Intensification of Polyphenols Extraction from Eryngium creticum Leaves Using Ired-Irrad® and Evaluation of Antibiofilm and Antibacterial Activities
Abstract
:1. Introduction
2. Results
2.1. Selection of Particle Size and Solid to Liquid Ratio
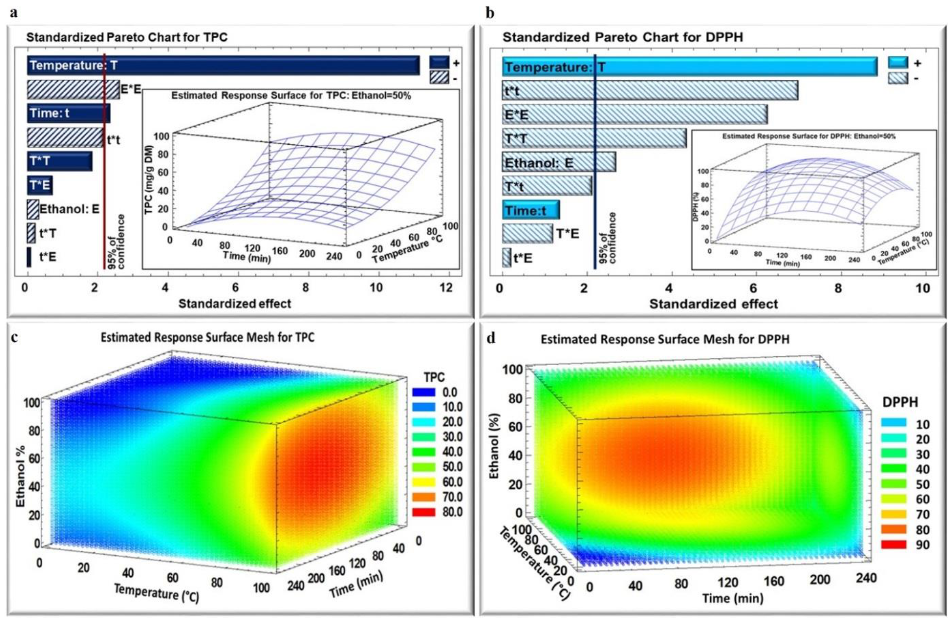
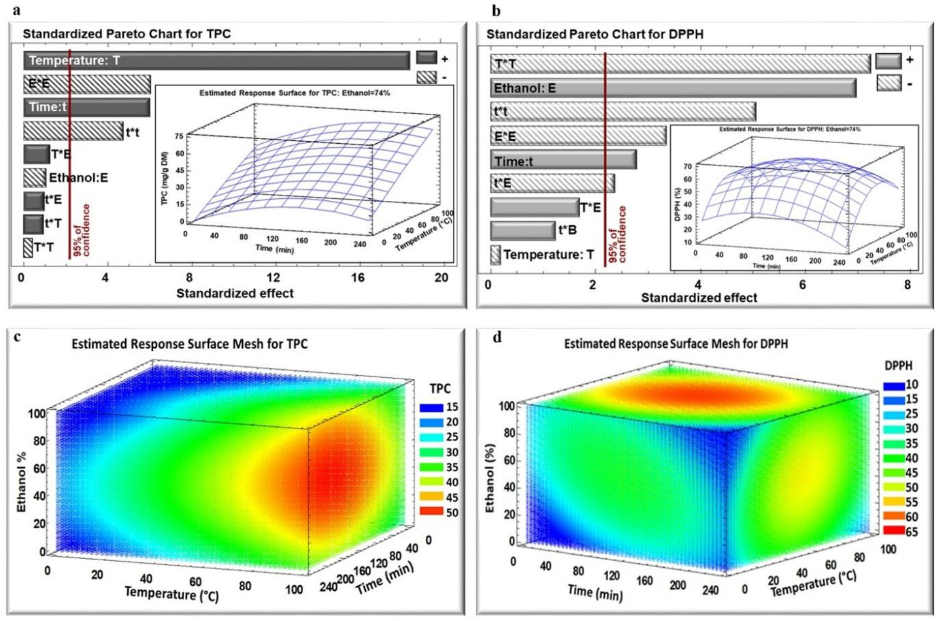
2.2. Influence of Time, Temperature, and Ethanol Percentage on TPC Yield, and DPPH Inhibition Percentage
2.3. Optimization of Extraction
2.4. Identification of Polyphenols by UHPLC Analysis of the Optimal Conditions for IR and WB Extracts
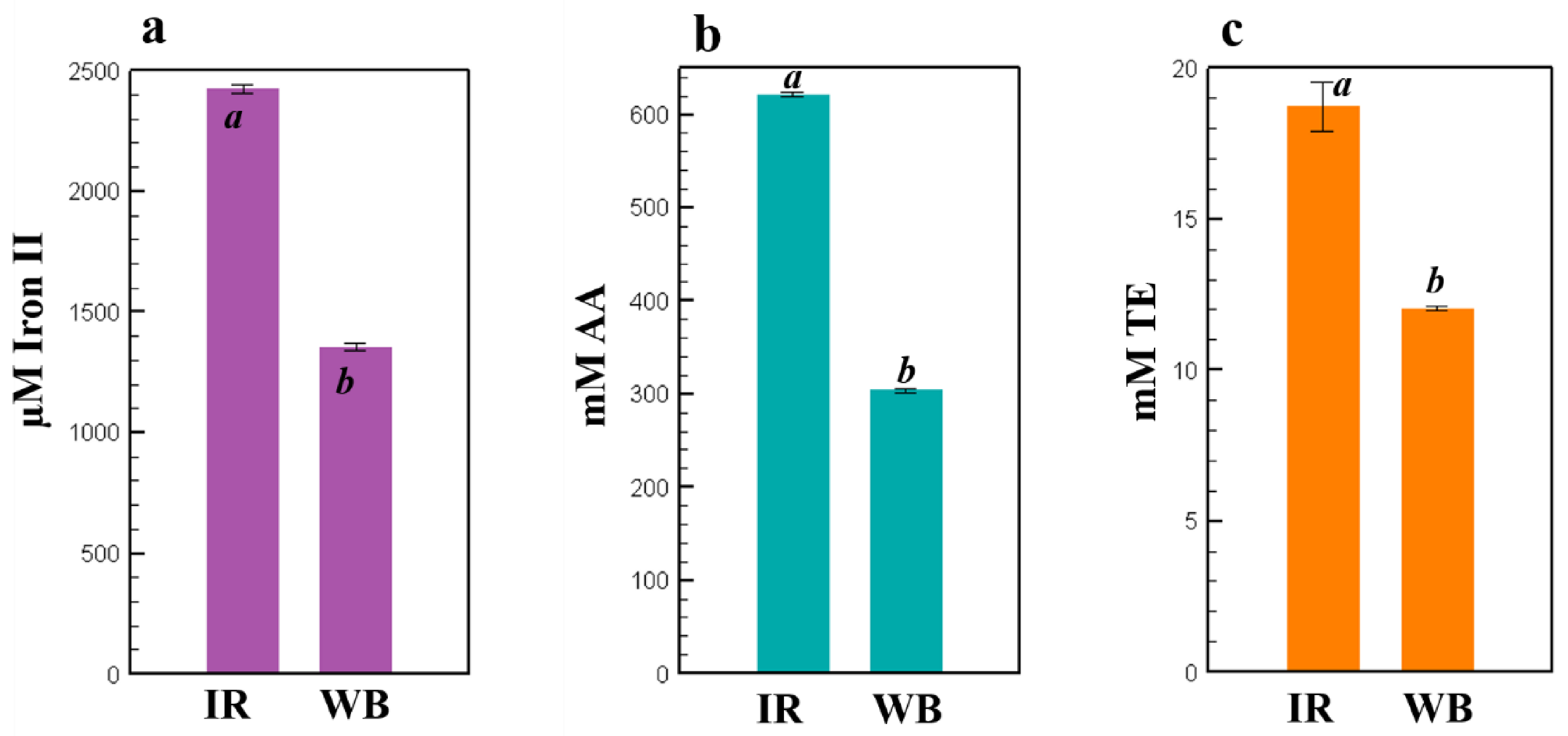
2.5. Antioxidant Activities of IR and WB Extracts Obtained under the Optimal Extraction Conditions
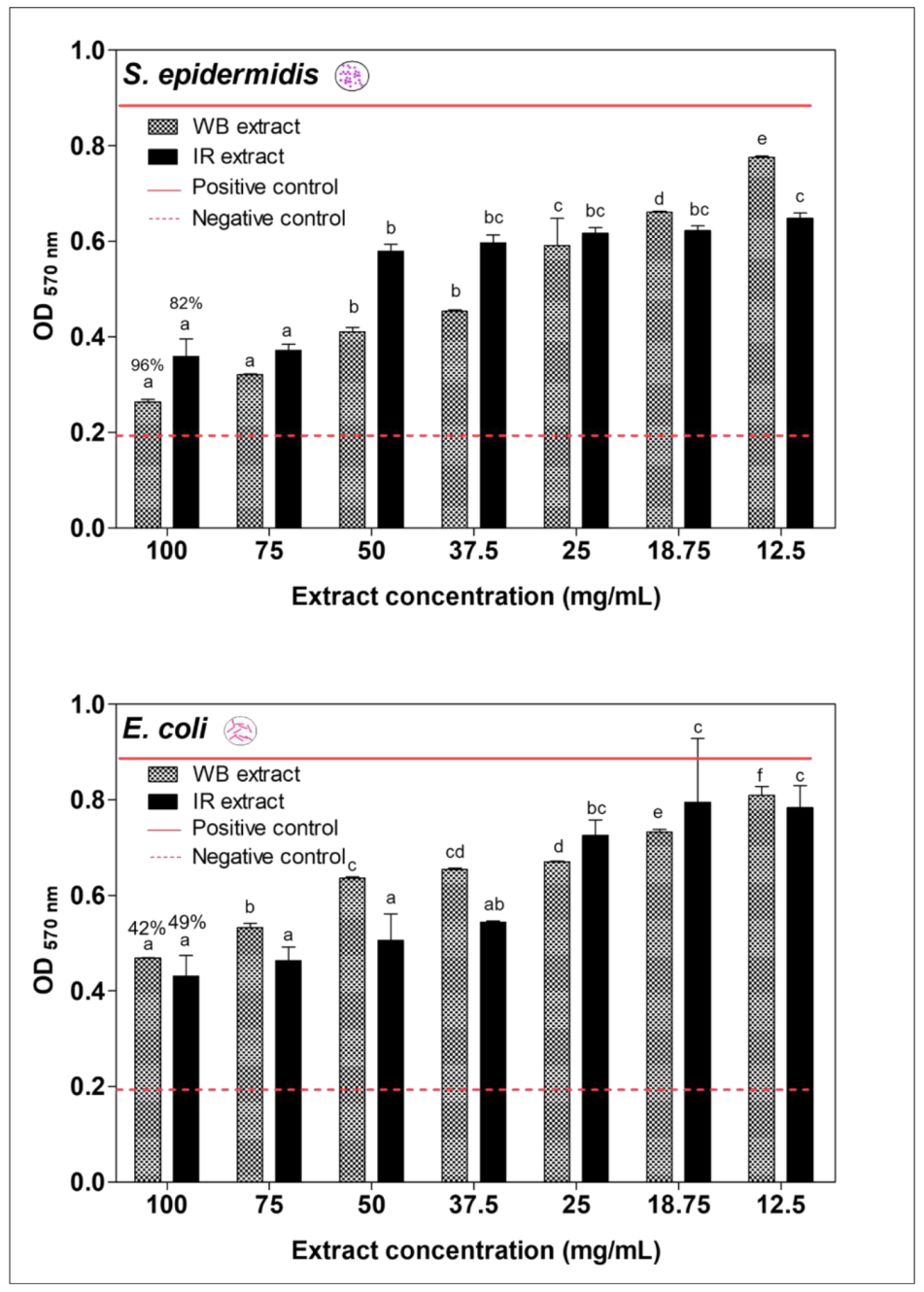
2.6. Antibacterial Activities of the Optimal Conditions for IR and WB Extracts
2.7. Antibiofilm Prevention Activity of IR and WB Extracts Obtained at Optimal Extraction Condition
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals, Media, and Bacterial Strains
4.3. Dry Matter Content
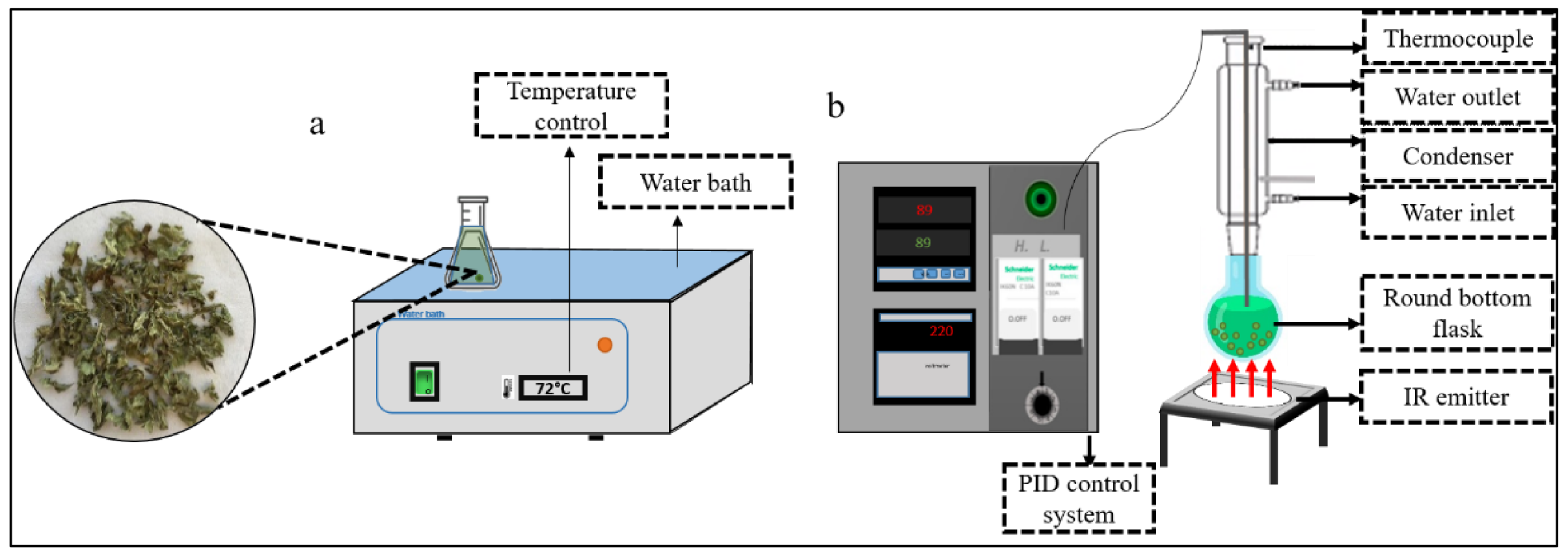
4.4. Extraction Procedures
4.4.1. Water Bath Extraction (WB)
4.4.2. Infrared-Assisted Extraction (IR)
4.5. Experimental Design
4.6. Determination of TPC
4.7. Ultra–High–Performance Liquid Chromatography (UHPLC)
4.8. Antiradical Activity
4.9. Antioxidant Activity
4.9.1. Ferric Reducing Antioxidant Power Assay (FRAP)
4.9.2. 2,2′-Azinobis (3-Ethylbenzothiazoline-6-Sulphonic Acid) Radical Scavenging Assay (ABTS)
4.9.3. Cupric Ion Reducing Antioxidant Capacity Assay (CUPRAC)
4.10. Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC) Assays
4.11. Biofilm Prevention Assay
4.12. Statistical Analysis
5. Conclusions
6. Patent
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Küpeli, E.; Kartal, M.; Aslan, S.; Yesilada, E. Comparative Evaluation of the Anti-Inflammatory and Antinociceptive Activity of Turkish Eryngium Species. J. Ethnopharmacol. 2006, 107, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Kikowskaa, M.; Dworacka, M.; Kędziora, I.; Thiem, B. Eryngium Creticum – Ethnopharmacology, Phytochemistry and Pharmacological Activity. A Review. Braz. J. Pharmacogn. 2016, 26, 392–399. [Google Scholar] [CrossRef]
- Kartal, M.; Mitaine-Offer, A.-C.; Abu-Asaker, M.; Miyamoto, T.; Calis, I.; Wagner, H.; Lacaille-Dubois, M.-A. Two New Triterpene Saponins from Eryngium Campestre. Chem. Pharm. Bull. 2005, 53, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Su, Z.; Yuan, W.; Deng, G.; Li, S. Phytochemical Constituents and Pharmacological Activities of Eryngium L. (Apiaceae). Pharm. Crop. 2012, 3, 99–120. [Google Scholar] [CrossRef]
- Erdelmeier, C.A.J.; Sticher, O. Coumarin Derivatives from Eryngium Campestre. Planta Med. 1985, 51, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Aires, A. Phenolics in Foods: Extraction, Analysis and Measurements. In Henolic Compounds—Natural Sources, Importance and Applications; Soto-Hernandez, M., Palma-Tenango, M., Garcia-Mateos, M.d.R., Eds.; IntechOpen: London, UIK, 2017. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Rajha, H.N.; Debs, E.; Maroun, R.G.; Louka, N. Système d’extraction, de Séparation Ou de Prétraitement Assisté Par Rayonnement Infrarouge. Adéquation Entre Les Caractéristiques Du Rayonnement et Celles de La Matière Traitée. Lebanese Patent 29 November 2017. [Google Scholar]
- Abi-Khattar, A.-M.; Rajha, H.N.; Abdel-Massih, R.M.; Maroun, R.G.; Louka, N.; Debs, E. Intensification of Polyphenol Extraction from Olive Leaves Using Ired-Irrad®, an Environmentally-Friendly Innovative Technology. Antioxidants 2019, 8, 227. [Google Scholar] [CrossRef] [Green Version]
- Escobedo, R.; Miranda, R.; Martínez, J. Infrared Irradiation: Toward Green Chemistry: A Review. Int. J. Mol. Sci. 2016, 17, 453. [Google Scholar] [CrossRef]
- Rajha, H.N.; Mhanna, T.; El Kantar, S.; El Khoury, A.; Louka, N.; Maroun, R.G. Innovative Process of Polyphenol Recovery from Pomegranate Peels by Combining Green Deep Eutectic Solvents and a New Infrared Technology. LWT Food Sci. Technol. 2019, 111, 138–146. [Google Scholar] [CrossRef]
- El Kantar, S.; Rajha, H.N.; Maroun, R.G.; Louka, N. Intensification of Polyphenols Extraction from Orange Peels Using Infrared as a Novel and Energy Saving Pretreatment. Food Eng. Mater. Sci. Nanotechnol. Sci. Nanotechnol. 2020, 85, 414–420. [Google Scholar] [CrossRef]
- Pinelo, M.; Rubilar, M.; Jerez, M.; Sineiro, J.; Núñez, M.J. Effect of Solvent, Temperature, and Solvent-to-Solid Ratio on the Total Phenolic Content and Antiradical Activity of Extracts from Different Components of Grape Pomace. J. Agric. Food Chem. 2005, 53, 2111–2117. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; Faveri, D.M. De Effects of Extraction Time, Temperature and Solvent on Concentration and Antioxidant Activity of Grape Marc Phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Rajha, H.N.; El Darra, N.; Vorobiev, E.; Louka, N.; Maroun, R.G. An Environment Friendly, Low-Cost Extraction Process of Phenolic Compounds from Grape Byproducts. Optimization by Multi-Response Surface Methodology. Food Nutr. Sci. 2013, 04, 650–659. [Google Scholar] [CrossRef]
- Cacace, J.E.; Mazza, G. Mass Transfer Process during Extraction of Phenolic Compounds from Milled Berries. J. Food Eng. 2003, 59, 379–389. [Google Scholar] [CrossRef]
- Rajha, H.N.; El Darra, N.; Hobaika, Z.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Extraction of Total Phenolic Compounds, Flavonoids, Anthocyanins and Tannins from Grape Byproducts by Response Surface Methodology. Influence of Solid-Liquid Ratio, Particle Size, Time, Temperature and Solvent Mixtures on the Optimization Process. Food Nutr. Sci. 2014, 5, 397–409. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, G.; Xie, M.; Chen, B.; Li, Y. Infrared-Assisted Extraction Coupled with High-Performance Liquid Chromatography for Simultaneous Determination of Eight Active Compounds in Radix Salviae Miltiorrhizae. J. Sep. Sci. 2010, 33, 2888–2897. [Google Scholar] [CrossRef]
- Richardson, P. Thermal Technologies in Food Processing; Elsevier: Amsterdam, The Netherlands, 2001; ISBN 0849312167. [Google Scholar]
- Baldosano, H.; Castillo, M.G.; Elloran, C.; Bacani, F.T. Effect of Particle Size, Solvent and Extraction Time on Tannin Extract from Spondias Purpurea Bark Through Soxhlet Extraction. Proc. DLSU Res. Congr. 2015, 3, 4–9. [Google Scholar]
- Dent, M.; Uzelac, V.; Penić, M.; Brnčić, M.; Bosiljkov, T.; Levaj, B. The Effect of Extraction Solvents, Temperature and Time on the Composition and Mass Fraction of Polyphenols in Dalmatian Wild Sage (Salvia officinalis L.) Extracts. Food Technol. Biotechnol. 2012, 51, 84–91. [Google Scholar]
- Cai, Y.; Yu, Y.; Duan, G.; Li, Y. Study on Infrared-Assisted Extraction Coupled with High Performance Liquid Chromatography (HPLC) for Determination of Catechin, Epicatechin, and Procyanidin B2 in Grape Seeds. Food Chem. 2011, 127, 1872–1877. [Google Scholar] [CrossRef]
- Paun, G.; Neagu, E.; Moroeanu, V.; Albu, C.; Savin, S.; Lucian Radu, G. Chemical and Bioactivity Evaluation of Eryngium Planum and Cnicus Benedictus Polyphenolic-Rich Extracts. Biomed Res. Int. 2019, 2019, 3692605. [Google Scholar] [CrossRef]
- Conea, S.; Vlase, L.; Chirila, I. Comparative Study on the Polyphenols and Pectin of Three Eryngium Species and Their Antimicrobial Activity. Cellul. Chem. Technol. 2016, 50, 473–481. [Google Scholar]
- Kartal, M.; Mitaine-Offer, A.-C.; Abu-Asaker, M.; Miyamoto, T.; Calis., I.; Wagner, H. Phytochemical Constituents and Pharmacological Activities of Eryngium L. (Apiaceae). Planta Med. 2005, 63, 99–120. [Google Scholar]
- Elsbaey, M.; Ibrahim, M.A.A.; Shawky, A.M.; Miyamoto, T. Eryngium Creticum L.: Chemical Characterization, SARS-CoV-2 Inhibitory Activity, and In Silico Study. ACS Omega 2022, 7. [Google Scholar] [CrossRef] [PubMed]
- Maroun, R.G.; Rajha, H.N.; El Darra, N.; El Kantar, S.; Chacar, S.; Debs, E.; Vorobiev, E.; Louka, N. Emerging Technologies for the Extraction of Polyphenols from Natural Sources; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128135723. [Google Scholar]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A Review on Extraction, Identification and Purification Methods, Biological Activities and Approaches to Enhance Its Bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Martinović, N.; Abramovi, H. Sinapic Acid and Its Derivatives: Natural Sources and Bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar]
- Amarowicz, R.; Fornal, J.; Karamac, M. Antioxidative and Bactericidal Properties of Phenolic Compounds in Rapeseeds. Eur. J. Lipid Sci. Technol. 1992, 94, 149–152. [Google Scholar]
- Damaj, R.; Sabbah, A.; Nasser, G.; Francis, M.B.; Hijazi, A.; Annan, H.; Al Rekaby, A.A.A.; Rammal, H. Antioxidant Activity and Chemical Composition of the Ethanolic Extract from Leaves and Stems of the Lebanese E. Cretcium. J. Multidiscip. Eng. Sci. Technol. 2016, 3, 5813–5823. [Google Scholar]
- Makki, R.; Ze, D.; Rammal, H.; Sweidan, A.; Al Bazzal, A.; Chokr, A. Antibacterial Activity of Two Lebanese Plants: Eryngium Creticum and Centranthus Longiflorus. Nanomedicine Nanotechnol. 2016, 6. [Google Scholar] [CrossRef]
- Meot-Duros, L.; Le Floch, G.; Magné, C. Radical Scavenging, Antioxidant and Antimicrobial Activities of Halophytic Species. J. Ethnopharmacol. 2008, 116, 258–262. [Google Scholar] [CrossRef]
- Tohmé, G.; Tohmé, H. Illustrated Flora of Lebanon; National Council for Scientific Research: Beirut, Lebanon, 2007. [Google Scholar]
- Slinkard, K.; Singleton, V. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Kallithraka, S.; Mohdaly, A.A.A.; Makris, D.P.; Kefalas, P. Determination of Major Anthocyanin Pigments in Hellenic Native Grape Varieties (Vitis vinifera sp.): Association with Antiradical Activity. J. Food Compos. Anal. 2005, 18, 375–386. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 7th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2006; ISBN 1-56238-989-0. [Google Scholar]
- Kanaan, H.; El-Mestrah, M.; Sweidan, A.; As-Sadi, F.; Al Bazzal, A.; Chokr, A. Screening for Antibacterial and Antibiofilm Activities in Astragalus Angulosus. J. Intercult. Ethnopharmacol. 2017, 6, 50–57. [Google Scholar] [CrossRef]
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of Coagulase-Negative Staphylococci to Plastic Tissue Culture Plates: A Quantitative Model for the Adherence of Staphylococci to Medical Devices. J. Clin. Microbiol. 1985, 22, 966–1006. [Google Scholar] [CrossRef]
- Chokr, A.; Watier, D.; Eleaume, H.; Pangon, B.; Ghnassia, J.-C.; Dietrich, M.; Jabbouri, S. Correlation between Biofilm Formation and Production of Polysaccharide Intercellular Adhesin in Clinical Isolates of Coagulase-Negative Staphylococci. Int. J. Med. Microbiol. 2006, 296, 381–388. [Google Scholar] [CrossRef]

| Run | Variable | Responses | |||||
|---|---|---|---|---|---|---|---|
| Time (min) | Temperature (°C) | Ethanol Percentage (%) | IR | WB | |||
| TPC (mg GAE/g DM) | DPPH Inhibition Percentage | TPC (mg GAE/g DM) | DPPH Inhibition Percentage | ||||
| 1 | 70 | 35 | 30 | 23.39 | 45.15 | 17.46 | 25.45 |
| 2 | 170 | 35 | 30 | 26.08 | 53.10 | 19.42 | 38.93 |
| 3 | 70 | 75 | 30 | 44.59 | 81.80 | 35.56 | 7.42 |
| 4 | 170 | 75 | 30 | 47.73 | 77.71 | 40.87 | 28.65 |
| 5 | 70 | 35 | 70 | 18.13 | 33.53 | 12.55 | 61.53 |
| 6 | 170 | 35 | 70 | 23.04 | 44.70 | 17.99 | 41.21 |
| 7 | 70 | 75 | 70 | 45.23 | 65.50 | 35.02 | 56.51 |
| 8 | 170 | 75 | 70 | 47.14 | 55.04 | 43.14 | 56.96 |
| 9 | 36 | 55 | 50 | 30.69 | 41.41 | 20.19 | 11.98 |
| 10 | 204 | 55 | 50 | 44.93 | 55.29 | 38.29 | 53.05 |
| 11 | 120 | 21 | 50 | 26.28 | 34.95 | 15.68 | 16.21 |
| 12 | 120 | 88 | 50 | 73.53 | 83.20 | 57.20 | 23.17 |
| 13 | 120 | 55 | 16 | 35.41 | 50.33 | 28.36 | 14.52 |
| 14 | 120 | 55 | 83 | 37.41 | 52.26 | 25.76 | 70.43 |
| 15 | 120 | 55 | 50 | 39.42 | 80.81 | 36.42 | 66.71 |
| 16 | 120 | 55 | 50 | 39.17 | 80.84 | 35.97 | 69.63 |
| 17 | 120 | 55 | 50 | 40.93 | 80.46 | 35.51 | 67.09 |
| 18 | 120 | 55 | 50 | 40.58 | 80.36 | 36.40 | 67.81 |
| 19 | 120 | 55 | 50 | 39.42 | 80.94 | 34.68 | 68.15 |
| 20 | 120 | 55 | 50 | 40.48 | 79.19 | 35.27 | 68.72 |
| 21 | 120 | 55 | 50 | 40.82 | 78.42 | 34.23 | 69.63 |
| 22 | 120 | 55 | 50 | 40.73 | 79.71 | 34.68 | 67.33 |
| Extraction Technique | Equations |
|---|---|
| IR | TPC = − 10.21 + 0.29 t + 0.0015 T + 0.52 E − 0.0009 t2 − 0.0003 t T + 0.0001 t E + 0.005 T2 + 0.003 T E − 0.007 E2 DPPH = − 159.174 + 1.25 t + 3.19 T + 2.41 E − 0.004 t2 − 0.004 t T − 0.0004 t E − 0.015 T2 − 0.0056 T E − 0.02 E2 |
| WB | TPC = − 26.83 + 0.26 t + 0.42 T + 0.62 E − 0.00111123 t2 + 0.0007 t T + 0.0008 t E − 0.0006 T2 + 0.002 T E − 0.009 E2 DPPH = − 184.92 + 1.27 t + 3.06 T + 2.65 E− 0.004 t2 + 0.0035 t T− 0.007 t E − 0.03 T2 + 0.012 T E − 0.017 E2 |
| Parameters | Optimum Conditions | |||
|---|---|---|---|---|
| WB | IR | |||
| TPC | DPPH | TPC | DPPH | |
| Time (min) | 167 | 116 | 130 | 113 |
| Temperature (°C) | 89 | 58 | 88 | 79 |
| Ethanol (%) | 75 | 74 | 55 | 42 |
| TPC optimal value (mg GAE/g DM) | 56.6 | 77 | ||
| DPPH optimal value (%) | 76 | 88.8 | ||
| R-squared | 97.3 | 92.65 | 92.4 | 94.17 |
| Parameters | Multiple Optimization | |||
| WB | IR | |||
| Time (min) | 162 | 109 | ||
| Temperature (°C) | 91 | 89 | ||
| Ethanol (%) | 75 | 50 | ||
| TPC value predicted (mg GAE/g DM) | 45.5 | 76 | ||
| TPC value observed (mg GAE/g DM) | 44.60 ± 1.6 | 75.83 ± 1.3 | ||
| DPPH inhibition percentage predicted (%) | 66.2 | 83.7 | ||
| DPPH inhibition percentage observed (%) | 63.10 ± 1.3 | 82.80 ± 0.5 | ||
| Extraction Method | Concentration (μg/mL) | |
|---|---|---|
| Rutin | Sinapic Acid | |
| IR | 9.8 | 4 |
| WB | 6.4 | 1.3 |
| Plant extract | MIC (mg/mL) | MBC (mg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| E. coli | P. aeruginosa | S. epidermidis | S. aureus | E. coli | P. aeruginosa | S. epidermidis | S. aureus | |
| IR | 75 | 100 | 75 | 100 | 100 | >100 | 100 | >100 |
| WB | 100 | 100 | 50 | 100 | 100 | 100 | 75 | 100 |
| Bacterial Strains | Reference | |
|---|---|---|
| Gram-positive | Staphylococcus aureus | ATCC 49619 |
| Staphylococcus epidermidis RP62A | ATCC 35984 | |
| Gram-negative | Escherichia coli | ATCC 35218 |
| Pseudomonas aeruginosa | ATCC 27853 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammoud, M.; Chokr, A.; Rajha, H.N.; Safi, C.; Walsem, M.v.; Broek, L.A.M.v.d.; Debs, E.; Maroun, R.G.; Louka, N.; Rammal, H. Intensification of Polyphenols Extraction from Eryngium creticum Leaves Using Ired-Irrad® and Evaluation of Antibiofilm and Antibacterial Activities. Plants 2022, 11, 2458. https://doi.org/10.3390/plants11192458
Hammoud M, Chokr A, Rajha HN, Safi C, Walsem Mv, Broek LAMvd, Debs E, Maroun RG, Louka N, Rammal H. Intensification of Polyphenols Extraction from Eryngium creticum Leaves Using Ired-Irrad® and Evaluation of Antibiofilm and Antibacterial Activities. Plants. 2022; 11(19):2458. https://doi.org/10.3390/plants11192458
Chicago/Turabian StyleHammoud, Mariam, Ali Chokr, Hiba N. Rajha, Carl Safi, Martijn van Walsem, Lambertus A. M. van den Broek, Espérance Debs, Richard G. Maroun, Nicolas Louka, and Hassan Rammal. 2022. "Intensification of Polyphenols Extraction from Eryngium creticum Leaves Using Ired-Irrad® and Evaluation of Antibiofilm and Antibacterial Activities" Plants 11, no. 19: 2458. https://doi.org/10.3390/plants11192458
APA StyleHammoud, M., Chokr, A., Rajha, H. N., Safi, C., Walsem, M. v., Broek, L. A. M. v. d., Debs, E., Maroun, R. G., Louka, N., & Rammal, H. (2022). Intensification of Polyphenols Extraction from Eryngium creticum Leaves Using Ired-Irrad® and Evaluation of Antibiofilm and Antibacterial Activities. Plants, 11(19), 2458. https://doi.org/10.3390/plants11192458











