1. Introduction
Current estimates indicate that the global population could reach 10 billion people by 2050. This increase in population will require a much more efficient and sustainable food production system. Our food production system relies heavily on chemical inputs, such as N-containing fertilizer and pesticides, for maximum plant yield. In a more integrated food production system, the soil microbial communities will likely play a significant role in achieving and maintaining system sustainability. Soil organisms can play a pivotal role in nutrient cycling. Soil microflora (bacteria and fungi) and fauna (protozoa and invertebrates such as nematodes, mites, and earthworms) can influence the availability of nutrients for crop production through the decomposition of crop residues, mineralization and immobilization of nutrients, biological N fixation, and bioturbation. Mineralization or immobilization of nutrients by soil microbes influences temporal nutrient availability, soil nutrient status, and net productivity of agroecosystems [
1]. Soil microbial biomass is regarded as an early indicator of changes in soil fertility and agroecosystem properties [
2]. The impact of N fertilization on soil microbial biomass and activity is still not fully understood. Understanding how fertilizer application (particularly N, the most used chemical fertilizer worldwide) interacts with soil microbes is of utmost importance for the development of best management practices that target improved microbial activity for efficient, sustainable food production.
The adverse effects of long-term N fertilizer application on soil enzyme activity was reported from a 20-year fertilization regime in a vegetable greenhouse production system [
3] because the application of 300 and 600 kg N ha
−1 decreased α- and β-glucosidase activities compared with 75 Mg ha
−1 of composted manure [
3]. However, a meta-analysis of long-term trials from around the world concluded that the application of mineral fertilizers (N, P, and K fertilizers) increased microbial biomass by 15% compared to no fertilization [
4]. The addition of mineral fertilizer also increased soil organic C by 12% compared with no fertilization and was a major factor contributing to increased microbial biomass [
4]. The duration of experiments has also been reported to affect the response of soil microbial biomass, with the greatest increase in microbial activity in experiments longer than 20 years [
4]. In contrast, a different meta-analysis reported that microbial biomass declined 15% with N fertilization (the decline in microbial biomass increased as N load increased) [
5].
The activity of soil enzymes involved in the degradation of soil organic matter can be used to provide an early indication of changes in soil health as a result of changes in soil management [
6,
7,
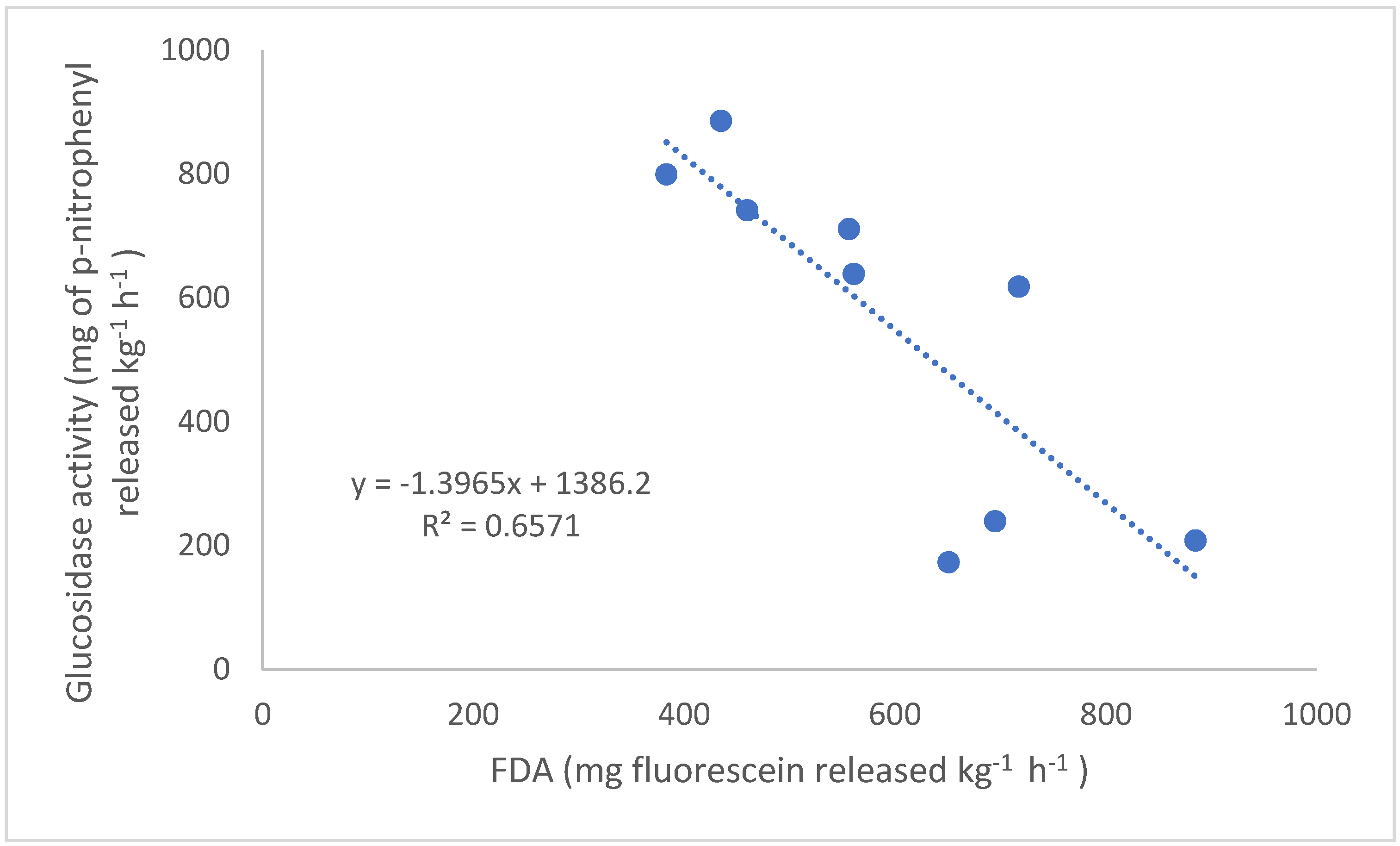
8]. The hydrolysis of FDA has been found to represent a wide range of soil enzyme activity, as many enzymes can hydrolyze FDA, including lipases, esterases, and proteases [
9]. To our knowledge there is no research that closely looks at the effects of N fertilizer addition on FDA activity. β-Glucosidase activity is a useful enzyme for soil quality monitoring because of its central role in the enzymatic degradation of cellulose, the main component of plant polysaccharides, and soil organic matter cycling [
7]. ß-Glucosidase activity can provide a reflection of past biological activity and the capacity of soils to stabilize organic matter [
10]. To our knowledge, there have been few studies that compared the effects of N fertilizer rate and timing of application on β-glucosidase in maize cultivated under continuous production. A greenhouse vegetable trial reported that β-glucosidase activity increased when 75 Mg ha
−1 of composted manure was applied at planting followed by a sidedress of 138 or 276 kg N ha
−1, due to a greater turnover rate of soil C [
3]. This supports the findings of the meta-analysis by Geisseler and Scow [
4] and Jian et al. [
11], where the application of N fertilizer increased β-glucosidase activity by 11 to 15%, likely also as a result of improved soil C. Since microbial activity is influenced by changes in soil organic C, increased crop productivity should increase the amount of crop residue returned to the soil, thus increasing soil organic matter content over time [
4]. In contrast, an incubation study using soils from 28 ecosystems across North America where no C inputs were added concluded that β-glucosidase activity decreased by 12% and soil microbial biomass decreased by 35% when N was added [
12]. The addition of N may lead to increased soil C sequestration rates, due to a shift in the metabolic capabilities of soil bacterial communities and the favoring of microbial communities less capable of decomposing more recalcitrant soil C pools [
12].
Similar to β-glucosidase activity, soil phosphomonoesterase increases with the addition of organic matter, likely as a result of increased organic P, which must be first mineralized before it can be used by microbes and plants [
13,
14]. In a rice (
Oryza sativa) wheat (
Triticum aestivum) rotation system on a sandy loam soil in China, the application of different N rates and sources increased phosphomonoesterase activity by 8 to 71% compared with no fertilization [
15]. In addition, by reducing traditional N fertilizer doses by 20% and replacing 50% of N fertilizer with organic matter, phosphomonoesterase activity increased by 35 to 74% compared with traditional N fertilizer doses [
15]. Nitrogen fertilization was reported to have had no effect on soil phosphomonoesterase activity in a grazed-pasture system [
16]. Similarly, a field experiment assessing different rates of urea-N and broiler litter-N on phosphomonoesterase activity reported that alkaline phosphomonoesterase activity increased with increasing rates of broiler litter but not with increasing rates of urea [
17]. In contrast, incubation studies have reported an increase in soil phosphomonoesterase activity with N fertilization [
18]. The application of increasing N fertilizer (0 to 300 mg N kg
−1 applied as NO
3−-N or NH
4+-N) increased phosphomonoesterase activity but only if the N source was nitrate, whereas ammonium had no effect [
18]. The lack of increased phosphomonoesterase activity with ammonium application was attributed to microbial reallocation of C to biomass or enzyme production [
19]. The lack of a response with ammonium suggests that either microbes are mineralizing less C from protein sources, due to reduced protease production, or that ammonium restricts microbial respiration rates [
20]. Allison and Vitousek [
20] also found that β-glucosidase and acid phosphomonoesterase activities increased in treatments where only carbon and nitrogen were added, while the activities of enzymes such as acid phosphomonoesterase and glycine aminopeptidase declined in response to ammonium and phosphomonoesterase additions.
Arylsulfatase is secreted to release soil available S from organic S (mainly ester sulfates); this enzyme can be a useful indicator of soil health [
21]. Similar to the activities of β-glucosidase and phosphomonoesterase, arylsulfatase is highly correlated with soil organic C content [
22,
23]. A three-year greenhouse study compared the application of different rates of ammonium sulfate and organic fertilizer and reported that arylsulfatase activity was greatest when the soil organic matter level was high and also when 540 kg N ha
−1 yr
−1 was applied as compost [
24]. Only a limited number of studies have investigated the effect of N fertilizer on arylsulfatase activity. Soil acidification due to N addition decreased arylsulfatase activity by 40%, while the addition of water increased arylsulfatase activity in a semi-arid grassland [
21]. In contrast, a field study in Iowa reported that N fertilization had no effect on arylsulfatase activity [
23]. The effect of N and water addition on arylsulfatase activity highlights the complex relationship of soil enzyme activity with the hydrolysis of urea.
Over the years, correlations have been established on the effect of tillage, pH, and residue management on soil enzyme activities involved in C, N, P, and S cycling in soils [
22,
25]. More information is needed to better understand the complex relationship between soil enzyme activity and N fertilizer management. In particular, little research has explored the effect of different N rates and timing of application in maize on soil enzyme activities. This study was designed to provide an opportunity to assess how the application of different urea-N rates and timing of application impacted soil enzyme activities under a soybean [
Glycine max L. (Merr.)]-maize-maize-maize (SB-M-M-M) production system. Our hypothesis is that the addition of N fertilizer would stimulate microbial growth, resulting in an increase in the activity of the enzymes targeted in this research. The enzyme activities selected for study are responsible for C turnover (β-glucosidase), organic P turnover (acid phosphomonoesterase), and organic S turnover (arylsulfatase); in addition, fluorescein diacetate (FDA) was studied as a surrogate for overall microbial activity.