Co-Inoculation with Azospirillum brasilense and Bradyrhizobium sp. Enhances Nitrogen Uptake and Yield in Field-Grown Cowpea and Did Not Change N-Fertilizer Recovery
Abstract
1. Introduction
2. Results
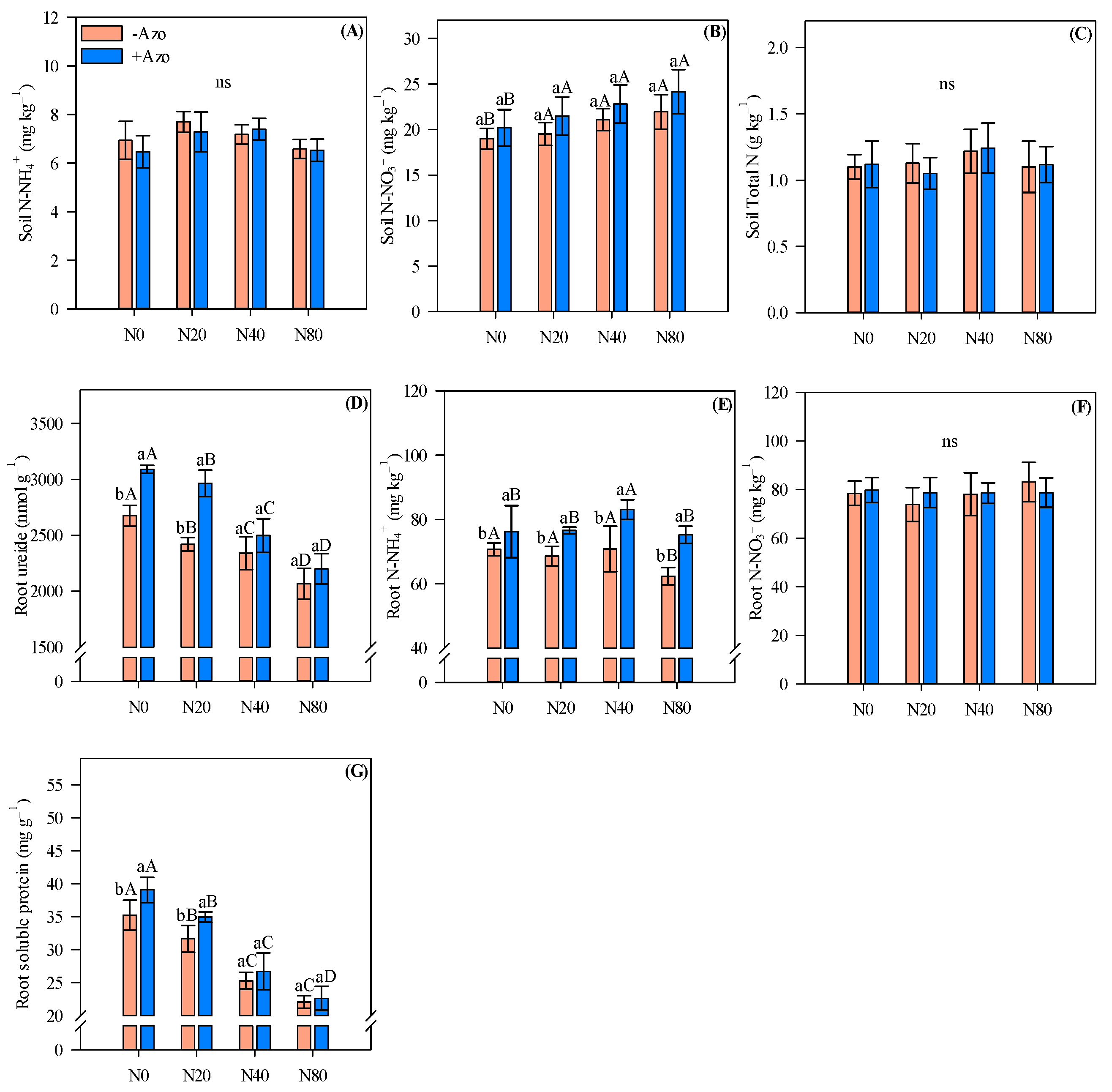
2.1. Soil and Root Nitrogen Fractions
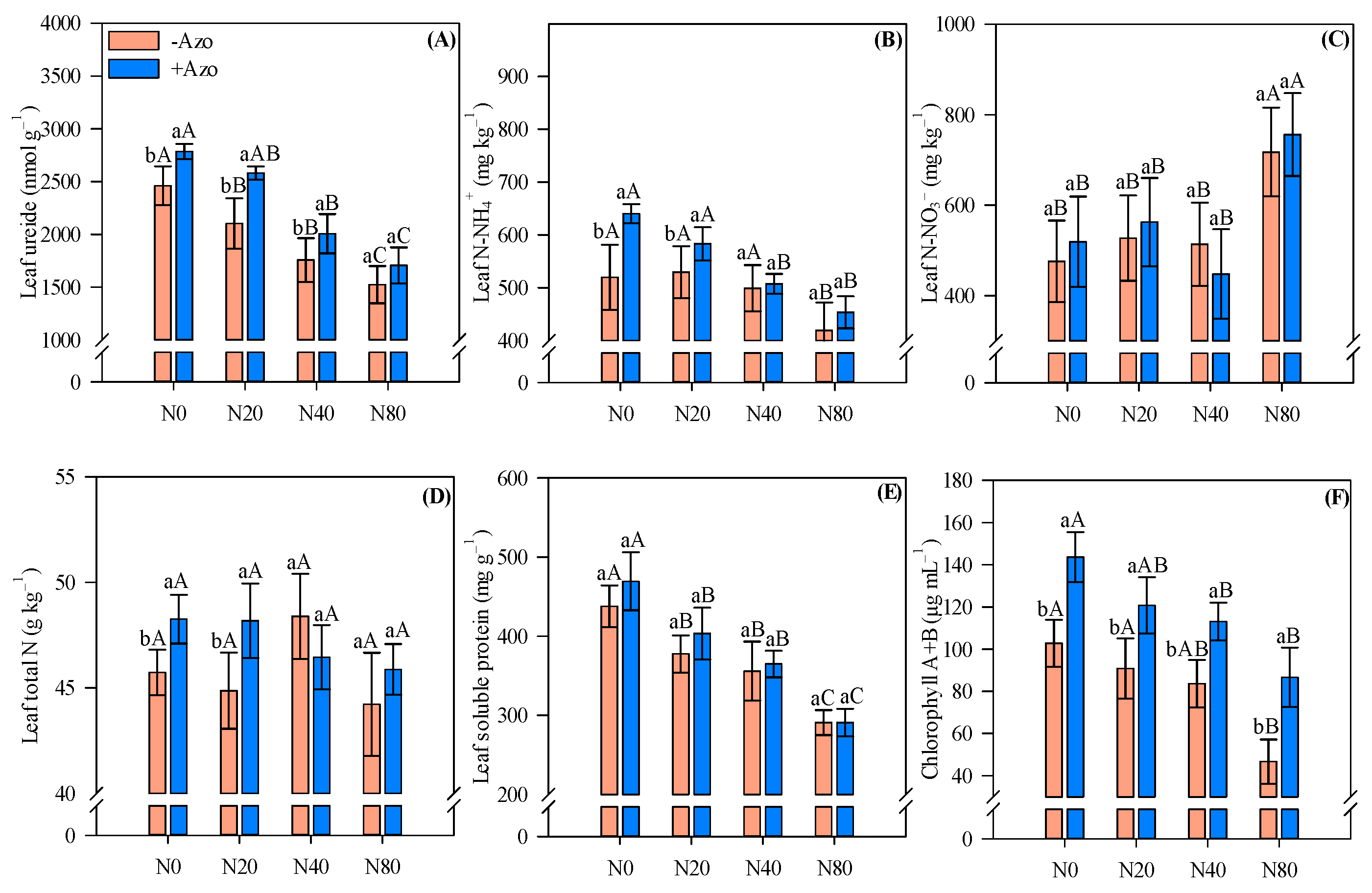
2.2. Leaf Nitrogen Fractions and Chlorophyll Pigment
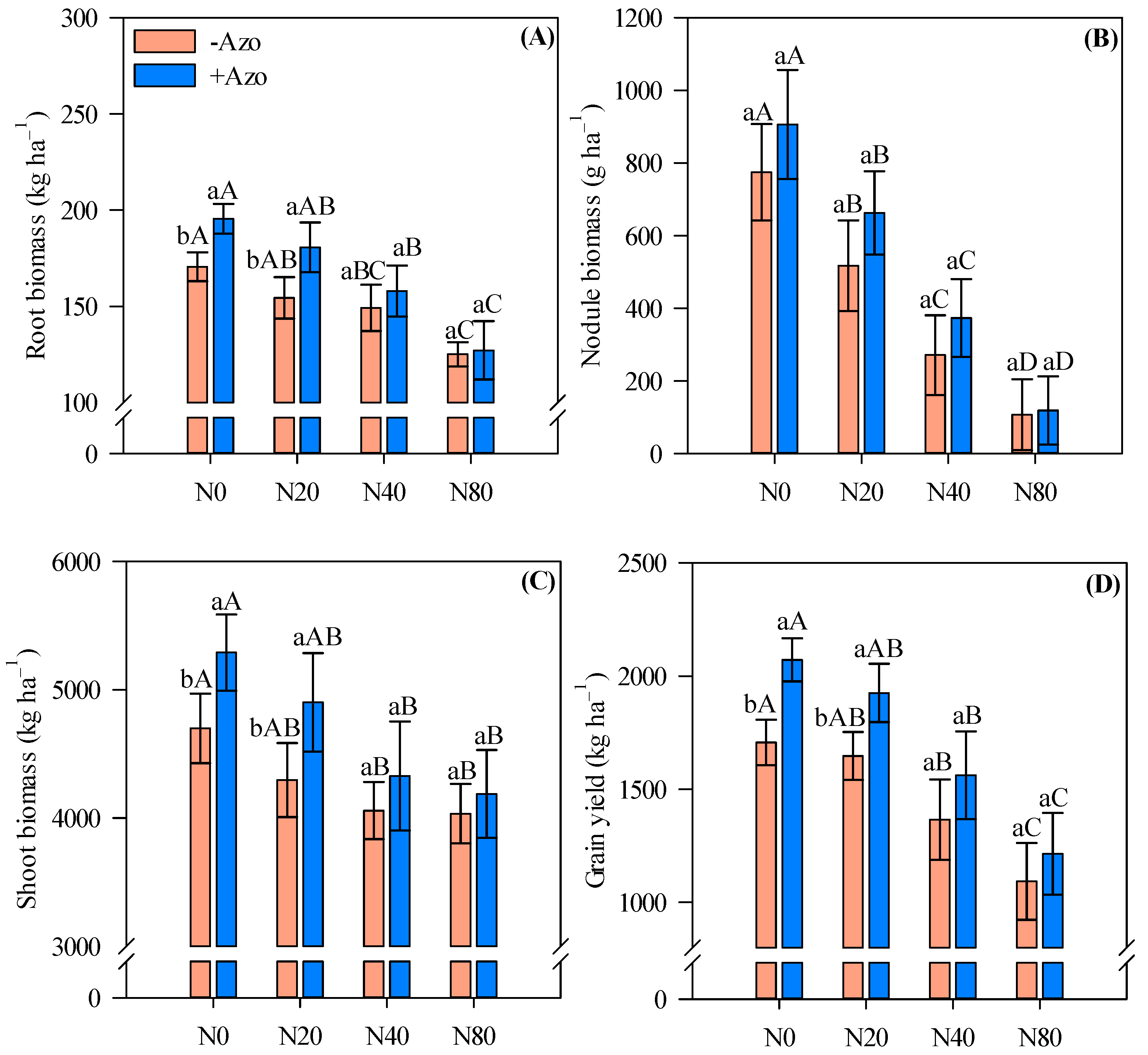
2.3. Biomass Production and Grain Yield
2.4. Shoot and Root Nitrogen Accumulation and 15N-Fertilizer Recovery
3. Discussion
4. Materials and Methods
4.1. Field-Grown Site Characterization
4.2. Experimental Design and Treatments
4.3. Cowpea Crop Management
4.4. Samplings, Measurements and Analysis
4.4.1. Leaf, Root, Nodule and Soil Collection
4.4.2. Nitrogen Fractions in Plant Tissue and Soluble Protein Analysis
4.4.3. Chlorophyll Pigment Analysis
4.4.4. Shoot Biomass and Cowpea Grain Yield
4.4.5. Total N Accumulation and 15N-Fertilizer Recovery in Cowpea Shoot and Grain
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jayathilake, C.; Visvanathan, R.; Deen, A.; Bangamuwage, R.; Jayawardana, B.C.; Nammi, S.; Liyanage, R. Cowpea: An overview on its nutritional facts and health benefits. J. Sci. Food Agric. 2018, 98, 4793–4806. [Google Scholar] [PubMed]
- Domínguez-Perles, R.; Machado, N.; Abraão, A.S.; Carnide, V.; Ferreira, L.; Rodrigues, M.; Rosa, E.A.D.S.; Barros, A.I.R.N.A. Chemometric analysis on free amino acids and proximate compositional data for selecting cowpea (Vigna unguiculata L.) diversity. J. Food Compos. Anal. 2016, 53, 69–76. [Google Scholar]
- Carvalho, M.; Castro, I.; Moutinho-Pereira, J.; Correia, C.; Egea-Cortines, M.; Matos, M.; Rosa, E.; Carnide, V.; Lino-Net, T. Evaluating stress responses in cowpea under drought stress. Plant Physiol. 2019, 153, 1600–1617. [Google Scholar] [CrossRef] [PubMed]
- Boukar, O.; Belko, N.; Chamarthi, S.; Togola, A.; Batieno, J.; Owusu, E.; Haruna, M.; Diallo, S.; Umar, M.L.; Olufajo, O.; et al. Cowpea (Vigna unguiculata): Genetics, genomics and breeding. Plant Breed. 2019, 138, 415–424. [Google Scholar] [CrossRef]
- Owade, J.O.; Abong, G.; Okoth, M.; Mwang’ombe, A.W. A review of the contribution of cowpea leaves to food and nutrition security in East Africa. Food Sci. Nutr. 2020, 8, 36–47. [Google Scholar] [CrossRef]
- Wilker, J.; Navabi, A.; Rajcan, I.; Marsolais, F.; Hill, B.; Torkamaneh, D.; Pauls, K.P. Agronomic performance and nitrogen fixation of heirloom and conventional dry bean varieties under low-nitrogen field conditions. Front. Plant Sci. 2019, 10, 952. [Google Scholar] [CrossRef]
- Adams, M.A.; Turnbull, T.L.; Sprent, J.I.; Buchmann, N. Legume are different: Leaf nitrogen, photosynthesis, and water use efficiency. Proc. Natl. Acad. Sci. USA 2016, 113, 4098–4103. [Google Scholar] [CrossRef]
- Reinprecht, Y.; Schram, L.; Marsolais, F.; Smith, T.H.; Hill, B.; Pauls, K.P. Effects of nitrogen application on nitrogen fixation in common bean production. Front. Plant Sci. 2020, 11, 1172. [Google Scholar] [CrossRef]
- Elowad, H.O.A.; Hall, A.E. Influences of early and late nitrogen fertilization on yield and nitrogen fixation of cowpea under well-watered and dry field conditions. Field Crops Res. 1987, 15, 229–244. [Google Scholar] [CrossRef]
- Awonaike, K.O.; Kumarasinghe, K.S.; Danso, S.K.A. Nitrogen fixation and yield of cowpea as influenced by cultivar and Bradyrhizobium strain. Field Crops Res. 1990, 24, 163–171. [Google Scholar] [CrossRef]
- Martins, L.M.V.; Xavier, G.R.; Rangel, F.W.; Ribeiro, J.R.A.; Neves, M.C.P.; Morgado, L.B.; Rumjanek, N.G. Contribution of biological nitrogen fixation to cowpea: A strategy for improving grain yield in the semi-arid region of Brazil. Biol. Fertil. Soils 2003, 38, 333–339. [Google Scholar] [CrossRef]
- Freitas, V.F.; Cerezini, P.; Hungria, M.; Nogueira, M.A. Strategies to deal with drought-stress in biological nitrogen fixation in soybean. Appl. Soil Ecol. 2022, 172, 104352. [Google Scholar] [CrossRef]
- Nguyen, T.; Atieno, M.; Herrmann, L.; Nakasathien, S.; Sarobol, E.; Wongkaew, A.; Nguyen, K.T.; Lesueur, D. Does inoculation with native rhizobia enhance nitrogen fixation and yield of cowpea through legume-based intercropping in the northern mountainous areas of Vietnam? Exp. Agric. 2020, 56, 825–836. [Google Scholar]
- Graham, P.H. Some problems of nodulation and symbiotic nitrogen fixation in Phaseolus vulgaris L.: A review. Field Crops Res. 1981, 4, 93–112. [Google Scholar]
- Naab, J.B.; Chimphango, S.M.B.; Dakora, F.D. N2 fixation in cowpea plants grown in farmers’ fields in the upper west region of Ghana, measured using 15N natural abundance. Symbiosis 2009, 48, 37–46. [Google Scholar] [CrossRef]
- Amorim, M.R.; Mendes, L.W.; Antunes, J.E.L.; Oliveira, L.M.S.; Melo, V.M.M.; Oliveira, F.A.S.; Aquino, J.P.A.; Rocha, S.M.B.; De Araujo Pereira, A.P.; Da Costa, A.F.; et al. Cowpea nodules host a similar bacterial community regardless of soil properties. Appl. Soil Ecol. 2022, 172, 104354. [Google Scholar] [CrossRef]
- Muldoon, J.F.; Hume, D.J.; Beversdorf, W.D. Effects of seed-and soil-applied Rhizobium japonicum inoculants on soybeans in Ontario. Can. J. Plant Sci. 1980, 60, 399–409. [Google Scholar] [CrossRef]
- Ravuri, V.; Hume, D.J. Performance of a superior Bradyrhizobium japonicum and a selected Sinorhizobium fredii strain with soybean cultivars. Agron. J. 1992, 84, 1051–1056. [Google Scholar] [CrossRef]
- Peoples, M.B.; Gault, R.R.; Lean, B.; Sykes, J.D.; Brockwell, J. Nitrogen fixation by soybean in commercial irrigated crops of central and southern New South Wales. Soil Biol. Biochem. 1995, 27, 553–561. [Google Scholar] [CrossRef]
- Goss, M.J.; de Varennes, A.; Smith, P.S.; Ferguson, J.A. N2 fixation by soybeans grown with different levels of mineral nitrogen, and the fertilizer replacement value for a following crop. Can. J. Soil Sci. 2002, 82, 139–145. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Salvagiotti, F. New insights into soybean biological nitrogen fixation. Agron. J. 2018, 110, 1185–1196. [Google Scholar] [CrossRef]
- Barbosa, J.Z.; Hungria, M.; Sena, J.V.S.; Poggere, G.; Reis, A.R.; Corrêa, R.S. Meta-analysis reveals benefits of co-inoculation of soybean with Azospirillum brasilense and Bradyrhizobium spp. in Brazil. Appl. Soil Ecol. 2021, 163, 103913. [Google Scholar] [CrossRef]
- Jemo, M.; Sulieman, S.; Bekkaoui, F.; Olomide, O.A.K.; Hashem, A.; Abd Allah, E.F.; Alqarawi, A.A.; Tran, L.S.P. Comparative analysis of the combined effects of different water and phosphate levels on growth and biological nitrogen fixation of nine cowpea varieties. Front. Plant Sci. 2017, 8, 2111. [Google Scholar] [CrossRef] [PubMed]
- Hungria, M.; Campo, R.J.; Souza, E.M.; Pedrosa, F.O. Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant Soil 2010, 331, 413–425. [Google Scholar] [CrossRef]
- Hungria, M.; Ribeiro, R.A.; Nogueira, M.A. Draft genome sequences of Azospirillum brasilense strains Ab-V5 and Ab-V6, commercially used in inoculants for grasses and legumes in Brazil. Genome Announc. 2018, 6, e00393-18. [Google Scholar] [CrossRef] [PubMed]
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits that go far beyond biological nitrogen fixation. AMB Express 2018, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Galindo, F.S.; Teixeira Filho, M.C.M.; Buzetti, S.; Pagliari, P.H.; Santini, J.M.K.; Alves, C.J.; Megda, M.M.; Nogueira, T.A.R.; Andreotti, M.; Arf, O. Maize yield response to nitrogen rates and sources associated with Azospirillum brasilense. Agron. J. 2019, 111, 1985–1997. [Google Scholar]
- Galindo, F.S.; Buzetti, S.; Rodrigues, W.L.; Boleta, E.H.M.; Silva, V.M.; Tavanti, R.F.R.; Fernandes, G.C.; Biagini, A.L.C.; Rosa, P.A.L.; Teixeira Filho, M.C.M. Inoculation of Azospirillum brasilense associated with silicon as a liming source to improve nitrogen fertilization in wheat crops. Sci. Rep. 2020, 10, 6160. [Google Scholar] [CrossRef]
- Galindo, F.S.; Pagliari, P.H.; Buzetti, S.; Rodrigues, W.L.; Santini, J.M.K.; Boleta, E.H.M.; Rosa, P.A.L.; Nogueira, T.A.R.; Lazarini, E.; Teixeira Filho, M.C.M. Can silicon applied to correct soil acidity in combination with Azospirillum brasilense inoculation improve nitrogen use efficiency in maize? PLoS ONE 2020, 15, e0230954. [Google Scholar]
- Cassán, F.; Coniglio, A.; López, G.; Molina, R.; Nievas, S.; Carlan, C.N.; Donadio, F.; Torres, D.; Rosas, S.; Pedrosa, F.O.; et al. Everything you must know about Azospirillum and its impact on agriculture and beyond. Biol. Fertil. Soils 2020, 56, 461–479. [Google Scholar] [CrossRef]
- Zeffa, D.M.; Fantin, L.H.; Koltun, A.; de Oliveira, A.L.M.; Nunes, M.P.B.A.; Canteri, M.G.; Gonçalves, L.S.A. Effects of plant growth-promoting rhizobacteria on co-inoculation with Bradyrhizobium in soybean crop: A meta-analysis of studies from 1987 to 2018. PeerJ 2020, 8, e7905. [Google Scholar] [CrossRef] [PubMed]
- Sibponkrung, S.; Kondo, T.; Tanaka, K.; Tittabutr, P.; Boonkerd, N.; Yoshida, K.-I.; Teaumroong, N. Co-inoculation of Bacillus velezensis strain S141 and Bradyrhizobium strains promotes nodule growth and nitrogen fixation. Microorganisms 2020, 8, 678. [Google Scholar] [CrossRef] [PubMed]
- Prakamhang, J.; Tittabutr, P.; Boonkerd, N.; Teamtisong, K.; Uchiumi, T.; Abe, M.; Teaumroong, N. Proposed some interactions at molecular level of PGPR coinoculated with Bradyrhizobium diazoefficiens USDA110 and B. japonicum THA6 on soybean symbiosis and its potential of field application. Appl. Soil Ecol. 2015, 85, 38–49. [Google Scholar] [CrossRef]
- Hungria, M.; Nogueira, M.A.; Araujo, R.S. Co-inoculation of soybeans and common beans with rhizobia and azospirilla: Strategies to improve sustainability. Biol. Fertil. Soils 2013, 49, 791–801. [Google Scholar]
- Moretti, L.G.; Crusciol, C.A.; Kuramae, E.E.; Bossolani, J.W.; Moreira, A.; Costa, N.R.; Alves, C.J.; Pascoaloto, I.M.; Rondina, A.B.L.; Hungria, M. Effects of growth-promoting bacteria on soybean root activity, plant development, and yield. Agron. J. 2020, 112, 418–428. [Google Scholar] [CrossRef]
- Galindo, F.S.; Silva, E.C.; Pagliari, P.H.; Fernandes, G.C.; Rodrigues, W.L.; Biagini, A.L.C.; Baratella, E.B.; Silva Júnior, C.A.; Moretti Neto, M.J.; Silva, V.M.; et al. Nitrogen recovery from fertilizer and use efficiency response to Bradyrhizobium sp. and Azospirillum brasilense combined with N rates in cowpea-wheat crop sequence. Appl. Soil Ecol. 2021, 157, 103764. [Google Scholar] [CrossRef]
- Pedrosa, F.O.; Oliveira, A.L.M.; Guimarães, V.F.; Etto, R.M.; Souza, E.M.; Furmam, F.G.; Gonçalves, D.R.P.; Santos, O.J.A.P.; Gonçalves, L.S.A.; Battistus, A.G.; et al. The ammonium excreting Azospirillum brasilense strain HM053: A new alternative inoculant for maize. Plant Soil 2020, 451, 45–56. [Google Scholar] [CrossRef]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Outstanding impact of Azospirillum brasilense strains Ab-V5 and Ab-V6 on the Brazilian agriculture: Lessons that farmers are receptive to adopt new microbial inoculants. R. Bras. Ciência Solo. 2021, 45, e0200128. [Google Scholar] [CrossRef]
- Bashan, Y.; De-Bashan, L.E. How the plant growth-promoting bacterium Azospirillum promotes plant growth—A critical assessment. Adv. Agron. 2010, 108, 77–136. [Google Scholar]
- Cassán, F.; Diaz-Zorita, M. Azospirillum sp. in current agriculture: From the laboratory to the field. Soil Biol. Biochem. 2016, 103, 117–130. [Google Scholar] [CrossRef]
- Caires, E.F.; Bini, A.R.; Barão, L.F.C.; Haliski, A.; Duart, V.M.; Ricardo, K.S. Seed inoculation with Azospirillum brasilense and nitrogen fertilization for no-till cereal production. Agron. J. 2020, 113, 560–576. [Google Scholar] [CrossRef]
- Pankievicz, V.C.S.; Irving, T.B.; Maia, L.G.S.; Ané, J.-M. Are we there yet? The long walk towards the development of efficient symbiotic associations between nitrogen-fixing bacteria and non-leguminous crops. BMC Biol. 2019, 17, 99. [Google Scholar]
- Rafi, M.M.; Krishnaveni, M.S.; Charyulu, P.B.B.N. Phosphate-solubilizing microorganisms and their emerging role in sustainable agriculture. In Recent Developments in Applied Microbiology and Biochemistry; Buddolla, V., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2019; Volume 17, pp. 223–233. [Google Scholar]
- Chibeba, A.M.; Guimarães, M.D.F.; Brito, O.R.; Nogueira, M.A.; Araujo, R.S.; Hungria, M. Co-inoculation of soybean with Bradyrhizobium and Azospirillum promotes early nodulation. Am. J. Plant Sci. 2015, 6, 1641–1649. [Google Scholar] [CrossRef]
- Rondina, A.B.L.; Sanzovo, A.W.S.; Guimarães, G.S.; Wendling, J.R.; Nogueira, M.A.; Hungria, M. Changes in root morphological traits in soybean co-inoculated with Bradyrhizobium spp. and Azospirillum brasilense or treated with A. brasilense exudates. Biol. Fertil. Soils 2020, 56, 537–549. [Google Scholar] [CrossRef]
- Yoneyama, T.; Karasuyama, M.; Kouchi, H.; Ishizuka, J. Occurrence of ureide accumulation in soybean plants. Soil Sci. Plant Nutr. 1985, 31, 133–140. [Google Scholar] [CrossRef]
- Bosse, M.A.; Silva, M.B.; Oliveira, N.G.R.M.; Araujo, M.A.; Rodrigues, C.; Azevedo, J.P.; Reis, A.R. Physiological impact of flavonoids on nodulation and ureide metabolism in legume plants. Plant Physiol. Biochem. 2021, 166, 512–521. [Google Scholar] [CrossRef]
- Dhanapal, A.P.; Ray, J.D.; Singh, S.K.; Hoyos-Villegas, V.; Smith, J.R.; Purcell, L.C.; King, C.A.; Fritschi, F.B. Genome-wide association analysis of diverse soybean genotypes reveals novel markers for nitrogen traits. Plant Genome 2015, 8, plantgenome2014.11.0086. [Google Scholar] [CrossRef]
- Santachiara, G.; Borrás, L.; Salvagiotti, F.; Gerde, J.A.; Rotundo, J.L. Relative importance of biological nitrogen fixation and mineral uptake in high yielding soybean cultivars. Plant Soil 2017, 418, 191–203. [Google Scholar] [CrossRef]
- Brito, M.M.P.; Muraoka, T.; Silva, E.C. Uptake rate of nitrogen from soil and fertilizer, and N derived from symbiotic fixation in cowpea (Vigna unguiculata (L.) walp.) and common bean (Phaseolus vulgaris L.) determined using the 15N isotope. R. Bras. Ciência Solo 2009, 33, 895–905, (In Portuguese, with English Abstract). [Google Scholar] [CrossRef]
- Belane, A.K.; Dakora, F.D. Assessing the relationship between photosynthetic C accumulation and symbiotic N nutrition in leaves of field-grown nodulated cowpea (Vigna unguiculata L. Walp.) genotypes. Photosynthetica 2015, 53, 562–571. [Google Scholar] [CrossRef]
- Mapope, N.; Dakora, F.D. N2 fixation, C accumulation, and plant water relations in soybean (Glycine max L.) varieties sampled from farmers’ fields in South Africa, measured using 15N and 13C natural abundance. Agric. Ecosyst. Environ. 2016, 221, 174–186. [Google Scholar] [CrossRef]
- Kaschuk, G.; Kuyper, T.W.; Leffelaar, P.A.; Hungria, M.; Giller, K.E. Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biol. Biochem. 2009, 41, 1233–1244. [Google Scholar] [CrossRef]
- Cerezini, P.; Kuwano, B.H.; Grunvald, A.K.; Hungria, M.; Nogueira, M.A. Soybean tolerance to drought depends on the associated Bradyrhizobium strain. Braz. J. Microbiol. 2020, 51, 1977–1986. [Google Scholar] [PubMed]
- Helliwell, J.R.; Sturrock, C.J.; Mairhofer Craigon, S.J.; Ashton, R.W.; Miller, A.J.; Whalley, W.R.; Mooney, S.J. The emergent rhizosphere: Imaging the development of the porous architecture at the root-soil interface. Sci. Rep. 2017, 7, 14875. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; White, P.J.; Whalley, W.R.; Shen, J.; Shi, L. Shaping an optimal soil by root-soil interaction. Trends Plant Sci. 2017, 22, 823–829. [Google Scholar] [CrossRef]
- Erktan, A.; McCormack, M.L.; Roumet, C. Frontiers in root ecology: Recent advances and future challenges. Plant Soil 2018, 424, 1–9. [Google Scholar] [CrossRef]
- Jin, X.; Yang, G.; Tan, C.; Zhao, C. Effects of nitrogen stress on the photosynthetic CO2 assimilation, chlorophyll fluorescence and sugar-nitrogen ratio in corn. Sci. Rep. 2015, 5, 9311. [Google Scholar] [CrossRef]
- Bassi, D.; Menossi, M.; Mattiello, L. Nitrogen supply influences photosynthesis establishment along the sugarcane leaf. Sci. Rep. 2018, 8, 2327. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Chang. Biol. 2017, 23, 3513–3524. [Google Scholar]
- Busch, F.A. Photorespiration in the context of Rubisco biochemistry, CO2 diffusion and metabolism. Plant J. 2020, 101, 919–939. [Google Scholar] [CrossRef]
- Ansari, F.A.; Ahmad, I.; Pichtel, J. Growth stimulation and alleviation of salinity stress to wheat by the biofilm forming Bacillus pumilus strain FAB10. Appl. Soil Ecol. 2019, 143, 45–54. [Google Scholar]
- Galindo, F.S.; Teixeira Filho, M.C.M.; Buzetti, S.; Santini, J.M.K.; Ludkiewicz, M.G.Z.; Baggio, G. Modes of application of cobalt, molybdenum and Azospirillum brasilense on soybean yield and profitability. R. Bras. Eng. Agr. Amb. 2017, 21, 180–185. [Google Scholar] [CrossRef]
- Galindo, F.S.; Teixeira Filho, M.C.M.; Buzetti, S.; Ludkiewicz, M.G.Z.; Rosa, P.A.L.; Tritapepe, C.A. Technical and economic viability of co-inoculation with Azospirillum brasilense in soybean cultivars in the Cerrado. R. Bras. Eng. Agr. Amb. 2018, 22, 51–56. [Google Scholar] [CrossRef]
- Garcia, M.V.C.; Nogueira, M.A.; Hungria, M. Combining microorganisms in inoculants is agronomically important but industrially challenging: Case study of a composite inoculant containing Bradyrhizobium and Azospirillum for the soybean crop. AMB Express 2021, 11, 71. [Google Scholar] [CrossRef]
- Salvagiotti, F.; Cassman, K.G.; Specht, J.E.; Walters, D.T.; Weiss, A.; Dobermann, A. Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crops Res. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Santachiara, G.; Salvagiotti, F.; Rotundo, J.L. Nutritional and environmental effects on biological nitrogen fixation in soybean: A meta-analysis. Field Crops Res. 2019, 240, 106–115. [Google Scholar] [CrossRef]
- Streeter, J.G. Inhibition of legume nodule formation and N2 fixation by nitrate. Crit. Rev. Plant Sci. 1988, 7, 1–23. [Google Scholar] [CrossRef]
- Vessey, J.K.; Waterer, J. In search of the mechanism of nitrate inhibition of nitrogenase activity in legume nodules: Recent developments. Physiol. Plant. 1992, 84, 171–176. [Google Scholar] [CrossRef]
- Paulo, E.N.; Galindo, F.S.; Rabêlo, F.H.S.; Frazão, J.J.; Lavres, J. Nitrification inhibitor 3,4-Dimethylpyrazole phosphate improves nitrogen recovery and accumulation in cotton plants by reducing NO3- leaching under 15N-urea fertilization. Plant Soil 2021, 469, 259–272. [Google Scholar]
- Stougaard, J. Regulators and regulation of legume root nodule development. Plant Physiol. 2000, 124, 531–540. [Google Scholar]
- Barbosa, J.M.; Roberto, L.A.; Hungria, M.; Corrêa, R.S.; Magri, E.; Correia, T.D. Meta-analysis of maize responses to Azospirillum brasilense inoculation in Brazil: Benefits and lessons to improve inoculation efficiency. Appl. Soil Ecol. 2022, 170, 104276. [Google Scholar] [CrossRef]
- Soares, A.L.L.; Pereira, J.P.A.R.; Ferreira, P.A.A.; Vale, H.M.M.; Lima, A.S.; Andrade, M.J.B.; Moreira, F.M.S. Agronomic efficiency of selected rhizobia strains and diversity of native nodulating populations in perdões (MG–Brazil). I—Cowpea. R. Bras. Ciência Solo 2006, 30, 795–802, (In Portuguese, with English Abstract). [Google Scholar] [CrossRef]
- Ulzen, J.; Abaidoo, R.C.; Mensah, N.E.; Masso, C.; AbdelGadir, A.H. Bradyrhizobium inoculants enhance grain yields of soybean and cowpea in Northern Ghana. Front. Plant Sci. 2016, 7, 1770. [Google Scholar] [CrossRef] [PubMed]
- Brito, M.M.P.; Muraoka, T.; Silva, E.C. Contribuition of nitrogen from biological nitrogen fixation, nitrogen fertilizer and soil nitrogen on the growth of the common bean and cowpea. Bragantia 2011, 70, 206–215, (In Portuguese, with English Abstract). [Google Scholar] [CrossRef]
- Craswell, E.; Lefroy, R. The role and function of organic matter in tropical soils. Nutr. Cycl. Agroecosyst. 2001, 61, 7–18. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA, Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Van Raij, B.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Chemical Analysis for Fertility Assessment of Tropical Soils; IAC: Campinas, Brazil, 2001; p. 285. (In Portuguese) [Google Scholar]
- Bremner, J. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Andrade, D.S.; Hamakawa, P.J. Estimation of the number of viable rhizobia cells in soil and inoculants for plant infection. In Manual of Methods Used in Agricultural Microbiology Studies; Hungria, M., Araújo, R.S., Eds.; Embrapa-SPI: Brasília, Brazil, 1994; pp. 63–94. (In Portuguese) [Google Scholar]
- Zilli, J.E.; Neto, M.L.; França Júnior, I.; Perin, L.; De Melo, A.R. Cowpea response to inoculation with Bradyrhizobium strains recommended for soybean. R. Bras. Ciência Solo 2011, 35, 739–742, (In Portuguese, with English Abstract). [Google Scholar] [CrossRef][Green Version]
- Vincent, J.M. A Manual for the Practical Study of Root-Nodule Bacteria; IBP Handbook 15; Blackwell Scientific: Oxford, UK, 1970. [Google Scholar]
- Mendes, I.C.; Hungria, M.; Vargas, M.A.T. Establishment of Bradyrhizobium japonicum and B. elkanii strains in a Brazilian Cerrado oxisol. Biol. Fertil. Soils 2004, 40, 28–35. [Google Scholar] [CrossRef]
- Döbereiner, J.; Baldani, V.L.D.; Baldani, J.I. How to Isolate and Identify Diazotrophic Bacteria from Non-Leguminous Plants; Embrapa-SPI e Seropédica, Embrapa-CNPAB: Brasília, Brazil, 1995; 60p. (In Portuguese) [Google Scholar]
- Abdul Rahman, N.; Larbi, A.; Kotu, B.; Marthy Tetteh, F.; Hoeschle-Zeledon, I. Does nitrogen matter for legumes? Starter nitrogen effects on biological and economic benefits of cowpea (Vigna unguiculata L.) in Guinea and Sudan Savanna of West Africa. Agronomy 2018, 8, 120. [Google Scholar] [CrossRef]
- De Andrade Júnior, A.S.; Santos, A.A.; Sobrinhos, C.A.; Bastos, E.A.; De Melo, F.B.; Pinto Viana, F.M.; Freire Filho, F.R.; Da Carneiro, J.S.; Rocha, M.M.; Cardoso, M.J.; et al. Cowpea Cultivation Teresina: Embrapa Meio-Norte; Embrapa Meio-Norte, Sistemas de Produção, 2: Teresina, Brazil, 2002; 102p. (In Portuguese) [Google Scholar]
- Cantarella, H.; Trivelin, P.C.O. Determination of total nitrogen in soil. In Chemical Analysis for Fertility Assessment in Tropical Soils; Van Raij, B., Andrade, J.C., Cantarella, H., Quaggio, J.A., Eds.; Instituto Agronômico: Campinas, Brazil, 2001; pp. 262–269. (In Portuguese) [Google Scholar]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Evaluation of the Nutritional Status of Plants: Principles and Applications, 2nd ed.; Potafos: Piracicaba, Brazil, 1997; 319p. (In Portuguese) [Google Scholar]
- Bielesk, R.L.; Turner, N.A. Separation and estimation of amino acids in crude plant extracts by thin-layer electrophoresis and chromatography. Anal. Biochem. 1966, 17, 278–293. [Google Scholar] [CrossRef]
- Vogels, G.D.; Van Der Drift, C. Differential analyses of glyoxylate derivates. Anal. Biochem. 1970, 33, 143–157. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing principle of protein-dye-binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids pigments of photosynthetic biomembranes. Methods Enzymol. 1997, 148, 350–382. [Google Scholar]
- Barrie, A.; Prosser, S.J. Automated analysis of light-element stable isotopes by isotope ratio mass spectrometry. In Mass Spectrometry of Soils; Boutton, T.W., Yamasaki, S., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 1–46. [Google Scholar]
- Cabrera, M.L.; Kissel, D.E. Review and simplification of calculations in 15N tracer studies. Fertil. Res. 1989, 20, 11–15. [Google Scholar] [CrossRef]
- Högberg, P. Tansley Review No. 95. 15N natural abundance in soil-plant systems. New Phytol. 1997, 137, 179–203. [Google Scholar] [CrossRef] [PubMed]
- Trivelin, P.C.O.; Lara Cabezas, W.A.R.; Victoria, R.L.; Reichardt, K. Evaluation of a 15N plot design for estimating plant recovery of fertilizer nitrogen applied to sugarcane. Sci. Agric. 1994, 51, 226–234. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: https://www.R-project.org/ (accessed on 12 June 2022).

| Soil Chemical Attributes | 0–0.20 m Layer |
|---|---|
| Total N | 1.04 g kg−1 |
| P (resin) | 39.0 mg kg−1 |
| S (SO4) | 30.0 mg kg−1 |
| Organic matter | 21.0 g kg−1 |
| pH (CaCl2) | 5.1 |
| K | 2.3 mmolc kg−1 |
| Ca | 31.0 mmolc kg−1 |
| Mg | 33.0 mmolc kg−1 |
| H + Al | 34.0 mmolc kg−1 |
| Al | 0.0 mmolc kg−1 |
| B (hot water) | 0.23 mg kg−1 |
| Cu (DTPA) | 3.7 mg kg−1 |
| Fe (DTPA) | 25.0 mg kg−1 |
| Mn (DTPA) | 30.1 mg kg−1 |
| Zn (DTPA) | 1.7 mg kg−1 |
| Cation exchange capacity (pH 7.0) | 100.3 mmolc kg−1 |
| Base saturation (%) | 66 |
| Bradyrhizobia populations in soil | 3.5 × 104 cells g−1 soil |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galindo, F.S.; Pagliari, P.H.; da Silva, E.C.; Silva, V.M.; Fernandes, G.C.; Rodrigues, W.L.; Céu, E.G.O.; de Lima, B.H.; Jalal, A.; Muraoka, T.; et al. Co-Inoculation with Azospirillum brasilense and Bradyrhizobium sp. Enhances Nitrogen Uptake and Yield in Field-Grown Cowpea and Did Not Change N-Fertilizer Recovery. Plants 2022, 11, 1847. https://doi.org/10.3390/plants11141847
Galindo FS, Pagliari PH, da Silva EC, Silva VM, Fernandes GC, Rodrigues WL, Céu EGO, de Lima BH, Jalal A, Muraoka T, et al. Co-Inoculation with Azospirillum brasilense and Bradyrhizobium sp. Enhances Nitrogen Uptake and Yield in Field-Grown Cowpea and Did Not Change N-Fertilizer Recovery. Plants. 2022; 11(14):1847. https://doi.org/10.3390/plants11141847
Chicago/Turabian StyleGalindo, Fernando Shintate, Paulo Humberto Pagliari, Edson Cabral da Silva, Vinicius Martins Silva, Guilherme Carlos Fernandes, Willian Lima Rodrigues, Elaine Garcia Oliveira Céu, Bruno Horschut de Lima, Arshad Jalal, Takashi Muraoka, and et al. 2022. "Co-Inoculation with Azospirillum brasilense and Bradyrhizobium sp. Enhances Nitrogen Uptake and Yield in Field-Grown Cowpea and Did Not Change N-Fertilizer Recovery" Plants 11, no. 14: 1847. https://doi.org/10.3390/plants11141847
APA StyleGalindo, F. S., Pagliari, P. H., da Silva, E. C., Silva, V. M., Fernandes, G. C., Rodrigues, W. L., Céu, E. G. O., de Lima, B. H., Jalal, A., Muraoka, T., Buzetti, S., Lavres, J., & Teixeira Filho, M. C. M. (2022). Co-Inoculation with Azospirillum brasilense and Bradyrhizobium sp. Enhances Nitrogen Uptake and Yield in Field-Grown Cowpea and Did Not Change N-Fertilizer Recovery. Plants, 11(14), 1847. https://doi.org/10.3390/plants11141847










