A Novel Soy Isoflavone Derivative, 3′-Hydroxyglycitin, with Potent Antioxidant and Anti-α-Glucosidase Activity
Abstract
:1. Introduction
2. Results and Discussion
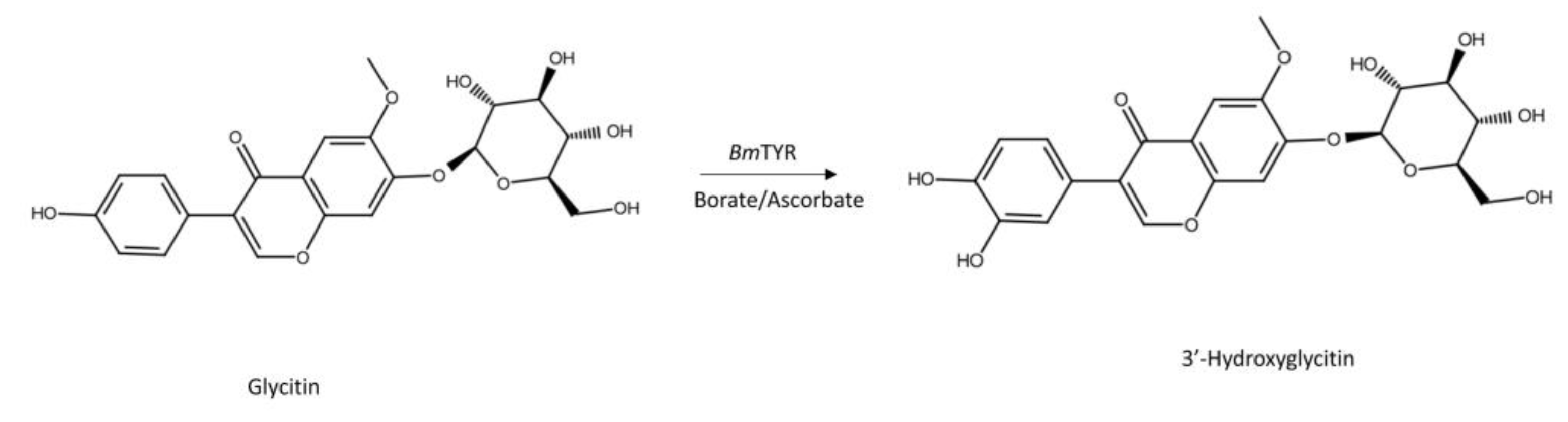
2.1. Biotransformation of Glycitin by BmTYR
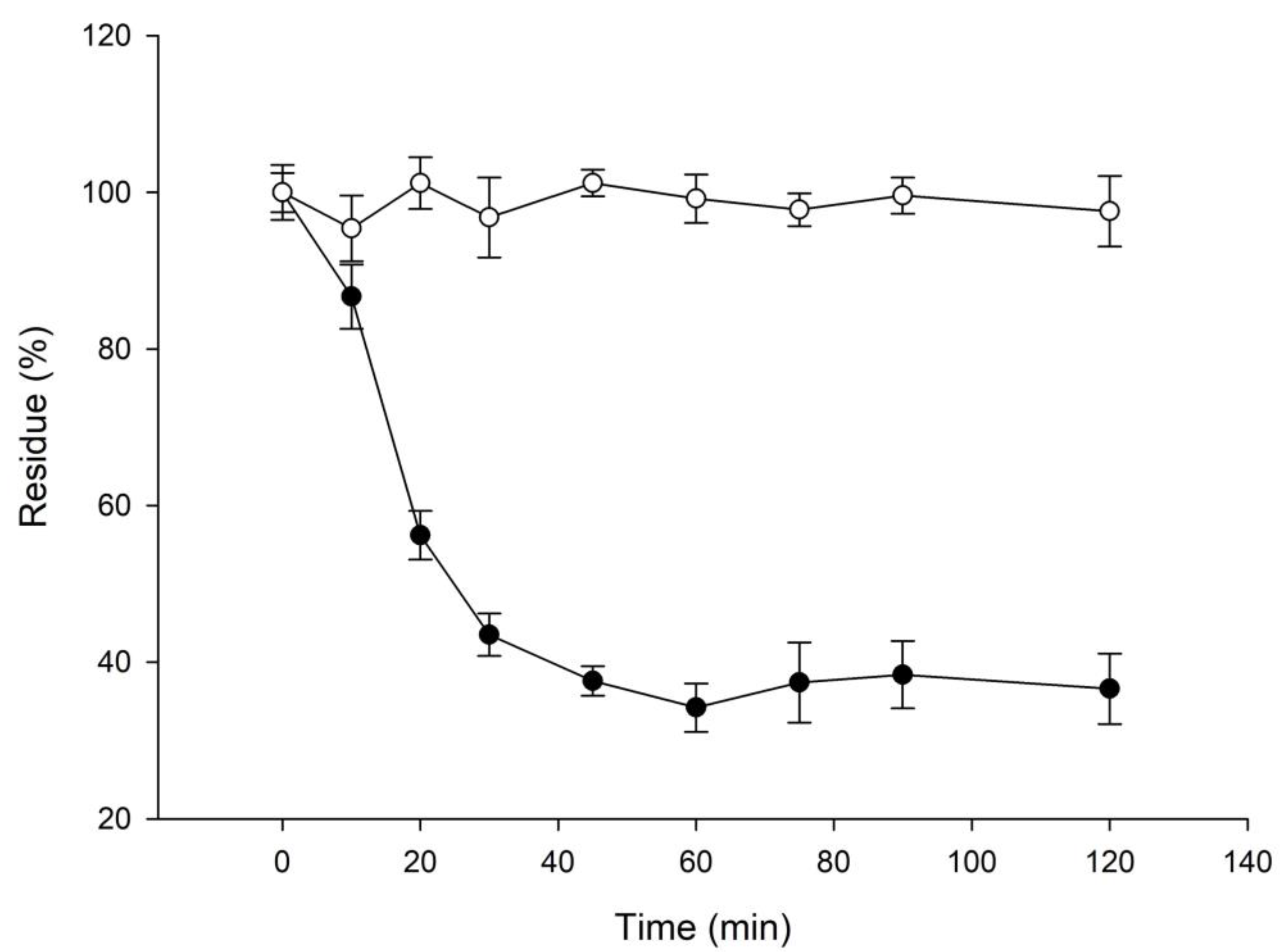
2.2. 3′-Hydroxylglycitin Possesses Potent Antioxidant Activity
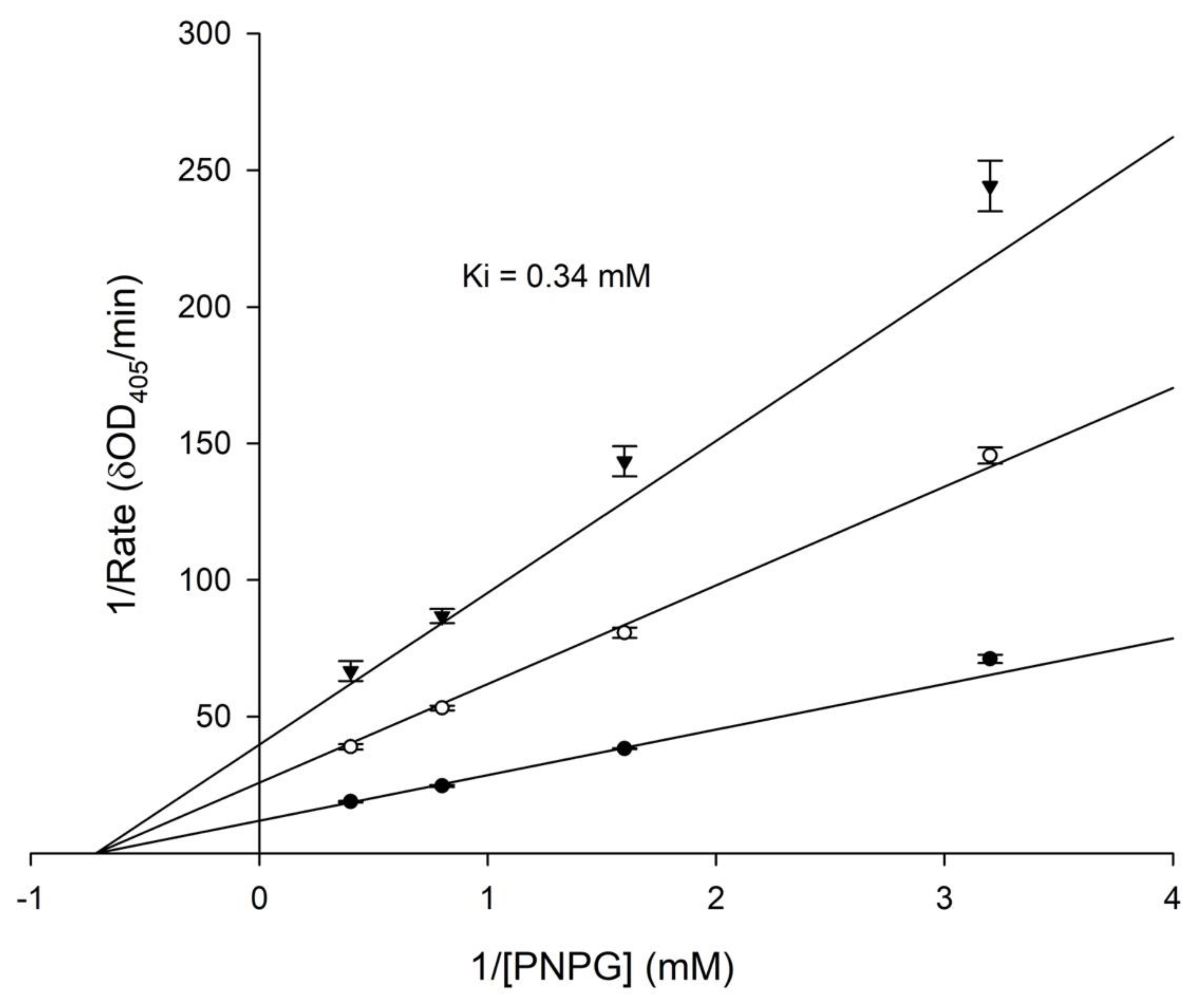
2.3. 3′-Hydroxylglycitin Possesses Potent Anti-α-Glucosidase Activity
3. Materials and Methods
3.1. Cells, Chemicals, and Microorganisms
3.2. Biotransformation Using BmTYR
3.3. HPLC Analysis
3.4. Purification and Identification of the Biotransformation Metabolite
3.5. Determination of Antiradical Activity Using a DPPH Assay
3.6. Determination of Stability
3.7. Determination of Anti-α-Glucosidase Activity
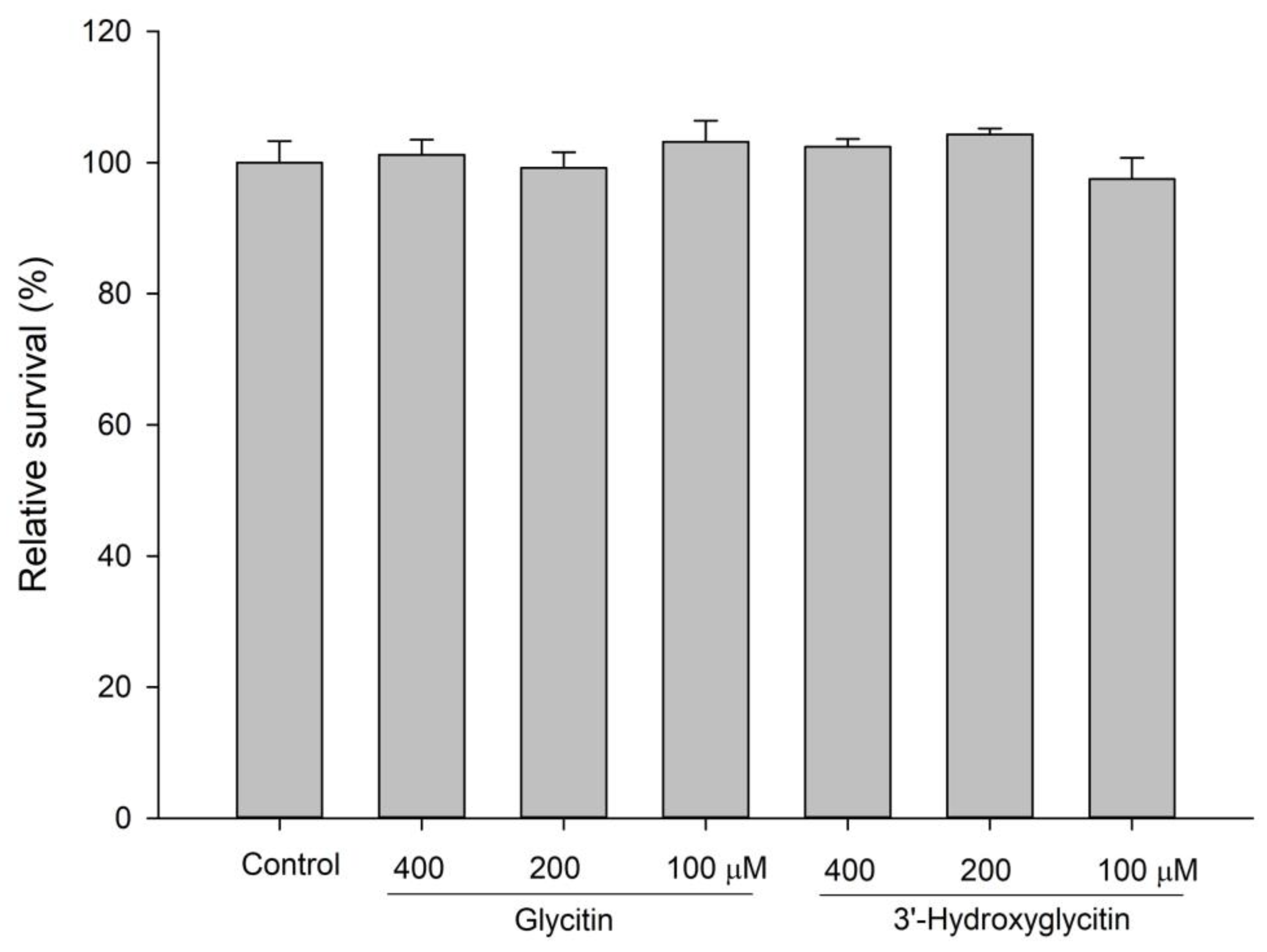
3.8. Cytotoxicity by Determination of Cell Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sohn, S.I.; Pandian, S.; Oh, Y.J.; Kang, H.J.; Cho, W.S.; Cho, Y.S. Metabolic engineering of isoflavones: An updated overview. Front. Plant. Sci. 2021, 12, 670103. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S. Current perspectives on the beneficial effects of soybean isoflavones and their metabolites for humans. Antioxidants 2021, 10, 1064. [Google Scholar] [CrossRef]
- Chang, T.S. Isolation, bioactivity, and production of ortho-hydroxydaidzein and ortho-hydroxygenistein. Int. J. Mol. Sci. 2014, 15, 5699–5716. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Baek, K.; Lee, J.E.; Kim, B.G. Using tyrosinase as a monophenol monooxygenase: A combined strategy for effective inhibition of melanin formation. Biotechnol. Bioeng. 2016, 113, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.M.; Wang, D.S.; Chang, T.S. Improving free radical scavenging activity of soy isoflavone glycosides daidzin and genistin by 3′-Hydroxylation Using Recombinant Escherichia coli. Molecules 2016, 21, 1723. [Google Scholar] [CrossRef] [PubMed]
- Sadegh, R.; Maryam, S.; Ali, M.; Mostafa, R.T.; Shokoofe, N. Anti-breast cancer activities of 8-hydroxydaidzein by targeting breast cancer stem-like cells. J. Pharm. Pharm. Sci. 2020, 32, 47–57. [Google Scholar]
- Lee, D.E.; Lee, K.W.; Byun, S.; Jung, S.K.; Song, N.; Lim, S.H.; Heo, Y.S.; Kim, J.E.; Kang, N.J.; Kim, B.Y.; et al. 7,3′,4′-Trihydroxyisoflavone, a metabolite of the soy isoflavone daidzein, suppresses ultraviolet B-induced skin cancer by targeting Cot and MKK4. J. Biol. Chem. 2011, 286, 14246–14256. [Google Scholar] [CrossRef]
- Goh, M.J.; Park, J.S.; Bae, J.H.; Kim, D.H.; Kim, H.K.; Na, Y.J. Effects of ortho-dihydroxyisoflavone derivatives from Korean fermented soybean paste on melanogenesis in B16 melanoma cells and human skin equivalents. Phytother Res. 2012, 26, 1107–1112. [Google Scholar] [CrossRef]
- Tai, S.S.K.; Lin, C.G.; Wu, M.H.; Chang, T.S. Evaluation of depigmenting activity by 8-hydroxydaidzein in mouse B16 melanoma cells and human volunteers. Int. J. Mol. Sci. 2009, 10, 4257–4266. [Google Scholar]
- Kim, E.; Kang, Y.G.; Kim, J.H.; Kim, Y.J.; Lee, T.R.; Lee, J.; Kim, D.; Cho, J.Y. The antioxidant and anti-Inflammatory activities of 8-hydroxydaidzein (8-HD) in activated macrophage—Like RAW264.7 Cells. Int. J. Mol. Sci 2018, 19, 1828. [Google Scholar]
- Wu, P.S.; Ding, H.Y.; Yen, J.H.; Chen, S.F.; Lee, K.H.; Wu, M.J. Anti-inflammatory activity of 8-hydroxydaidzein in LPS-stimulated BV2 microglial cells via activation of Nrf2-antioxidant and attenuation of Akt/NF-κB-inflammatory signaling pathways, as well as inhibition of COX-2 activity. J. Agric. Food Chem. 2018, 66, 5790–5801. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Morikawa, T.; Xu, F.; Ninomiya, K.; Yoshikawa, M. New isoflavones and pterocarpane with hepatoprotective activity from the stems of Erycibe expansa. Planta Med. 2004, 70, 1201–1209. [Google Scholar] [CrossRef]
- Tsuchihashi, R.; Kodera, M.; Sakamoto, S.; Nakajima, Y.; Yamazaki, T.; Niiho, Y.; Nohara, T.; Kinjo, J. Microbial transformation and bioconversion of isoflavones from Pueraria flowers by human intestinal bacterial strains. J. Nat. Med. 2009, 63, 254–260. [Google Scholar] [CrossRef]
- Tasdemir, D.; Kaiser, M.; Brun, R.; Yardley, V.; Schmidt, T.J.; Tosun, F.; Ruedi, P. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: In vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.M.; Werbovetz, K.A. Isoflavonoids and other compounds from Psorothamnus arborescens with antiprotozoal activities. J. Nat. Prod. 2006, 69, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Sohretoglu, D.; Sari, S. Flavonoids as alpha-glucosidase inhibitors: Mechanistic approaches merged with enzyme kinetics and molecular modelling. Phytochem. Rev. 2020, 19, 1081–1092. [Google Scholar] [CrossRef]
- Abbas, G.; Al Harrasi, A.; Hussain, H.; Hamaed, A.; Supuran, C.T. The management of diabetes mellitus-imperative role of natural products against dipeptidyl peptidase-4, alpha-glucosidase and sodium-dependent glucose co-transporter 2 (SGLT2). Bioorg. Chem. 2019, 86, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. Alpha-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S.; Chao, S.Y.; Chen, Y.C. Production of ortho-hydroxydaidzein derivatives by a recombinant strain of Pichia pastoris harboring a cytochrome P450 fusion gene. Process Biochem. 2013, 48, 426–429. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Ding, H.-Y.; Wang, T.-Y.; Cai, C.-Z.; Chang, T.-S. Application of biotransformation-guided purification in Chinese medicine: An example to produce butin from licorice. Catalysts 2022, 12, 718. [Google Scholar] [CrossRef]
- Treml, J.; Smejkal, K. Flavonoids as potent scavengers of hydroxyl radicals. Compr. Rev. Food. Sci. Food. Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-S.; Wang, T.-Y.; Yang, S.-Y.; Kao, Y.-H.; Wu, Y.-W.; Chiang, C.-M. Potential industrial production of a well-soluble, alkaline-stable, and anti-inflammatory isoflavone glucoside from 8-hydroxydaidzein glucosylated by recombinant amylosucrase of Deinococcus geothermalis. Molecules 2019, 24, 2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.-Y.; Wang, T.-Y.; Ding, H.-Y.; Lee, C.-C.; Chang, T.-S. A Novel Soy Isoflavone Derivative, 3′-Hydroxyglycitin, with Potent Antioxidant and Anti-α-Glucosidase Activity. Plants 2022, 11, 2202. https://doi.org/10.3390/plants11172202
Wu J-Y, Wang T-Y, Ding H-Y, Lee C-C, Chang T-S. A Novel Soy Isoflavone Derivative, 3′-Hydroxyglycitin, with Potent Antioxidant and Anti-α-Glucosidase Activity. Plants. 2022; 11(17):2202. https://doi.org/10.3390/plants11172202
Chicago/Turabian StyleWu, Jiumn-Yih, Tzi-Yuan Wang, Hsiou-Yu Ding, Chuan-Che Lee, and Te-Sheng Chang. 2022. "A Novel Soy Isoflavone Derivative, 3′-Hydroxyglycitin, with Potent Antioxidant and Anti-α-Glucosidase Activity" Plants 11, no. 17: 2202. https://doi.org/10.3390/plants11172202
APA StyleWu, J.-Y., Wang, T.-Y., Ding, H.-Y., Lee, C.-C., & Chang, T.-S. (2022). A Novel Soy Isoflavone Derivative, 3′-Hydroxyglycitin, with Potent Antioxidant and Anti-α-Glucosidase Activity. Plants, 11(17), 2202. https://doi.org/10.3390/plants11172202







