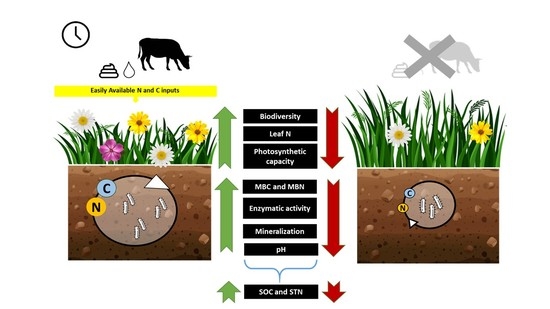
Consequences of Grazing Cessation for Soil Environment and Vegetation in a Subalpine Grassland Ecosystem
Abstract
:1. Introduction
2. Results
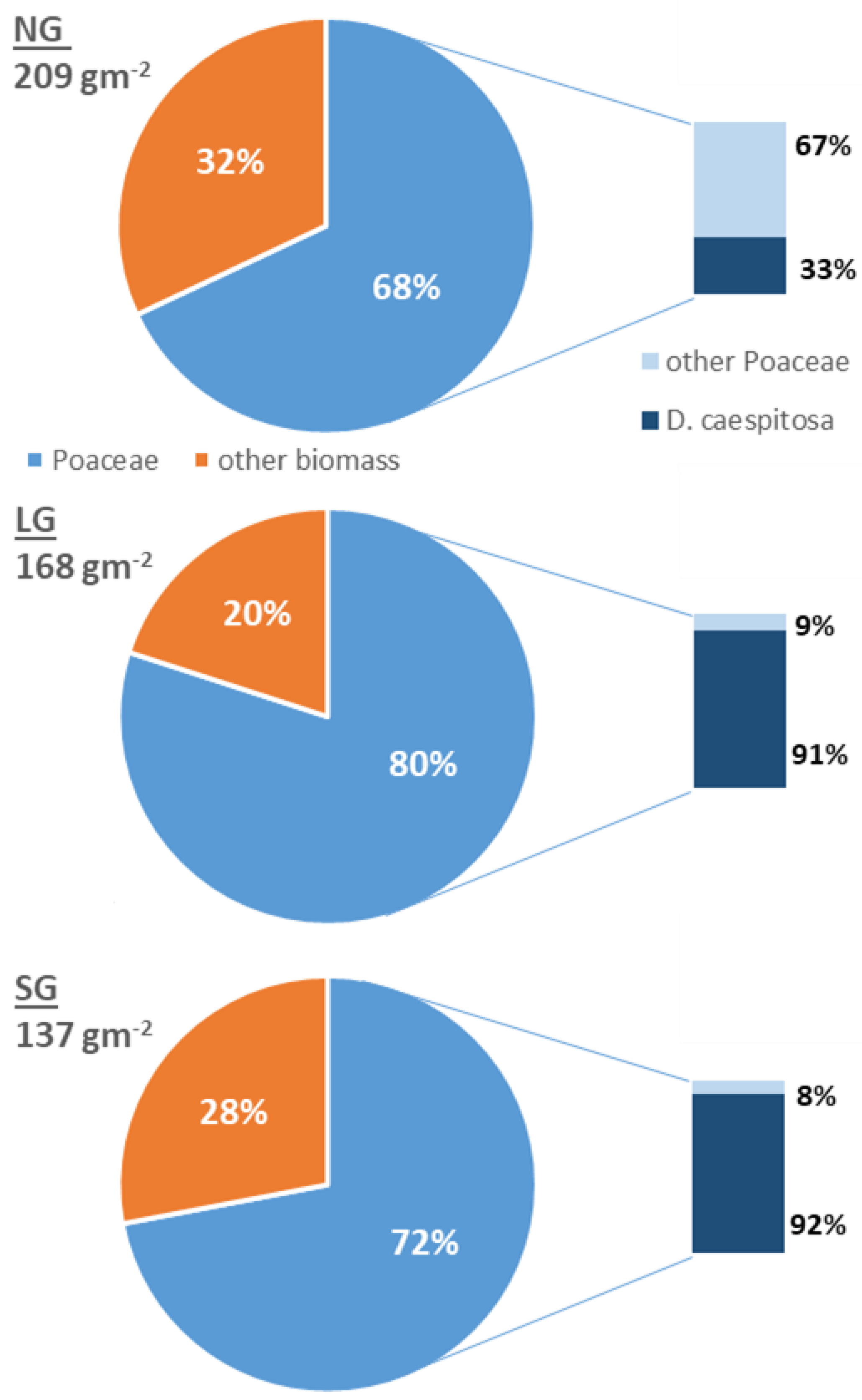
2.1. Vegetation Diversity
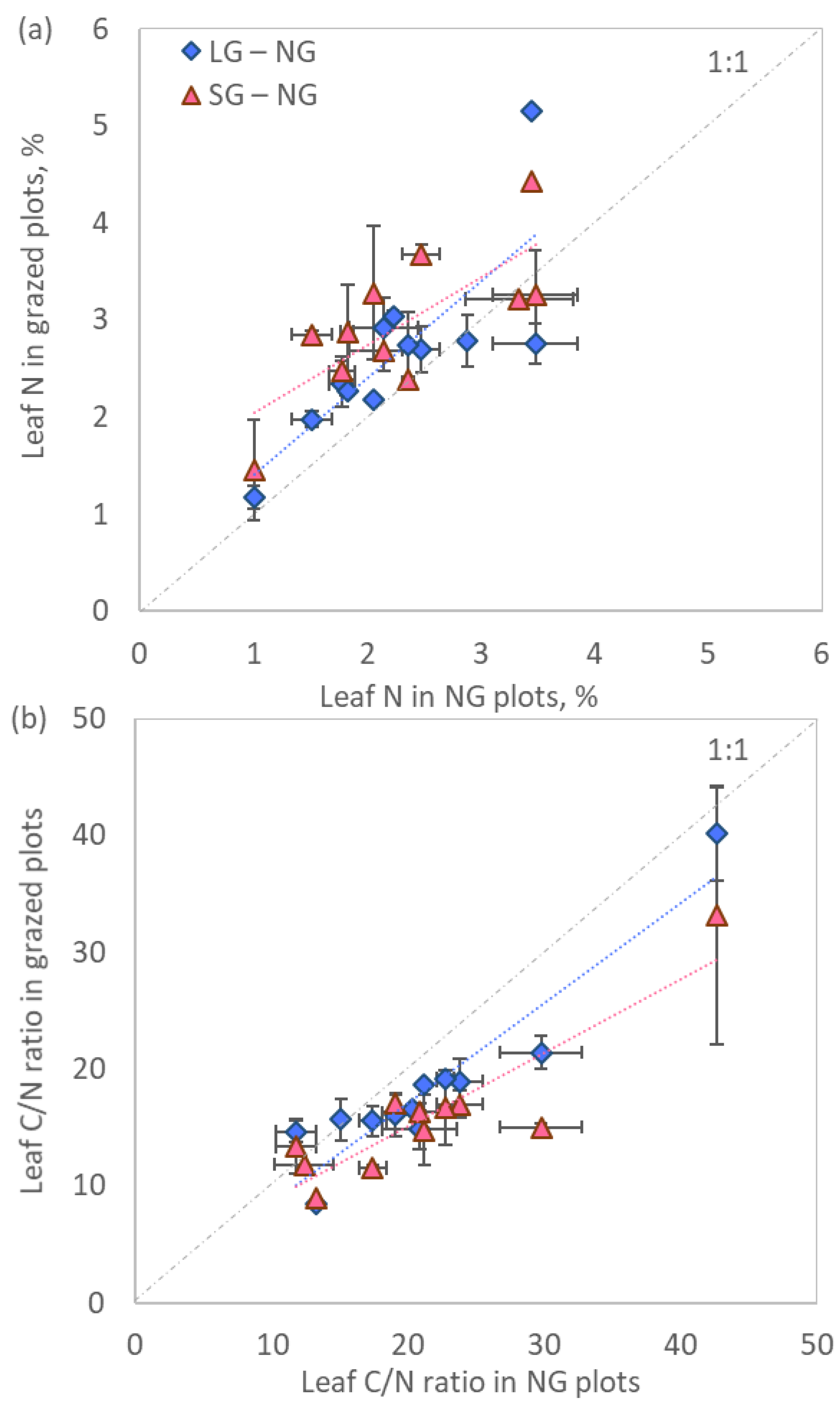
2.2. Vegetation Functioning
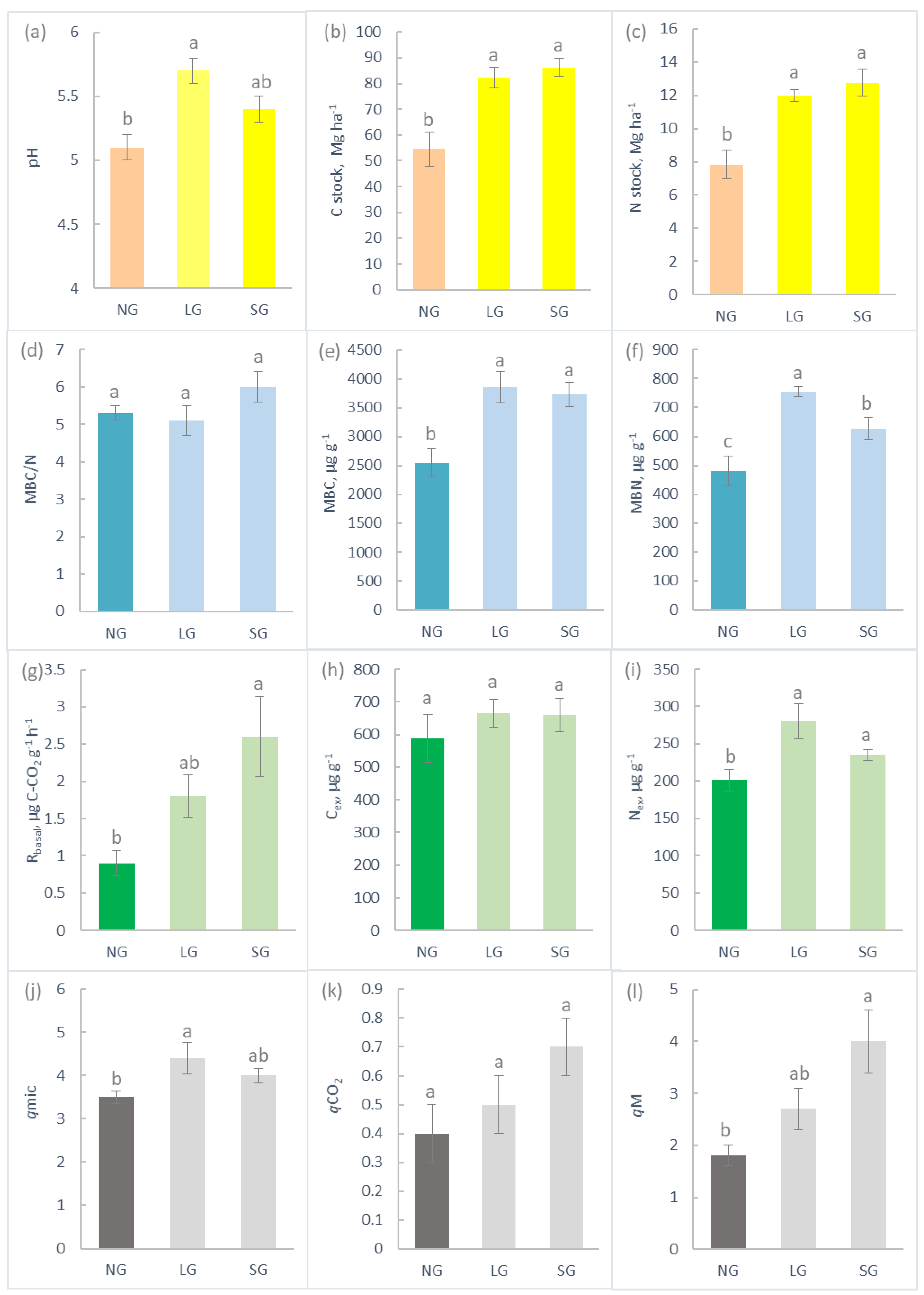
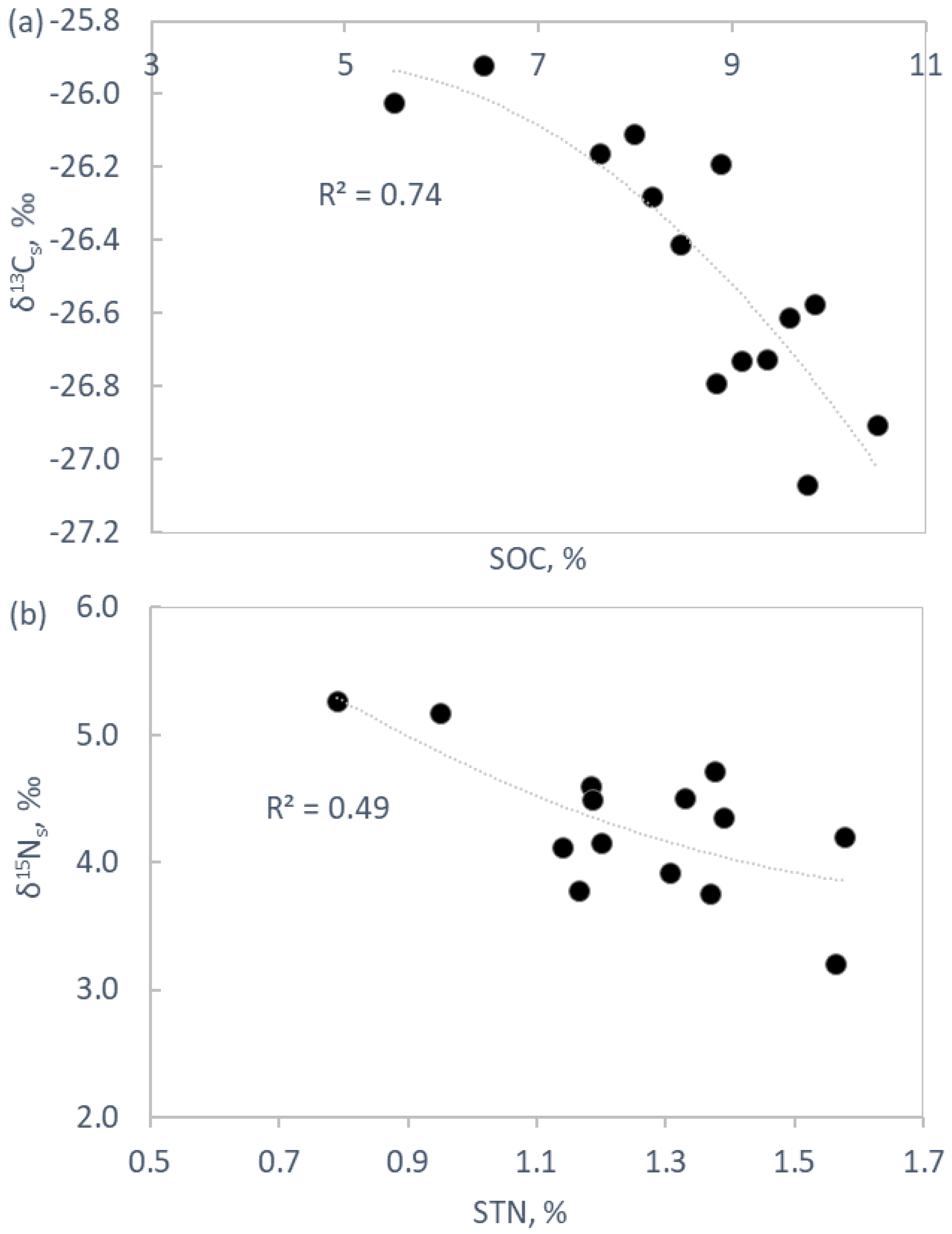
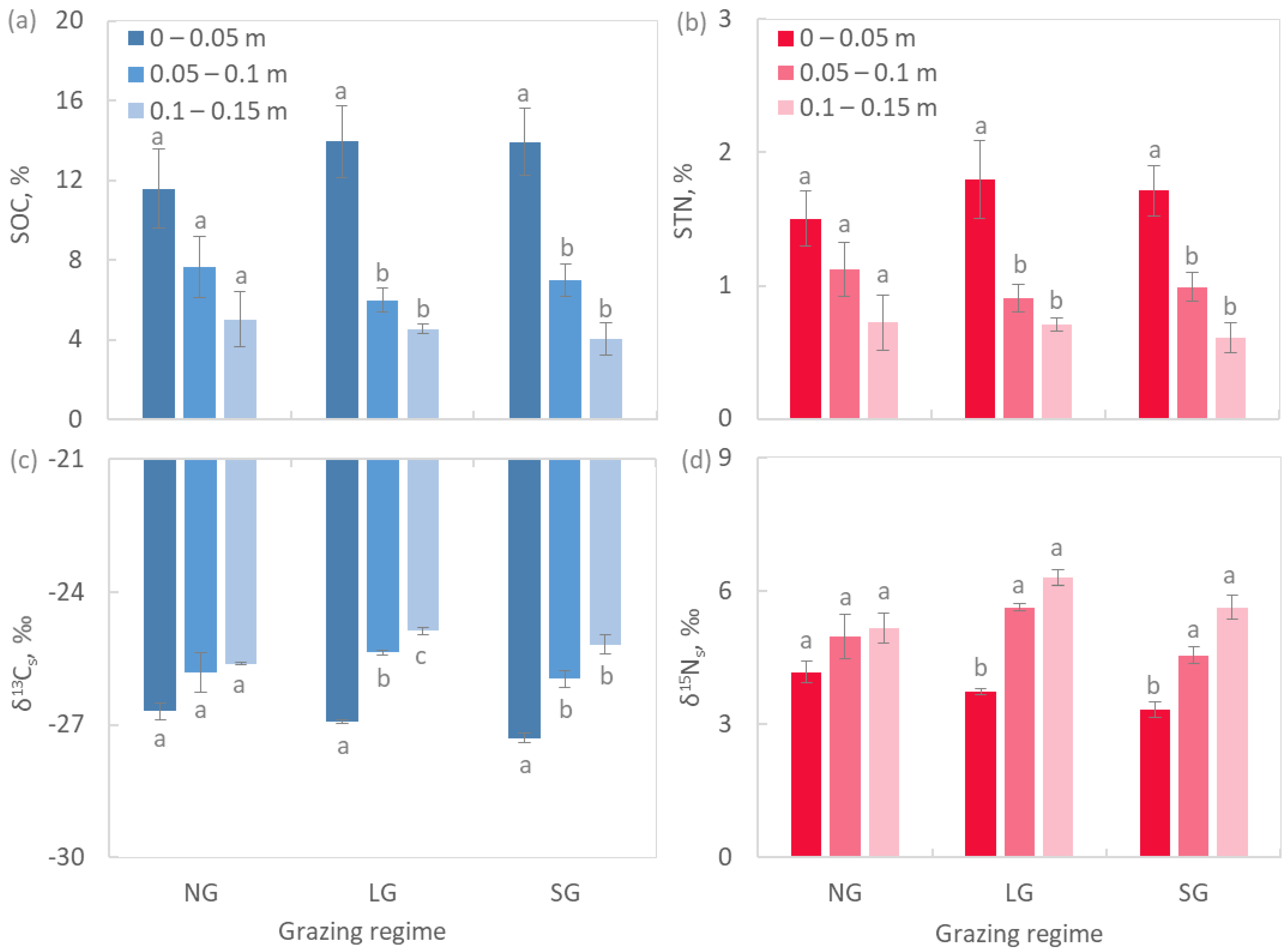
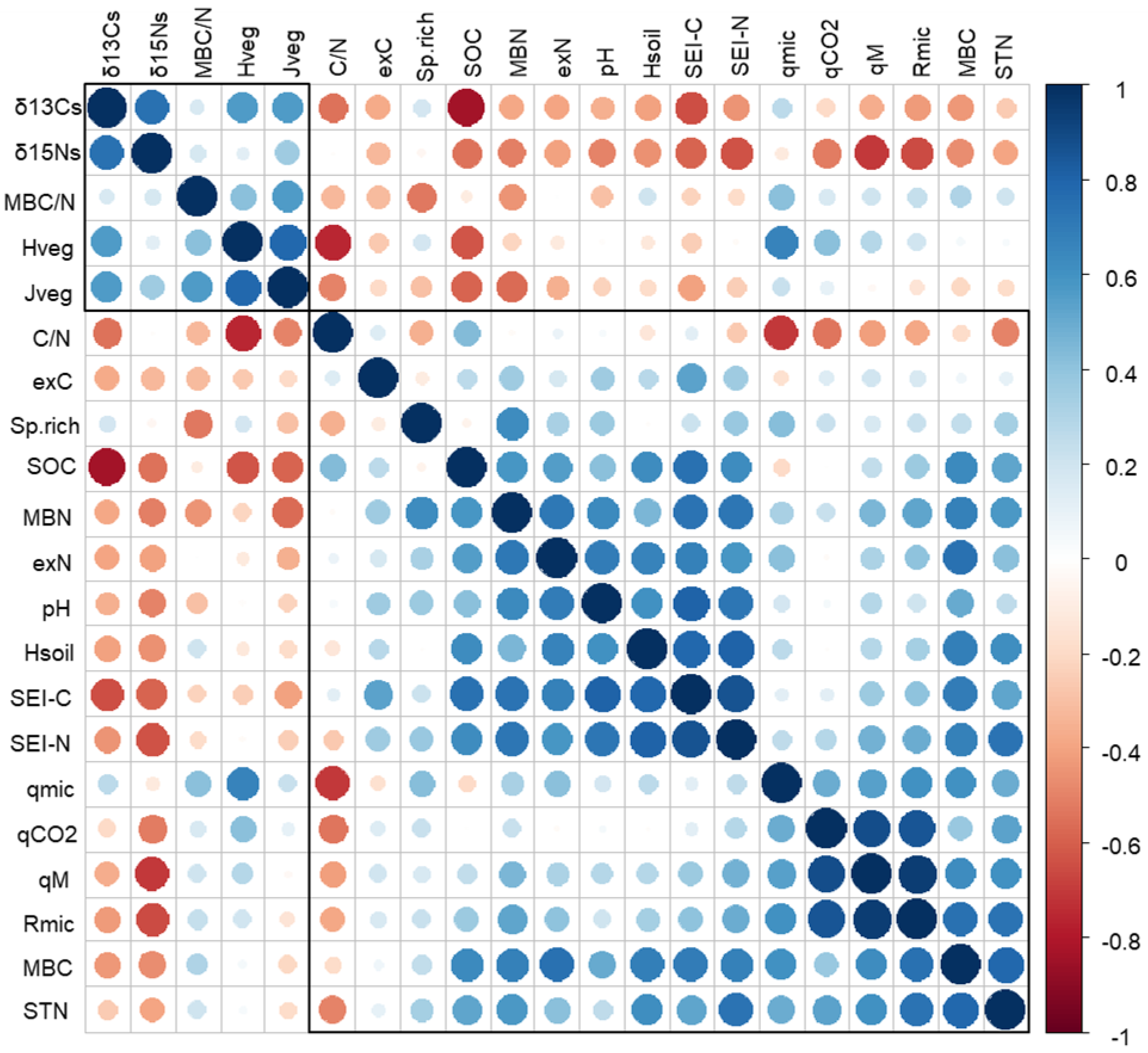
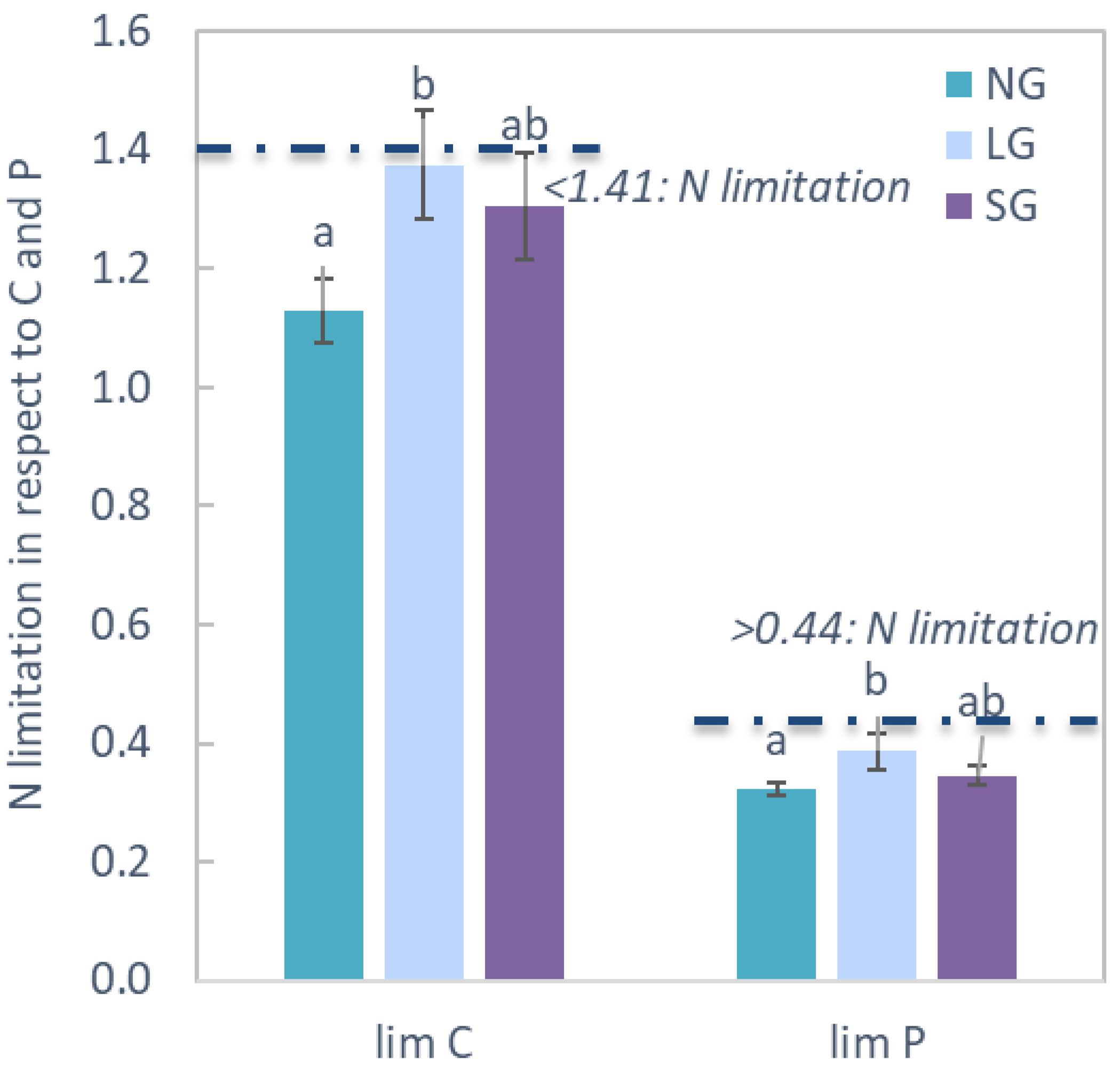
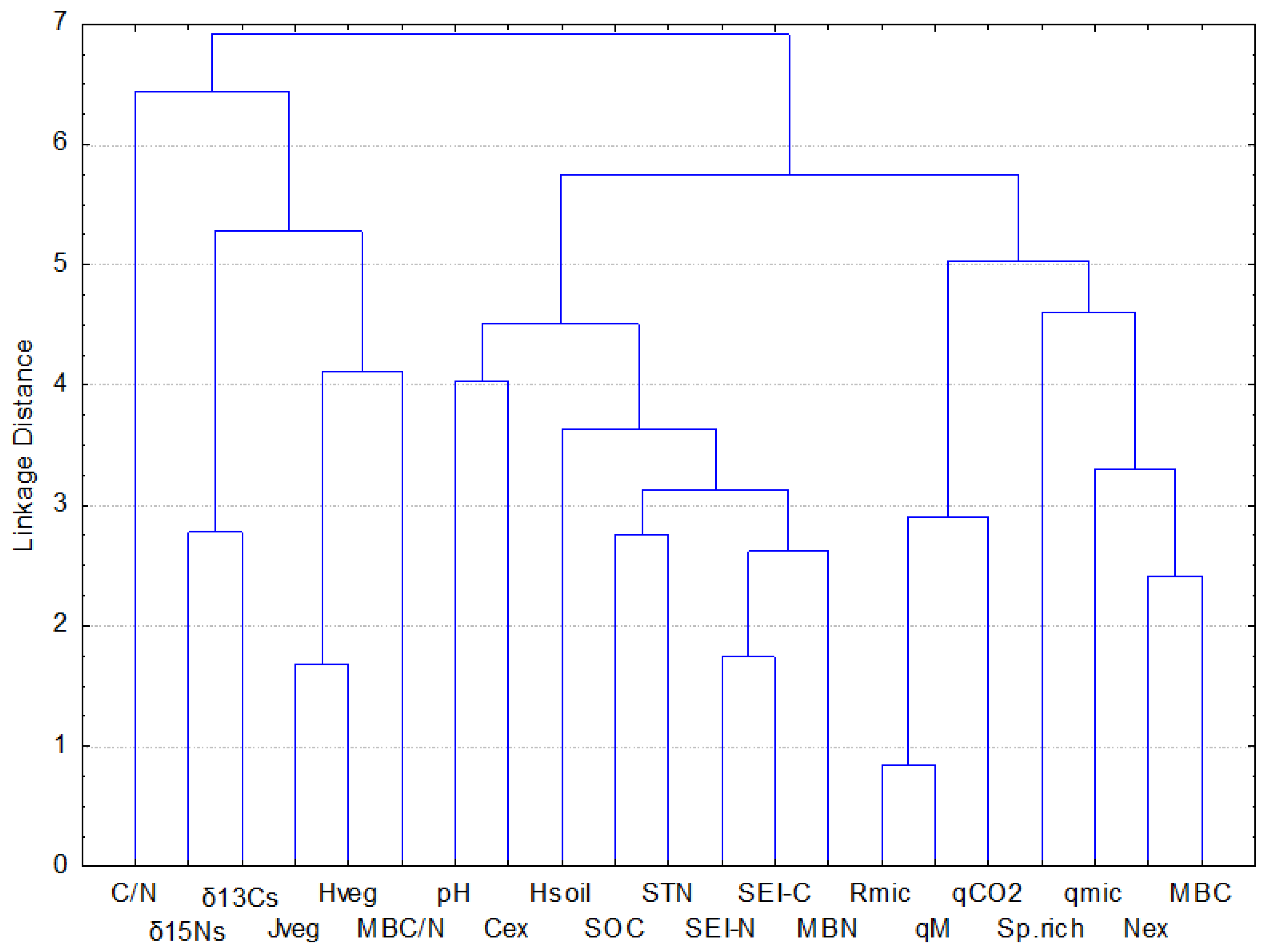
2.3. Soil Chemistry and Biochemistry
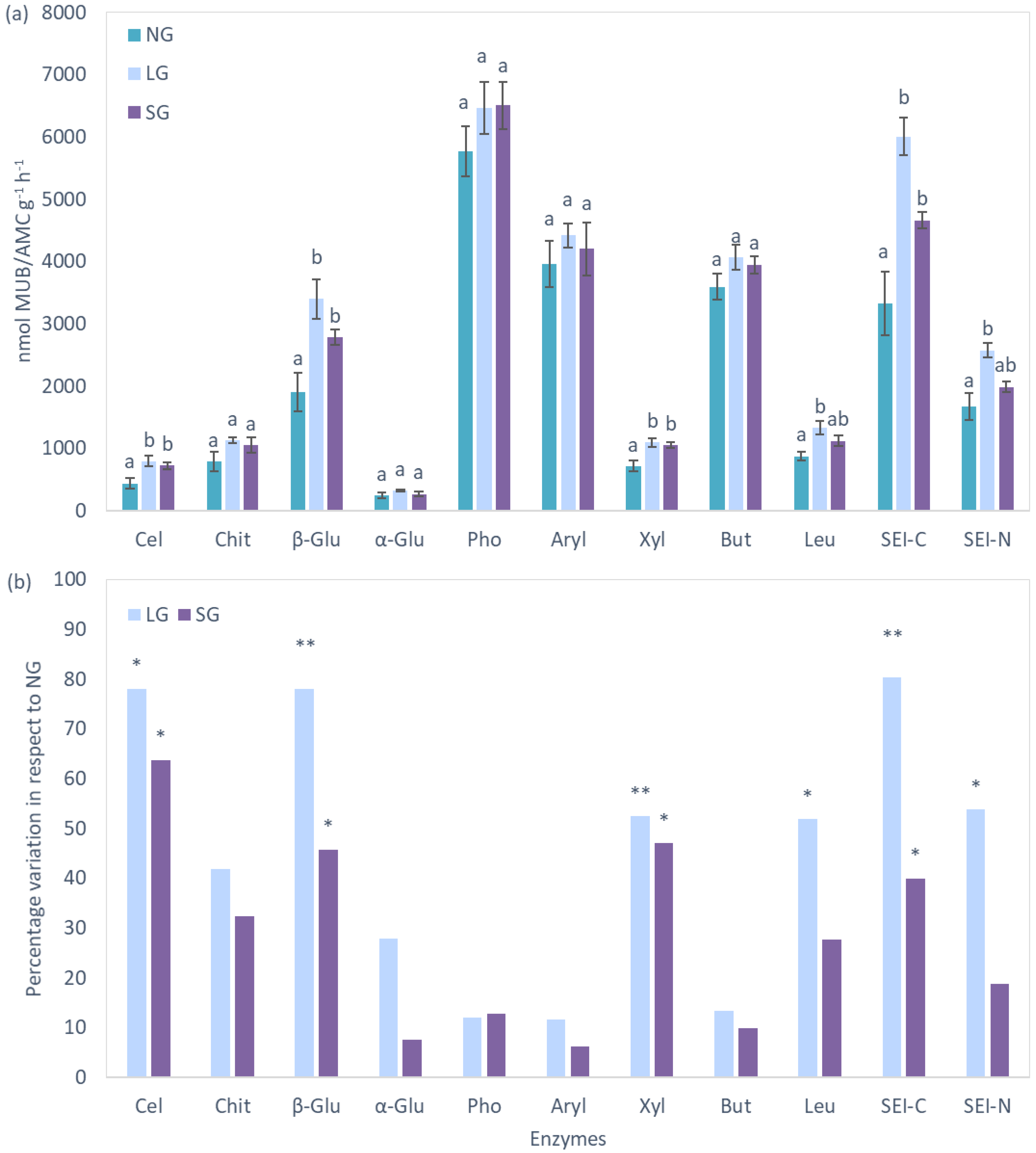
2.4. Extracellular Enzymes
3. Discussions
3.1. Biodiversity
3.2. Vegetation Functioning
3.3. Soil
4. Materials and Methods
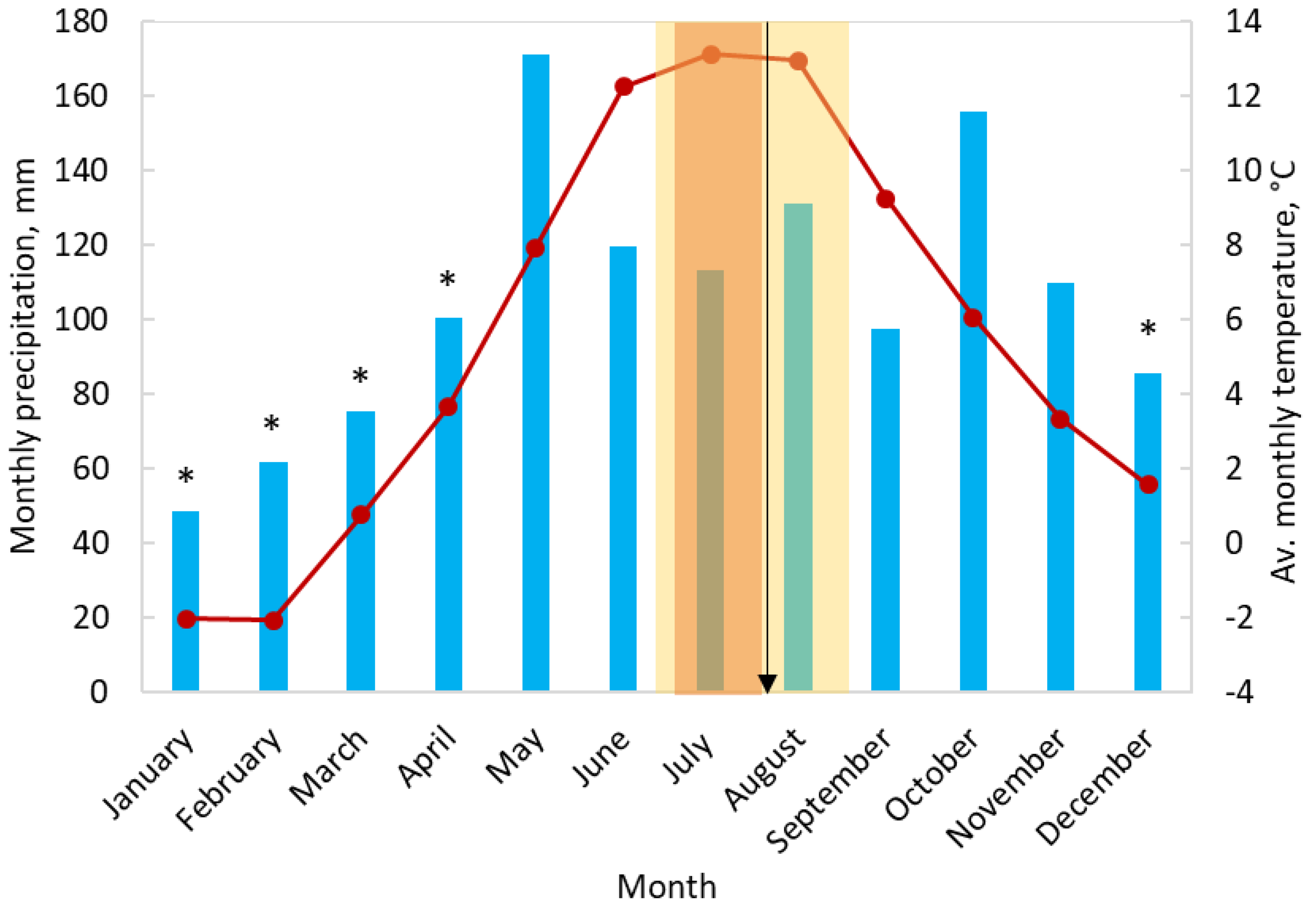
4.1. Site Description
4.2. Sampling
4.3. Isotope Characterization of Vegetation
4.4. Soil Chemical and Biochemical Analyses
4.5. Indices
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carrer, F. Archeologia della pastorizia nelle Alpi: Nuovi dati e vecchi dubbi. Preist. Alp. 2013, 47, 49–56. [Google Scholar]
- Mlekuž, D. The ethnography of the Cyclops: Neolithic pastoralists in the eastern Adriatic. Doc. Praehist. 2005, 32, 15–51. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeler, R.A.; Mittermeler, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Väre, H.; Lampinen, R.; Humphries, C.; Williams, P. Taxonomic Diversity of Vascular Plants in the European Alpine Areas. In Alpine Biodiversity in Europe; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Fischer, M.; Rudmann-Maurer, K.; Weyand, A.; Stöcklin, J. Agricultural Land Use and Biodiversity in the Alps. Mt. Res. Dev. 2008, 28, 148–155. [Google Scholar] [CrossRef]
- Zoller, H.; Bischof, N. Intensity of cultivation and its influence of species richness in vegetation. Phytocoenologia 1980, 7, 35–51. [Google Scholar] [CrossRef]
- MacDonald, D.; Crabtree, J.R.; Wiesinger, G.; Dax, T.; Stamou, N.; Fleury, P.; Gutierrez Lazpita, J.; Gibon, A. Agricultural abandonment in mountain areas of Europe: Environmental consequences and policy response. J. Environ. Manag. 2000, 59, 47–69. [Google Scholar] [CrossRef]
- Niedertscheider, M.; Erb, K. Land system change in Italy from 1884 to 2007: Analysing the North-South divergence on the basis of an integrated indicator framework. Land Use Policy 2014, 39, 366–375. [Google Scholar] [CrossRef]
- González Díaz, J.A.; Celaya, R.; Fernández García, F.; Osoro, K.; Rosa García, R. Dynamics of rural landscapes in marginal areas of northern Spain: Past, present, and future. Land Degrad. Dev. 2019, 30, 141–150. [Google Scholar] [CrossRef]
- Améztegui, A.; Brotons, L.; Coll, L. Land-use changes as major drivers of mountain pine (Pinus uncinata Ram.) expansion in the Pyrenees. Glob. Ecol. Biogeogr. 2010, 19, 632–641. [Google Scholar] [CrossRef]
- Zimmermann, P.; Tasser, E.; Leitinger, G.; Tappeiner, U. Effects of land-use and land-cover pattern on landscape-scale biodiversity in the European Alps. Agric. Ecosyst. Environ. 2010, 139, 13–22. [Google Scholar] [CrossRef]
- Campagnaro, T.; Frate, L.; Carranza, M.L.; Sitzia, T. Multi-scale analysis of alpine landscapes with different intensities of abandonment reveals similar spatial pattern changes: Implications for habitat conservation. Ecol. Indic. 2017, 74, 147–159. [Google Scholar] [CrossRef]
- Food and Agricultural Organization of the United Nations. State of the World’s Forests; Food and Agricultural Organization of the United Nations: Rome, Italy, 2007. [Google Scholar]
- Wang, W.; Fang, J. Soil respiration and human effects on global grasslands. Glob. Planet. Chang. 2009, 67, 20–28. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ranalli, M.G.; Haddix, M.L.; Six, J.; Lugato, E. Soil carbon storage informed by particulate and mineral-associated organic matter. Nat. Geosci. 2019, 12, 989–994. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Chang. Biol. 2002, 8, 345–370. [Google Scholar] [CrossRef]
- Alberti, G.; Leronni, V.; Piazzi, M.; Petrella, F.; Mairota, P.; Peressotti, A.; Piussi, P.; Valentini, R.; Gristina, L.; Mantia, T.L.; et al. Impact of woody encroachment on soil organic carbon and nitrogen in abandoned agricultural lands along a rainfall gradient in Italy. Reg. Environ. Chang. 2011, 11, 917–924. [Google Scholar] [CrossRef]
- Guidi, C.; Vesterdal, L.; Gianelle, D.; Rodeghiero, M. Changes in soil organic carbon and nitrogen following forest expansion on grassland in the Southern Alps. For. Ecol. Manag. 2014, 328, 103–116. [Google Scholar] [CrossRef]
- Fino, E.; Blasi, E.; Perugini, L.; Pellis, G.; Valentini, R.; Chiti, T. Is soil contributing to climate change mitigation during woody encroachment? A case study on the italian alps. Forests 2020, 11, 887. [Google Scholar] [CrossRef]
- Pellis, G.; Chiti, T.; Rey, A.; Curiel Yuste, J.; Trotta, C.; Papale, D. The ecosystem carbon sink implications of mountain forest expansion into abandoned grazing land: The role of subsoil and climatic factors. Sci. Total Environ. 2019, 672, 106–120. [Google Scholar] [CrossRef]
- Tommaso, C.; Emanuele, B.; Guido, P.; Lucia, P.; Vincenza, C.M.; Riccardo, V. Soil organic carbon pool’s contribution to climate change mitigation on marginal land of a Mediterranean montane area in Italy. J. Environ. Manag. 2018, 218, 593–601. [Google Scholar] [CrossRef]
- Wang, X.; McConkey, B.G.; VandenBygaart, A.J.; Fan, J.; Iwaasa, A.; Schellenberg, M. Grazing improves C and N cycling in the Northern Great Plains: A meta-analysis. Sci. Rep. 2016, 6, 33190. [Google Scholar] [CrossRef] [PubMed]
- Conant, R.T.; Cerri, C.E.P.; Osborne, B.B.; Paustian, K. Grassland management impacts on soil carbon stocks: A new synthesis: A. Ecol. Appl. 2017, 27, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.; Hastings, A.; Chadwick, D.R.; Jones, D.L.; Evans, C.D.; Jones, M.B.; Rees, R.M.; Smith, P. Critical review of the impacts of grazing intensity on soil organic carbon storage and other soil quality indicators in extensively managed grasslands. Agric. Ecosyst. Environ. 2018, 253, 62–82. [Google Scholar] [CrossRef]
- Minasny, B.; Malone, B.P.; McBratney, A.B.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.S.; Cheng, K.; Das, B.S.; et al. Soil carbon 4 per mille. Geoderma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- Hörtnagl, L.; Barthel, M.; Buchmann, N.; Eugster, W.; Butterbach-Bahl, K.; Díaz-Pinés, E.; Zeeman, M.; Klumpp, K.; Kiese, R.; Bahn, M.; et al. Greenhouse gas fluxes over managed grasslands in Central Europe. Glob. Chang. Biol. 2018, 24, 1843–1872. [Google Scholar] [CrossRef]
- Soussana, J.F.; Lemaire, G. Coupling carbon and nitrogen cycles for environmentally sustainable intensification of grasslands and crop-livestock systems. Agric. Ecosyst. Environ. 2014, 190, 9–17. [Google Scholar] [CrossRef]
- Sun, Y.; Schleuss, P.-M.; Pausch, J.; Xu, X.; Kuzyakov, Y. Nitrogen pools and cycles in Tibetan Kobresia pastures depending on grazing. Biol. Fertil. Soils 2018, 54, 569–581. [Google Scholar] [CrossRef]
- Shen, H.; Dong, S.; Li, S.; Xiao, J.; Han, Y.; Yang, M.; Zhang, J.; Gao, X.; Xu, Y.; Li, Y.; et al. Grazing enhances plant photosynthetic capacity by altering soil nitrogen in alpine grasslands on the Qinghai-Tibetan plateau. Agric. Ecosyst. Environ. 2019, 280, 161–168. [Google Scholar] [CrossRef]
- Viglizzo, E.F.; Ricard, M.F.; Taboada, M.A.; Vázquez-Amábile, G. Reassessing the role of grazing lands in carbon-balance estimations: Meta-analysis and review. Sci. Total Environ. 2019, 661, 531–642. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Y.; Sun, W.; Huang, Y. Effects of grazing exclusion on soil carbon dynamics in alpine grasslands of the Tibetan Plateau. Geoderma 2019, 353, 133–143. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; Han, G.; Schellenberg, M.P.; Wu, Q.; Gu, C. Grazing induced changes in plant diversity is a critical factor controlling grassland productivity in the Desert Steppe, Northern China. Agric. Ecosyst. Environ. 2018, 265, 73–83. [Google Scholar] [CrossRef]
- Pykälä, J. Cattle grazing increases plant species richness of most species trait groups in mesic semi-natural grasslands. Plant Ecol. 2005, 175, 216–217. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Delgado-Baquerizo, M.; Travers, S.K.; Val, J.; Oliver, I.; Dorrough, J.W.; Soliveres, S. Livestock activity increases exotic plant richness, but wildlife increases native richness, with stronger effects under low productivity. J. Appl. Ecol. 2018, 55, 766–776. [Google Scholar] [CrossRef]
- Klimek, S.; Richter gen. Kemmermann, A.; Hofmann, M.; Isselstein, J. Plant species richness and composition in managed grasslands: The relative importance of field management and environmental factors. Biol. Conserv. 2007, 134, 559–570. [Google Scholar] [CrossRef]
- Borer, E.T.; Seabloom, E.W.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Lind, E.M.; Adler, P.B.; Alberti, J.; Anderson, T.M.; Bakker, J.D.; et al. Herbivores and nutrients control grassland plant diversity via light limitation. Nature 2014, 508, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Schuster, B.; Diekmann, M. Changes in species density along the soil pH gradient—Evidence from German plant communities. Folia Geobot. 2003, 38, 367–379. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Zvereva, E.L. A second life for old data: Global patterns in pollution ecology revealed from published observational studies. Environ. Pollut. 2011, 159, 1067–1075. [Google Scholar] [CrossRef]
- Azevedo, L.B.; Van Zelm, R.; Hendriks, A.J.; Bobbink, R.; Huijbregts, M.A.J. Global assessment of the effects of terrestrial acidification on plant species richness. Environ. Pollut. 2013, 174, 10–15. [Google Scholar] [CrossRef]
- Gavrichkova, O.; Galvagno, M.; Brugnoli, E.; Chiti, T.; Cremonese, E.; Filippa, G.; Mattioni, M.; Morra di Cella, U.; Calfapietra, C. Carbon fluxes in Alpine grasslands. In NextData Volume; NextData: Rome, Italy, 2019. [Google Scholar]
- Lüscher, A. Land Use Systems in Grassland Dominated Regions. In Proceedings of the 20th General Meeting of the European Grassland Federation, Luzern, Switzerland, 21–24 June 2004. [Google Scholar]
- Soussana, J.-F.; Soussana, J.-F.; Loiseau, P.; Vuichard, N.; Ceschia, E.; Balesdent, J.; Chevallier, T.; Arrouays, D. Carbon cycling and sequestration opportunities in temperate grasslands. Soil Use Manag. 2004, 20, 219–230. [Google Scholar] [CrossRef]
- Uchida, K.; Ushimaru, A. Biodiversity declines due to abandonment and intensification of agricultural lands: Patterns and mechanisms. Ecol. Monogr. 2014, 84, 637–658. [Google Scholar] [CrossRef]
- Tälle, M.; Deák, B.; Poschlod, P.; Valkó, O.; Westerberg, L.; Milberg, P. Grazing vs. mowing: A meta-analysis of biodiversity benefits for grassland management. Agric. Ecosyst. Environ. 2016, 222, 200–212. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Wardle, D.A. Herbivore-mediated linkages between aboveground and belowground communities. Ecology 2003, 84, 2258–2268. [Google Scholar] [CrossRef]
- Fauci, M.F.; Dick, R.P. Soil Microbial Dynamics: Short- and Long-Term Effects of Inorganic and Organic Nitrogen. Soil Sci. Soc. Am. J. 1994, 58, 801–806. [Google Scholar] [CrossRef]
- Khan, K.S.; Mack, R.; Castillo, X.; Kaiser, M.; Joergensen, R.G. Microbial biomass, fungal and bacterial residues, and their relationships to the soil organic matter C/N/P/S ratios. Geoderma 2016, 271, 115–123. [Google Scholar] [CrossRef]
- Gavrichkova, O.; Brykova, R.A.; Brugnoli, E.; Calfapietra, C.; Cheng, Z.; Kuzyakov, Y.; Liberati, D.; Moscatelli, M.C.; Pallozzi, E.; Vasenev, V.I. Secondary soil salinization in urban lawns: Microbial functioning, vegetation state, and implications for carbon balance. Land Degrad. Dev. 2020, 31, 2591–2604. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhang, X.; Wang, X.; Fu, S.; Wu, S.; Lu, X.; Ren, C.; Han, X.; Yang, G. Soil bacteria and fungi respond differently to plant diversity and plant family composition during the secondary succession of abandoned farmland on the Loess Plateau, China. Plant Soil 2020, 448, 183–200. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, L.; Lin, Q.; Yuan, M.; Xu, D.; Yu, H.; Hu, Y.; Duan, J.; Li, X.; He, Z.; et al. Responses of the functional structure of soil microbial community to livestock grazing in the Tibetan alpine grassland. Glob. Chang. Biol. 2013, 19, 637–648. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.; Song, Z.; Wang, J.; Guo, L. Interactions of soil bacteria and fungi with plants during long-term grazing exclusion in semiarid grasslands. Soil Biol. Biochem. 2018, 124, 47–58. [Google Scholar] [CrossRef]
- Clark, C.M.; Flynn, D.F.B.; Butterfield, B.J.; Reich, P.B. Testing the Link between Functional Diversity and Ecosystem Functioning in a Minnesota Grassland Experiment. PLoS ONE 2012, 7, e52821. [Google Scholar] [CrossRef]
- Ivashchenko, K.; Sushko, S.; Selezneva, A.; Ananyeva, N.; Zhuravleva, A.; Kudeyarov, V.; Makarov, M.; Blagodatsky, S. Soil microbial activity along an altitudinal gradient: Vegetation as a main driver beyond topographic and edaphic factors. Appl. Soil Ecol. 2021, 168, 104197. [Google Scholar] [CrossRef]
- Kandeler, E.; Kampichler, C.; Horak, O. Influence of heavy metals on the functional diversity of soil microbial communities. Biol. Fertil. Soils 1996, 23, 299–306. [Google Scholar] [CrossRef]
- Caldwell, B.A. Enzyme activities as a component of soil biodiversity: A review. Pedobiologia 2005, 49, 637–644. [Google Scholar] [CrossRef]
- Moscatelli, M.C.; Secondi, L.; Marabottini, R.; Papp, R.; Stazi, S.R.; Mania, E.; Marinari, S. Assessment of soil microbial functional diversity: Land use and soil properties affect CLPP-MicroResp and enzymes responses. Pedobiologia 2018, 66, 36–42. [Google Scholar] [CrossRef]
- Brimecombe, M.J.; De Leij, F.A.A.M.; Lynch, J.M. Rhizodeposition and microbial populations. In The Rhizosphere: Biochemistry and Organic Substances at the Soil-Plant Interface; RC Press: Boca Raton, FL, USA; London, UK; Taylor & Francis Group: New York, NY, USA, 2007; pp. 73–109. [Google Scholar]
- Williams, A.; Langridge, H.; Straathof, A.L.; Muhamadali, H.; Hollywood, K.A.; Goodacre, R.; de Vries, F.T. Root functional traits explain root exudation rate and composition across a range of grassland species. J. Ecol. 2021, 110, 21–33. [Google Scholar] [CrossRef]
- Gavrichkova, O.; Moscatelli, M.C.; Kuzyakov, Y.; Grego, S.; Valentini, R. Influence of defoliation on CO2 efflux from soil and microbial activity in a Mediterranean grassland. Agric. Ecosyst. Environ. 2010, 136, 87–96. [Google Scholar] [CrossRef]
- Zhou, G.; Zhou, X.; He, Y.; Shao, J.; Hu, Z.; Liu, R.; Zhou, H.; Hosseinibai, S. Grazing intensity significantly affects belowground carbon and nitrogen cycling in grassland ecosystems: A meta-analysis. Glob. Chang. Biol. 2017, 23, 1167–1179. [Google Scholar] [CrossRef]
- Mikola, J.; Setälä, H.; Virkajärvi, P.; Saarijärvi, K.; Ilmarinen, K.; Voigt, W.; Vestberg, M. Defoliation and patchy nutrient return drive grazing effects on plant and soil properties in a dairy cow pasture. Ecol. Monogr. 2009, 79, 221–244. [Google Scholar] [CrossRef]
- Klumpp, K.; Fontaine, S.; Attard, E.; Le Roux, X.; Gleixner, G.; Soussana, J.F. Grazing triggers soil carbon loss by altering plant roots and their control on soil microbial community. J. Ecol. 2009, 97, 876–885. [Google Scholar] [CrossRef]
- Grigulis, K.; Lavorel, S.; Krainer, U.; Legay, N.; Baxendale, C.; Dumont, M.; Kastl, E.; Arnoldi, C.; Bardgett, R.D.; Poly, F.; et al. Relative contributions of plant traits and soil microbial properties to mountain grassland ecosystem services. J. Ecol. 2013, 101, 47–57. [Google Scholar] [CrossRef]
- Kelly, J.F. Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can. J. Zool. 2000, 78, 1–27. [Google Scholar] [CrossRef]
- Tonn, B.; Porath, I.; Lattanzi, F.A.; Isselstein, J. Urine effects on grass and legume nitrogen isotopic composition: Pronounced short-term dynamics of δ 15 N. PLoS ONE 2019, 14, e0210623. [Google Scholar] [CrossRef] [PubMed]
- Sitters, J.; Olde Venterink, H. Stoichiometric impact of herbivore dung versus urine on soils and plants. Plant Soil 2021, 462, 59–65. [Google Scholar] [CrossRef]
- Cai, Y.; Akiyama, H. Nitrogen loss factors of nitrogen trace gas emissions and leaching from excreta patches in grassland ecosystems: A summary of available data. Sci. Total Environ. 2016, 572, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, J.; He, X.Z.; Hou, F. Different effects of sheep excrement type and supply level on plant and soil C:N:P stoichiometry in a typical steppe on the loess plateau. Plant Soil 2021, 462, 45–58. [Google Scholar] [CrossRef]
- Cotching, W.E.; Taylor, L.; Corkrey, S.R. Spatial variation of soil nutrients in dairy pasture paddocks. New Zeal. J. Agric. Res. 2020, 63, 492–504. [Google Scholar] [CrossRef]
- Witte, C.P. Urea metabolism in plants. Plant Sci. 2011, 180, 431–438. [Google Scholar] [CrossRef]
- Seibt, U.; Rajabi, A.; Griffiths, H.; Berry, J.A. Carbon isotopes and water use efficiency: Sense and sensitivity. Oecologia 2008, 155, 441–454. [Google Scholar] [CrossRef]
- Brüggemann, N.; Gessler, A.; Kayler, Z.; Keel, S.G.; Badeck, F.; Barthel, M.; Boeckx, P.; Buchmann, N.; Brugnoli, E.; Esperschütz, J.; et al. Carbon allocation and carbon isotope fluxes in the plant-soil-atmosphere continuum: A review. Biogeosciences 2011, 8, 3457–3489. [Google Scholar] [CrossRef]
- Cernusak, L.A.; Ubierna, N.; Winter, K.; Holtum, J.A.M.; Marshall, J.D.; Farquhar, G.D. Environmental and physiological determinants of carbon isotope discrimination in terrestrial plants. New Phytol. 2013, 200, 950–965. [Google Scholar] [CrossRef]
- Wilson, C.H.; Strickland, M.S.; Hutchings, J.A.; Bianchi, T.S.; Flory, S.L. Grazing enhances belowground carbon allocation, microbial biomass, and soil carbon in a subtropical grassland. Glob. Chang. Biol. 2018, 24, 2997–3009. [Google Scholar] [CrossRef]
- Lai, L.; Kumar, S. A global meta-analysis of livestock grazing impacts on soil properties. PLoS ONE 2020, 15, e0236638. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; He, Z. Effects of grazing patterns on grassland biomass and soil environments in China: A meta-analysis. PLoS ONE 2019, 14, e0215223. [Google Scholar] [CrossRef] [PubMed]
- Aldezabal, A.; Moragues, L.; Odriozola, I.; Mijangos, I. Impact of grazing abandonment on plant and soil microbial communities in an Atlantic mountain grassland. Appl. Soil Ecol. 2015, 96, 251–260. [Google Scholar] [CrossRef]
- Xun, W.; Yan, R.; Ren, Y.; Jin, D.; Xiong, W.; Zhang, G.; Cui, Z.; Xin, X.; Zhang, R. Grazing-induced microbiome alterations drive soil organic carbon turnover and productivity in meadow steppe. Microbiome 2018, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Goenster-Jordan, S.; Ingold, M.; Jannoura, R.; Buerkert, A.; Joergensen, R.G. Soil microbial properties of subalpine steppe soils at different grazing intensities in the Chinese Altai Mountains. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Li, X.G.; Li, F.M.; Singh, B.; Rengel, Z.; Zhan, Z.Y. Soil management changes organic carbon pools in alpine pastureland soils. Soil Tillage Res. 2007, 93, 186–196. [Google Scholar] [CrossRef]
- Güsewell, S.; Jewell, P.L.; Edwards, P.J. Effects of heterogeneous habitat use by cattle on nutrient availability and litter decomposition in soils of an Alpine pasture. Plant Soil 2005, 268, 135–149. [Google Scholar] [CrossRef]
- Hiltbrunner, D.; Schulze, S.; Hagedorn, F.; Schmidt, M.W.I.; Zimmmermann, S. Cattle trampling alters soil properties and changes soil microbial communities in a Swiss sub-alpine pasture. Geoderma 2012, 170, 369–377. [Google Scholar] [CrossRef]
- O’Callaghan, M.; Gerard, E.M.; Carter, P.E.; Lardner, R.; Sarathchandra, U.; Burch, G.; Ghani, A.; Bell, N. Effect of the nitrification inhibitor dicyandiamide (DCD) on microbial communities in a pasture soil amended with bovine urine. Soil Biol. Biochem. 2010, 42, 1425–1436. [Google Scholar] [CrossRef]
- Kahmen, A.; Wanek, W.; Buchmann, N. Foliar δ15N values characterize soil N cycling and reflect nitrate or ammonium preference of plants along a temperate grassland gradient. Oecologia 2008, 156, 861–870. [Google Scholar] [CrossRef]
- Seeber, J.; Seeber, G.U.H. Effects of land-use changes on humus forms on alpine pastureland (Central Alps, Tyrol). Geoderma 2005, 124, 215–222. [Google Scholar] [CrossRef]
- Zeglin, L.H.; Bottomley, P.J.; Jumpponen, A.; Rice, C.W.; Arango, M.; Lindsley, A.; McGowan, A.; Mfombep, P.; Myrold, D.D. Altered precipitation regime affects the function and composition of soil microbial communities on multiple time scales. Ecology 2013, 94, 2334–2335. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Barthelemy, H.; Stark, S.; Olofsson, J. Strong responses of subarctic plant communities to long-term reindeer feces manipulation. Ecosystems 2015, 18, 740–751. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Ouimette, A.P. Controls of nitrogen isotope patterns in soil profiles. Biogeochemistry 2009, 95, 89–98. [Google Scholar] [CrossRef]
- Boström, B.; Comstedt, D.; Ekblad, A. Isotope fractionation and 13C enrichment in soil profiles during the decomposition of soil organic matter. Oecologia 2007, 153, 89–98. [Google Scholar] [CrossRef]
- Sponheimer, M.; Robinson, T.; Ayliffe, L.; Passey, B.; Roeder, B.; Shipley, L.; Lopez, E.; Cerling, T.; Dearing, D.; Ehleringer, J. An experimental study of carbon-isotope fractionation between diet, hair, and feces of mammalian herbivores. Can. J. Zool. 2003, 81, 871–876. [Google Scholar] [CrossRef]
- Lee, C.; Hristov, A.N.; Cassidy, T.; Heyler, K. Nitrogen isotope fractionation and origin of ammonia nitrogen volatilized from cattle manure in simulated storage. Atmosphere 2011, 2, 256–270. [Google Scholar] [CrossRef]
- Michener, R.; Lajtha, K. Stable Isotopes in Ecology and Environmental Science, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Vargas, R.S.; Bataiolli, R.; da Costa, P.B.; Lisboa, B.; Passaglia, L.M.P.; Beneduzi, A.; Vargas, L.K. Microbial quality of soil from the Pampa biome in response to different grazing pressures. Genet. Mol. Biol. 2015, 38, 205–212. [Google Scholar] [CrossRef]
- Curtin, D.; Peterson, M.E.; Qiu, W.; Fraser, P.M. Predicting soil pH changes in response to application of urea and sheep urine. J. Environ. Qual. 2020, 49, 1445–1452. [Google Scholar] [CrossRef]
- Curtin, D.; Campbell, C.A.; Jalil, A. Effects of acidity on mineralization: pH-dependence of organic matter mineralization in weakly acidic soils. Soil Biol. Biochem. 1998, 30, 57–64. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; IUSS: Vienna, Austria, 2014. [Google Scholar]
- Macoon, B.; Sollenberger, L.E.; Moore, J.E.; Staples, C.R.; Fike, J.H.; Portier, K.M. Comparison of three techniques for estimating the forage intake of lactating dairy cows on pasture1. J. Anim. Sci. 2003, 81, 2357–2366. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.M.; Gray, A. Don’t let spurious accusations of pseudoreplication limit our ability to learn from natural experiments (and other messy kinds of ecological monitoring). Ecol. Evol. 2015, 5, 5295–5304. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.; Bodart, J. Changes in vegetation diversity and composition following livestock removal along an upland elevational gradient. Iforest—Biogeosciences For. 2015, 8, 582–589. [Google Scholar] [CrossRef]
- Violante, P. Metodi di Analisi Chimica del Suolo; Franco Angeli: Milano, Italy, 2000. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Badalucco, L.; Grego, S.; Dell’Orco, S.; Nannipieri, P. Effect of liming on some chemical, biochemical, and microbiological properties of acid soils under spruce (Picea abies L.). Biol. Fertil. Soils 1992, 14, 76–83. [Google Scholar] [CrossRef]
- Marx, M.C.; Wood, M.; Jarvis, S.C. A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol. Biochem. 2001, 33, 1633–1640. [Google Scholar] [CrossRef]
- Vepsäläinen, M.; Kukkonen, S.; Vestberg, M.; Sirviö, H.; Maarit Niemi, R. Application of soil enzyme activity test kit in a field experiment. Soil Biol. Biochem. 2001, 33, 1665–1672. [Google Scholar] [CrossRef]
- Wittmann, C.; Kähkönen, M.A.; Ilvesniemi, H.; Kurola, J.; Salkinoja-Salonen, M.S. Areal activities and stratification of hydrolytic enzymes involved in the biochemical cycles of carbon, nitrogen, sulphur and phosphorus in podsolized boreal forest soils. Soil Biol. Biochem. 2004, 36, 425–433. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. Ratios of microbial biomass carbon to total organic carbon in arable soils. Soil Biol. Biochem. 1989, 21, 471–479. [Google Scholar] [CrossRef]
- Dilly, O.; Munch, J.C. Ratios between estimates of microbial biomass content and microbial activity in soils. Biol. Fertil. Soils 1998, 27, 374–379. [Google Scholar] [CrossRef]
- Marinari, S.; Liburdi, K.; Fliessbach, A.; Kalbitz, K. Effects of organic management on water-extractable organic matter and C mineralization in European arable soils. Soil Tillage Res. 2010, 106, 211–217. [Google Scholar] [CrossRef]
- Kooch, Y.; Ehsani, S.; Akbarinia, M. Stoichiometry of microbial indicators shows clearly more soil responses to land cover changes than absolute microbial activities. Ecol. Eng. 2019, 131, 99–106. [Google Scholar] [CrossRef]
- Waldrop, M.P.; Balser, T.C.; Firestone, M.K. Linking microbial community composition to function in a tropical soil. Soil Biol. Biochem. 2000, 32, 1837–1846. [Google Scholar] [CrossRef]
- Shannon, C.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1949. [Google Scholar]
- Vonlanthen, C.M.; Kammer, P.M.; Eugster, W.; Bühler, A.; Veit, H. Alpine vascular plant species richness: The importance of daily maximum temperature and pH. Plant Ecol. 2006, 184, 13–25. [Google Scholar] [CrossRef]
- Bending, G.D.; Turner, M.K.; Rayns, F.; Marx, M.C.; Wood, M. Microbial and biochemical soil quality indicators and their potential for differentiating areas under contrasting agricultural management regimes. Soil Biol. Biochem. 2004, 36, 1785–1792. [Google Scholar] [CrossRef]
| Family | Specie | Patch | ||
|---|---|---|---|---|
| NG | LG | SG | ||
| Asteraceae | Achillea millefolium L. | 1.11% a | 5.63% a | 2.90% a |
| Centaurea nigrescens Willd. | ||||
| Cirsium eriophorum (L.) Scop. | ||||
| Apiaceae | sp. | 0% a | 0% a | 1.96% a |
| Campanulaceae | Campanula rotundifolia L. | 0% a | 0.03% a | 0% a |
| Caryophyllaceae | Stellaria graminea L. | 0.17% a | 0.14% a | 0% a |
| Clusiaceae | Hypericum perforatum L. | 5.47% a | 0.20% a | 0% a |
| Fabaceae | Trifolium pratense L. | 0% a | 0.68% a | 0.36% a |
| Trifolium repens L. | ||||
| Poaceae | Deschampsia caespitosa (L.) Beauv. | 67.64% b | 79.50% a | 72.00% a |
| Festuca sp. | ||||
| Phleum alpinum L. | ||||
| Poa alpina L. | ||||
| Polygonaceae | Rumex acetosa L. | 18.47% a | 1% b | 6.71% ab |
| Rumex alpinum L. | ||||
| Primulaceae | Primula elatior (L.) Hill. | 0% | 0.18% | 0% |
| Ranunculaceae | Ranunculus acris L. | 0% b | 3.86% ab | 7.17% a |
| Rosaceae | Alchemilla vulgaris L. | 1.54% a | 7.93% a | 5.98% a |
| Urticacae | Urtica dioica L. | 5.02% a | 0% a | 2.47% a |
| other | 0.58% a | 0.84% a | 0.05% | |
| Number of species m−2 | 8.00 ± 0.71 b | 11.80 ± 1.39 a | 8.40 ± 0.40 b | |
| Indices of Shannon | 1.24 ± 0.05 a | 1.17 ± 0.13 a | 1.15 ± 0.08 a | |
| Indices of Eveness | 0.64 ± 0.03 a | 0.49 ± 0.08 a | 0.55 ± 0.04 a | |
| Species | Patch | N, | C/N Ratio | δ13Cveg | δ15Nveg | Biomass | N Stock |
|---|---|---|---|---|---|---|---|
| % | ‰ | ‰ | g m−2 | kg ha−1 | |||
| Deschampsia caespitosa (L.) Beauv. | NG | 1.8 ± 0.1 b | 23.8 ± 1.7a | −27.8 ± 0.4 a | 1.4 ± 0.2 a | 45.0 ± 15.1 b | 6.1 ± 0.9 b |
| LG | 2.3 ± 0.2 ab | 18.9 ± 2.0 ab | −26.9 ±0.3 a | 1.6 ± 0.9 a | 108.2 ± 23.0 a | 26.5 ± 7.6 a | |
| SG | 2.5 ± 0.1 a | 17.0 ± 1.1 b | −26.9 ± 0.2 a | 2.0 ± 0.4 a | 91.3 ± 17.9 a | 22.3 ± 3.5 a | |
| Festuca sp. | NG | 1.9 ± 0.1 b | 22.3 ± 0.6a | −27.8 ± 0.2 a | 1.4 ± 0.3 a | 94.0 ± 32.1 a | 17.8 ± 6.3 a |
| LG | 2.3 ± 0.2 ab | 19.7 ± 1.7 ab | −28.0 ± 0.3 a | 0.9 ± 0.7 a | 10.9 ± 8.2 b | 2.5 ± 1.8 b | |
| SG | 3.1 ± 0.3 a | 14.0 ± 1.1 b | −28.0 ± 0.2 a | 2.3 ± 0.5 a | 7.1 ± 1.9 b | 2.2 ± 1.9 b | |
| Achillea millefolium L. | NG | 2.2 ± 0.3 a | 20.8 ± 2.7 a | −30.3 ± 0.1 a | 0.7 ± 0.5 a | 3.1 ± 2.1 a | 0.5 ± 0.3 |
| LG | 2.9 ± 0.3 a | 14.8 ± 1.7 a | −29.6 ± 0.1 b | 1.6 ± 0.7 a | 4.7 ± 1.3 a | 1.3 ± 0.2 | |
| SG | 2.7 ± 0.3 a | 16.1 ± 1.1 a | −29.7 ± 0.1 b | 1.6 ± 0.4 a | 4.6 ± 1.7 a | 0.8 ± 0.1 | |
| Alchemilla vulgaris L. | NG | 2.5 ± 0.2 b | 17.4 ± 1.1 a | −28.6 ± 0.1a | 1.1 ± 0.1 a | 4.3 ± 1.3 c | 1.1 ± 0.4 c |
| LG | 2.7 ± 0.2 b | 15.6 ± 1.3 ab | −27.3 ± 0.2 b | 0.8 ± 0.6 a | 13.3 ± 3.7 b | 3.4 ± 0.8 b | |
| SG | 3.9 ± 0.1 a | 11.5 ± 0.2 b | −26.7 ± 0.1 c | −1.7 ± 0.3 a | 20.4 ± 2.6 a | 7.5 ± 0.8 a | |
| Rumex acetosa L. | NG | 3.9 ± 0.4 a | 11.8 ± 1.5 a | −27.5 ± 0.1 a | 1.9 ± 0.4 a | 39.8 ±13.5 a | 13.5 ± 0.2 a |
| LG | 2.8 ± 0.2 a | 14.6 ± 0.9 a | −28.4 ± 0.8 a | 3.4 ± 1.4 a | 1.9 ± 0.6 b | 0.6 ± 0.3 b | |
| SG | 3.3 ± 0.5 a | 13.4 ± 2.3 a | −27.9 ± 0.2 a | 4.4 ± 1.3 a | 8.2 ± 3.2 b | 3.1 ±1.3 b |
| Characteristics | Av. Value |
|---|---|
| δ13C, % | −28.65 ± 0.12 |
| δ15N, % | 1.91 ± 0.41 |
| N, % | 1.89 ± 0.19 |
| C, % | 43.68 ± 1.43 |
| C:N | 23.42 ± 1.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavrichkova, O.; Pretto, G.; Brugnoli, E.; Chiti, T.; Ivashchenko, K.V.; Mattioni, M.; Moscatelli, M.C.; Scartazza, A.; Calfapietra, C. Consequences of Grazing Cessation for Soil Environment and Vegetation in a Subalpine Grassland Ecosystem. Plants 2022, 11, 2121. https://doi.org/10.3390/plants11162121
Gavrichkova O, Pretto G, Brugnoli E, Chiti T, Ivashchenko KV, Mattioni M, Moscatelli MC, Scartazza A, Calfapietra C. Consequences of Grazing Cessation for Soil Environment and Vegetation in a Subalpine Grassland Ecosystem. Plants. 2022; 11(16):2121. https://doi.org/10.3390/plants11162121
Chicago/Turabian StyleGavrichkova, Olga, Gaia Pretto, Enrico Brugnoli, Tommaso Chiti, Kristina V. Ivashchenko, Michele Mattioni, Maria Cristina Moscatelli, Andrea Scartazza, and Carlo Calfapietra. 2022. "Consequences of Grazing Cessation for Soil Environment and Vegetation in a Subalpine Grassland Ecosystem" Plants 11, no. 16: 2121. https://doi.org/10.3390/plants11162121
APA StyleGavrichkova, O., Pretto, G., Brugnoli, E., Chiti, T., Ivashchenko, K. V., Mattioni, M., Moscatelli, M. C., Scartazza, A., & Calfapietra, C. (2022). Consequences of Grazing Cessation for Soil Environment and Vegetation in a Subalpine Grassland Ecosystem. Plants, 11(16), 2121. https://doi.org/10.3390/plants11162121