Jatropha curcas L. as a Plant Model for Studies on Vegetative Propagation of Native Forest Plants
Abstract
Highlights
- Jatropha curcas L. has great potential to be used as a model plant in several studies involving native forest species.
- The immersion in the 2,4-D solution accelerated the emission of primary roots in hardwood cuttings.
- Studies on vegetative propagation of native species can use Jatropha curcas L. species as a model for obtaining important information in a short time and reducing labor costs.
- The immersion of cuttings of native species in solutions with low concentrations of 2,4-D can favor the rooting process and vegetative propagation.
Abstract
1. Introduction
1.1. Why Can Jatropha Be an Excellent Plant Model for Studies on Vegetative Propagation of Native Forest Plants?
1.2. Plant Regulators and Vegetative Propagation of Jatropha Plants
2. Material and Methods
2.1. Experimental Conditions
2.2. Experimental Design
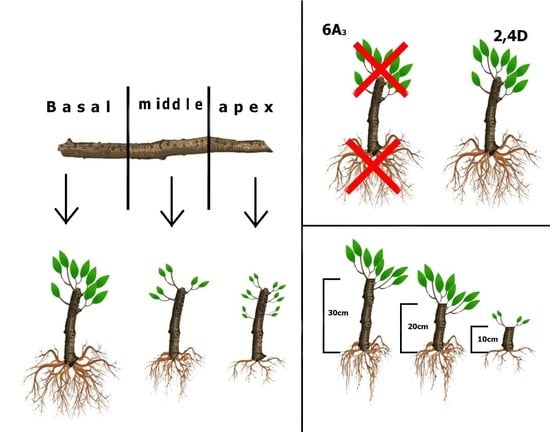
2.2.1. Experiment I—Hardwood Cutting Length
2.2.2. Experiment II—Micronutrients and Plant Regulators
2.3. Implementation and Conduction of Experiments
2.4. Statistical Analysis
3. Results
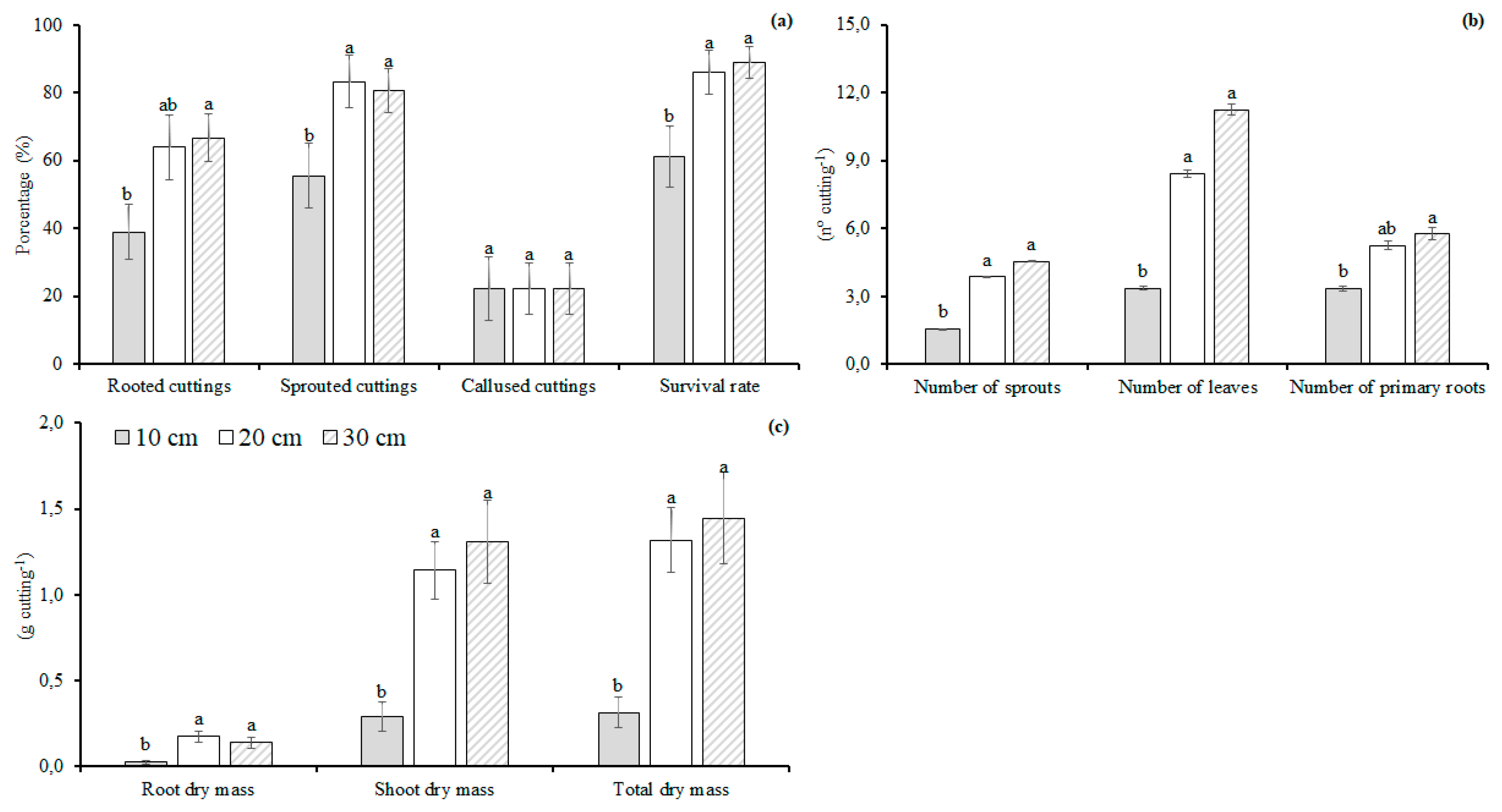
3.1. Hardwood Cutting Length
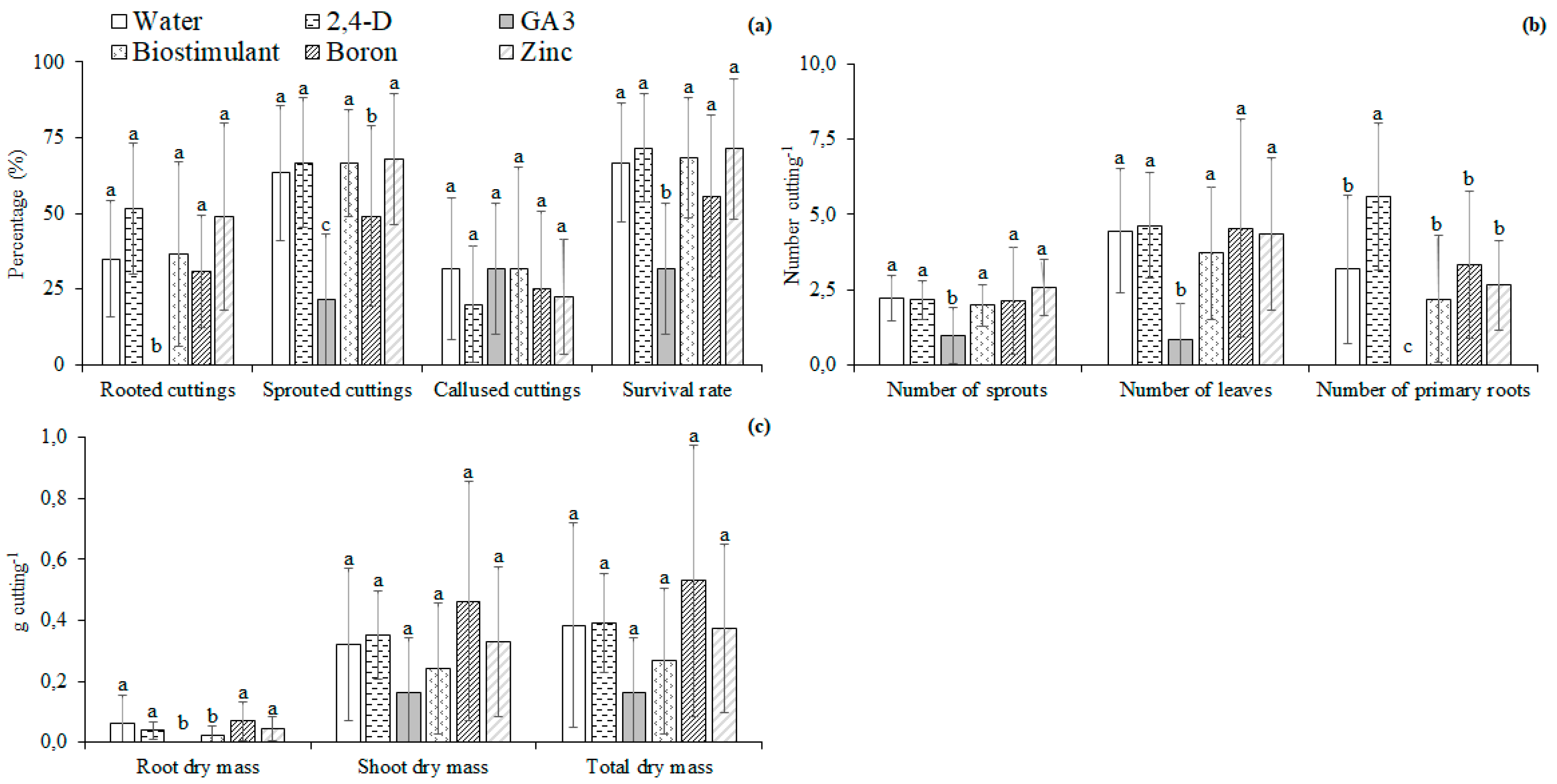
3.2. Micronutrients and Plant Regulators
4. Discussion
4.1. Hardwood Cutting Length
4.2. Micronutrients and Plant Regulators
4.3. Effect of Nutrients and Hormonal Treatment Was the Part-Specific
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neupane, D.; Bhattarai, D.; Ahmed, Z.; Das, B.; Pandey, S.; Solomon, J.K.Q.; Qin, R.; Adhikari, P. Growing Jatropha (Jatropha curcas L.) as a Potential Second-Generation Biodiesel Feedstock. Inventions 2021, 6, 60. [Google Scholar] [CrossRef]
- Chauhan, K.C.; Scholar, H.S.; Pradesh, U.; Kumar, I.N.; Sharma, H.; Kumar, N. Study of growth parameter on different genotype of Jatropha curcas in clustering pattern to improve yield and oil contant. Pharma Innov. J. 2021, 10, 1030–1033. Available online: http://www.thepharmajournal.com (accessed on 5 April 2022).
- Ewunie, G.A.; Morken, J.; Lekang, O.I.; Yigezu, Z.D. Factors affecting the potential of Jatropha curcas for sustainable bio-diesel production: A critical review. Renew. Sustain. Energy Rev. 2020, 137, 110500. [Google Scholar] [CrossRef]
- Ranucci, C.R.; Alves, H.J.; Monteiro, M.R.; Kugelmeier, C.L.; Bariccatti, R.A.; de Oliveira, C.R.; da Silva, E.A. Potential alter-native aviation fuel from jatropha (Jatropha curcas L.), babassu (Orbignya phalerata) and palm kernel (Elaeis guineensis) as blends with Jet-A1 kerosene. J. Clean. Prod. 2018, 185, 860–869. [Google Scholar] [CrossRef]
- Hashimoto, N.; Nishida, H.; Kimoto, M.; Tainaka, K.; Ikeda, A.; Umemoto, S. Effects of Jatropha oil blending with C-heavy oil on soot emissions and heat absorption balance characteristics for boiler combustion. Renew. Energy 2018, 126, 924–932. [Google Scholar] [CrossRef]
- Mitra, S.; Ghose, A.; Gujre, N.; Senthilkumar, S.; Borah, P.; Paul, A.; Rangan, L. A review on environmental and socioeco-nomic perspectives of three promising biofuel plants Jatropha curcas, Pongamia pinnata and Mesua ferrea. Biomass-Bioenergy 2021, 151, 106173. [Google Scholar] [CrossRef]
- Faller, E.M.; Brian, J.F.; Grant, A.S.; Isabel, J.G.; Rose, W.L. A review on the medicinal uses and toxicological effects of herbal plant Jatropha curcas L. Int. J. Res. Publ. Rev. 2022, 3, 3389–3408. Available online: https://ijrpr.com/uploads/V3ISSUE5/IJRPR4389.pdf (accessed on 5 May 2022).
- Pereira, I.R.; D’Abadia, P.L.; Prado, A.D.L.D.; Matos, F.; Nabout, J.; Gonçalves, P.; Almeida, L. Trends and gaps in the global scientific literature about Jatropha curcas L. (Euphorbiaceae), a tropical plant of economic importance. Semin. Ciências Agrárias 2018, 39, 7–18. [Google Scholar] [CrossRef]
- Oliveira, M.D.F.D.C.; Santos, D.P.; Figueredo, K.S.; Reis, F.D.O.; Ferraz, T.M.; Braun, H.; Campostrini, E.; Figueiredo, F.A.M.M.D.A. Production and quality of eucalyptus mini-cuttings using kaolin-based particle films. J. For. Res. 2020, 32, 953–965. [Google Scholar] [CrossRef]
- Investigating, H.; Rihn, A.L.; Knuth, M.J.; Peterson, B.J.; Torres, A.P.; Campbell, J.H.; Boyer, C.R.; Palma, M.A.; Khacha-tryan, H. Investigating Drivers of Native Plant Production in the United States Green Industry. Sustainability 2022, 14, 6774. [Google Scholar] [CrossRef]
- McCormick, M.L.; Carr, A.N.; Massatti, R.; Winkler, D.E.; De Angelis, P.; Olwell, P. How to increase the supply of native seed to improve restoration success: The US native seed development process. Restor. Ecol. 2021, 29, e13499. [Google Scholar] [CrossRef]
- Ingvarsson, P.K.; Dahlberg, H. The effects of clonal forestry on genetic diversity in wild and domesticated stands of forest trees. Scand. J. For. Res. 2018, 34, 370–379. [Google Scholar] [CrossRef]
- Da, J.F.; Junior, S.; Vidigal, F.; Souza, D.; Pádua, J.G. EMBRAPA. A Arca de Noé das Frutas Nativas Brasileiras. 2021. Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1136847/a-arca-de-noe-das-frutas-nativas-brasileiras?link-banner-inicial (accessed on 5 May 2022).
- Meinke, D.W.; Cherry, J.M.; Dean, C.; Rounsley, S.D.; Koornneef, M. Arabidopsis thaliana: A Model Plant for Genome Analy-sis. Science 1998, 282, 662–682. [Google Scholar] [CrossRef]
- De-Farias, L.F.; Silva, S.A.; Aona, L.Y.; DE Oliveira, F.F. Floral biology and reproductive system of physic nut (Jatropha curcas L., Euphorbiaceae) in ’Recôncavo da Bahia’, Brazil. An. Acad. Bras. Ciências 2022, 94, 20201814. [Google Scholar] [CrossRef]
- Nair, K.P. Rubber. In Tree Crops; Springer: Cham, Switzerland, 2021; pp. 287–332. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Clarindo, W.R.; Praça, M.M.; Araújo, F.S.; Carels, N. Genome size, base composition and karyotype of Jatropha curcas L., an important biofuel plant. Plant Sci. 2008, 174, 613–617. [Google Scholar] [CrossRef]
- Arockiasamy, S.; Kumpatla, J.; Hadole, S.; Yepuri, V.; Patil, M.; Shrivastava, V.; Rao, C.; Kancharla, N.; Jalali, S.; Varshney, A.; et al. Breeding and biotechnological efforts in Jatropha curcas L. for sustainable yields. Oil Crop Sci. 2021, 6, 180–191. [Google Scholar] [CrossRef]
- Chun-Yun, L.; Yang, Z.; Xiu-Rong, W. Characterization of the complete chloroplast genome of Jatropha curcas var. ni-groviensrugosus. Mitochondrial DNA Part B 2022, 7, 222–223. [Google Scholar] [CrossRef]
- Drumond, M.A.; De Arruda, F.P.; Dos Anjos, J.B.; Semi-Árido, E. PINHÃO-MANSO-Jatropha curcas L. Empresa Brasileira de Pesquisa Agropecuária Embrapa Semi-Árido Ministério da Agricultura, Pecuária e Abastecimento. 2008. Available online: http://www.cpatsa.embrapa.br (accessed on 5 May 2022).
- Kettenhuber, P.W.; Sousa, R.; Sutili, F. Vegetative Propagation of Brazilian Native Species for Restoration of Degraded Areas. Floresta Ambient. 2019, 26, 20170956. [Google Scholar] [CrossRef]
- Reddy, M.C.; Indu, K.; Bhargavi, C.; Rajendra, M.P.; Babu, B.H. A review on vegetative propagation and applications in forestry. J. Plant Dev. Sci. 2022, 14, 265–272. [Google Scholar]
- Sandhya, S.; Mehta, S.; Pandey, S.; Husen, A. Adventitious root formation in cuttings as influenced by genotypes, leaf area, and types of cuttings. Environ. Physiol. Chem. Control. Adventitious Rooting Cut. 2022, 381–395. [Google Scholar] [CrossRef]
- Guirra, B.S.; da Silva, M.F.; da Silva, J.E.S.B.; Guirra, K.S. Nutrição da planta matriz determina a qualidade de propágulos. Cad. Ciências Agrárias 2021, 13, 1–6. [Google Scholar] [CrossRef]
- Saini, K.; Markakis, M.N.; Zdanio, M.; Balcerowicz, D.M.; Beeckman, T.; De Veylder, L.; Prinsen, E.; Beemster, G.T.S.; Vissen-berg, K. Alteration in Auxin Homeostasis and Signaling by Overexpression of PINOID Kinase Causes Leaf Growth Defects in Arabidopsis thaliana. Front. Plant Sci. 2017, 8, 1009. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.T., Kester; Kester, D.E.; Davies, F.T.; Geneve, R.L. Hartmann & Kester’s Plant Propagation: Principles and Practices; Prentice Hall/Pearson: Hoboken, NJ, USA, 2011. [Google Scholar]
- Ferreira, D.F. SISVAR: A computer analysis system to fixed effects split plot type designs. Braz. J. Biom. 2019, 37, 529–535. Available online: https://des.ufla.br/~danielff/programas/sisvar.html (accessed on 5 May 2022).
- da Conceição, S.J.L.; do Carmo, V.M.; Regina, B.D.S.E.; Neves, G.R.; Veloso, N.R. Estaquia em frutíferas do Cerrado/Cutting in fruit of the Cerrado|Souza. Braz. J. Dev. 2020, 6, 15531–15544. Available online: https://www.brazilianjournals.com/index.php/BRJD/article/view/8164 (accessed on 5 May 2022).
- Dias, P.C.; De Oliveira, L.S.; Xavier, A.; Wendling, I. Estaquia e miniestaquia de espécies florestais lenhosas do Brasil. Pesqui. Florest. Bras. 2012, 32, 453–462. [Google Scholar] [CrossRef]
- Hammer, K.; Heller, J. Promoting the conservation and use of underutilized and neglected crops. Schriften Genet. Resour. 1998, 8, 223–227. [Google Scholar]
- Barbante, K.B. Fisiologia Vegetal, 3rd ed.; Guanabara Koogan: Rio De Janeiro, Brazil, 2019. [Google Scholar]
- Severino, L.S.; Lima, R.L.S.; Lucena, A.M.A.; Freire, M.A.O.; Sampaio, L.R.; Veras, R.P.; Medeiros, K.A.A.L.; Sofiatti, V.; Arriel, N.H.C. Propagation by stem cuttings and root system structure of Jatropha curcas. Biomass Bioenergy 2011, 35, 3160–3166. [Google Scholar] [CrossRef]
- De Lima, R.L.S.; Severino, L.S.; Pereira, W.E.; De Lucena, A.M.A.; Gheyi, H.R.; Arriel, N.H.C. Comprimento das estacas e parte do ramo para formação de mudas de pinhão-manso. Rev. Bras. Eng. Agric. Ambient. 2010, 14, 1234–1239. [Google Scholar] [CrossRef]
- Kathiravan, M.; Ponnuswamy, A.S.; Chinnapaiyan, V. Determination of suitable cutting size for vegetative propagation and comparison of propagules to evaluate the seed quality attributes in Jatropha curcas Limn. Nat. Prod. Radiance 2009, 8, 162–166. [Google Scholar]
- De Lima, R.L.S.; de Siqueira, D.L.; Weber, O.B.; Cazetta, J.O. Comprimento de estacas e parte do ramo na formação de mu-das de aceroleira. SciELO 2006, 28, 83–86. [Google Scholar] [CrossRef]
- Gomes, E.N.; Krinski, D. Propagação vegetativa de Piper umbellatum L. (Piperaceae) em função de substratos e comprimentos de estacas. Sci. Agrar. 2017, 17, 31. [Google Scholar] [CrossRef][Green Version]
- Dos Santos, A.R.; Gonçalves, E.D.O.; Gibson, E.L.; Araújo, E.F.; Wendling, I.; Tertuliano, L.A.; Caldeira, M.V.W. Mini-cuttings technique for vegetative propagation of Dalbergia nigra. CERNE 2020, 26, 427–434. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Elsevier: Frisco, CO, USA, 1955. [Google Scholar]
- Goss, M.J.; Miller, M.H.; Bailey, L.D.; Grant, C.A. Root growth and distribution in relation to nutrient availability and uptake. Eur. J. Agron. 1993, 2, 57–67. [Google Scholar] [CrossRef]
- Overvoorde, P.; Fukaki, H.; Beeckman, T. Auxin Control of Root Development. Cold Spring Harb. Perspect. Biol. 2010, 2, a001537. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.O.; Júnior, J.F.G.; Rodrigues, J.D. Reguladores vegetais na quebra da dominância apical de mamoeiro (Carica papaya L.). Rev. Bras. Frutic. 2004, 26, 348–350. [Google Scholar] [CrossRef]
- Jarvis, B.C.; Booth, A. Influence of indole-butyric acid, boron, myo-inositol, vitamin D2 and seedling age on adventitious root development in cuttings of Phaseolus aureus. Physiol. Plant. 1981, 53, 213–218. [Google Scholar] [CrossRef]
- Ono, E.G.; Rodrigues, J.D.; de Pinho, S.Z. Enraizamento de estacas caulinares de kiwi (Actinidia chinensis Planch cv Abbott) tratadas com auxinas e boro. Sci. Agricola 1995, 52, 462–468. [Google Scholar] [CrossRef]
- Josten, P.; Kutschera, U. The Micronutrient Boron Causes the Development of Adventitious Roots in Sunflower Cuttings. Ann. Bot. 1999, 84, 337–342. [Google Scholar] [CrossRef]
- Nicoloso, F.T.; Lazzari, M.; Fortunato, R.P. Propagação vegetativa de Platanus acerifolia Ait: (II) efeito da aplicação de zinco, boro e ácido indolbutírico no enraizamento de estacas. Ciência Rural. 1999, 29, 487–492. [Google Scholar] [CrossRef]
- Yamashita, K.; Okamura, S.; Honsho, C.; Tetsumura, T. Zinc Treatment in Combination with Auxin Enhances Rooting of Cuttings in Taiwan Native Strain of Mango (Mangifera indica L.). Jpn. J. Trop. Agric. 2006, 50, 76–81. [Google Scholar]
- Mengel, K.; Kirkby, E.A. Principles of Plant Nutrition. In Principles of Plant Nutrition; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar] [CrossRef]
- Brian, P.W.; Hemming, H.G.; Lowe, D. Inhibition of Rooting of Cuttings by Gibberellic Acid: With one Figure in the Text. Ann. Bot. 1960, 24, 407–419. [Google Scholar] [CrossRef]
- Mitsuhashi, M.; Shibaoka, H.; Shimokoriyama, M. Portulal: A rooting promoting substance in Portulaca leaves1. Plant Cell Physiol. 1969, 10, 715–723. [Google Scholar] [CrossRef]
- Ernstsen, A.; Hansen, J. Influence of gibberellic acid and stock plant irradiance on carbohydrate content and rooting in cuttings of Scots pine seedlings (Pinus sylvestris L.). Tree Physiol. 1986, 1, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.D.; Haissig, B.E. Chemical control of adventitious root formation in cuttings. Bull. Plant Growth Regul. Soc. Am. 1990, 18, 1–17. [Google Scholar]
- Keeley, K.; Preece, J.E.; Taylor, B.H. Increased Rooting of ‘Norton’ Grape Cuttings Using Auxins and Gibberellin Biosynthesis Inhibitors. HortScience 2003, 38, 281–283. [Google Scholar] [CrossRef]
- Kawai, Y. Effects of Exogenous BAP, GA3, and ABA on Endogenous Auxin and Rooting of Grapevine Hardwood Cuttings. J. Jpn. Soc. Hortic. Sci. 1997, 66, 93–98. [Google Scholar] [CrossRef][Green Version]
- Gundersen, K. Some experiments with gibberellic acid. Acta Hortigotoburgensis 1959, 22, 87–110. [Google Scholar]
- Henrique, A.; Campinhos, E.N.; Ono, E.O.; de Pinho, S.Z. Effect of plant growth regulators in the rooting of Pinus cuttings. Braz. Arch. Biol. Technol. 2006, 49, 189–196. [Google Scholar] [CrossRef]
- Hansen, J. Light dependent promotion and inhibition of adventitious root formation by gibberellic acid. Planta 1975, 123, 203–205. [Google Scholar] [CrossRef]
- Hansen, J. Adventitious Root Formation Induced by Gibberellic Acid and Regulated by the Irradiance to the Stock Plants. Physiol. Plant. 1976, 36, 77–81. [Google Scholar] [CrossRef]
- Ljung, K.; Bhalerao, R.; Sandberg, G. Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J. 2002, 28, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Rosier, C.L.; Frampton, J.; Goldfarb, B.; Blazich, F.A.; Wise, F.C. Growth Stage, Auxin Type, and Concentration Influence Rooting of Stem Cuttings of Fraser Fir. HortScience 2004, 39, 1397–1402. [Google Scholar] [CrossRef]
- Štefančič, M.; Štampar, F.; Osterc, G. Influence of IAA and IBA on root development and quality of Prunus ‘GiSelA 5’ leafy cuttings. HortScience 2005, 40, 2052–2055. [Google Scholar] [CrossRef]
- Aseesh, P.; Sushma, T.; Dinesh, G. Role of auxin on adventitious root formation and subsequent growth of cutting raised plantlets of Ginkgo biloba L. Int. J. Biodivers. Conserv. 2011, 3, 142–146. [Google Scholar]
- Gatineau, F.; Fouché, J.; Kevers, C.; Hausman, J.; Gaspar, T. Quantitative variations of indolyl compounds including IAA, IAA-aspartate and serotonin in walnut microcuttings during root induction. Biol. Plant. 1997, 39, 131–137. [Google Scholar] [CrossRef]
- Pop, T.I.; Pamfil, D.; Bellini, C. Auxin Control in the Formation of Adventitious Roots. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 307–316. [Google Scholar] [CrossRef]
- Kochhar, S.; Singh, S.P.; Kochhar, V.K. Effect of auxins and associated biochemical changes during clonal propagation of the biofuel plant—Jatropha curcas. Biomass-Bioenergy 2008, 32, 1136–1143. [Google Scholar] [CrossRef]
- Altman, A.; Wareing, P.F. The Effect of IAA on Sugar Accumulation and Basipetal Transport of 14C-labelled Assimilates in Relation to Root Formation in Phaseolus vulgaris Cuttings. Physiol. Plant. 1975, 33, 32–38. [Google Scholar] [CrossRef]
- Camellia, N.A.N.; Thohirah, L.A.; Abdullah, N.A.P.; Khidir, O.M. Improvement on Rooting Quality of Jatropha curcas Us-ing Indole Butyric Acid (IBA). Res. J. Agric. Biol. Sci. 2009, 5, 338–343. [Google Scholar]
- Dhillon, R.; Hooda, M.; Pundeer, J.; Ahlawat, K.; Chopra, I. Effects of auxins and thiamine on the efficacy of techniques of clonal propagation in Jatropha curcas L. Biomass-Bioenergy 2011, 35, 1502–1510. [Google Scholar] [CrossRef]
- Skoog, F.; Miller, C.O. Chemical Regulation of Growth and Organ Formation in Plant Tissues Cultured in Vitro. In Molecular and Cellular Aspects of Development; Bell, E., Ed.; Harper and Row: New York, NY, USA, 1965. [Google Scholar]
- Ongaro, V.; Leyser, O. Hormonal control of shoot branching. J. Exp. Bot. 2008, 59, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-H.; Liu, Y.-B.; Zhang, X.-S. Auxin–Cytokinin Interaction Regulates Meristem Development. Mol. Plant 2011, 4, 616–625. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lustosa Sobrinho, R.; Zoz, T.; Finato, T.; Oliveira, C.E.d.S.; Neto, S.S.d.O.; Zoz, A.; Alaraidh, I.A.; Okla, M.K.; Alwasel, Y.A.; Beemster, G.; et al. Jatropha curcas L. as a Plant Model for Studies on Vegetative Propagation of Native Forest Plants. Plants 2022, 11, 2457. https://doi.org/10.3390/plants11192457
Lustosa Sobrinho R, Zoz T, Finato T, Oliveira CEdS, Neto SSdO, Zoz A, Alaraidh IA, Okla MK, Alwasel YA, Beemster G, et al. Jatropha curcas L. as a Plant Model for Studies on Vegetative Propagation of Native Forest Plants. Plants. 2022; 11(19):2457. https://doi.org/10.3390/plants11192457
Chicago/Turabian StyleLustosa Sobrinho, Renato, Tiago Zoz, Taciane Finato, Carlos Eduardo da Silva Oliveira, Sebastião Soares de Oliveira Neto, André Zoz, Ibrahim A. Alaraidh, Mohammad K. Okla, Yasmeen A. Alwasel, Gerrit Beemster, and et al. 2022. "Jatropha curcas L. as a Plant Model for Studies on Vegetative Propagation of Native Forest Plants" Plants 11, no. 19: 2457. https://doi.org/10.3390/plants11192457
APA StyleLustosa Sobrinho, R., Zoz, T., Finato, T., Oliveira, C. E. d. S., Neto, S. S. d. O., Zoz, A., Alaraidh, I. A., Okla, M. K., Alwasel, Y. A., Beemster, G., & AbdElgawad, H. (2022). Jatropha curcas L. as a Plant Model for Studies on Vegetative Propagation of Native Forest Plants. Plants, 11(19), 2457. https://doi.org/10.3390/plants11192457