Carbon Sequestration in Turfgrass–Soil Systems
Abstract
1. Introduction
2. Turfgrass Systems
2.1. Soil Organic Carbon Stocks
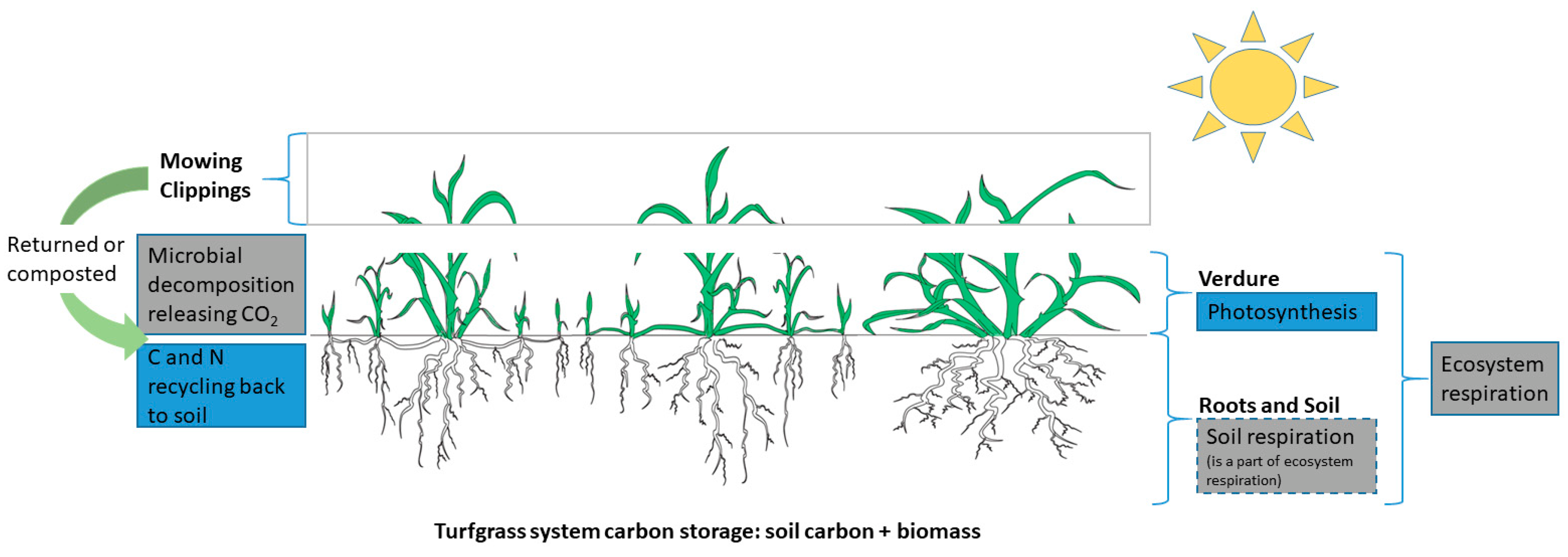
2.2. Biomass and Net Primary Productivity
2.3. Ecosystem Respiration
2.4. Hidden Carbon Cost and Net Greenhouse Gas Emissions
2.4.1. Lawns
2.4.2. Golf Courses
3. System Comparison
4. Age of Turfgrass
5. Grass Species Selection
6. Turf Use and Management Intensity
7. Management Practices
7.1. Irrigation
7.2. Nitrogen Fertilization
7.3. Mowing
7.4. Plant Growth Regulator
8. Methods for Carbon Research and Limitations
9. Best Management Practices for Carbon Sequestration
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- US Environmental Protection Agency. Inventory of U.S. Greenhouse Gas Emissions and Sinks Fast Facts: 1990–2018; EPA 430-F-20-002; US Environmental Protection Agency: Washington, DC, USA, 2020.
- Morgan, J.A.; Follett, R.F.; Allen, L.H.; Del Grosso, S.; Derner, J.D.; Dijkstra, F.; Franzluebbers, A.; Fry, R.; Paustian, K.; Schoeneberger, M.M. Carbon sequestration in agricultural lands of the United States. J. Soil Water Conserv. 2010, 65, 6A–13A. [Google Scholar] [CrossRef]
- Neubauer, S.C. Global warming potential is not an ecosystem property. Ecosystems 2021, 24, 2079–2089. [Google Scholar] [CrossRef]
- Chapin, F.S.; Woodwell, G.M.; Randerson, J.T.; Rastetter, E.B.; Lovett, G.M.; Baldocchi, D.D.; Clark, D.A.; Harmon, M.E.; Schimel, D.S.; Valentini, R.; et al. Reconciling carbon-cycle concepts, terminology, and methods. Ecosystems 2006, 9, 1041–1050. [Google Scholar] [CrossRef]
- Milesi, C.; Running, S.W.; Elvidge, C.D.; Dietz, J.B.; Tuttle, B.T.; Nemani, R.R. Mapping and Modeling the Biogeochemical Cycling of Turf Grasses in the United States. Environ. Manag. 2005, 36, 426–438. [Google Scholar] [CrossRef]
- Pouyat, R.V.; Yesilonis, I.D.; Nowak, D.J. Carbon storage by urban soils in the United States. J. Environ. Qual. 2006, 35, 1566–1575. [Google Scholar] [CrossRef]
- Pouyat, R.V.; Yesilonis, I.D.; Golubiewski, N.E. A comparison of soil organic carbon stocks between residential turf grass and native soil. Urban Ecosyst. 2009, 12, 45–62. [Google Scholar] [CrossRef]
- Zirkle, G.; Lal, R.; Augustin, B. Modeling carbon sequestration in home lawns. HortScience 2011, 46, 808–814. [Google Scholar] [CrossRef]
- Van Delden, L.; Larsen, E.; Rowlings, D.; Scheer, C.; Grace, P. Establishing turf grass increases soil greenhouse gas emissions in peri-urban environments. Urban Ecosyst. 2016, 19, 749–762. [Google Scholar] [CrossRef]
- Gu, C.; Crane, J.; Hornberger, G.; Carrico, A. The effects of household management practices on the global warming potential of urban lawns. J. Environ. Manag. 2015, 151, 233–242. [Google Scholar] [CrossRef]
- Braun, R.C.; Bremer, D.J. Nitrous oxide emissions in turfgrass systems: A review. Agron. J. 2018, 110, 2222–2232. [Google Scholar] [CrossRef]
- Jo, H.-K.; McPherson, G.E. Carbon storage and flux in urban residential greenspace. J. Environ. Manag. 1995, 45, 109–133. [Google Scholar] [CrossRef]
- Huang, B.; DaCosta, M.; Jiang, Y. Research advances in mechanisms of turfgrass tolerance to abiotic stresses: From physiology to molecular biology. Crit. Rev. Plant Sci. 2014, 33, 141–189. [Google Scholar] [CrossRef]
- Falk, J.H. Energetics of a suburban lawn ecosystem. Ecology 1976, 57, 141–150. [Google Scholar] [CrossRef]
- Falk, J.H. The primary productivity of lawns in a temperate environment. J. Appl. Ecol. 1980, 17, 689–695. [Google Scholar] [CrossRef]
- Huyler, A.; Chappelka, A.H.; Prior, S.A.; Somers, G.L. Drivers of soil carbon in residential ‘pure lawns’ in Auburn, Alabama. Urban Ecosyst. 2014, 17, 205–219. [Google Scholar] [CrossRef]
- Huyler, A.; Chappelka, A.H.; Prior, S.A.; Somers, G.L. Influence of aboveground tree biomass, home age, and yard maintenance on soil carbon levels in residential yards. Urban Ecosyst. 2014, 17, 787–805. [Google Scholar] [CrossRef]
- Selhorst, A.; Lal, R. Net carbon sequestration potential and emissions in home lawn turfgrasses of the United States. Environ. Manag. 2013, 51, 198–208. [Google Scholar] [CrossRef]
- Selhorst, A.L.; Lal, R. Carbon budgeting in golf course soils of Central Ohio. Urban Ecosyst. 2011, 14, 771–781. [Google Scholar] [CrossRef]
- Raciti, S.M.; Groffman, P.M.; Jenkins, J.C.; Pouyat, R.V.; Fahey, T.J.; Pickett, S.T.A.; Cadenasso, M.L. Accumulation of carbon and nitrogen in residential soils with different land-use histories. Ecosystems 2011, 14, 287–297. [Google Scholar] [CrossRef]
- Qian, Y.; Follett, R.F. Assessing soil carbon sequestration in turfgrass systems using long-term soil testing data. Agron. J. 2002, 94, 930–935. [Google Scholar] [CrossRef]
- Riches, D.; Porter, I.; Dingle, G.; Gendall, A.; Grover, S. Soil greenhouse gas emissions from Australian sports fields. Sci. Total Environ. 2020, 707, 134420. [Google Scholar] [CrossRef]
- Townsend-Small, A.; Czimczik, C.I. Correction to “Carbon sequestration and greenhouse gas emissions in urban turf”. Geophys. Res. Lett. 2010, 37, L06707. [Google Scholar] [CrossRef]
- Contosta, A.R.; Lerman, S.B.; Xiao, J.; Varner, R.K. Biogeochemical and socioeconomic drivers of above- and below-ground carbon stocks in urban residential yards of a small city. Landsc. Urban Plan. 2020, 196, 103724. [Google Scholar] [CrossRef]
- Smith, R.M.; Williamson, J.C.; Pataki, D.E.; Ehleringer, J.; Dennison, P. Soil carbon and nitrogen accumulation in residential lawns of the Salt Lake Valley, Utah. Oecologia 2018, 187, 1107–1118. [Google Scholar] [CrossRef]
- Burghardt, W.; Schneider, T. Bulk density and content, density and stock of carbon, nitrogen and heavy metals in vegetable patches and lawns of allotments gardens in the northwestern Ruhr area, Germany. J. Soils Sediments 2018, 18, 407–417. [Google Scholar] [CrossRef]
- Campbell, C.D.; Seiler, J.R.; Wiseman, P.E.; Strahm, B.D.; Munsell, J.F. Soil carbon dynamics in residential lawns converted from Appalachian mixed oak stands. Forests 2014, 5, 425–438. [Google Scholar] [CrossRef]
- Velasco, E.; Segovia, E.; Choong, A.M.F.; Lim, B.K.Y.; Vargas, R. Carbon dioxide dynamics in a residential lawn of a tropical city. J. Environ. Manag. 2021, 280, 111752. [Google Scholar] [CrossRef]
- Weissert, L.F.; Salmond, J.A.; Schwendenmann, L. Variability of soil organic carbon stocks and soil CO2 efflux across urban land use and soil cover types. Geoderma 2016, 271, 80–90. [Google Scholar] [CrossRef]
- Livesley, S.J.; Ossola, A.; Threlfall, C.G.; Hahs, A.K.; Williams, N.S.G. Soil carbon and carbon/nitrogen ratio change under tree canopy, tall grass, and turf grass areas of urban green space. J. Environ. Qual. 2016, 45, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Shi, Z.; Chu, L.M. Carbon emission and sequestration of urban turfgrass systems in Hong Kong. Sci. Total Environ. 2014, 473–474, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Mestdagh, I.; Sleutel, S.; Lootens, P.; Van Cleemput, O.; Carlier, L. Soil organic carbon stocks in verges and urban areas of Flanders, Belgium. Grass Forage Sci. 2005, 60, 151–156. [Google Scholar] [CrossRef]
- Trammell, T.L.E.; Pataki, D.E.; Pouyat, R.V.; Groffman, P.M.; Rosier, C.; Bettez, N.; Cavender-Bares, J.; Grove, M.J.; Hall, S.J.; Heffernan, J.; et al. Urban soil carbon and nitrogen converge at a continental scale. Ecol. Monogr. 2020, 90, e01401. [Google Scholar] [CrossRef]
- Golubiewski, N.E. Urbanization increases grassland carbon pools: Effects of landscaping in Colorado’s Front Range. Ecol. Appl. 2006, 16, 555–571. [Google Scholar] [CrossRef]
- Kaye, J.P.; McCulley, R.L.; Burke, I.C. Carbon fluxes, nitrogen cycling, and soil microbial communities in adjacent urban, native and agricultural ecosystems. Glob. Chang. Biol. 2005, 11, 575–587. [Google Scholar] [CrossRef]
- Pérez-Ruiz, E.R.; Vivoni, E.R.; Templeton, N.P. Urban land cover type determines the sensitivity of carbon dioxide fluxes to precipitation in Phoenix, Arizona. PLoS ONE 2020, 15, e0228537. [Google Scholar] [CrossRef]
- Trammell, T.L.E.; Pouyat, R.V.; Carreiro, M.M.; Yesilonis, I. Drivers of soil and tree carbon dynamics in urban residential lawns: A modeling approach. Ecol. Appl. 2017, 27, 991–1000. [Google Scholar] [CrossRef]
- Qian, Y.; Follett, R.F.; Kimble, J.M. Soil organic carbon input from urban turfgrasses. Soil Sci. Soc. Am. J. 2010, 74, 366–371. [Google Scholar] [CrossRef]
- Hamido, S.A.; Guertal, E.; Wood, C.W. Carbon sequestration under warm season turfgrasses in home lawns. J. Geosci. Environ. Prot. 2016, 4, 53–63. [Google Scholar] [CrossRef]
- Shchepeleva, A.S.; Vasenev, V.I.; Mazirov, I.M.; Vasenev, I.I.; Prokhorov, I.S.; Gosse, D.D. Changes of soil organic carbon stocks and CO2 emissions at the early stages of urban turf grasses’ development. Urban Ecosyst. 2017, 20, 309–321. [Google Scholar] [CrossRef]
- Amoatey, P.; Sulaiman, H. Quantifying carbon storage potential of urban plantations and landscapes in Muscat, Oman. Environ. Dev. Sustain. 2020, 22, 7969–7984. [Google Scholar] [CrossRef]
- Qian, Y.L.; Bandaranayake, W.; Parton, W.J.; Mecham, B.; Harivandi, M.A.; Mosier, A.R. Long-term effects of clipping and nitrogen management in turfgrass on soil organic carbon and nitrogen dynamics. J. Environ. Qual. 2003, 32, 1694–1700. [Google Scholar] [CrossRef]
- Lilly, P.J.; Jenkins, J.C.; Carroll, M.J. Management alters C allocation in turfgrass lawns. Landsc. Urban Plan. 2015, 134, 119–126. [Google Scholar] [CrossRef]
- Casler, M.D.; Duncan, R.R. Turfgrass Biology, Genetics, and Breeding; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Beard, J.B. Turfgrass: Science and Culture; Prentice-Hall: Englewood Cliffs, NJ, USA, 1973. [Google Scholar]
- Evers, M.; de Kroon, H.; Visser, E.; de Caluwe, H. Carbon accumulation of cool season sports turfgrass species in distinctive soil layers. Agron. J. 2020, 112, 3435–3449. [Google Scholar] [CrossRef]
- Raturi, S.; Islam, K.R.; Carroll, M.J.; Hill, R.L. Thatch and soil characteristics of cool- and warm-season turfgrasses. Commun. Soil Sci. Plant Anal. 2005, 35, 2161–2176. [Google Scholar] [CrossRef]
- Ledeboer, F.B.; Skogley, C.R. Investigations into the nature of thatch and methods for its decomposition. Agron. J. 1967, 59, 320–323. [Google Scholar] [CrossRef]
- Gautam, P.; Young, J.R.; Sapkota, M.; Longing, S.; Weindorf, D.C. Soil carbon sequestration in bermudagrass golf course fairways in Lubbock, Texas. Agron. J. 2020, 112, 148–157. [Google Scholar] [CrossRef]
- Acuña E., A.A.; Pastenes V., C.; Villalobos G., L. Carbon sequestration and photosynthesis in newly established turfgrass cover in central Chile. Agron. J. 2017, 109, 397–405. [Google Scholar] [CrossRef]
- López-Bellido, R.J.; Lal, R.; Danneberger, T.K.; Street, J.R. Plant growth regulator and nitrogen fertilizer effects on soil organic carbon sequestration in creeping bentgrass fairway turf. Plant Soil 2010, 332, 247–255. [Google Scholar] [CrossRef]
- Law, Q.D.; Patton, A.J. Biogeochemical cycling of carbon and nitrogen in cool-season turfgrass systems. Urban For. Urban Green. 2017, 26, 158–162. [Google Scholar] [CrossRef]
- Rogers, J.N.; Waddington, D.V. Impact absorption characteristics on turf and soil surfaces. Agron. J. 1992, 84, 203–209. [Google Scholar] [CrossRef]
- Aldahir, P.C.F.; McElroy, J.S. A review of sports turf research techniques related to playability and safety standards. Agron. J. 2014, 106, 1297–1308. [Google Scholar] [CrossRef]
- Ng, B.J.L.; Hutyra, L.R.; Nguyen, H.; Cobb, A.R.; Kai, F.M.; Harvey, C.; Gandois, L. Carbon fluxes from an urban tropical grassland. Environ. Pollut. 2015, 203, 227–234. [Google Scholar] [CrossRef]
- Song, Y.; Burgess, P.; Han, H.; Huang, B. Carbon balance of turfgrass systems in response to seasonal temperature changes under different mowing heights. J. Amer. Soc. Hort. Sci. 2015, 140, 317–322. [Google Scholar] [CrossRef]
- McPhillips, L.E.; Groffman, P.M.; Schneider, R.L.; Walter, M.T. Nutrient cycling in grassed roadside ditches and lawns in a suburban watershed. J. Environ. Qual. 2016, 45, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Hamido, S.A.; Wood, C.W.; Guertal, E.A. Carbon dioxide flux from bermudagrass turf as affected by nitrogen rate. Agron. J. 2016, 108, 1000–1006. [Google Scholar] [CrossRef]
- Bae, J.; Ryu, Y. Spatial and temporal variations in soil respiration among different land cover types under wet and dry years in an urban park. Landsc. Urban Plan. 2017, 167, 378–385. [Google Scholar] [CrossRef]
- Decina, S.M.; Hutyra, L.R.; Gately, C.K.; Getson, J.M.; Reinmann, A.B.; Short Gianotti, A.G.; Templer, P.H. Soil respiration contributes substantially to urban carbon fluxes in the greater Boston area. Environ. Pollut. 2016, 212, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Lerman, S.B.; Contosta, A.R. Lawn mowing frequency and its effects on biogenic and anthropogenic carbon dioxide emissions. Landsc. Urban Plan. 2019, 182, 114–123. [Google Scholar] [CrossRef]
- Kowalewski, A.; Schmid, C.; Wang, R.; Braithwaite, E. Advances in managing organic matter in turfgrass ecosystems. In Achieving Sustainable Turfgrass Management; Fidanza, M., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2022. [Google Scholar]
- Hurto, K.A.; Turgeon, A.J.; Spomer, L.A. Physical characteristics of thatch as a turfgrass growing medium. Agron. J. 1980, 72, 165–167. [Google Scholar] [CrossRef]
- Upadhyay, S.; Singh, R.; Verma, P.; Raghubanshi, A.S. Spatio-temporal variability in soil CO2 efflux and regulatory physicochemical parameters from the tropical urban natural and anthropogenic land use classes. J. Environ. Manag. 2021, 295, 113141. [Google Scholar] [CrossRef]
- Byrne, L.B.; Bruns, M.A.; Kim, K.C. Ecosystem properties of urban land covers at the aboveground–belowground interface. Ecosystems 2008, 11, 1065–1077. [Google Scholar] [CrossRef]
- Bowne, D.R.; Johnson, E.R. Comparison of soil carbon dioxide efflux between residential lawns and corn fields. Soil Sci. Soc. Am. J. 2013, 77, 856–859. [Google Scholar] [CrossRef]
- Huyler, A.; Chappelka, A.H.; Fan, Z.; Prior, S.A. A comparison of soil carbon dynamics in residential yards with and without trees. Urban Ecosyst. 2017, 20, 87–96. [Google Scholar] [CrossRef]
- Livesley, S.J.; Dougherty, B.J.; Smith, A.J.; Navaud, D.; Wylie, L.J.; Arndt, S.K. Soil-atmosphere exchange of carbon dioxide, methane and nitrous oxide in urban garden systems: Impact of irrigation, fertiliser and mulch. Urban Ecosyst. 2010, 13, 273–293. [Google Scholar] [CrossRef]
- Singh, S.; Yan, S.; Sorochan, J.; Stier, J.; Mayes, M.A.; Zhuang, J.; Jagadamma, S. Soil carbon accumulation and nutrient availability in managed and unmanaged ecosystems of east Tennessee. Soil Sci. Soc. Am. J. 2019, 83, 458–465. [Google Scholar] [CrossRef]
- Groffman, P.M.; Pouyat, R.V. Methane uptake in urban forests and lawns. Environ. Sci. Technol. 2009, 43, 5229–5235. [Google Scholar] [CrossRef]
- Kaye, J.P.; Burke, I.C.; Mosier, A.R.; Pablo Guerschman, J. Methane and nitrous oxide fluxes from urban soils to the atmosphere. Ecol. Appl. 2004, 14, 975–981. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, Y.; Bremer, D.J.; Kaye, J.P. Simulation of nitrous oxide emissions and estimation of global warming potential in turfgrass systems using the DAYCENT model. J. Environ. Qual. 2013, 42, 1100–1108. [Google Scholar] [CrossRef]
- Henry, J.M.; Gibeault, V.A.; Lazaneo, V.F. Practical Lawn Fertilization; Agriculture and Natural Resources Publication 8065; University of California: Berkeley, CA, USA, 2002. [Google Scholar]
- US Environmental Protection Agency. Golf Course Adjustments Factors for Modifying Estimated Drinking Water Concentrations and Estimated Environmental Concentrations Generated by Tier I (FIRST) and Tier II (PRZM/EXAMS) Models; US Environmental Protection Agency: Washington, DC, USA, 2005. [Google Scholar]
- Braun, R.C.; Bremer, D.J. Carbon sequestration in zoysiagrass turf under different irrigation and fertilization management regimes. Agrosyst. Geosci. Environ. 2019, 2, 180060. [Google Scholar] [CrossRef]
- Bartlett, M.D.; James, I.T. Corrigendum to “A model of greenhouse gas emissions from the management of turf on two golf courses” [Sci Total Environ 409 (2011) 1357–1367]. Sci. Total Environ. 2011, 409, 5136. [Google Scholar] [CrossRef]
- Dobbs, E.K.; Potter, D.A. Naturalized habitat on golf courses: Source or sink for natural enemies and conservation biological control? Urban Ecosyst. 2016, 19, 899–914. [Google Scholar] [CrossRef]
- Gelernter, W.D.; Stowell, L.J.; Johnson, M.E.; Brown, C.D. Documenting trends in land-use characteristics and environmental stewardship programs on US golf courses. Crop Forage Turfgrass Manag. 2017, 3, 1–12. [Google Scholar] [CrossRef]
- Poeplau, C.; Marstorp, H.; Thored, K.; Kätterer, T. Effect of grassland cutting frequency on soil carbon storage—A case study on public lawns in three Swedish cities. Soil 2016, 2, 175–184. [Google Scholar] [CrossRef]
- Bartlett, M.D.; James, I.T. Are golf courses a source or sink of atmospheric carbon dioxide? A modelling approach. Proc. Inst. Mech. Eng. Part P J. Sports Eng. Technol. 2011, 225, 75–83. [Google Scholar] [CrossRef]
- Bekken, M.A.H.; Soldat, D.J. Estimated energy use and greenhouse gas emissions associated with golf course turfgrass maintenance in the Northern USA. Int. Turfgrass Soc. Res. J. 2022, 14, 58–75. [Google Scholar] [CrossRef]
- Tidåker, P.; Wesström, T.; Kätterer, T. Energy use and greenhouse gas emissions from turf management of two Swedish golf courses. Urban For. Urban Green. 2017, 21, 80–87. [Google Scholar] [CrossRef]
- Turgeon, A.J.; Kaminski, J.E. Turfgrass Management; Turfpath: State College, PA, USA, 2019. [Google Scholar]
- Lal, R.; Augustin, B. (Eds.) Carbon Sequestration in Urban Ecosystems; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Beard, J.B.; Green, R.L. The role of turfgrasses in environmental protection and their benefits to humans. J. Environ. Qual. 1994, 23, 452–460. [Google Scholar] [CrossRef]
- Selhorst, A.; Lal, R. Effects of climate and soil properties on U.S. home lawn soil organic carbon concentration and pool. Environ. Manag. 2012, 50, 1177–1192. [Google Scholar] [CrossRef]
- Gordon, A.M.; Surgeoner, G.A.; Hall, J.C.; Ford-Robertson, J.B.; Vyn, T.J. Comments on “the role of turfgrasses in environmental protection and their benefits to humans,” by J.B. Beard and R.L. Green. J. Environ. Qual. 23:452–460. J. Environ. Qual. 1996, 25, 206–208. [Google Scholar] [CrossRef]
- Pahari, R.; Leclerc, M.Y.; Zhang, G.; Nahrawi, H.; Raymer, P. Carbon dynamics of a warm season turfgrass using the eddy-covariance technique. Agric. Ecosyst. Environ. 2018, 251, 11–25. [Google Scholar] [CrossRef]
- Dass, P.; Houlton, B.Z.; Wang, Y.; Warlind, D. Grasslands may be more reliable carbon sinks than forests in California. Environ. Res. Lett. 2018, 13, 074027. [Google Scholar] [CrossRef]
- Huh, K.Y.; Deurer, M.; Sivakumaran, S.; McAuliffe, K.; Bolan, N.S. Carbon sequestration in urban landscapes: The example of a turfgrass system in New Zealand. Soil Res. 2008, 46, 610–616. [Google Scholar] [CrossRef]
- Carley, D.S.; Goodman, D.; Sermons, S.; Shi, W.; Bowman, D.; Miller, G.; Rufty, T. Soil organic matter accumulation in creeping bentgrass greens: A chronosequence with implications for management and carbon sequestration. Agron. J. 2011, 103, 604–610. [Google Scholar] [CrossRef]
- Bandaranayake, W.; Qian, Y.L.; Parton, W.J.; Ojima, D.S.; Follett, R.F. Estimation of soil organic carbon changes in turfgrass systems using the CENTURY model. Agron. J. 2003, 95, 558–563. [Google Scholar] [CrossRef]
- Sapkota, M.; Young, J.; Coldren, C.; Slaughter, L.; Longing, S. Soil physiochemical properties and carbon sequestration of Urban landscapes in Lubbock, TX, USA. Urban For. Urban Green. 2020, 56, 126847. [Google Scholar] [CrossRef]
- Shi, W.; Bowman, D.; Rufty, T. Microbial control of soil carbon accumulation in turfgrass systems. In Carbon Sequestration in Urban Ecosystems; Lal, R., Augustin, B., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 215–231. [Google Scholar]
- Yao, H.; Shi, W. Soil organic matter stabilization in turfgrass ecosystems: Importance of microbial processing. Soil Biol. Biochem. 2010, 42, 642–648. [Google Scholar] [CrossRef]
- Shi, W.; Yao, H.; Bowman, D. Soil microbial biomass, activity and nitrogen transformations in a turfgrass chronosequence. Soil Biol. Biochem. 2006, 38, 311–319. [Google Scholar] [CrossRef]
- Shi, W.; Dell, E.; Bowman, D.; Iyyemperumal, K. Soil enzyme activities and organic matter composition in a turfgrass chronosequence. Plant Soil 2006, 288, 285–296. [Google Scholar] [CrossRef]
- Lemus, R.; Lal, R. Bioenergy crops and carbon sequestration. Crit. Rev. Plant Sci. 2005, 24, 1–21. [Google Scholar] [CrossRef]
- Rasse, D.P.; Rumpel, C.; Dignac, M.-F. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 2005, 269, 341–356. [Google Scholar] [CrossRef]
- Law, Q.D.; Trappe, J.M.; Jiang, Y.; Turco, R.F.; Patton, A.J. Turfgrass selection and grass clippings management influence soil carbon and nitrogen dynamics. Agron. J. 2017, 109, 1719–1725. [Google Scholar] [CrossRef]
- White, L.M. Carbohydrate reserves of grasses: A review. J. Range Manag. 1973, 26, 13–18. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, Y.; Mecham, B.; Parton, W.J. Development of best turfgrass management practices using the DAYCENT model. Agron. J. 2013, 105, 1151–1159. [Google Scholar] [CrossRef]
- Frank, K.W.; Guertal, E.A. Nitrogen research in turfgrass. In Turfgrass: Biology, Use, and Management; Stier, J.C., Horgan, B.P., Bonos, S.A., Eds.; American Society of Agronomy Soil Science Society of America Crop Science Society of America, Inc.: Madison, WI, USA, 2013; pp. 457–491. [Google Scholar]
- Brandani, G.; Baldi, A.; Caturegli, L.; Gaetani, M.; Grossi, N.; Magni, S.; Pardini, A.; Volterrani, M.; Orlandini, S.; Verdi, L. Carbon dioxide and methane emissions by urban turfgrasses under different nitrogen rates: A comparison between tall fescue (Festuca arundinacea Schreb.) and hybrid bermudagrass (Cynodon dactylon [L.] Pers. var. dactylon × Cynodon transvaalensis Burtt-Davy. Appl. Ecol. Environ. Res. 2021, 19, 1–12. [Google Scholar] [CrossRef]
- Kauer, K.; Kõlli, R.; Viiralt, R.; Köster, T.; Noormets, M.; Laidna, T.; Keres, I.; Parol, A.; Varul, T.; Selge, A.; et al. Effect of cut plant residue management and fertilization on the dry-matter yield of swards and on carbon content of soil. Commun. Soil Sci. Plant Anal. 2013, 44, 205–218. [Google Scholar] [CrossRef]
- Shi, W.; Muruganandam, S.; Bowman, D. Soil microbial biomass and nitrogen dynamics in a turfgrass chronosequence: A short-term response to turfgrass clipping addition. Soil Biol. Biochem. 2006, 38, 2032–2042. [Google Scholar] [CrossRef]
- Allaire, S.E.; Dufour-L’Arrivée, C.; Lafond, J.A.; Lalancette, R.; Brodeur, J. Carbon dioxide emissions by urban turfgrass areas. Can. J. Soil Sci. 2008, 88, 529–532. [Google Scholar] [CrossRef]
- Grossi, N.; Fontanelli, M.; Garramone, E.; Peruzzi, A.; Raffaelli, M.; Pirchio, M.; Martelloni, L.; Frasconi, C.; Caturegli, L.; Gaetani, M.; et al. Autonomous mower saves energy and improves quality of tall fescue lawn. HortTechnology 2016, 26, 825. [Google Scholar] [CrossRef]
- Sivaraman, D.; Lindner, A.S. A comparative life cycle analysis of gasoline-, battery-, and electricity-powered lawn mowers. Environ. Eng. Sci. 2004, 21, 768–785. [Google Scholar] [CrossRef]
- Pirchio, M.; Fontanelli, M.; Labanca, F.; Sportelli, M.; Frasconi, C.; Martelloni, L.; Raffaelli, M.; Peruzzi, A.; Gaetani, M.; Magni, S.; et al. Energetic aspects of turfgrass mowing: Comparison of different rotary mowing systems. Agriculture 2019, 9, 178. [Google Scholar] [CrossRef]
- Bakker, D.M.; Javed, H.; Ashfaq, Z. Implementation and modelling of turf grass management options to improve soil carbon sequestration in a semi-arid environment. Environ. Sustain. 2022, 5, 185–195. [Google Scholar] [CrossRef]
- Kopp, K.L.; Guillard, K. Clipping management and nitrogen fertilization of turfgrass. Crop Sci. 2002, 42, 1225–1231. [Google Scholar] [CrossRef]
- Cevenini, L.; Corradini, M.; Pasini, I.; Volterrani, M.; Zuffa, D.; Minelli, A. Estimated net ecosystem exchange (NEE) of turfgrass at different management intensities in a golf course in the province of Verona. J. Environ. Sci. Eng. B 2016, 5, 601–614. [Google Scholar] [CrossRef][Green Version]
- Zhou, X.; Wang, X.; Tong, L.; Zhang, H.; Lu, F.; Zheng, F.; Hou, P.; Song, W.; Ouyang, Z. Soil warming effect on net ecosystem exchange of carbon dioxide during the transition from winter carbon source to spring carbon sink in a temperate urban lawn. J. Environ. Sci. 2012, 24, 2104–2112. [Google Scholar] [CrossRef]
- Hiller, R.V.; McFadden, J.P.; Kljun, N. Interpreting CO2 fluxes over a suburban lawn: The influence of traffic emissions. Bound.-Layer Meteorol. 2011, 138, 215–230. [Google Scholar] [CrossRef]
- Parton, W.J.; Schimel, D.S.; Cole, C.V.; Ojima, D.S. Analysis of factors controlling soil organic matter levels in great plains grasslands. Soil Sci. Soc. Am. J. 1987, 51, 1173–1179. [Google Scholar] [CrossRef]
- Parton, W.J.; Rasmussen, P.E. Long-term effects of crop management in wheat-fallow: II. CENTURY model simulations. Soil Sci. Soc. Am. J. 1994, 58, 530–536. [Google Scholar] [CrossRef]
- Parton, W.J.; Hartman, M.; Ojima, D.; Schimel, D. DAYCENT and its land surface submodel: Description and testing. Glob. Planet. Change 1998, 19, 35–48. [Google Scholar] [CrossRef]
- Beesley, L. Carbon storage and fluxes in existing and newly created urban soils. J. Environ. Manag. 2012, 104, 158–165. [Google Scholar] [CrossRef]
- Azeem, M.; Hale, L.; Montgomery, J.; Crowley, D.; McGiffen, M.E., Jr. Biochar and compost effects on soil microbial communities and nitrogen induced respiration in turfgrass soils. PLoS ONE 2020, 15, e0242209. [Google Scholar] [CrossRef]

| Unit | To Covert Other Units to Mg C ha−1 yr−1, Multiply by |
|---|---|
| Mg CO2 ha−1 yr−1 | 0.2727 |
| kg CO2 ha−1 yr−1 | 0.0002727 |
| kg C ha−1 yr−1 | 0.001 |
| kg CO2 m−2 yr−1 | 2.727 |
| kg C m−2 yr−1 | 10 |
| g CO2 m−2 yr−1 | 0.002727 |
| g C m−2 yr−1 | 0.01 |
| Mg CO2 km−2 yr−1 | 0.002727 |
| Reference | Location | Comparison * |
|---|---|---|
| Carbon gain in the system | ||
| Acuña E. et al. [50] | Central Chile | SOC: turfgrass > bare soil |
| Bae and Ryu [59] | Seoul, South Korea | SOC: mixed forest > wetland > lawn > bare soil |
| Upadhyay et al. [64] | Varanasi, India | SOC: urban plantation ≈ lawn> agriculture ≈ grassland > bare soil |
| Bowne and Johnson [66] | Elizabethtown, PA, USA | SOC: lawn ≈ corn field |
| Burghardt and Schneider [26] | Ruhr, Germany | SOC: vegetable garden ≈ lawn > meadow |
| Byrne et al. [65] | Central PA, USA | SOC: lawn ≈ bark > unmanaged vegetation> gravel |
| Campbell et al. [27] | Virginia, USA | Soil carbon: forest ≈ lawn |
| Golubiewski [34] | Colorado, USA | SOC: turfgrass ≈ tree SOC: urban green space > native grassland > agricultural field |
| Huyler et al. [67] | Auburn, AL, USA | SOC (only at 0–15 cm): lawn with tree > lawn without tree |
| Livesley et al. [68] | Victoria, Australia | SOC: wood chip mulched bed ≈ lawn |
| Livesley et al. [30] | Melbourne, Australia | SOC: tree > fairway |
| Raciti et al. [20] | Baltimore, MD, USA | SOC: lawn > forest |
| Singh et al. [69] | Knoxville, TN, USA | SOC: unmanaged system > lawn >row crop |
| Pouyat et al. [7] | Baltimore, MD, USA | SOC: lawn ≈ urban forest > rural forest |
| Pouyat et al. [7] | Denver, CO, USA | SOC: lawn > native grassland |
| Weissert et al. [29] | Auckland, New Zealand | SOC: parkland > urban forest |
| Kaye et al. [35] | Fort Collins, CO, USA | SOC: lawn > native grassland > corn ANPP: corn > lawn > native grassland |
| Jo and McPherson [12] | Chicago, IL, USA | Biomass: tress & shrubs> turfgrass > herbaceous plants |
| Groffman and Pouyat [70] | Baltimore, MD, USA | Atmospheric CH4 uptake: rural forest > urban forest > lawn |
| Livesley et al. [68] | Victoria, Australia | Atmospheric CH4 uptake: wood chip mulched bed > lawn |
| Kaye et al. [71] | Fort Collins, CO, USA | Atmospheric CH4 uptake: native grassland > lawn |
| van Delden et al. [9] | Samford Valley, Australia | Atmospheric CH4 uptake: forest > turfgrass > fallow > pasture |
| Carbon loss in the system | ||
| Bae and Ryu [59] | Seoul, South Korea | Rs: mixed forest > wetland ≈ lawn > bare soil |
| Ng et al. [55] | Singapore | Rs: lawn > bare soil |
| Upadhyay et al. [64] | Varanasi, India | Rs: lawn > grassland ≈ urban plantation > agriculture > bare soil |
| Bowne and Johnson [66] | Elizabethtown, PA, USA | Rs: lawn > corn field |
| Byrne et al. [65] | Central PA, USA | Mean Rs: lawn ≈ bark > unmanaged vegetation ≈ gravel |
| Decina et al. [60] | Boston, MA, USA | Rs: urban landscape > lawn > urban forest |
| Livesley et al. [68] | Victoria, Australia | Rs: wood chip mulched bed ≈ lawn |
| Kaye et al. [35] | Fort Collins, CO, USA | Rs: lawn > corn ≈ native grassland |
| Weissert et al. [29] | Auckland, New Zealand | Rs: parkland ≈ urban forest |
| Reference | Turf Use | Location | Turf Age (Year) | Soil Depth (cm) | Regression Response | Number of Years to Reach Max SOC * | SOC Accumulation Rate (Mg C ha−1 yr−1) |
|---|---|---|---|---|---|---|---|
| Townsend-Small and Czimczik [23] | Lawn | Irvine, CA | 2–33 | 20 | Linear | 33 | 1.4 |
| Raciti et al. [20] | Lawn | Baltimore, MD | 4–44 | 100 | Linear | 44 | 0.82 |
| Smith et al. [25] | Lawn | Salt Lake City, UT | 7–100 | 40 | Linear | 100 | 0.30 |
| Sapkota et al. [93] | Lawn | Lubbock, TX | 0–63 | 10 | Quadratic | 53.6 | 0.21 |
| Huh et al. [90] | Green | Palmerston North, New Zealand | 5–40 | 25 | Linear | 40 | 0.69 |
| Carley et al. [91] | Green | North Carolina, USA | 0–25 | 7.6 | Hyperbolic | 25 | 0.59 |
| Qian and Follett [21] | Green | Colorado, USA | 1.5–45 | 11.4 | Quadratic | 45 | 1.0 |
| Qian and Follett [21] | Fairway | Colorado, USA | 4–45 | 11.4 | Quadratic with plateau | 31 | 0.9 |
| Gautam et al. [49] | Fairway | Lubbock, TX | 13–93 | 7.5 | Quadratic | 46.4 | 0.22 |
| Shi et al. [94] | Fairway | North Carolina, USA | 2–100 | 15 | Hyperbolic | 100 | 0.5–6 |
| Selhorst and Lal [19] | Fairway | Central Ohio, USA | 2–97 | 15 | Quadratic | 14 (0–2.5 cm) | 3.55 |
| 30 (2.5–5 cm) | |||||||
| 62 (5–10 cm) | |||||||
| 81 (10–15 cm) | |||||||
| Selhorst and Lal [19] | Rough | Central Ohio, USA | 2–97 | 15 | Quadratic | 12 (0–2.5 cm) | 2.64 |
| 24 (2.5–5 cm) | |||||||
| 68 (5–10 cm) | |||||||
| 91 (10–15 cm) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Mattox, C.M.; Phillips, C.L.; Kowalewski, A.R. Carbon Sequestration in Turfgrass–Soil Systems. Plants 2022, 11, 2478. https://doi.org/10.3390/plants11192478
Wang R, Mattox CM, Phillips CL, Kowalewski AR. Carbon Sequestration in Turfgrass–Soil Systems. Plants. 2022; 11(19):2478. https://doi.org/10.3390/plants11192478
Chicago/Turabian StyleWang, Ruying, Clint M. Mattox, Claire L. Phillips, and Alec R. Kowalewski. 2022. "Carbon Sequestration in Turfgrass–Soil Systems" Plants 11, no. 19: 2478. https://doi.org/10.3390/plants11192478
APA StyleWang, R., Mattox, C. M., Phillips, C. L., & Kowalewski, A. R. (2022). Carbon Sequestration in Turfgrass–Soil Systems. Plants, 11(19), 2478. https://doi.org/10.3390/plants11192478






