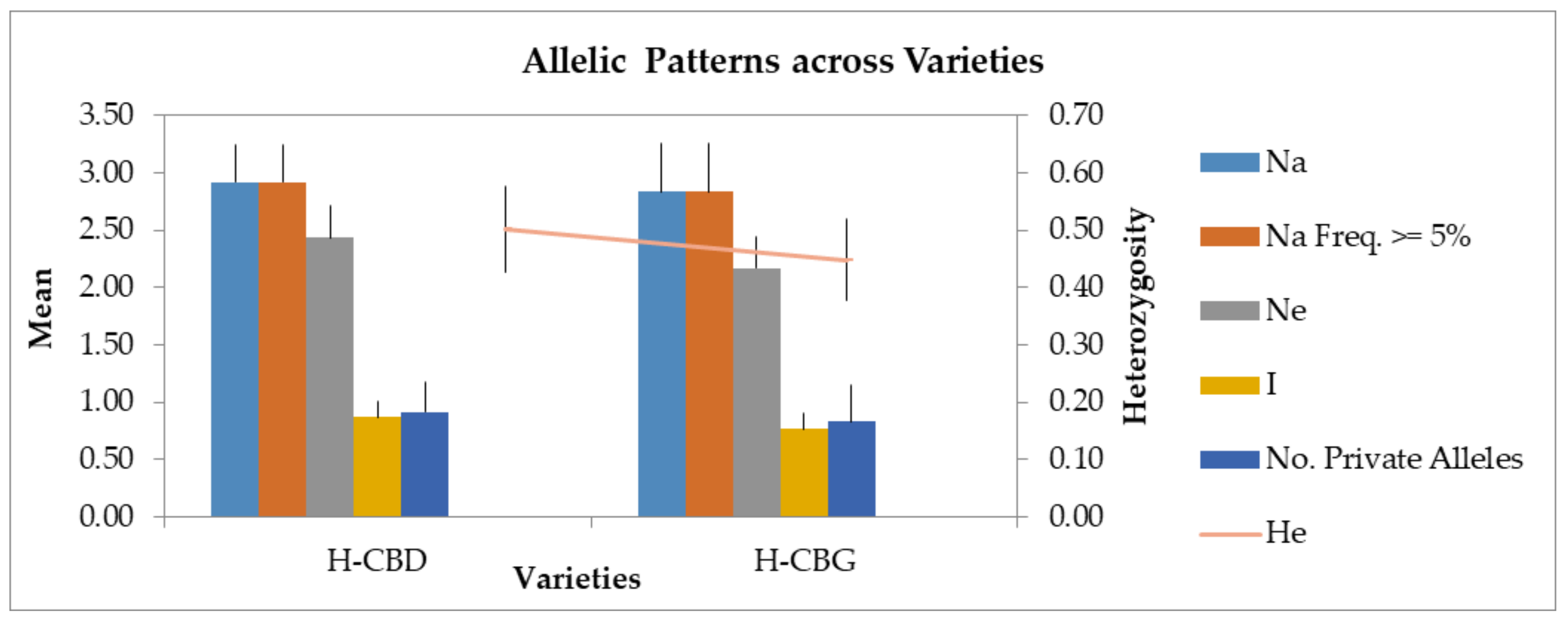
2.2. Genetic Data and Homogeneity Assessment
3. Discussion
3.1. Plant Regeneration through Callus Formation
3.2. Genetic Data and Homogeneity Assessment
4. Materials and Methods
4.1. Plant Material
4.2. Plant Regeneration through Callus Formation
4.3. DNA Isolation and Quantification
4.4. Microsatellite Loci
4.5. PCR Reaction Mix and Amplification
4.6. Capillary Electrophoresis, Genotyping and Statistical Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, K. The Archaeology of Ancient China; Yale University Press: New Haven, CT, USA, 1986; ISBN 0-300-03782-1. [Google Scholar]
- Kung, C. Archeology in China. Tor. Univ. Tor. Press 1959, 1, 131. [Google Scholar]
- Vavilov, N.I. Studies on the Origin of Cultivated Plants. Bull. Appl. Bot. Plant Breed. 1926, 1, 1–248. [Google Scholar]
- Schultes, R.E.; Klein, W.M.; Plowman, T.; Lockwood, T.E. Cannabis: An Example of Taxonomic Neglect. Bot. Mus. Leafl. Harv. Univ. 1974, 23, 337–367. [Google Scholar] [CrossRef]
- Salami, S.A.; Martinelli, F.; Giovino, A.; Bachari, A.; Arad, N.; Mantri, N. It Is Our Turn to Get Cannabis High: Put Cannabinoids in Food and Health Baskets. Molecules 2020, 25, 4036. [Google Scholar] [CrossRef]
- McPartland, J.M.; Russo, E.B. Cannabis and Cannabis Extracts: Greater Than the Sum of Their Parts? J. Cannabis Ther. 2001, 1, 103–132. [Google Scholar] [CrossRef]
- Gill, E.W.; Paton, W.D.M.; Pertwee, R.G. Preliminary Experiments on the Chemistry and Pharmacology of Cannabis. Nature 1970, 228, 134–136. [Google Scholar] [CrossRef]
- Mechoulam, R.; Gaoni, Y. Recent Advances in the Chemistry of Hashish. In Fortschritte der Chemie Organischer Naturstoffe / Progress in the Chemistry of Organic Natural Products / Progrès dans la Chimie des Substances Organiques Naturelles; Ashurst, P.R., Bohlmann, F., Farkas, L., Gaoni, Y., Kling, H., Mechoulam, R., Morrison, G.A., Pallos, L., Romo, J., De Vivar, A.R., et al., Eds.; Springer Vienna: Vienna, Austria, 1967; pp. 175–213. ISBN 978-3-7091-8164-5. [Google Scholar]
- Chandra, S.; Lata, H.; ElSohly, M.A. Cannabis sativa L.-Botany and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 3-319-54564-7. [Google Scholar]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A Unified Critical Inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef]
- Citti, C.; Linciano, P.; Panseri, S.; Vezzalini, F.; Forni, F.; Vandelli, M.A.; Cannazza, G. Cannabinoid Profiling of Hemp Seed Oil by Liquid Chromatography Coupled to High-Resolution Mass Spectrometry. Front. Plant Sci. 2019, 10, 120. [Google Scholar] [CrossRef]
- Pavlovic, R.; Panseri, S.; Giupponi, L.; Leoni, V.; Citti, C.; Cattaneo, C.; Cavaletto, M.; Giorgi, A. Phytochemical and Ecological Analysis of Two Varieties of Hemp (Cannabis sativa L.) Grown in a Mountain Environment of Italian Alps. Front. Plant Sci. 2019, 10, 1265. [Google Scholar] [CrossRef]
- Tzadok, M.; Uliel-Siboni, S.; Linder, I.; Kramer, U.; Epstein, O.; Menascu, S.; Nissenkorn, A.; Yosef, O.B.; Hyman, E.; Granot, D.; et al. CBD-Enriched Medical Cannabis for Intractable Pediatric Epilepsy. Seizure 2016, 35, 41–44. [Google Scholar] [CrossRef]
- Brierley, D.I.; Samuels, J.; Duncan, M.; Whalley, B.J.; Williams, C.M. A Cannabigerol-Rich Cannabis sativa Extract, Devoid of ∆9-Tetrahydrocannabinol, Elicits Hyperphagia in Rats. Behav. Pharmacol. 2017, 28, 280–284. [Google Scholar] [CrossRef]
- Ioannidis, K.; Dadiotis, E.; Mitsis, V.; Melliou, E.; Magiatis, P. Biotechnological Approaches on Two High CBD and CBG Cannabis sativa L. (Cannabaceae) Varieties: In Vitro Regeneration and Phytochemical Consistency Evaluation of Micropropagated Plants Using Quantitative 1H-NMR. Molecules 2020, 25, 5298. [Google Scholar] [CrossRef]
- Ioannidis, K.; Tomprou, I.; Mitsis, V. An Alternative In Vitro Propagation Protocol of Cannabis sativa L. (Cannabaceae) Presenting Efficient Rooting, for Commercial Production. Plants 2022, 11, 1333. [Google Scholar] [CrossRef]
- Fournier, G.; Richez-Dumanois, C.; Duvezin, J.; Mathieu, J.-P.; Paris, M. Identification of a New Chemotype in Cannabis sativa : Cannabigerol—Dominant Plants, Biogenetic and Agronomic Prospects. Planta Med. 1987, 53, 277–280. [Google Scholar] [CrossRef]
- De Meijer, E.P.M.; Hammond, K.M. The Inheritance of Chemical Phenotype in Cannabis sativa L. (II): Cannabigerol Predominant Plants. Euphytica 2005, 145, 189–198. [Google Scholar] [CrossRef]
- De Meijer, E.P.M.; Bagatta, M.; Carboni, A.; Crucitti, P.; Moliterni, V.M.C.; Ranalli, P.; Mandolino, G. The Inheritance of Chemical Phenotype in Cannabis sativa L. Genetics 2003, 163, 335–346. [Google Scholar] [CrossRef]
- Coogan, T.A. Analysis of the Cannabinoid Content of Strains Available in the New Jersey Medicinal Marijuana Program. J. Cannabis Res. 2019, 1, 11. [Google Scholar] [CrossRef]
- Barcaccia, G.; Palumbo, F.; Scariolo, F.; Vannozzi, A.; Borin, M.; Bona, S. Potentials and Challenges of Genomics for Breeding Cannabis Cultivars. Front. Plant Sci. 2020, 11, 1472. [Google Scholar] [CrossRef] [PubMed]
- Citti, C.; Linciano, P.; Russo, F.; Luongo, L.; Iannotta, M.; Maione, S.; Laganà, A.; Capriotti, A.L.; Forni, F.; Vandelli, M.A.; et al. A Novel Phytocannabinoid Isolated from Cannabis sativa L. with an in Vivo Cannabimimetic Activity Higher than Δ9-Tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol. Sci. Rep. 2019, 9, 20335. [Google Scholar] [CrossRef]
- Vasil, I.K. Progress in the Regeneration and Genetic Manipulation of Cereal Crops. Bio/Technol. 1988, 6, 397–402. [Google Scholar] [CrossRef]
- Monthony, A.S.; Page, S.R.; Hesami, M.; Jones, A.M.P. The Past, Present and Future of Cannabis sativa Tissue Culture. Plants 2021, 10, 185. [Google Scholar] [CrossRef]
- Phillips, R.L.; Kaeppler, S.M.; Olhoft, P. Genetic Instability of Plant Tissue Cultures: Breakdown of Normal Controls. Proc. Natl. Acad. Sci. USA 1994, 91, 5222–5226. [Google Scholar] [CrossRef]
- Cullis, C.A. The Molecular Biology of Plant Cells and Cultures. In Plant Biotechnology; Fowler, M.W., Warren, G.S., Eds.; Pergamon: Amsterdam, The Netherlands, 1992; pp. 19–32. ISBN 978-0-08-034731-8. [Google Scholar]
- Pierik, R.L.M. In Vitro Culture of Higher Plants; Springer Science & Business Media: Berlin, Germany, 1997; ISBN 0-7923-4527-4. [Google Scholar]
- Rawls, B.; Harris-Shultz, K.; Dhekney, S.; Forrester, I.; Sitther, V. Clonal Fidelity of Micropropagated Psidium Guajava L. Plants Using Microsatellite Markers. Am. J. Plant Sci. 2015, 6, 2385. [Google Scholar] [CrossRef][Green Version]
- Lata, H.; Chandra, S.; Techen, N.; Khan, I.A.; ElSohly, M.A. Molecular Analysis of Genetic Fidelity in Cannabis sativa L. Plants Grown from Synthetic (Encapsulated) Seeds Following in Vitro Storage. Biotechnol. Lett. 2011, 33, 2503–2508. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Lata, H.; Mehmedic, Z.; Khan, I.A.; ElSohly, M.A. Assessment of Cannabinoids Content in Micropropagated Plants of Cannabis sativa and Their Comparison with Conventionally Propagated Plants and Mother Plant during Developmental Stages of Growth. Planta Med. 2010, 76, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Sarmento, D.; Oliveira, M.M. Genetic Stability of Micropropagated Almond Plantlets, as Assessed by RAPD and ISSR Markers. Plant Cell Rep. 2004, 23, 492–496. [Google Scholar] [CrossRef]
- Saker, M.M.; Bekheet, S.A.; Taha, H.S.; Fahmy, A.S.; Moursy, H.A. Detection of Somaclonal Variations in Tissue Culture-Derived Date Palm Plants Using Isoenzyme Analysis and RAPD Fingerprints. Biol. Plant. 2000, 43, 347–351. [Google Scholar] [CrossRef]
- Datwyler, S.L.; Weiblen, G.D. Genetic Variation in Hemp and Marijuana (Cannabis sativa L.) According to Amplified Fragment Length Polymorphisms. J. Forensic Sci. 2006, 51, 371–375. [Google Scholar] [CrossRef]
- Debnath, S. Bioreactors and Molecular Analysis in Berry Crop Micropropagation —A Review. Can. J. Plant Sci. 2011, 91, 147–157. [Google Scholar] [CrossRef]
- Debnath, S.C.; Vyas, P.; Goyali, J.C.; Igamberdiev, A.U. Morphological and Molecular Analyses in Micropropagated Berry Plants Acclimatized under Ex Vitro Condition. Can. J. Plant Sci. 2012, 92, 1065–1073. [Google Scholar] [CrossRef]
- Patel, D.A.; Zander, M.; Dalton-Morgan, J.; Batley, J. Advances in Plant Genotyping: Where the Future Will Take Us. In Plant Genotyping; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–11. [Google Scholar]
- Borin, M.; Palumbo, F.; Vannozzi, A.; Scariolo, F.; Sacilotto, G.B.; Gazzola, M.; Barcaccia, G. Developing and Testing Molecular Markers in Cannabis sativa (Hemp) for Their Use in Variety and Dioecy Assessments. Plants 2021, 10, 2174. [Google Scholar] [CrossRef]
- Kovalchuk, I.; Pellino, M.; Rigault, P.; van Velzen, R.; Ebersbach, J.; Ashnest, J.R.; Mau, M.; Schranz, M.E.; Alcorn, J.; Laprairie, R.B.; et al. The Genomics of Cannabis and Its Close Relatives. Annu. Rev. Plant Biol. 2020, 71, 713–739. [Google Scholar] [CrossRef]
- Mehboob-ur-Rahman; Zafar, Y.; Paterson, A.H. Gossypium DNA Markers: Types, Numbers, and Uses. In Genetics and Genomics of Cotton; Paterson, A.H., Ed.; Springer US: New York, NY, USA, 2009; pp. 101–139. ISBN 978-0-387-70810-2. [Google Scholar]
- Vieira, M.L.C.; Santini, L.; Diniz, A.L.; de Freitas Munhoz, C. Microsatellite Markers: What They Mean and Why They Are so Useful. Genet. Mol. Biol. 2016, 39, 312–328. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, M.; Tao, A.; Xu, J.; Lin, L.; Fang, P.; Qi, J. Genetic Structure and Relationship Analysis of an Association Population in Jute (Corchorus Spp.) Evaluated by SSR Markers. PLoS ONE 2015, 10, e0128195. [Google Scholar] [CrossRef]
- Gonzaga, Z.J.; Aslam, K.; Septiningsih, E.M.; Collard, B.C. Evaluation of SSR and SNP Markers for Molecular Breeding in Rice. Plant Breed. Biotechnol. 2015, 139–152. [Google Scholar] [CrossRef]
- Faeti, V.; Mandolino, G.; Ranalli, P. Genetic Diversity of Cannabis sativa Germplasm Based on RAPD Markers. Plant Breed. 1996, 115, 367–370. [Google Scholar] [CrossRef]
- Kayis, S.A.; Hakki, E.E.; Pinarkara, E. Comparison of Effectiveness of ISSR and RAPD Markers in Genetic Characterization of Seized Marijuana (Cannabis sativa L.) in Turkey. Afr. J. Agric. Res. 2010, 5, 2925–2933. [Google Scholar]
- Cirovic, N.; Kecmanovic, M.; Keckarevic, D.; Keckarevic Markovic, M. Differentiation of Cannabis Subspecies by THCA Synthase Gene Analysis Using RFLP. J. Forensic Leg. Med. 2017, 51, 81–84. [Google Scholar] [CrossRef]
- Kojoma, M.; Iida, O.; Makino, Y.; Sekita, S.; Satake, M. DNA Fingerprinting of Cannabis sativa Using Inter-Simple Sequence Repeat (ISSR) Amplification. Planta Med. 2002, 68, 60–63. [Google Scholar] [CrossRef]
- Lata, H.; Chandra, S.; Techen, N.; Khan, I.A.; ElSohly, M.A. Assessment of the Genetic Stability of Micropropagated Plants of Cannabis sativa by ISSR Markers. Planta Med. 2010, 76, 97–100. [Google Scholar] [CrossRef]
- Alghanim, H.J.; Almirall, J.R. Development of Microsatellite Markers in Cannabis sativa for DNA Typing and Genetic Relatedness Analyses. Anal. Bioanal. Chem. 2003, 376, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, S.; Peakall, R. Isolation of Microsatellite Markers in Cannabis sativa L. (Marijuana). Mol. Ecol. Notes 2003, 3, 105–107. [Google Scholar] [CrossRef]
- Mendoza, M.A.; Mills, D.K.; Lata, H.; Chandra, S.; ElSohly, M.A.; Almirall, J.R. Genetic Individualization of Cannabis sativa by a Short Tandem Repeat Multiplex System. Anal. Bioanal. Chem. 2009, 393, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Dufresnes, C.; Jan, C.; Bienert, F.; Goudet, J.; Fumagalli, L. Broad-Scale Genetic Diversity of Cannabis for Forensic Applications. PLoS ONE 2017, 12, e0170522. [Google Scholar] [CrossRef] [PubMed]
- Houston, R.; Birck, M.; Hughes-Stamm, S.; Gangitano, D. Evaluation of a 13-Loci STR Multiplex System for Cannabis sativa Genetic Identification. Int. J. Leg. Med. 2016, 130, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Valverde, L.; Lischka, C.; Scheiper, S.; Nedele, J.; Challis, R.; de Pancorbo, M.M.; Pfeiffer, H.; Köhnemann, S. Characterization of 15 STR Cannabis Loci: Nomenclature Proposal and SNPSTR Haplotypes. Forensic Sci. Int. Genet. 2014, 9, 61–65. [Google Scholar] [CrossRef]
- Köhnemann, S.; Nedele, J.; Schwotzer, D.; Morzfeld, J.; Pfeiffer, H. The Validation of a 15 STR Multiplex PCR for Cannabis Species. Int. J. Leg. Med. 2012, 126, 601–606. [Google Scholar] [CrossRef]
- Howard, C.; Gilmore, S.; Robertson, J.; Peakall, R. A Cannabis sativa STR Genotype Database for Australian Seizures: Forensic Applications and Limitations*. J. Forensic Sci. 2009, 54, 556–563. [Google Scholar] [CrossRef]
- Coyle, H.; Shutler, G.; Abrams, S.; Hanniman, J.; Neylon, S.; Ladd, C.; Palmbach, T.; Lee, H. A Simple DNA Extraction Method for Marijuana Samples Used in Amplified Fragment Length Polymorphism (AFLP) Analysis. J. Forensic Sci. 2003, 48, 343–347. [Google Scholar] [CrossRef]
- Howard, C.; Gilmore, S.; Robertson, J.; Peakall, R. Developmental Validation of a Cannabis sativa STR Multiplex System for Forensic Analysis. J. Forensic Sci. 2008, 53, 1061–1067. [Google Scholar] [CrossRef]
- Gilmore, S.; Peakall, R.; Robertson, J. Short Tandem Repeat (STR) DNA Markers Are Hypervariable and Informative in Cannabis sativa: Implications for Forensic Investigations. Forensic Sci. Int. 2003, 131, 65–74. [Google Scholar] [CrossRef]
- Hsieh, H.-M.; Hou, R.-J.; Tsai, L.-C.; Wei, C.-S.; Liu, S.-W.; Huang, L.-H.; Kuo, Y.-C.; Linacre, A.; Lee, J.C.-I. A Highly Polymorphic STR Locus in Cannabis sativa. Forensic Sci. Int. 2003, 131, 53–58. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Mandolino, G.; Ranalli, P. Advances in Biotechnological Approaches for Hemp Breeding. Adv. Hemp Res. 1999, 185. [Google Scholar]
- Ślusarkiewicz-Jarzina, A.S.; Ponitka, A.; Kaczmarek, Y. Influence of Cultivar, Explant Source and Plant Growth Regulator on Callus Induction and Plant Regeneration of Cannabis sativa L. Acta Biol. Cracov. Ser. Bot. 2005, 47, 145–151. [Google Scholar]
- Thacker, X.; Thomas, K.; Fuller, M.; Smith, S.; DuBois, J. Determination of Optimal Hormone and Mineral Salts Levels in Tissue Culture Media for Callus Induction and Growth of Industrial Hemp (Cannabis sativa L.). Agric. Sci. 2018, 9, 1250–1268. [Google Scholar] [CrossRef][Green Version]
- Feeney, M.; Punja, Z.K. Tissue Culture and Agrobacterium-Mediated Transformation of Hemp (Cannabis sativa L.). In Vitro Cell. Dev. Biol. Plant 2003, 39, 578–585. [Google Scholar] [CrossRef]
- Lata, H.; Chandra, S.; Khan, I.A.; ElSohly, M.A. High Frequency Plant Regeneration from Leaf Derived Callus of High Δ9-Tetrahydrocannabinol Yielding Cannabis sativa L. Planta Med. 2010, 76, 1629–1633. [Google Scholar] [CrossRef]
- Monthony, A.S.; Kyne, S.T.; Grainger, C.M.; Jones, A.M.P. Recalcitrance of Cannabis sativa to de Novo Regeneration; a Multi-Genotype Replication Study. bioRxiv 2020, 2020.06.23.167478. [Google Scholar] [CrossRef]
- Wielgus, K.; Luwanska, A.; Lassocinski, W.; Kaczmarek, Z. Estimation of Cannabis sativa L. Tissue Culture Conditions Essential for Callus Induction and Plant Regeneration. J. Nat. Fibers 2008, 5, 199–207. [Google Scholar] [CrossRef]
- Fisse, J.; Braut, F.; Cosson, L.; Paris, M. Étude in Vitro Des Capacités Organogénétiques de Tissus de Cannabis sativa L.; Effet de Différentes Substances de Croissance. Pl. Méd. Phytoth. 1981, 15, 217–223. [Google Scholar]
- Lata, H.; Chandra, S.; Techen, N.; Khan, I.A.; ElSohly, M.A. In Vitro Mass Propagation of Cannabis sativa L.: A Protocol Refinement Using Novel Aromatic Cytokinin Meta-Topolin and the Assessment of Eco-Physiological, Biochemical and Genetic Fidelity of Micropropagated Plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 18–26. [Google Scholar] [CrossRef]
- Ferreira, C.M.; Handro, W. Production, Maintenance and Plant Regeneration from Cell Suspension Cultures of Stevia Rebaudiana (Bert.) Bertoni. Plant Cell Rep. 1988, 7, 123–126. [Google Scholar] [CrossRef]
- Aminah, H.; Dick, J. McP.; Leakey, R.R.B.; Grace, J.; Smith, R.I. Effect of Indole Butyric Acid (IBA) on Stem Cuttings of Shorea Leprosula. For. Ecol. Manag. 1995, 72, 199–206. [Google Scholar] [CrossRef]
- Nadeem, M.; Palni, L.M.S.; Purohit, A.N.; Pandey, H.; Nandi, S.K. Propagation and Conservation of Podophyllum Hexandrum Royle: An Important Medicinal Herb. Biol. Conserv. 2000, 92, 121–129. [Google Scholar] [CrossRef]
- Zygomala, A.M.; Ioannidis, C.; Koropouli, X. IN VITRO PROPAGATION OF CISTUS CRETICUS L. Acta Hortic. 2003, 391–396. [Google Scholar] [CrossRef]
- Lata, H.; Chandra, S.; Khan, I.; ElSohly, M.A. Thidiazuron-Induced High-Frequency Direct Shoot Organogenesis of Cannabis sativa L. In Vitro Cell. Dev. Biol. Plant 2009, 45, 12–19. [Google Scholar] [CrossRef]
- Thomas, T.D.; Philip, B. Thidiazuron-Induced High-Frequency Shoot Organogenesis from Leaf-Derived Callus of Ia Medicinal Climber, Tylophora Indica (Burm. F.) Merrill. In Vitro Cell. Dev. Biol. Plant 2005, 41, 124–128. [Google Scholar] [CrossRef]
- Patel, R.M.; Shah, R.R. Regeneration of Stevia Plant through Callus Culture. Indian J. Pharm. Sci. 2009, 71, 46–50. [Google Scholar] [CrossRef]
- Wright, S. Evolution and the Genetics of Populations, Volume 4: Variability within and among Natural Populations. University of Chicago Press: Chicago, IL, USA, 1978. [Google Scholar]
- Nei, M. Estimation of Average Heterozygosity and Genetic Distance from a Small Number of Individuals. Genetics 1978, 89, 583–590. [Google Scholar] [CrossRef]
- Nei, M. Genetic Distance between Populations. Am. Nat. 1972, 106, 283–292. [Google Scholar] [CrossRef]
- Wright, S. The Interpretation of Population Structure by F-Statistics with Special Regard to Systems of Mating. Evolution 1965, 19, 395–420. [Google Scholar] [CrossRef]
- Bairu, M.W.; Aremu, A.O.; Van Staden, J. Somaclonal Variation in Plants: Causes and Detection Methods. Plant Growth Regul. 2011, 63, 147–173. [Google Scholar] [CrossRef]
- Larkin, P.J.; Scowcroft, W.R. Somaclonal Variation—A Novel Source of Variability from Cell Cultures for Plant Improvement. Theor. Appl. Genet. 1981, 60, 197–214. [Google Scholar] [CrossRef]
- Benson, E. Plant Conservation Biotechnology; CRC Press: Boca Raton, FL, USA, 1999; ISBN 1-4822-7303-9. [Google Scholar]
- Panis, B.; Nagel, M.; Van den Houwe, I. Challenges and Prospects for the Conservation of Crop Genetic Resources in Field Genebanks, in in Vitro Collections and/or in Liquid Nitrogen. Plants 2020, 9, 1634. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Sheng Gerald, L.T.; Teixeira da Silva, J.A. Micropropagation in the Twenty-First Century. In Plant Cell Culture Protocols; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer New York: New York, NY, USA, 2018; pp. 17–46. ISBN 978-1-4939-8594-4. [Google Scholar]
- Hazarika, B.N. Acclimatization of Tissue-Cultured Plants. Curr. Sci. 2003, 85, 1704–1712. [Google Scholar]
- Dobránszki, J.; Teixeira da Silva, J.A. Micropropagation of Apple—A Review. Biotechnol. Adv. 2010, 28, 462–488. [Google Scholar] [CrossRef]
- Sahijram, L.; Soneji, J.R.; Bollamma, K.T. Analyzing Somaclonal Variation in Micropropagated Bananas (Musa Spp.). Vitr. Cell. Dev. Biol. Plant 2003, 39, 551–556. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Gulyás, A.; Magyar-Tábori, K.; Wang, M.-R.; Wang, Q.-C.; Dobránszki, J. In Vitro Tissue Culture of Apple and Other Malus Species: Recent Advances and Applications. Planta 2019, 249, 975–1006. [Google Scholar] [CrossRef]
- Sharma, U.; Rai, M.K.; Shekhawat, N.; Kataria, V. Genetic Homogeneity Revealed in Micropropagated Bauhinia Racemosa Lam. Using Gene Targeted Markers CBDP and SCoT. Physiol. Mol. Biol. Plants 2019, 25, 581–588. [Google Scholar] [CrossRef]
- Palombi, M.; Damiano, C. Comparison between RAPD and SSR Molecular Markers in Detecting Genetic Variation in Kiwifruit (Actinidia deliciosa A. Chev). Plant Cell Rep. 2002, 20, 1061–1066. [Google Scholar] [CrossRef]
- Van Bakel, H.; Stout, J.M.; Cote, A.G.; Tallon, C.M.; Sharpe, A.G.; Hughes, T.R.; Page, J.E. The Draft Genome and Transcriptome of Cannabis sativa. Genome Biol. 2011, 12, R102. [Google Scholar] [CrossRef]
- Braich, S.; Baillie, R.C.; Spangenberg, G.C.; Cogan, N.O. A New and Improved Genome Sequence of Cannabis sativa. BioRxiv 2020. [Google Scholar] [CrossRef]
- Hurgobin, B.; Tamiru-Oli, M.; Welling, M.T.; Doblin, M.S.; Bacic, A.; Whelan, J.; Lewsey, M.G. Recent Advances in Cannabis sativa Genomics Research. New Phytol. 2021, 230, 73–89. [Google Scholar] [CrossRef]
- Nowakowska, M.; Pavlović, Ž.; Nowicki, M.; Boggess, S.L.; Trigiano, R.N. In Vitro Propagation of an Endangered Helianthus Verticillatus by Axillary Bud Proliferation. Plants 2020, 9, 712. [Google Scholar] [CrossRef] [PubMed]
- Kakimzhanova, A.; Dyussembekova, D.; Nurtaza, A.; Yessimseitova, A.; Shevtsov, A.; Lutsay, V.; Ramankulov, Y.; Kabieva, S. An Efficient Micropropagation System for the Vulnerable Wild Apple Species, Malus Sieversii, and Confirmation of Its Genetic Homogeneity. Erwerbs-Obstbau 2022, 1–12. [Google Scholar] [CrossRef]
- Nurtaza, A.; Magzumova, G.; Yessimseitova, A.; Karimova, V.; Shevtsov, A.; Silayev, D.; Lutsay, V.; Ramankulov, Y.; Kakimzhanova, A. Micropropagation of the Endangered Species Malus Niedzwetzkyana for Conservation Biodiversity in Kazakhstan. In Vitro Cell. Dev. Biol. Plant 2021, 57, 965–976. [Google Scholar] [CrossRef]
- Asadi-Aghbolaghi, M.; Dedicova, B.; Ranade, S.S.; Le, K.-C.; Sharifzadeh, F.; Omidi, M.; Egertsdotter, U. Protocol Development for Somatic Embryogenesis, SSR Markers and Genetic Modification of Stipagrostis pennata (Trin.) De Winter. Plant Methods 2021, 17, 70. [Google Scholar] [CrossRef] [PubMed]
- Bandupriya, H.; Iroshini, W.; Perera, S.; Vidhanaarachchi, V.; Fernando, S.; Santha, E.; Gunathilake, T. Genetic Fidelity Testing Using SSR Marker Assay Confirms Trueness to Type of Micropropagated Coconut (Cocos nucifera L.) Plantlets Derived from Unfertilized Ovaries. Open Plant Sci. J. 2017, 10. [Google Scholar] [CrossRef][Green Version]
- Wanmei, J.; Jing, D.; Yuanhua, W. Genetic Fidelity of Regeneration Adventitious Shoots in Grape through Organogenesis. Mol. Plant Breed. 2009, 7, 375–379. [Google Scholar]
- Pandey, R.; Singh, S.; Rastogi, J.; Sharma, M.; Singh, R. Early Assessment of Genetic Fidelity in Sugarcane (’Saccharum Officinarum’) Plantlets Regenerated through Direct Organogenesis with RAPD and SSR Markers. Aust. J. Crop Sci. 2012, 6, 618–624. [Google Scholar]
- Rai, M.K.; Phulwaria, M.; Gupta, A.K.; Shekhawat, N.; Jaiswal, U. Genetic Homogeneity of Guava Plants Derived from Somatic Embryogenesis Using SSR and ISSR Markers. Plant Cell Tissue Organ Cult. (PCTOC) 2012, 111, 259–264. [Google Scholar] [CrossRef]
- Wanmei, J.; Wang, Y.; Wang, H. Adventitious Shoot Regeneration from Leaves of Apple Rootstock ‘Pingyitiancha’ (Malus hupehensis Var. Pinyiensis) and Genetic Fidelity of Regenerated Plantlets Using SSR Markers. Can. J. Plant Sci. 2014, 94, 1345–1354. [Google Scholar] [CrossRef]
- Tiwari, J.K.; Chandel, P.; Gupta, S.; Gopal, J.; Singh, B.P.; Bhardwaj, V. Analysis of Genetic Stability of in Vitro Propagated Potato Microtubers Using DNA Markers. Physiol. Mol. Biol. Plants 2013, 19, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Castillo, N.R.F.; Bassil, N.V.; Wada, S.; Reed, B.M. Genetic Stability of Cryopreserved Shoot Tips of Rubus Germplasm. In Vitro Cell. Dev. Biol. Plant 2010, 46, 246–256. [Google Scholar] [CrossRef]
- Chandra, S.; Lata, H.; Techen, N.; Mehmadic, Z.; Khan, I.; ElSohly, M. Analysis of Genetic Diversity Using SSR Markers and Cannabinoid Contents in Different Varieties of Cannabis sativa L. Planta Med. 2011, 77, P_5. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a Genetic Linkage Map in Man Using Restriction Fragment Length Polymorphisms. Am. J. Hum. Genet. 1980, 32, 314. [Google Scholar] [PubMed]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising How the Computer Program Cervus Accommodates Genotyping Error Increases Success in Paternity Assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef] [PubMed]




| STR Locus 1 | H-CBD | H-CBG | Overall | |||
|---|---|---|---|---|---|---|
| Number of Alleles | Range (bp 2) | Number of Alleles | Range (bp 2) | Number of Alleles | Range (bp 2) | |
| ANUCS 202 | 4 | 150–178 | 2 | 150–156 | 4 | 150–178 |
| ANUCS 301 | 2 | 250–256 | 1 | 256–256 | 2 | 250–256 |
| B05 CANN1 | 4 | 244–256 | 2 | 247–256 | 4 | 244–256 |
| B01 CANN1 | 2 | 324–327 | 3 | 324–330 | 3 | 324–330 |
| ANUCS 303 | 1 | 147–147 | 2 | 144–147 | 2 | 144–147 |
| H09 CANN2 | 4 | 205–219 | 3 | 205–221 | 5 | 205–221 |
| ANUCS 305 | 3 | 144–156 | 3 | 144–156 | 3 | 144–156 |
| ANUCS 304 | 4 | 146–209 | 3 | 176–209 | 5 | 146–209 |
| ANUCS 201 | 3 | 182–204 | 5 | 156–186 | 6 | 156–204 |
| ANUCS 302 | 4 | 145–160 | 6 | 136–175 | 7 | 136–175 |
| ANUCS 501 | 1 | 90–90 | 1 | 90–90 | 1 | 90–90 |
| C11 CANN1 | 3 | 152–167 | 3 | 152–167 | 3 | 152–167 |
| Variety | n | Na | Ne | I | Ho | He | F | Percentage of Polymorphic Loci | Nei D | Nei I | Fst | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H-CBD | Mean | 8 | 2.92 | 2.44 | 0.87 | 0.48 | 0.50 | 0.05 | 83.33% | 0.145 | 0.865 | 0.075 |
| SE | 0.34 | 0.27 | 0.14 | 0.09 | 0.08 | 0.09 | ||||||
| H-CBG | Mean | 8 | 2.83 | 2.17 | 0.77 | 0.42 | 0.45 | 0.07 | 83.33% | |||
| SE | 0.42 | 0.28 | 0.13 | 0.11 | 0.07 | 0.18 | ||||||
| Overal | Mean | 8 | 2.88 | 2.30 | 0.82 | 0.45 | 0.48 | 0.06 | 83.33% | |||
| SE | 0.27 | 0.19 | 0.09 | 0.07 | 0.05 | 0.10 | 0.00% |
| Population | H-CBD | H-CBG | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Locus | Ho 1 | He 2 | PIC 3 | Ho 1 | He 2 | PIC | Ho 1 | He 2 | PIC |
| ANUCS 202 | 0.500 | 0.664 | 0.616 | 0.000 | 0.500 | 0.375 | 0.250 | 0.582 | 0.551 |
| ANUCS 301 | 0.250 | 0.469 | 0.359 | 0.000 | 0.000 | 0.000 | 0.125 | 0.234 | 0.258 |
| B05 CANN1 | 1.000 | 0.742 | 0.694 | 1.000 | 0.500 | 0.375 | 1.000 | 0.621 | 0.608 |
| B01 CANN1 | 0.500 | 0.500 | 0.375 | 0.875 | 0.586 | 0.520 | 0.688 | 0.543 | 0.482 |
| ANUCS 303 | 0.000 | 0.000 | 0.000 | 0.250 | 0.219 | 0.195 | 0.125 | 0.109 | 0.110 |
| H09 CANN2 | 0.500 | 0.695 | 0.645 | 0.375 | 0.617 | 0.544 | 0.438 | 0.656 | 0.693 |
| ANUCS 305 | 0.625 | 0.648 | 0.575 | 0.750 | 0.594 | 0.511 | 0.688 | 0.621 | 0.571 |
| ANUCS 304 | 0.500 | 0.672 | 0.612 | 0.250 | 0.656 | 0.582 | 0.375 | 0.664 | 0.669 |
| ANUCS 201 | 0.375 | 0.320 | 0.294 | 0.375 | 0.422 | 0.404 | 0.375 | 0.371 | 0.361 |
| ANUCS 302 | 1.000 | 0.688 | 0.630 | 1.000 | 0.781 | 0.748 | 1.000 | 0.734 | 0.717 |
| ANUCS 501 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| C11 CANN1 | 0.500 | 0.625 | 0.555 | 0.125 | 0.508 | 0.428 | 0.313 | 0.566 | 0.539 |
| Locus | Repeat Motif | Primer Sequences 5′→3′ | Expected Allele Size Range (bp *) | |
|---|---|---|---|---|
| Forward | Reverse | |||
| ANUCS 201 | (GA) | GGTTCAATGGAGATTCTCGT | CCACTAAACCAAAAGTACTCTTC | 209–265 |
| H09-CANN2 | (GA) | CGTACAGTGATCGTAGTTGAG | ACACATACAGAGAGAGCCC | 104–113 |
| ANUCS 303 | (GTG) | TAATCAACAATGACAATGGC | GATTAAGGTCCTCGACGATA | 314–349 |
| ANUCS 305 | (TGG) | AAAGTTGGTCTGAGAAGCAAT | CCTAGGAACTTTCGACAACA | 140–200 |
| ANUCS 301 | (TTA) | ATATGGTTGAAATCCATTGC | TAACAAAGTTTCGTGAGGGT | 141–162 |
| ANUCS 304 | (TCT)xTCA(TCT)y | TCTTCACTCACCTCCTCTCT | TCTTTAAGCGGGACTCGT | 235–245 |
| ANUCS 501 | (TTGTG) | AGCAATAATGGAGTGAGTGAAC | AGAGATCAAGAAATTGAGATTCC | 285–297 |
| ANUCS 302 | (ACA)x(ACA)y(ACA)z | AACATAAACACCAACAACTGC | ATGGTTGATGTTTTGATGGT | 163–189 |
| C11-CANN1 | (TGA)x(TGG)y | GTGGTGGTGATGATGATAATGG | TGAATTGGTTACGATGGCG | 120–242 |
| ANUCS 202 | (GA) | AGGACCAATTTTGAATATGC | AGAGAGGGAAGGGCTAACTA | 140–230 |
| B01-CANN1 | (GAA)xA(GAA)y | TGGAGTCAAATGAAAGGGAAC | CCATAGCATTATCCCACTCAAG | 140–230 |
| B05-CANN1 | (TTG) | TTGATGGTGGTGAAACGGC | CCCCAATCTCAATCTCAACCC | 120–267 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioannidis, K.; Tomprou, I.; Mitsis, V.; Koropouli, P. Genetic Evaluation of In Vitro Micropropagated and Regenerated Plants of Cannabis sativa L. Using SSR Molecular Markers. Plants 2022, 11, 2569. https://doi.org/10.3390/plants11192569
Ioannidis K, Tomprou I, Mitsis V, Koropouli P. Genetic Evaluation of In Vitro Micropropagated and Regenerated Plants of Cannabis sativa L. Using SSR Molecular Markers. Plants. 2022; 11(19):2569. https://doi.org/10.3390/plants11192569
Chicago/Turabian StyleIoannidis, Kostas, Ioanna Tomprou, Vangelis Mitsis, and Polyxeni Koropouli. 2022. "Genetic Evaluation of In Vitro Micropropagated and Regenerated Plants of Cannabis sativa L. Using SSR Molecular Markers" Plants 11, no. 19: 2569. https://doi.org/10.3390/plants11192569
APA StyleIoannidis, K., Tomprou, I., Mitsis, V., & Koropouli, P. (2022). Genetic Evaluation of In Vitro Micropropagated and Regenerated Plants of Cannabis sativa L. Using SSR Molecular Markers. Plants, 11(19), 2569. https://doi.org/10.3390/plants11192569








