Brassinosteroid Supplementation Alleviates Chromium Toxicity in Soybean (Glycine max L.) via Reducing Its Translocation
Abstract
:1. Introduction
2. Results
2.1. Effect of BRs on Seed Germination Parameters
2.2. Impact of Cr-Induced Stress on Phenotypical Trails of Soybean Seedlings
2.3. BRs Reduces the Chromium Stress by Improving Plant Growth, and Biomass Attributes
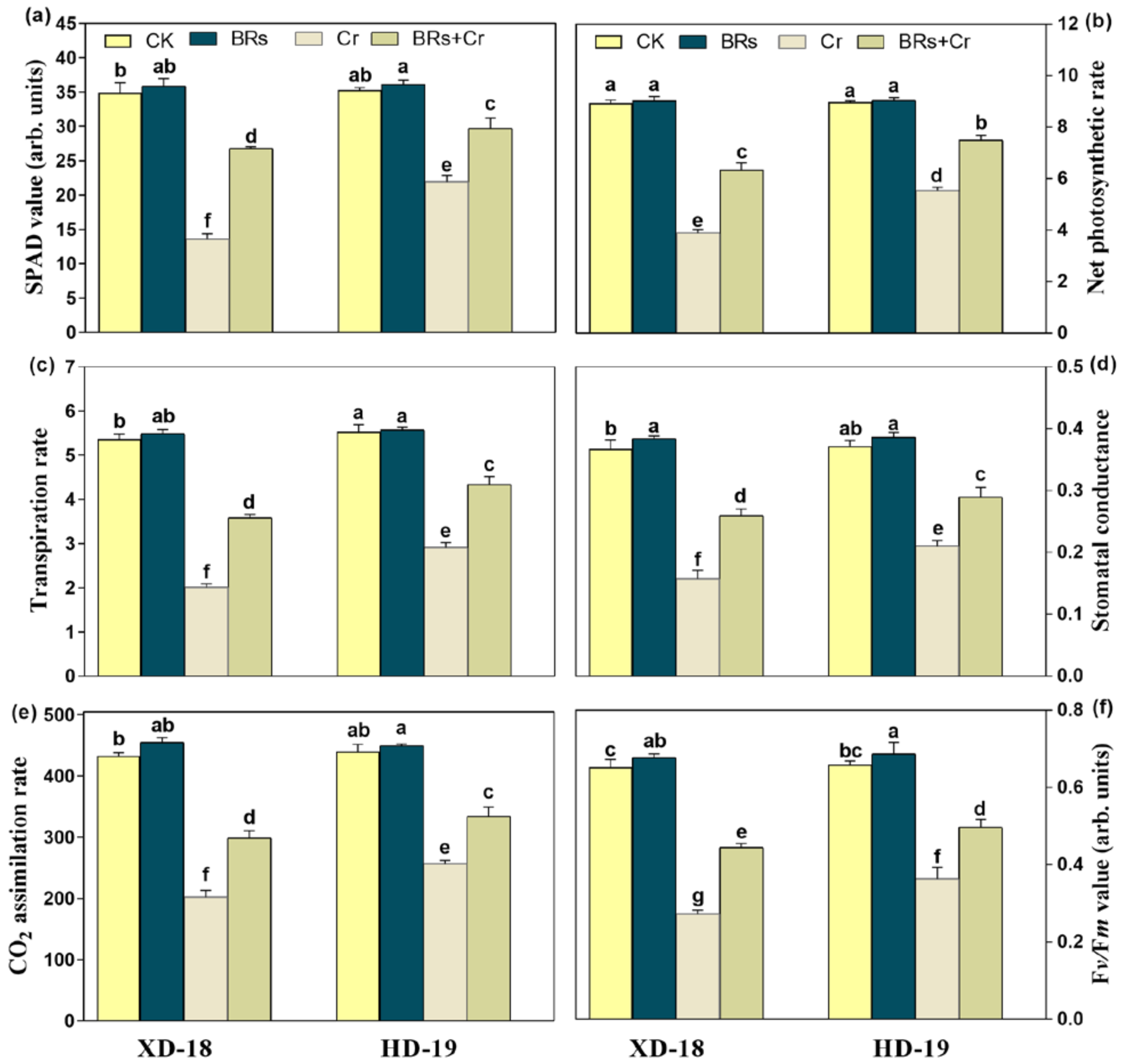
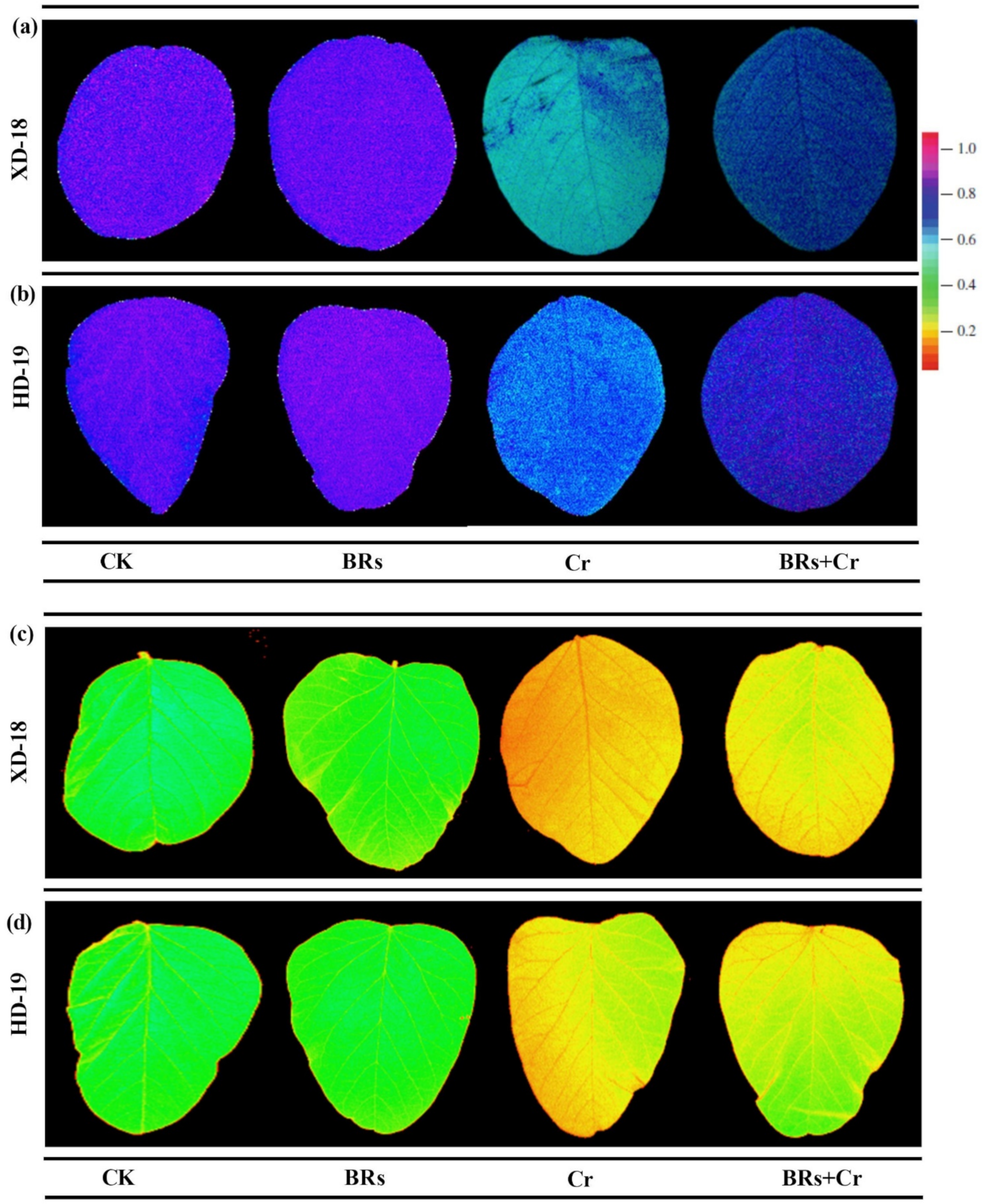
2.4. BRs Improves Plant Photosynthetic Traits under Chromium Stress
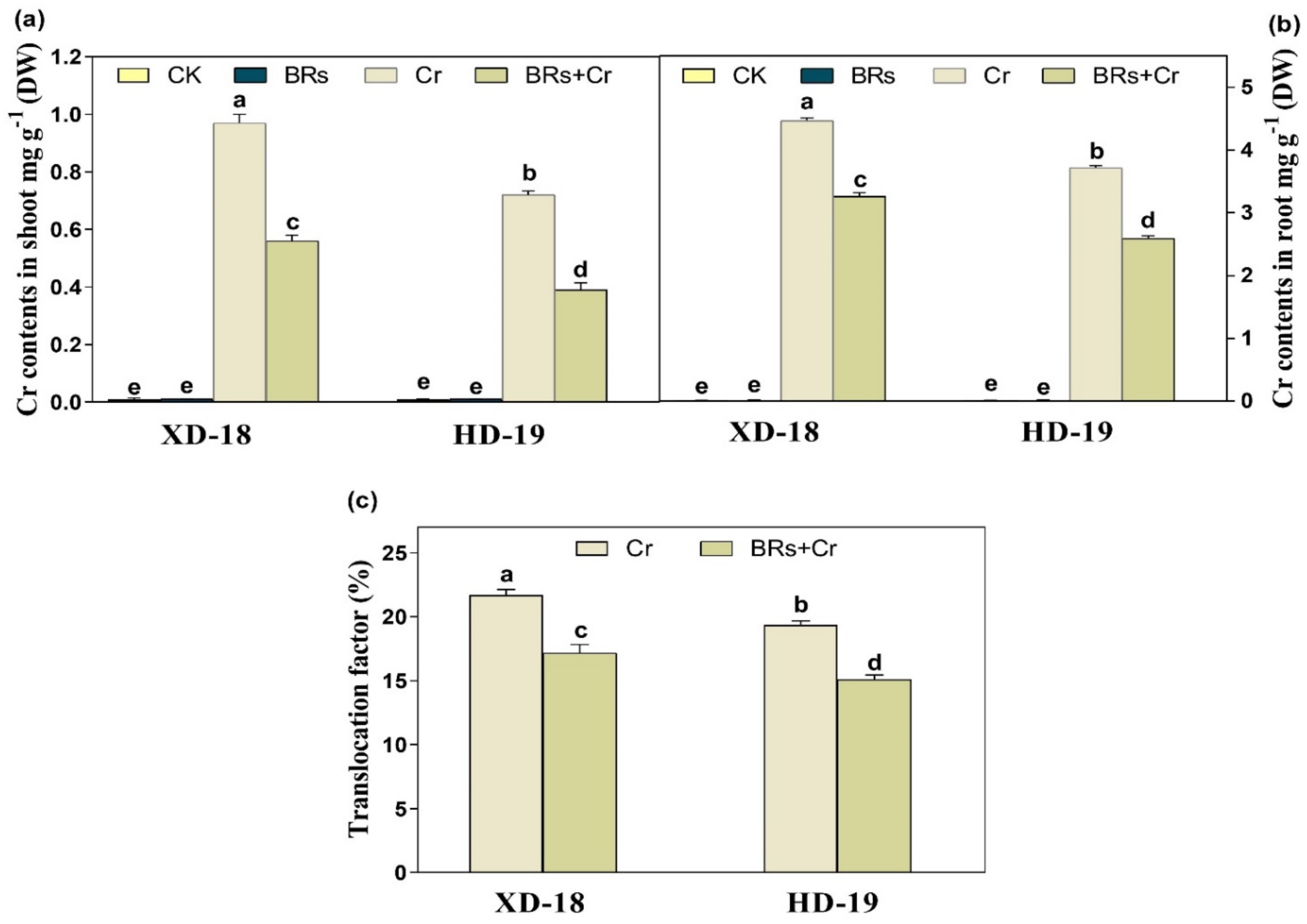
2.5. BRs Reduces Cr Uptake and Maintains Nutrient Homeostasis under Chromium Stress
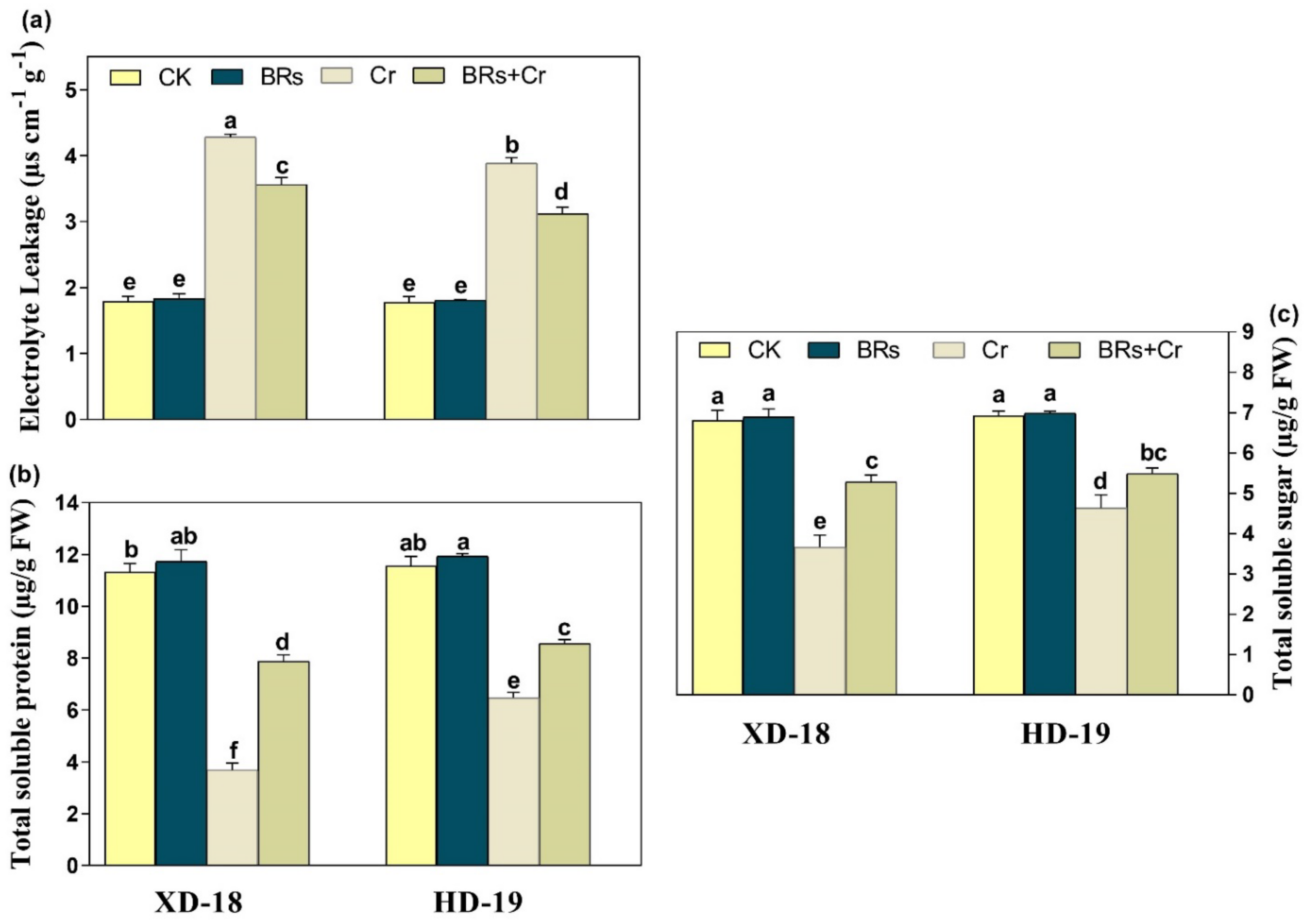
2.6. BRs Modulates the Electrolyte Leakage, Total Soluble Sugar, and Protein under Chromium Stress
2.7. BRs Decreases the ROS-Induced Cellular Oxidative Damage and Maintains Membrane Integrity by Lowering Chromium Stress
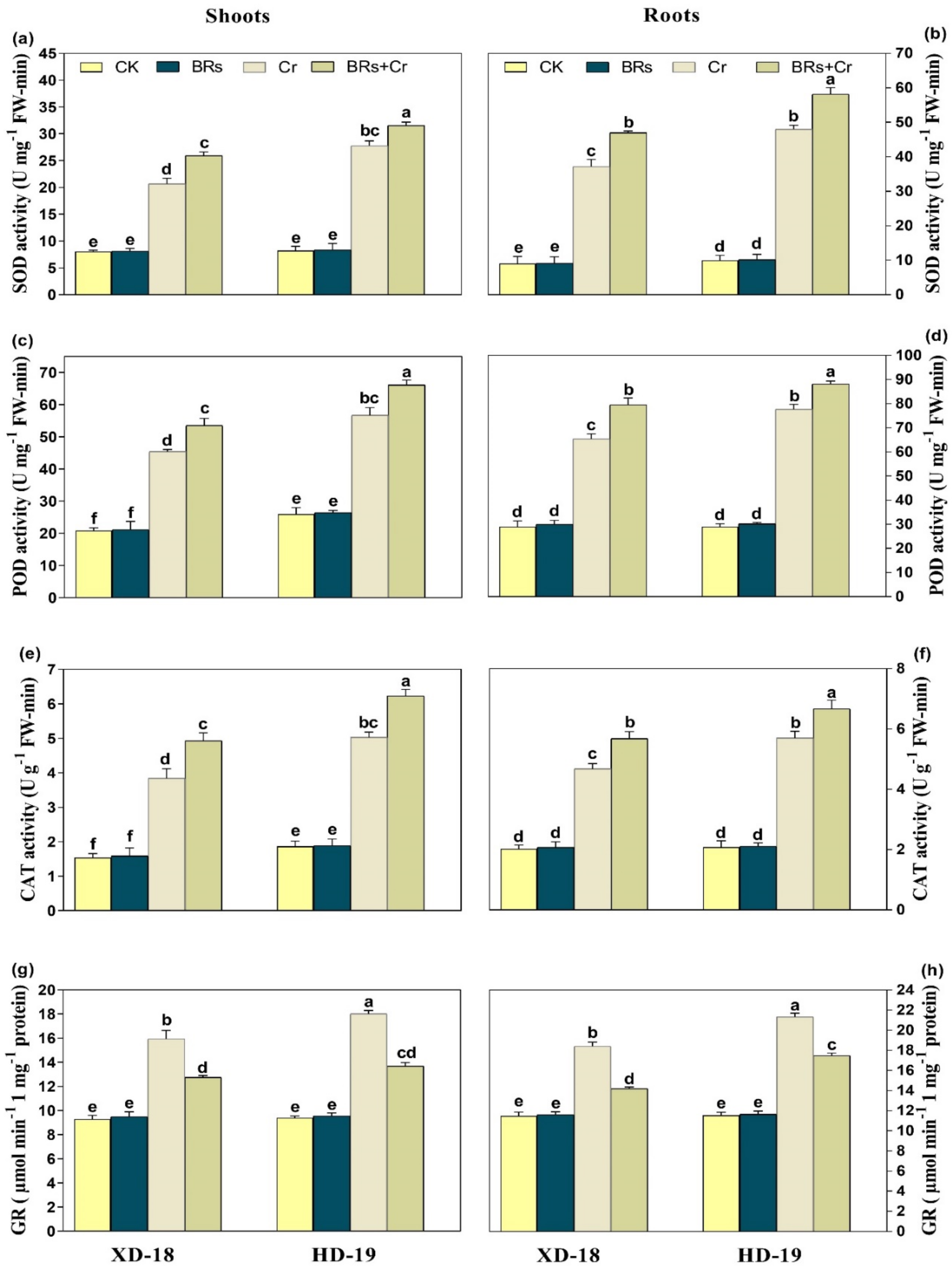
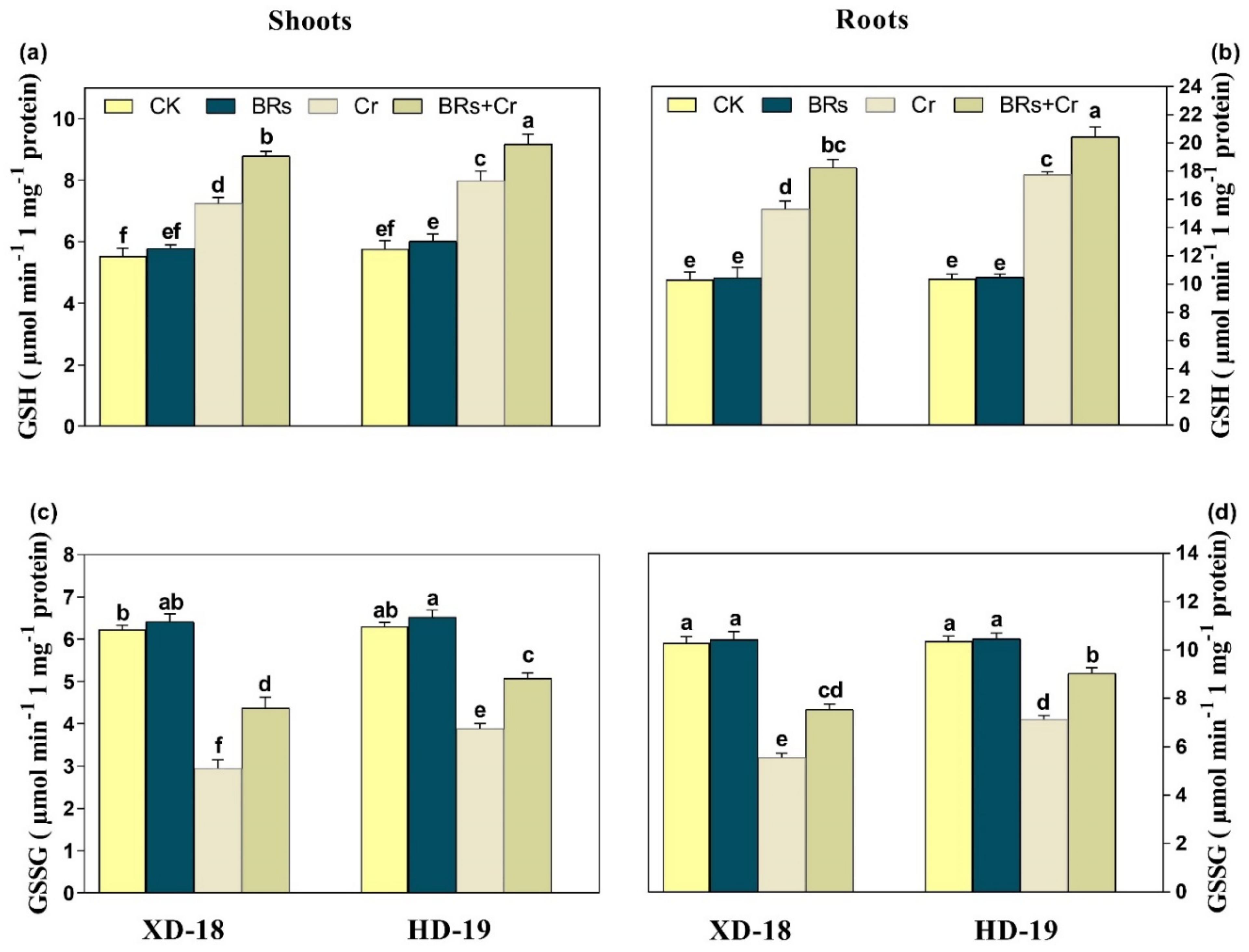
2.8. BRs Regulates Plant Antioxidants Enzymatic and Non-Enzymatic Activities under Chromium Stress
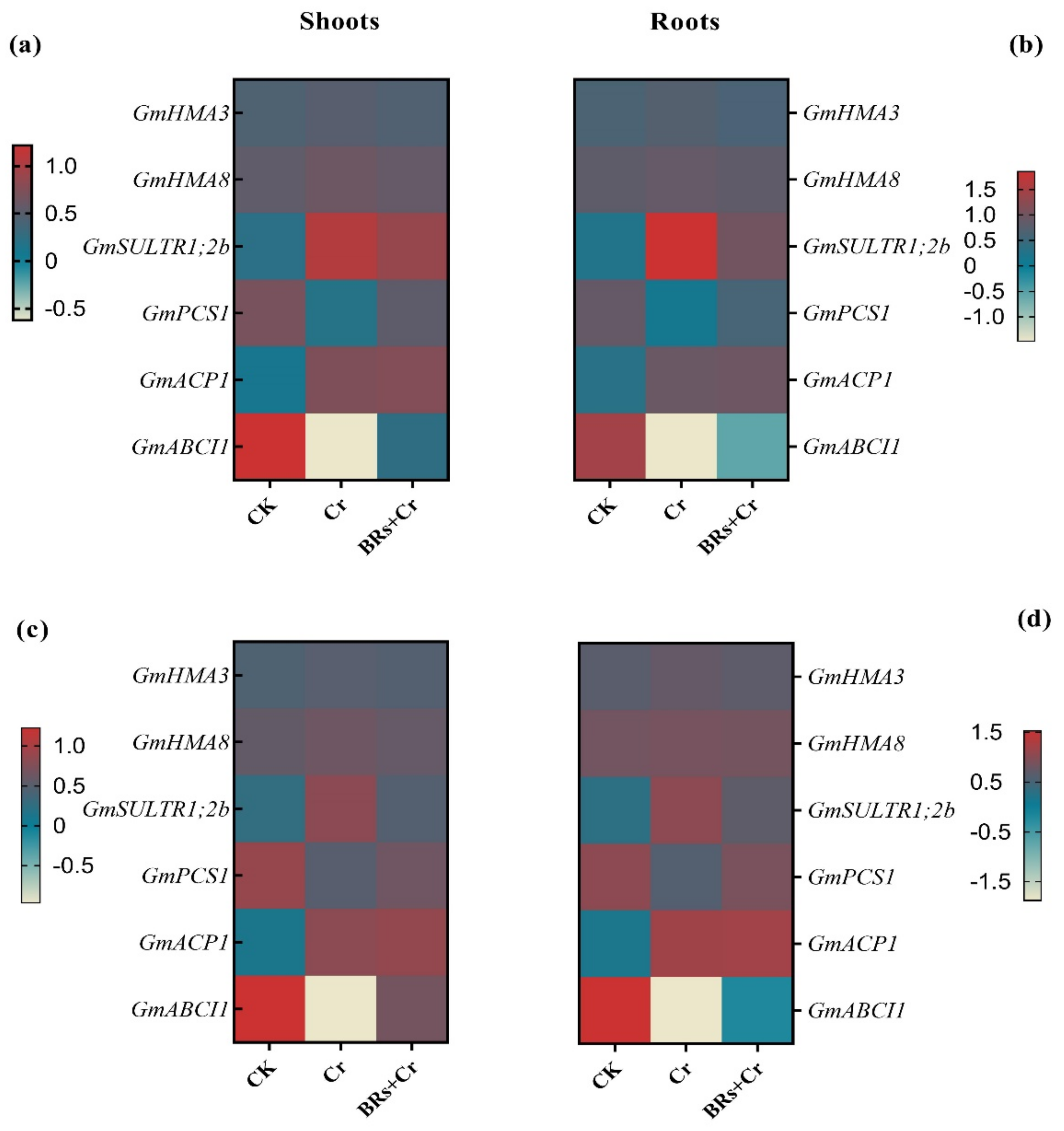
2.9. BRs Regulates Stress Responsive Genes under Chromium Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Seed Germination Analysis
4.3. Hydroponic Culture, Treatments, and Growth Conditions
4.4. Sampling, and Plant Growth Analysis
4.5. Determination of Photosynthetic, and Chlorophyll Fluorescence Measurements
4.6. Determination of Cr Accumulation, and Micronutrients Level
4.7. Determination of Total Soluble Sugar, Protein, and Electrolyte Leakage
4.8. Determination of Malondialdehyde Level, and Hydrogen Peroxidation Production
4.9. Antioxidative Enzymatic, and Non-Enzymatic Assay
4.10. Determination of Cr Stress-Responsive Gene Expression Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le, D.T.; Nishiyama, R.; Watanabe, Y.; Mochida, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.-S.P. Genome-wide survey and expression analysis of the plant-specific NAC transcription factor family in soybean during development and dehydration stress. DNA Res. 2011, 18, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.-H.; Du, W.-k.; Song, H.-N.; Shao, H.-B.; Qi, W.-C.; Sheteiwy, M.S.A.; Yu, D.-y. Identification of responsive miRNAs involved in combination stresses of phosphate starvation and salt stress in soybean root. Environ. Exp. Bot. 2019, 167, 103823. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Cunha-Oliveira, T.; Sobral, M.C.; Abreu, P.L.; Alpoim, M.C.; Urbano, A.M. Impact of carcinogenic chromium on the cellular response to proteotoxic stress. Int. J. Mol. Sci. 2019, 20, 4901. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Li, X.; Huang, R.; Nian, H. Application of exogenous glutathione decreases chromium translocation and alleviates its toxicity in soybean (Glycine max L.). Ecotoxicol. Environ. Saf. 2022, 234, 113405. [Google Scholar] [CrossRef]
- Tumolo, M.; Ancona, V.; De Paola, D.; Losacco, D.; Campanale, C.; Massarelli, C.; Uricchio, V.F. Chromium pollution in European water, sources, health risk, and remediation strategies: An overview. Int. J. Environ. Res. Public Health 2020, 17, 5438. [Google Scholar] [CrossRef]
- Basit, F.; Chen, M.; Ahmed, T.; Shahid, M.; Noman, M.; Liu, J.; An, J.; Hashem, A.; Fahad Al-Arjani, A.-B.; Alqarawi, A.A. Seed priming with brassinosteroids alleviates chromium stress in rice cultivars via improving ROS metabolism and antioxidant defense response at biochemical and molecular levels. Antioxidants 2021, 10, 1089. [Google Scholar] [CrossRef]
- Basit, F.; Ulhassan, Z.; Mou, Q.; Nazir, M.M.; Hu, J.; Hu, W.; Song, W.; Sheteiwy, M.S.; Zhou, W.; Bhat, J.A. Seed priming with nitric oxide and/or spermine mitigate the chromium toxicity in rice (Oryza sativa) seedlings by improving the carbon-assimilation and minimising the oxidative damages. Funct. Plant Biol. 2022. [Google Scholar] [CrossRef]
- Ertani, A.; Mietto, A.; Borin, M.; Nardi, S. Chromium in agricultural soils and crops: A review. Water Air Soil Pollut. 2017, 228, 190. [Google Scholar] [CrossRef]
- Basit, F.; Bhat, J.A.; Han, J.; Guan, Y.; Jan, B.L.; Shakoor, A.; Alansi, S. Screening of rice cultivars for Cr-stress response by using the parameters of seed germination, morpho-physiological and antioxidant analysis. Saudi J. Biol. Sci. 2022, 29, 3918–3928. [Google Scholar] [CrossRef]
- Kour, J.; Kohli, S.K.; Khanna, K.; Bakshi, P.; Sharma, P.; Singh, A.D.; Ibrahim, M.; Devi, K.; Sharma, N.; Ohri, P. Brassinosteroid signaling, crosstalk and, physiological functions in plants under heavy metal stress. Front. Plant Sci. 2021, 12, 608061. [Google Scholar] [CrossRef]
- Kumari, V.; Yadav, A.; Haq, I.; Kumar, S.; Bharagava, R.N.; Singh, S.K.; Raj, A. Genotoxicity evaluation of tannery effluent treated with newly isolated hexavalent chromium reducing Bacillus cereus. J. Environ. Manag. 2016, 183, 204–211. [Google Scholar] [CrossRef]
- Basit, F.; Bhat, J.A.; Dong, Z.; Mou, Q.; Zhu, X.; Wang, Y.; Hu, J.; Jan, B.L.; Shakoor, A.; Guan, Y. Chromium toxicity induced oxidative damage in two rice cultivars and its mitigation through external supplementation of brassinosteroids and spermine. Chemosphere 2022, 302, 134423. [Google Scholar] [CrossRef]
- Samantary, S. Biochemical responses of Cr-tolerant and Cr-sensitive mung bean cultivars grown on varying levels of chromium. Chemosphere 2002, 47, 1065–1072. [Google Scholar] [CrossRef]
- Knežević, M.M.; Stajković-Srbinović, O.S.; Assel, M.; Milić, M.D.; Mihajlovski, K.R.; Delić, D.I.; Buntić, A.V. The ability of a new strain of Bacillus pseudomycoides to improve the germination of alfalfa seeds in the presence of fungal infection or chromium. Rhizosphere 2021, 18, 100353. [Google Scholar] [CrossRef]
- Rout, G.R.; Samantaray, S.; Das, P. Effects of chromium and nickel on germination and growth in tolerant and non-tolerant populations of Echinochloa colona (L.) Link. Chemosphere 2000, 40, 855–859. [Google Scholar] [CrossRef]
- Parr, P.D.; Taylor, F.G., Jr. Germination and growth effects of hexavalent chromium in Orocol TL (a corrosion inhibitor) on Phaseolus vulgaris. Environ. Int. 1982, 7, 197–202. [Google Scholar] [CrossRef]
- Bajguz, A.; Hayat, S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009, 47, 1–8. [Google Scholar] [CrossRef]
- Sirhindi, G. Brassinosteroids: Biosynthesis and role in growth, development, and thermotolerance responses. In Molecular Stress Physiology of Plants; Springer: Berlin/Heidelberg, Germany, 2013; pp. 309–329. [Google Scholar]
- Ahammed, G.J.; Li, X.; Liu, A.; Chen, S. Brassinosteroids in plant tolerance to abiotic stress. J. Plant Growth Regul. 2020, 39, 1451–1464. [Google Scholar] [CrossRef]
- Basit, F.; Liu, J.; An, J.; Chen, M.; He, C.; Zhu, X.; Li, Z.; Hu, J.; Guan, Y. Brassinosteroids as a multidimensional regulator of plant physiological and molecular responses under various environmental stresses. Environ. Sci. Pollut. Res. 2021, 28, 44768–44779. [Google Scholar] [CrossRef]
- Bajguz, A. Metabolism of brassinosteroids in plants. Plant Physiol. Biochem. 2007, 45, 95–107. [Google Scholar] [CrossRef]
- Rajewska, I.; Talarek, M.; Bajguz, A. Brassinosteroids and response of plants to heavy metals action. Front. Plant Sci. 2016, 7, 629. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Sun, W.; Jin, X.; Yu, L.; Chen, C.; Yue, Z.; Dong, Y. Analysis of 2, 4-epibrassinolide created an enhancement tolerance on Cd toxicity in Solanum nigrum L. Environ. Sci. Pollut. Res. 2020, 27, 16784–16797. [Google Scholar] [CrossRef]
- Anwar, A.; Liu, Y.; Dong, R.; Bai, L.; Yu, X.; Li, Y. The physiological and molecular mechanism of brassinosteroid in response to stress: A review. Biol. Res. 2018, 51, 46. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Rattan, A.; Gautam, V.; Bhardwaj, R. Alleviation of cadmium and mercury stress by supplementation of steroid hormone to Raphanus sativus seedlings. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2016, 86, 661–666. [Google Scholar] [CrossRef]
- Xu, T.; Nan, F.; Jiang, X.; Tang, Y.; Zeng, Y.; Zhang, W.; Shi, B. Effect of soil pH on the transport, fractionation, and oxidation of chromium (III). Ecotoxicol. Environ. Saf. 2020, 195, 110459. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Jin, Z.; Han, Y.; Sun, P.; Li, G.; Li, W. Mitigation of chromium toxicity in Arabidopsis thaliana by sulfur supplementation. Ecotoxicol. Environ. Saf. 2019, 182, 109379. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Adhikari, S.; Ghosh, S.; Azahar, I.; Shaw, A.K.; Roy, D.; Roy, S.; Saha, S.; Hossain, Z. Imbalance of redox homeostasis and antioxidant defense status in maize under chromium (VI) stress. Environ. Exp. Bot. 2020, 169, 103873. [Google Scholar] [CrossRef]
- Wen, K.; Pan, H.; Li, X.; Huang, R.; Ma, Q.; Nian, H. Identification of an ATP-Binding Cassette Transporter Implicated in Aluminum Tolerance in Wild Soybean (Glycine soja). Int. J. Mol. Sci. 2021, 22, 13264. [Google Scholar] [CrossRef]
- Ma, M.; Chen, Q.; Dong, H.; Zhang, S.; Huang, X. Genome-wide identification and expression analysis of the bZIP transcription factors, and functional analysis in response to drought and cold stresses in pear (Pyrus breschneideri). BMC Plant Biol. 2021, 21, 583. [Google Scholar] [CrossRef]
- Qin, L.; Han, P.; Chen, L.; Walk, T.C.; Li, Y.; Hu, X.; Xie, L.; Liao, H.; Liao, X. Genome-wide identification and expression analysis of NRAMP family genes in soybean (Glycine max L.). Front. Plant Sci. 2017, 8, 1436. [Google Scholar] [CrossRef] [Green Version]
- Daud, M.; Mei, L.; Azizullah, A.; Dawood, M.; Ali, I.; Mahmood, Q.; Ullah, W.; Jamil, M.; Zhu, S. Leaf-based physiological, metabolic, and ultrastructural changes in cultivated cotton cultivars under cadmium stress mediated by glutathione. Environ. Sci. Pollut. Res. 2016, 23, 15551–15564. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Niazi, N.K.; Antunes, P. Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. In Reviews of Environmental Contamination and Toxicology Volume 241; Springer: Cham, Switzerland, 2016; pp. 73–137. [Google Scholar]
- Wani, P.A.; Khan, M.S. Bacillus species enhance growth parameters of chickpea (Cicer arietinum L.) in chromium stressed soils. Food Chem. Toxicol. 2010, 48, 3262–3267. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Arif, M.S.; Riaz, M.; Shahzad, S.M.; Imran, Q.; Ali, I. Combined ability of chromium (Cr) tolerant plant growth promoting bacteria (PGPB) and salicylic acid (SA) in attenuation of chromium stress in maize plants. Plant Physiol. Biochem. 2016, 108, 456–467. [Google Scholar] [CrossRef]
- Chauhan, R.; Awasthi, S.; Tripathi, P.; Mishra, S.; Dwivedi, S.; Niranjan, A.; Mallick, S.; Tripathi, P.; Pande, V.; Tripathi, R.D. Selenite modulates the level of phenolics and nutrient element to alleviate the toxicity of arsenite in rice (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2017, 138, 47–55. [Google Scholar] [CrossRef]
- Ganesh, K.S.; Baskaran, L.; Rajasekaran, S.; Sumathi, K.; Chidambaram, A.; Sundaramoorthy, P. Chromium stress induced alterations in biochemical and enzyme metabolism in aquatic and terrestrial plants. Colloids Surf. B Biointerfaces 2008, 63, 159–163. [Google Scholar] [CrossRef]
- Ulhassan, Z.; Gill, R.A.; Huang, H.; Ali, S.; Mwamba, T.M.; Ali, B.; Huang, Q.; Hamid, Y.; Khan, A.R.; Wang, J. Selenium mitigates the chromium toxicity in Brassicca napus L. by ameliorating nutrients uptake, amino acids metabolism and antioxidant defense system. Plant Physiol. Biochem. 2019, 145, 142–152. [Google Scholar] [CrossRef]
- Rodriguez, E.; Santos, C.; Azevedo, R.; Moutinho-Pereira, J.; Correia, C.; Dias, M.C. Chromium (VI) induces toxicity at different photosynthetic levels in pea. Plant Physiol. Biochem. 2012, 53, 94–100. [Google Scholar] [CrossRef]
- Qin, X.; Nie, Z.; Liu, H.; Zhao, P.; Qin, S.; Shi, Z. Influence of selenium on root morphology and photosynthetic characteristics of winter wheat under cadmium stress. Environ. Exp. Bot. 2018, 150, 232–239. [Google Scholar] [CrossRef]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Abd_Allah, E.F.; Alqarawi, A.A.; Ahmad, P. Selenium modulates dynamics of antioxidative defence expression, photosynthetic attributes and secondary metabolites to mitigate chromium toxicity in Brassica juncea L. plants. Environ. Exp. Bot. 2019, 161, 180–192. [Google Scholar] [CrossRef]
- Gill, R.A.; Ali, B.; Cui, P.; Shen, E.; Farooq, M.A.; Islam, F.; Ali, S.; Mao, B.; Zhou, W. Comparative transcriptome profiling of two Brassica napus cultivars under chromium toxicity and its alleviation by reduced glutathione. BMC Genom. 2016, 17, 885. [Google Scholar] [CrossRef] [Green Version]
- UdDin, I.; Bano, A.; Masood, S. Chromium toxicity tolerance of Solanum nigrum L. and Parthenium hysterophorus L. plants with reference to ion pattern, antioxidation activity and root exudation. Ecotoxicol. Environ. Saf. 2015, 113, 271–278. [Google Scholar] [CrossRef]
- Handa, N.; Kohli, S.; Sharma, A.; Thukral, A.; Bhardwaj, R.; Alyemeni, M.; Wijaya, L.; Ahmad, P. Selenium ameliorates chromium toxicity through modifications in pigment system, antioxidative capacity, osmotic system, and metal chelators in Brassica juncea seedlings. South Afr. J. Bot. 2018, 119, 1–10. [Google Scholar] [CrossRef]
- Basit, F.; Liu, J.; An, J.; Chen, M.; He, C.; Zhu, X.; Li, Z.; Hu, J.; Guan, Y. Seed priming with brassinosteroids alleviates aluminum toxicity in rice via improving antioxidant defense system and suppressing aluminum uptake. Environ. Sci. Pollut. Res. 2021, 29, 10183–10197. [Google Scholar] [CrossRef]
- Choudhary, S.P.; Kanwar, M.; Bhardwaj, R.; Yu, J.-Q.; Tran, L.-S.P. Chromium stress mitigation by polyamine-brassinosteroid application involves phytohormonal and physiological strategies in Raphanus sativus L. PLoS ONE 2012, 7, e33210. [Google Scholar] [CrossRef]
- Vincent, J.B. Effects of chromium supplementation on body composition, human and animal health, and insulin and glucose metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 483–489. [Google Scholar] [CrossRef]
- Singh, H.P.; Mahajan, P.; Kaur, S.; Batish, D.R.; Kohli, R.K. Chromium toxicity and tolerance in plants. Environ. Chem. Lett. 2013, 11, 229–254. [Google Scholar] [CrossRef]
- Jan, S.; Noman, A.; Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. 24-Epibrassinolide alleviates the injurious effects of Cr (VI) toxicity in tomato plants: Insights into growth, physio-biochemical attributes, antioxidant activity and regulation of Ascorbate–glutathione and Glyoxalase cycles. J. Plant Growth Regul. 2020, 39, 1587–1604. [Google Scholar] [CrossRef]
- Ahmad, R.; Ali, S.; Hannan, F.; Rizwan, M.; Iqbal, M.; Hassan, Z.; Akram, N.A.; Maqbool, S.; Abbas, F. Promotive role of 5-aminolevulinic acid on chromium-induced morphological, photosynthetic, and oxidative changes in cauliflower (Brassica oleracea botrytis L.). Environ. Sci. Pollut. Res. 2017, 24, 8814–8824. [Google Scholar] [CrossRef]
- Ali, S.; Chaudhary, A.; Rizwan, M.; Anwar, H.T.; Adrees, M.; Farid, M.; Irshad, M.K.; Hayat, T.; Anjum, S.A. Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.). Environ. Sci. Pollut. Res. 2015, 22, 10669–10678. [Google Scholar] [CrossRef]
- Singh, S.; Prasad, S.M. Regulation of chromium toxicity tolerance in tomato and brinjal by calcium and sulfur through nitric oxide: Involvement of enzymes of sulfur assimilation and the ascorbate-glutathione cycle. Environ. Exp. Bot. 2019, 166, 103789. [Google Scholar] [CrossRef]
- Santos, L.; Batista, B.; Lobato, A. Brassinosteroids mitigate cadmium toxicity in cowpea plants. Photosynthetica 2018, 56, 591–605. [Google Scholar] [CrossRef]
- Rodrigues, W.d.S.; Pereira, Y.C.; de Souza, A.L.M.; Batista, B.L.; Lobato, A.K.d.S. Alleviation of oxidative stress induced by 24-epibrassinolide in soybean plants exposed to different manganese supplies: UpRegulation of antioxidant enzymes and maintenance of photosynthetic pigments. J. Plant Growth Regul. 2020, 39, 1425–1440. [Google Scholar] [CrossRef]
- Gasic, K.; Korban, S.S. Expression of Arabidopsis phytochelatin synthase in Indian mustard (Brassica juncea) plants enhances tolerance for Cd and Zn. Planta 2007, 225, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Kwasniewski, M.; Chwialkowska, K.; Kwasniewska, J.; Kusak, J.; Siwinski, K.; Szarejko, I. Accumulation of peroxidase-related reactive oxygen species in trichoblasts correlates with root hair initiation in barley. J. Plant Physiol. 2013, 170, 185–195. [Google Scholar] [CrossRef]
- El-Shabrawi, H.; Kumar, B.; Kaul, T.; Reddy, M.K.; Singla-Pareek, S.L.; Sopory, S.K. Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 2010, 245, 85–96. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, J.; Zhang, S.; Gao, C.; Song, W. Identification of chilling-tolerance in maize inbred lines at germination and seedling growth stages. J. Zhejiang Univ. 2006, 32, 41–45. [Google Scholar]
- Schreiber, U.; Bilger, W. Progress in chlorophyll fluorescence research: Major developments during the past years in retrospect. Prog. Bot. Fortschr. Bot. 1993, 54, 151–173. [Google Scholar]
- Živčák, M.; Brestič, M.; Olšovská, K.; Slamka, P. Performance index as a sensitive indicator of water stress in Triticum aestivum L. Plant Soil Environ. 2008, 54, 133–139. [Google Scholar] [CrossRef]
- Kalaji, M.; Guo, P. Chlorophyll fluorescence: A useful tool in barley plant breeding programs. Photochem. Res. Prog. 2008, 29, 439–463. [Google Scholar]
- Aziz, H.; Sabir, M.; Ahmad, H.R.; Aziz, T.; Zia-ur-Rehman, M.; Hakeem, K.R.; Ozturk, M. Alleviating effect of calcium on nickel toxicity in rice. CLEAN–Soil Air Water 2015, 43, 901–909. [Google Scholar] [CrossRef]
- Ista, L.K.; Callow, M.E.; Finlay, J.A.; Coleman, S.E.; Nolasco, A.C.; Simons, R.H.; Callow, J.A.; Lopez, G.P. Effect of substratum surface chemistry and surface energy on attachment of marine bacteria and algal spores. Appl. Environ. Microbiol. 2004, 70, 4151–4157. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hasanuzzaman, M.; Fujita, M. Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol. Mol. Biol. Plants 2010, 16, 259–272. [Google Scholar] [CrossRef]
- Change, B.; Maehly, A. Assay of catalases and peroxidase. Methods Enzym. 1955, 2, 764–775. [Google Scholar]
- Schaedle, M.; Bassham, J.A. Chloroplast glutathione reductase. Plant Physiol. 1977, 59, 1011–1012. [Google Scholar] [CrossRef]
- Law, M.; Charles, S.A.; Halliwell, B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of paraquat. Biochem. J. 1983, 210, 899–903. [Google Scholar] [CrossRef]
- Sah, S.K.; Kaur, G.; Kaur, A. Rapid and reliable method of high-quality RNA extraction from diverse plants. Am. J. Plant Sci. 2014, 5, 3129. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

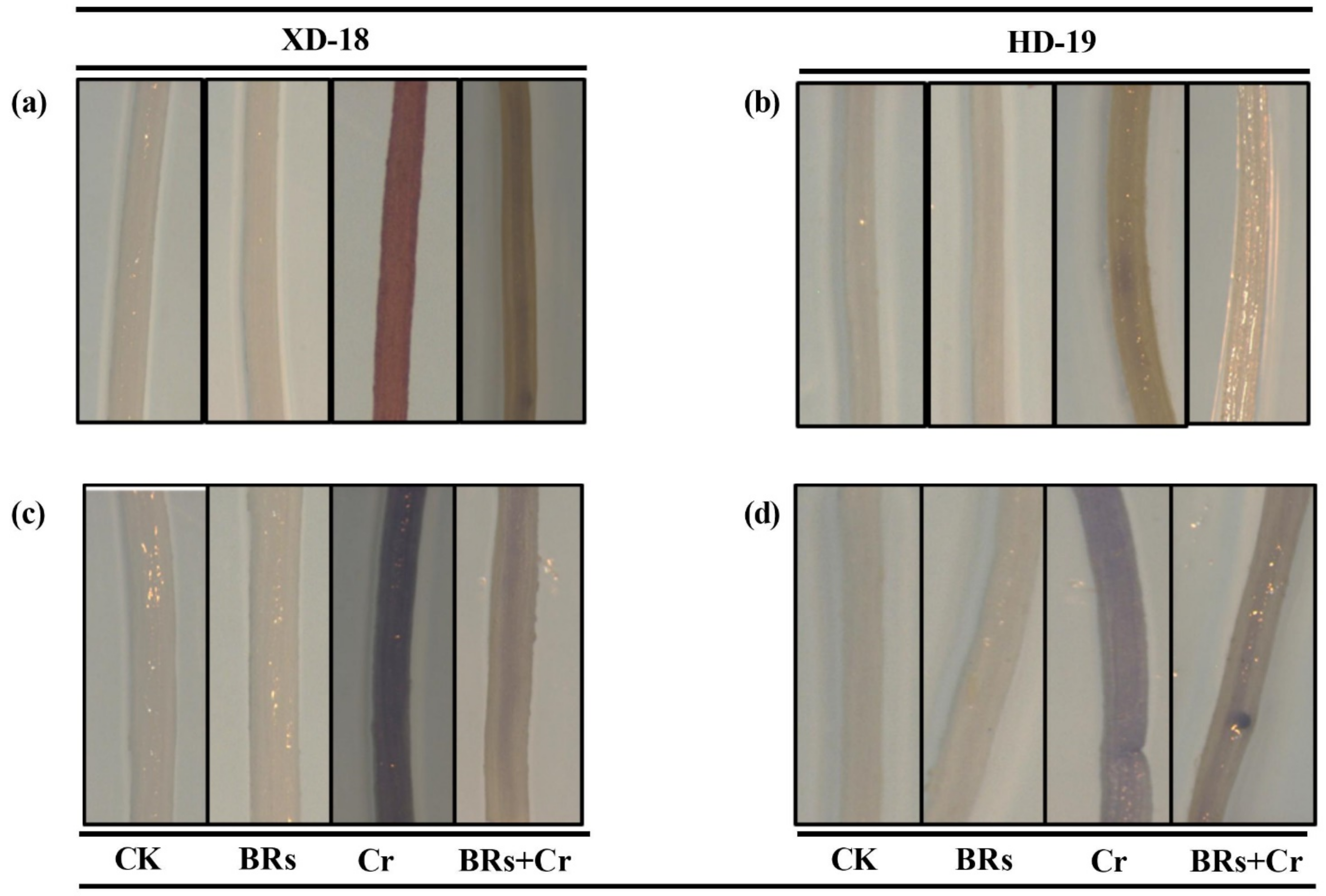
| Genotypes | Varieties Name | GE | GP | GI | MGT | VI | FW | DW | PL |
|---|---|---|---|---|---|---|---|---|---|
| XD-18 | CK | 98.67 ± 2.31a | 98.00 ± 2.00a | 35.40 ± 0.33b | 2.05 ± 0.05e | 4.03 ± 0.31a | 0.89 ± 0.02b | 0.11 ± 0.00b | 29.91 ± 0.22b |
| BRs | 99.67 ± 1.15a | 100.33 ± 1.15a | 36.04 ± 0.46ab | 1.92 ± 0.03ef | 4.33 ± 0.25a | 0.92 ± 0.02a | 0.12 ± 0.00ab | 30.92 ± 0.37ab | |
| Cr | 26.00 ± 2.00e | 27.33 ± 1.15e | 7.07 ± 0.04f | 2.71 ± 0.19a | 0.43 ± 0.01e | 0.23 ± 0.04f | 0.03 ± 0.00f | 13.59 ± 0.41f | |
| BRs + Cr | 55.33 ± 1.15c | 56.00 ± 0.00c | 18.79 ± 0.63d | 2.11 ± 0.08cd | 1.52 ± 0.31 c | 0.55 ± 0.01d | 0.08 ± 0.00d | 23.64 ± 0.13d | |
| HD-19 | CK | 99.67 ± 2.31a | 99.63 ± 1.15a | 35.71 ± 0.19b | 1.93 ± 0.04ef | 4.29 ± 0.19a | 0.91 ± 0.03ab | 0.12 ± 0.00ab | 30.25 ± 0.29ab |
| BRs | 100.00 ± 0.00a | 100.00 ± 0.00a | 36.67 ± 0.34a | 1.85 ± 0.02f | 4.41 ± 0.14a | 0.93 ± 0.21a | 0.13 ± 0.00a | 31.49 ± 0.35a | |
| Cr | 46.00 ± 2.00d | 46.67 ± 1.15d | 15.03 ± 0.71e | 2.32 ± 0.10b | 1.03 ± 0.09d | 0.46 ± 0.02e | 0.05 ± 0.00e | 19.52 ± 0.08e | |
| BRs + Cr | 64.00 ± 2.00b | 64.00 ± 2.00b | 22.99 ± 0.20c | 2.10 ± 0.14d | 1.93 ± 0.27b | 0.68 ± 0.12c | 0.09 ± 0.00cd | 25.78 ± 0.29c |
| Varieties | Treatments | Ca | Mg | Mn | Zn | Cu |
|---|---|---|---|---|---|---|
| Shoots (mg/g) | ||||||
| XD-18 | CK | 1.53b ± 0.47 | 0.41a ± 0.00 | 1.89c ± 0.56 | 0.47a ± 0.08 | 0.07a ± 0.003 |
| BRs | 1.58ab ± 0.18 | 0.42a ± 0.01 | 1.95ab ± 0.24 | 0.49a ± 0.06 | 0.07a ± 0.002 | |
| Cr | 0.39f ± 0.13 | 0.36a ± 0.03 | 0.31g ± 0.67 | 0.19e ± 0.01 | 0.02 ± 0.001 | |
| BRs + Cr | 1.09d ± 0.17 | 0.38a ± 0.02 | 0.71e ± 0.31 | 0.29cd ± 0.01 | 0.05a ± 0.002 | |
| HD-19 | CK | 1.57ab ± 0.78 | 0.41a ± 0.02 | 1.93bc ± 0.33 | 0.48a ± 0.03 | 0.07a ± 0.002 |
| BRs | 1.61a ± 0.58 | 0.43a ± 0.01 | 1.97a ± 0.72 | 0.49a ± 0.07 | 0.08a ± 0.010 | |
| Cr | 0.78e ± 0.14 | 0.38a ± 0.02 | 0.43f ± 0.20 | 0.27d ± 0.04 | 0.04a ± 0.001 | |
| BRs + Cr | 1.14cd ± 0.32 | 0.40a ± 0.04 | 0.74de ± 0.49 | 0.39b ± 0.04 | 0.05a ± 0.001 | |
| Varieties | Treatments | Roots (mg/g) | ||||
| XD-18 | CK | 0.39a ± 0.01 | 0.53a ± 0.05 | 0.34a ± 0.03 | 0.33b ± 0.03 | 0.09a ± 0.02 |
| BRs | 0.39a ± 0.00 | 0.54a ± 0.02 | 0.35a ± 0.07 | 0.36ab ± 0.04 | 0.10a ± 0.07 | |
| Cr | 0.17c ± 0.03 | 0.22d ± 0.01 | 0.09e ± 0.01 | 0.06e ± 0.01 | 0.02d ± 0.05 | |
| BRs + Cr | 0.36a ± 0.01 | 0.35b ± 0.03 | 0.27c ± 0.05 | 0.28c ± 0.11 | 0.06b ± 0.03 | |
| HD-19 | CK | 0.39a ± 0.02 | 0.53a ± 0.03 | 0.35ac ± 0.04 | 0.35ab ± 0.04 | 0.10a ± 0.03 |
| BRs | 0.40a ± 0.01 | 0.55a ± 0.02 | 0.36a ± 0.09 | 0.38a ± 0.06 | 0.10a ± 0.05 | |
| Cr | 0.31b ± 0.01 | 0.29c ± 0.01 | 0.21d ± 0.01 | 0.18d ± 0.01 | 0.04c ± 0.01 | |
| BRs + Cr | 0.37a ± 0.02 | 0.37b ± 0.02 | 0.29bc ± 0.17 | 0.30bc ± 0.30 | 0.07ab ± 0.02 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basit, F.; Bhat, J.A.; Hu, J.; Kaushik, P.; Ahmad, A.; Guan, Y.; Ahmad, P. Brassinosteroid Supplementation Alleviates Chromium Toxicity in Soybean (Glycine max L.) via Reducing Its Translocation. Plants 2022, 11, 2292. https://doi.org/10.3390/plants11172292
Basit F, Bhat JA, Hu J, Kaushik P, Ahmad A, Guan Y, Ahmad P. Brassinosteroid Supplementation Alleviates Chromium Toxicity in Soybean (Glycine max L.) via Reducing Its Translocation. Plants. 2022; 11(17):2292. https://doi.org/10.3390/plants11172292
Chicago/Turabian StyleBasit, Farwa, Javaid Akhter Bhat, Jin Hu, Prashant Kaushik, Ajaz Ahmad, Yajing Guan, and Parvaiz Ahmad. 2022. "Brassinosteroid Supplementation Alleviates Chromium Toxicity in Soybean (Glycine max L.) via Reducing Its Translocation" Plants 11, no. 17: 2292. https://doi.org/10.3390/plants11172292
APA StyleBasit, F., Bhat, J. A., Hu, J., Kaushik, P., Ahmad, A., Guan, Y., & Ahmad, P. (2022). Brassinosteroid Supplementation Alleviates Chromium Toxicity in Soybean (Glycine max L.) via Reducing Its Translocation. Plants, 11(17), 2292. https://doi.org/10.3390/plants11172292









